Abstract
In this review, we focus specifically on the role that the metalloproteinase, A Disintegrin and Metalloproteinase 17 [ADAM17] plays in the development and progression of the metabolic syndrome. There is a well-recognised link between the ADAM17 substrate tumour necrosis factor α (TNF-α) and obesity, inflammation and diabetes. In addition, knocking out ADAM17 in mice leads to an extremely lean phenotype. Importantly, ADAM17-deficient mice exhibit one of the most pronounced examples of hypermetabolism in rodents to date. It is vital to further understand the mechanistic role that ADAM17 plays in the metabolic syndrome. Such studies will demonstrate that ADAM17 is a valuable therapeutic target to treat obesity and diabetes.
Keywords: ADAM17, hypertension, metabolic syndromes, obesity, TACE, type 2 diabetes
Introduction
There is increasing evidence showing the link between metabolic syndrome and cardiovascular disease, chronic kidney disease, stroke and diabetes [1]. The metabolic syndrome is defined as a cluster of independent risk factors which coexist, leading to an increased risk of the above-mentioned diseases. Geographically, studies have shown varied levels of the prevalence of the metabolic syndrome, ranging from approximately 35% in the United States [2], 36% in Australia [3], up to 26% in Europe [1] and up to 37% in Asia [1].
There is a slightly different definition of metabolic syndrome between the NCEP ATPIII Criteria [4] and the World Health Organization (WHO) [5]. Although their criteria is very similar in many aspects, there are some slight differentiations based on what they believe to be the predominant causes of metabolic syndrome.
The NCEP [4] categorises an individual having metabolic syndrome when they have at least three out of five of the following markers:
-
1)
Waist circumference > 102 cm (40 inches) in males and >88 cm (35 inches) in females;
-
2)
Elevated triglycerides > 1.7 mmol/l (150mg/dl);
-
3)
Lowered high-density lipoprotein (HDL) cholesterol levels < 1.0 mmol/l (40 mg/dl) in males, <1.3 mmol/l (50 mg/dl) in females;
-
4)
Elevated fasting glucose > 5.6 mmol/l (100 mg/dl) (due to insulin resistance) and/or
-
5)
Elevated blood pressure > 130/85 mmHg.
WHO [4,5] categorises an individual as having metabolic syndrome when they have insulin resistance or diabetes, plus at least two out of five of the following markers:
-
1)
Waist/Hip ratio: >0.90 in males and >0.85 in females or body mass index (BMI) > 30 kg/m2;
-
2)
Elevated triglycerides > 1.7 mmol/l (150 mg/dl);
-
3)
Lowered HDL cholesterol levels < 0.9 mmol/l (35 mg/dl) in males, <1 mmol/l (39 mg/dl) in females;
-
4)
Elevated blood pressure > 140/90 mmHg;
-
5)
Microalbuminuria: Urinary albumin excretion rate ≥ 20 μg/min or albumin:creatinine ratio ≥ 30 mg/g.
Some lifestyle factors associated with metabolic syndrome include obesity, lack of physical activity and some genetic factors, such as mutations in genes regulating lipid metabolism [3].
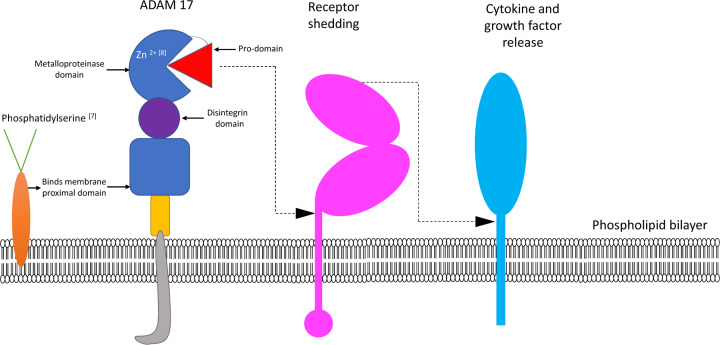
Obesity is a rapidly growing global pandemic which has almost tripled since 1975. As of 2016, more than 1.9 billion adults alone (>18 years) were overweight and of these, over 600 million were classified as obese [6]. Low-grade, chronic inflammation has been associated with the development of metabolic syndrome, mediated via activation of various cytokines. Here, we focus specifically on the role of the metalloproteinase A Disintegrin and Metalloproteinase 17 [ADAM17] (Figure 1) and how it may impact the metabolic syndrome.
Figure 1. Schematic representation of ADAM17-mediated shedding.
Phosphatidylserine transported to the outside of the membrane is required for ADAM17 activation [7]. ADAM enzymes are dependent on Zn2+ for activation and absence of Zn2+ renders the enzymes proteolytically inactive [8]. The function of ADAM17 includes shedding of receptors, growth factors and cytokines.
What is ADAM17?
To date, the mouse genome contains at least 34 Adam genes, while the human genome contains at least 27 Adam loci [9,10]. ADAM17 (also known as TACE or tumour necrosis factor α (TNF-α) converting enzyme), was first discovered by Black et al. in 1997 [11,12]. ADAM17 consists of numerous domains which include the pro-domain, metalloproteinase domain, disintegrin domain and membrane proximal domain. The pro-domain ensures that ADAM17 remains in an inactive state, until it is cleaved which thereby allows the metalloproteinase domain to become catalytically active [13]. Zinc binding to the metalloproteinase domain is also required for activity [8,14]. Interestingly, the disintegrin domain also possesses adhesive properties [14]. ADAM17 is a metalloproteinase that has been identified as the sheddase for a broad range of membrane-bound proteins expressed on numerous cell types including haematopoietic cells [15–17]. It plays a major role in chemokine/cytokine shedding, cell signalling, proliferation and growth [18]. As you will discover in this review, ADAM17 has both beneficial and detrimental effects. Although it has been shown to be beneficial for embryonic development, liver health and adipocyte differentiation, it is also implicated in the pathogenesis of many different diseases including, but not limited to cancer [19], heart disease [20], diabetes [21,22], rheumatoid arthritis [23] and Alzheimer’s disease [24]. In this review, we aim to highlight the impact of ADAM17 on the progression of Metabolic Syndrome.
Functional importance of ADAM17
Proteolysis usually occurs at the membrane-adjacent part of the substrate, many of which are receptors [25]. If the receptor shedding occurs before the ligand binding, the solubilised receptor can inhibit the ligand binding to cell surface receptors. This will be discussed in more detail later.
Although there are marked detrimental consequences of having excess amounts of ADAM17 activity, there is also a need for a balanced discussion on the important roles that it plays too.
Embryonic development
ADAM17 is particularly important for embryonic development as studies have shown that the embryos of ADAM17-deficient mice have defects in the mammary epithelium, vascular system, lung, eye, hair, heart and skin and therefore may die early on in pregnancy or even a few days after birth. Those mice that survived have reduced lymphocyte numbers, impaired T- and B-cell development and reduced body weight [8,26].
Liver health
The impact that ADAM17 has on liver health is controversial and it can act like ‘Jekyll and Hyde’ by playing both beneficial and detrimental roles in liver biology. Studies involving up-regulation of this metalloproteinase have been instrumental in highlighting that ADAM17 plays a major role in hepatosteatosis and liver inflammation, ultimately contributing to the development of metabolic syndrome [27]. However, there is also research indicating that ADAM17 plays a role in protecting hepatocytes from apoptosis in cases of drug-induced liver failure and that adenoviral delivery of ADAM17 prevented acetaminophen induced liver failure in a clinically relevant model of Fas-dependent fulminant hepatitis [28].
Adipocyte differentiation
ADAM17 may sometimes act like a ‘double-edged sword’ in relation to adipocyte differentiation. Although there are many studies demonstrating the detrimental effect of ADAM17 shedding on adiposity, one of its substrates, pre-adipocyte factor 1 (Pref-1) may be beneficial as discussed in more detail below. Interestingly, Pref-1 inhibits adipocyte differentiation [29].
Implications of ADAM17 in the metabolic syndrome
Obesity
ADAM17 was first identified as being responsible for shedding of the pro-inflammatory cytokine TNF-α [12]. There is a well-recognised link between TNF-α and obesity, inflammation and diabetes and an increased expression of TNF-α is found in the adipose tissue of obese and insulin-resistant animal and human models [30]. The TNF-α in human adipose tissue positively correlates with BMI, percentage of body fat and hyperinsulinaemia and studies have shown that weight loss decreases TNF-α levels [30].
Knocking out ADAM17 in mice leads to extremely lean animals. ADAM17-deficient mice exhibit one of the most pronounced examples of hypermetabolism reported in a rodent system to date. Elevated levels of uncoupling protein-1 in the brown adipose tissue of ADAM17-deficient mice compared with wildtype mice suggests that this lowered ADAM17 activity is linked to increased sympathetic outflow [31]. Interestingly, we have recently shown that sympathoexcitation in white adipose tissue is associated with beiging of adipose tissue [32].
In an independent study [33], high-fat diet (HFD) treated TaceMx1 mice (which have ADAM17 knocked out in haematopoietic cells) were found to have lower adipose tissue weights, systolic blood pressure, fasting glucose, fasting lipid levels and serum adiponectin levels. In addition, ADAM17 inactivation increased energy expenditure and oxidation of both fat and carbohydrate and improved glucose tolerance and insulin sensitivity when compared with the HFD wildtype (WT) mice.
Diabetes/insulin resistance
As mentioned previously, ADAM17 expression is significantly increased in the liver and adipose tissue of mice that have been fed HFD and it is positively associated with the development of insulin resistance and hepatosteatosis [33]. As increased ADAM17 expression is correlated with insulin resistance [30], it is likely that decreasing ADAM17 activity via various therapeutic strategies may increase insulin sensitivity and ultimately have a beneficial effect on obesity.
One study has highlighted that when ADAM17 is activated within the white adipocytes, it leads to the expression of inflammatory molecules such as Interleukin 6 (IL-6), Monocyte Chemotactic Protein 1 (MCP-1) and Suppressor of Cytokine Signalling 3 (SOCS3) [30,34]. This expression then leads to a low-grade inflammatory state that forces the macrophages to migrate into adipose tissue where they mediate enhanced insulin resistance [30].
Hypertension
Neurogenic hypertension is a form of high blood pressure which eventuates due to hyperactivation of the sympathetic nervous system. One mechanism by which neurogenic hypertension may occur is by ADAM17 mediated Angiotensin-converting enzyme type 2 (ACE2) shedding which results in loss of membrane-bound ACE2. This may promote high blood pressure as a consequence of a failure of ACE2 to convert angiotensin-II (vasoconstrictor) into angiotensin 1-7 (vasodilator) [35,36]. These findings are supported by studies highlighting that ADAM17 activation on glutamatergic neurons has been demonstrated to result in sympathoexcitation which may induce neurogenic hypertension [37].
Substrates for ADAM17 and implications for obesity and type 2 diabetes
Since the discovery of ADAM17, a vast array of proteins have been shown to be targets for shedding by this protease (Figure 2). It has been shown that the high glucose levels that exist during diabetes may be mediating increased expression of ADAM17 in cell types such as the mesangial cells [44] and therefore result in the subsequent cleavage of substrates. ADAM17 has also been shown to be governed by a number of cytokines. For instance IL-1β and TNFα may increase ADAM17 expression [45]. Additionally, external factors such as hypoxia have been shown to induce ADAM17 expression within human glioma cells by promoting Sp1 mediated transcription of the Adam17 gene [46]. The shedding event often promotes biological processes that may influence the metabolic syndrome. We will now discuss an important selection of ADAM17 substrates.
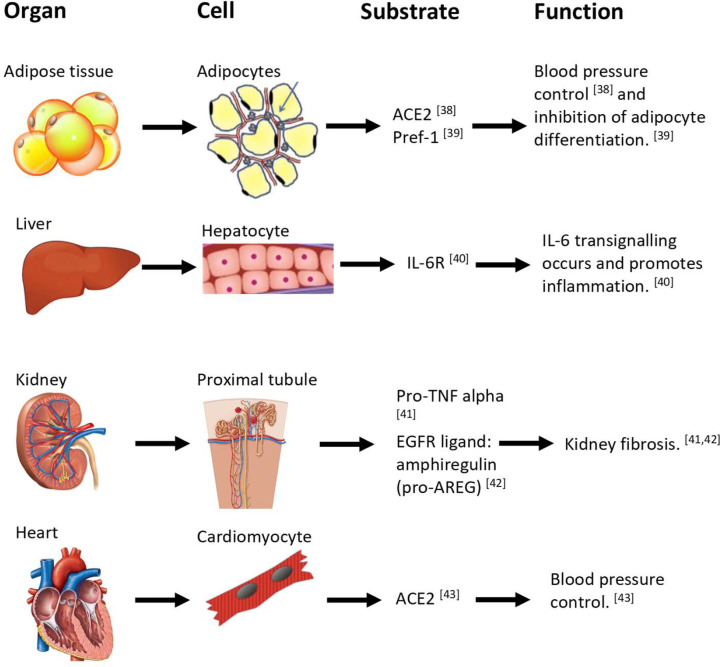
Figure 2. The effect of various ADAM17 substrates on bodily organs and cell tissue function.
ADAM17 shedding causes an alteration to homeostatic cell function due to the profound effects of ADAM17-mediated cleaved proteins [38–43] which circulate systemically and act at the molecular and cellular levels.
TNF-α
TNF-α was the first white adipose tissue derived inflammatory cytokine that was recognised to confer a link amongst obesity, inflammation and diabetes. It appears to be a crucial contributor to adipokine dysregulation in adipocytes [12,30]. Interestingly, ADAM17-mediated cleavage of TNF-α is implicated in both central and peripheral inflammation [18].
TNF-R1 and TNF-R2
The receptors for the cytokine TNF-α are TNF-R1 and TNF-R2. Both are shed by ADAM17 to release a soluble receptor which is between 30 and 40 kDa [47,48]. These soluble receptors act to inhibit the binding of circulating TNF-α to the membrane-bound TNF receptors [49,50].
Pre-adipocyte factor 1
As mentioned previously, ADAM17 may play a beneficial role in reducing adiposity as it is responsible for releasing Pref-1, which is known to inhibit adipose tissue differentiation. Pref-1 belongs to a ‘family of epidermal growth factor-like repeat containing proteins’ which are highly expressed in 3T3-L1 cells and it is reduced during adipocyte differentiation.
Although it is synthesised as a transmembrane protein, Pref-1 is processed to generate both a large 50-kDa soluble form, as well as small soluble forms. However, it is important to note that only the large soluble form is biologically active and inhibits adipogenesis. It is ADAM17 which releases this large 50-kDa soluble form. Mice lacking Pref-1 show accelerated fat deposition, but those mice that have overexpression of Pref-1 show reduced expression of adipocyte markers, as well as a decrease in fat mass [29].
IL-6R
IL-6 is a key regulator of a multitude of immune responses which range from bacterial infections to liver regeneration. ADAM17 is one of the metalloproteinases that mediates the release of the IL-6R from the cell membrane [51]. We and others have shown that when the 80-kDa membrane-bound form of the receptor is subjected to shedding by ADAM17, a 60-kDa agonistic soluble IL-6R (sIL-6R) is generated [52]. The process of sIL-6R binding to circulating IL-6 and then subsequently stimulating cells of the body is called trans-signalling [52]. Trans-signalling has been linked to cancer and we have also shown that trans-signalling promotes obesity-induced adipose tissue inflammation [53].
Epiderminal growth factor receptor ligands (in particular epiregulin)
A feedback signalling cascade known as the epiderminal growth factor receptor ligands (EGFR)/ADAM17 axis occurs through the shedding of the EGFR ligands by ADAM17. This cascade is particularly sensitive to external triggers such as cigarette smoke and bacterial toxins, resulting in increased shedding of many growth factors, cytokines and cytokine receptors which are all substrates of ADAM17 [54]. ADAM17 has been implicated in the shedding of the EGFR ligands TGF-α and HB-EGF. The other EGFR ligands amphiregulin and epiregulin have been identified as novel substrates of ADAM17 [55]. It was found that eNOS−/− db/db mice with advanced Diabetic Neuropathy who were treated with the EGFR inhibitor Erlotinib displayed decreases in fasting blood glucose levels, improved glucose tolerance and insulin sensitivity and lowered levels of adiponectin [56].
Fractalkine
Fractalkine (FKN) is the sole member of the CX3C chemokine family and is a potent chemoattractor for T cells, monocytes and natural killer cells. Although FKN is predominantly expressed in the epithelial cells, increased expression can also be seen in atherosclerotic lesions, psoriatic plaques and in human kidneys with glomerulonephritis. FKN cleavage occurs in response to inflammatory stimuli such as hypertension and diabetes, amongst other cardiovascular diseases. ADAM17 has been found to be responsible for the inducible cleavage of FKN. When FKN was transfected into host cells, inducible cleavage was blocked using the ADAM17 inhibitor TAPI-2 [57].
In human studies, plasma FKN levels were significantly higher in type 2 diabetes (T2D) patients when compared with non-diabetics and was found to correlate positively with many pro-inflammatory cytokines, including TNF-α [58]. It has been shown in human studies that those individuals with the highest FKN levels also have the highest BMI, Waist Circumference, Weight/Hip Ratio, % Fat, Blood Glucose, Insulin, HOMA-IR, Triglycerides and Total Cholesterol, as well as lowered HDL-c levels [59]. In murine studies [60], male C57BL/6 Cx3cr1−/− (FKN knockout) mice display improved glucose tolerance compared with WT mice, independent of obesity. The Cx3cr1−/− mice also possessed improved insulin sensitivity compared with the WT mice [60].
IL-1R
Interleukin 1 (IL-1α and IL-1β) are major proinflammatory cytokines which have metabolic consequences. IL-1β has been shown to promote β-cell destruction in Type 1 Diabetes [61] and increase insulin resistance. IL-1α has been shown to reduce insulin signalling, as well as increase plasma triglyceride levels [62]. In addition, IL-1β plays a role in T2D [63], by its involvement in the pathogenesis of insulin resistance and ultimately promoting islet cell death. The release of IL-1β from β-cells under metabolic stress and autocrine signalling via IL1R leads to NF-κB activation and subsequent synthesis and release of IL-1β and chemokines from β-cells. The latter promotes pancreatic immune cell infiltration and cytokine release from β-cells.
Interleukin 1 ligands also play an essential role in the regulation of innate immunity and they bind to two different receptors, IL-1R1 and IL-1R2. IL-1R1 is capable of transducing cellular signals due to its cytoplasmic domain, but IL-1R2 acts as a decoy receptor for IL-1 as it lacks this cytoplasmic domain [64]. ADAM17 indirectly enhances IL-1 signalling in cells by selectively cleaving the decoy receptor IL-1R2 and in turn promotes IL-1 binding the IL-1R1 which allows signalling [64]. By changing the balance between IL-1R1 and its decoy receptor IL-1R2, ADAM17 enhances sensitivity to IL-1.
Cofactors of ADAM17
Rhomboid protein
The Rhomboid Proteins (iRhoms), particularly iRhom 1 and 2 are a necessary component of ADAM17 biology. IRhom is a protease which is necessary for the maturation and trafficking of ADAM17 from the endoplasmic reticulum (ER) through the Golgi and absence of cellular iRhom will result in impaired exit of ADAM17 out of the ER. [65] The iRhoms play a number of different roles including intercellular signalling, mitochondrial dynamics, parasite invasion and protein quality control [66]. Both iRhom 1 and 2 are jointly responsible for all ADAM17 activity. Beyond this, iRhom 2 can actually control the substrate specificity of ADAM17 [66].
The iRhom 2 protein has been found to be increased in obese mice with adipose tissue inflammation. When iRhom 2 is knocked out, those mice fed upon HFD had a mitigation of obesity, insulin resistance and chronic adipose tissue inflammation in comparison with that in mice with iRhom 2 overexpression [67]. With metabolic disorder there is an up-regulation of iRhom2 in the macrophages which produces TNF-α production. The iRhom 2 in macrophages facilitates the trafficking of ADAM17 and thereby promotes inflammation [67].
ADAM17 inhibitors
Tissue inhibitor of metalloproteinase 3: the endogenous ADAM17 inhibitor
Tissue Inhibitor of Metalloproteinase 3 (TIMP-3) is the only known endogenous inhibitor of ADAM17. It has been found to control cytokine and growth factor bioavailability so as to regulate inflammation, cell death and survival in the liver [31]. While down-regulation of TIMP-3 increases ADAM17 activity, up-regulation of TIMP-3 conversely inhibits ADAM17 activity. TIMP-3 deficient mice have also been shown to possess a heightened level of inflammation and impaired glucose tolerance due to increased levels of TNF-α caused by uncontrolled shedding [31]. The inhibitor, TIMP-3 was found to be down-regulated in adipose tissue/obesity and this correlated with an increase in ADAM17 [31]. When coupled with insulin resistance, TIMP-3 down-regulation has also been found to accelerate liver inflammation and steatosis [31].
Exogenous ADAM17 inhibitors
ADAM17 has been found to be a promising therapeutic target for cancers of many tissues including breast, brain, colon, kidney, lung, liver, ovaries, pancreas and prostate. Thus far, exogenous ADAM17 inhibitors have been trialled in the setting of cancer.
Anti-ADAM17 antibody D1/GW280264X
ADAM17 is highly expressed in ovarian cancer cells. When ADAM17 was inhibited in ovarian cancer cell lines using either anti-ADAM17 antibody D1 or GW280264X, the cancer cells were sensitised to cisplatin-induced apoptosis, therefore significantly reducing cell viability [68].
TMI-005 (apratastat)
It has been found that ADAM17 can promote radiotherapy resistance in non-small-cell lung cancers. In in vitro studies, treating lung adenocarcinoma A549 cells with the ADAM17 inhibitor TMI-005, it was found that the inhibitor sensitised the tumorigenic cells to the radiotherapy. In murine studies, dual therapy with TMI-005 and radiotherapy prolonged survival in mice [69].
ZLDI-8
ZLDI-8 is one of the ADAM17 inhibitors that has been used to suppress the metastasis of Hepatocellular Carcinoma (HCC). It has been demonstrated that ZLDI-8 enhances the chemotherapeutic effects on tumor cell proliferation blockade, induction of apoptosis and cell cycle arrest by inhibiting the notch pathway and blocking chemical resistance [70].
TNF484
Aside from ZLDI-8, TNF484 is another ADAM17 inhibitor that has been shown to inhibit cell proliferation, migration and invasion of some HCC cell lines [71].
Although these exogenous ADAM17 inhibitors have been used mostly in the setting of cancer, it would be intriguing to assess their action in the setting of obesity and diabetes in animal models in the future.
Side effects of ADAM17 inhibitors
In human studies [72], the administration of ADAM17 inhibitors in the clinical setting has proven to effectively decrease inflammatory mediators without any known side effects. In other human studies, the inhibitor INCB7839, which was used to treat breast cancer, was discontinued due to it causing an increase in deep vein thrombosis in a number of patients [73]. There have also been side effects noted after using ADAM17 inhibitors, such as musculoskeletal and liver toxicity [73]. Further studies need to be conducted to discover other novel potential side effects.
Novel hypothesis: future research to unravel the role of ADAM17 in hyperglycaemia
Sodium Glucose Co-transporter 2 (SGLT2) helps to reabsorb up to 95% of glucose in the S1 and S2 segments of the proximal tubule. We hypothesise that in the setting of diabetes, an increase in ADAM17 elevates the renal sympathetic nervous system activity and in turn SGLT2 expression which promotes glucose reabsorption and hyperglycaemia. Glucose is an upstream mediator of hyperactivation of the sympathetic nervous system, which prevails in obesity and T2D [74]. It is interesting to note that glucose is also a stimulus for promoting expression of ADAM17 which contributes to the metabolic syndrome [55,75].
SGLT2 inhibition decreases glucose reabsorption, increases glucose excretion and is approved for use as a mode of treatment for diabetes [76]. The use of SGLT2 inhibitors has been shown to significantly reduce cardiovascular mortality and cardiovascular events [76]. Our team has shown that SGLT2 inhibition promotes sympathoinhibition and this may be a mechanism underlying cardiorenal benefits [77].
We anticipate that ADAM17 expression and activity may be reduced with SGLT2 inhibition which may decrease both sympathetic nervous system hyperactivation and hyperglycaemia.
We and others have conducted SGLT2 inhibition in mice [77]. We believe that SGLT2 inhibition should be conducted in diabetic mice and ADAM17 expression and activity should be further studied in this animal model.
Other ADAM family members and their involvement in the metabolic syndrome
Our group has previously sought to ascertain whether the metalloproteinases ADAM19 and ADAM28 correlate with parameters of the metabolic syndrome in mice and humans. We showed for the first time in humans that both ADAM19 and ADAM28 are strongly correlated with parameters of the metabolic syndrome, particularly BMI, relative fat and the index of insulin resistance (HOMA-IR) [78,79]. We also demonstrated in our diet-induced obesity mouse model that neutralising ADAM19 therapy results in weight loss and improves insulin sensitivity [78]. In addition, down-regulation of ADAM28 with siRNA technology resulted in a lack of weight gain, promotion of insulin sensitivity/glucose tolerance, decreased liver TNF-α levels and reduced blood urea nitrogen, alkaline phosphatase and aspartate aminotransferase in our diet-induced obesity mouse model. ADAM28 knockout mice also displayed reduced body weight, elevated HDL cholesterol levels and reduction in blood urea nitrogen, alkaline phosphatase and aspartate aminotransferase [80]. Therefore, neutralisation of ADAM19 and ADAM28 may be a potential therapeutic approach to treat obesity and T2D. Clinical trials should be conducted in humans using ADAM19 and ADAM28 inhibitors.
Conclusion
After considering our previous metabolic studies with regards to ADAM19 and ADAM28 and the fact that ADAM17 plays a multitude of roles in the pathogenesis of many diseases, it is vital to further understand the role ADAM17 plays in promoting features of the metabolic syndrome. Such studies will demonstrate that ADAM17 is a valuable therapeutic target to treat obesity and diabetes. It is highly likely that ADAM17, ADAM19 and ADAM28 work in concert to promote the metabolic syndrome.
Abbreviations
- ACE2
angiotensin-converting enzyme type 2
- ADAM17
a disintegrin and metalloproteinase 17
- BMI
body mass index
- FKN
Fractalkine
- HCC
hepatocellular carcinoma
- HDL
high-density lipoprotein
- HFD
high-fat diet
- IL-6
interleukin 6
- iRhom
Rhomboid protein
- Pref-1
pre-adipocyte factor 1
- SGLT2
sodium glucose co-transporter 2
- sIL-6R
soluble IL-6R
- TIMP-3
tissue inhibitor of metalloproteinase 3
- TNF-α
tumour necrosis factor α
- T2D
type 2 diabetes
- WHO
World Health Organization
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Open Access
Open access for this article was enabled by the participation of University of Western Australia in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with CAUL.
References
- 1.Sigit F.S., Tahapary D.L., Trompet S., Sartono E., Van Dijk K.W., Rosendaal F.R.et al. (2020) The prevalence of metabolic syndrome and its association with body fat distribution in middle-aged individuals from Indonesia and the Netherlands: a cross-sectional analysis of two population-based studies. Diabetol. Metab. Syndr. 12, 2 10.1186/s13098-019-0503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirode G. and Wong R.J. (2020) Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA 323, 2526–2528 10.1001/jama.2020.4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranasinghe P., Mathangasinghe Y., Jayawardena R., Hills A.P. and Misra A. (2017) Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: a systematic review. BMC Public Health 17, 101 10.1186/s12889-017-4041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy S., Brewer B., Cleeman J., Smith S. and Lenfant C. (2004) Definition of metabolic syndrome. Circulation 109, 433–438 10.1161/01.CIR.0000111245.75752.C6 [DOI] [PubMed] [Google Scholar]
- 5.Huang P. (2009) A comprehensive definition for metabolic syndrome. Dis. Model Mech. 2, 231–237 10.1242/dmm.001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skurski J., Penniman C.M., Geesala R., Dixit G., Pulipati P., Bhardwaj G.et al. (2020) Loss of iRhom2 accelerates fat gain and insulin resistance in diet-induced obesity despite reduced adipose tissue inflammation. Metabolism 106, 154–194 10.1016/j.metabol.2020.154194 [DOI] [PubMed] [Google Scholar]
- 7.Sommer A., Kordowski F., Büch J., Maretzky T., Evers A., Andrä J.et al. (2016) Phosphatidylserine exposure is required for ADAM17 sheddase function. Nat. Commun. 7, 11523 10.1038/ncomms11523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gooz M. (2010) ADAM-17: the enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 45, 146–169 10.3109/10409231003628015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho C. (2012) Testicular and epididymal ADAMs: expression and function during fertilization. Nat. Revol. Urol. 9, 550–560 10.1038/nrurol.2012.167 [DOI] [PubMed] [Google Scholar]
- 10.Bahudhanapati H., Bhattacharya S. and Wei S. (2015) Evolution of vertebrate adam genes; duplication of testicular adams from ancient adam9/9-like loci. PLoS ONE 10, e0136281 10.1371/journal.pone.0136281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black R.A., Rauch C.T., Kozlosky C.J., Peschon J.J., Slack J.L., Wolfson M.F.et al. (1997) A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385, 729–733 10.1038/385729a0 [DOI] [PubMed] [Google Scholar]
- 12.Seals D.F. and Courtneidge S.A. (2003) The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 17, 7–30 10.1101/gad.1039703 [DOI] [PubMed] [Google Scholar]
- 13.Lambrecht B.N., Vanderkerken M. and Hammad H. (2018) The emerging role of ADAM metalloproteinases in immunity. Nat. Rev. Immunol. 18, 745–758 10.1038/s41577-018-0068-5 [DOI] [PubMed] [Google Scholar]
- 14.Dreymueller D., Uhlig S. and Ludwig A. (2015) ADAM-family metalloproteinases in lung inflammation: potential therapeutic targets. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, 325–343 10.1152/ajplung.00294.2014 [DOI] [PubMed] [Google Scholar]
- 15.Peschon J.J., Slack J.L., Reddy P., Stocking K.L., Sunnarborg S.W., Lee D.C.et al. (1998) An essential role for ectodomain shedding in mammalian development. Science 282, 1281–1284 10.1126/science.282.5392.1281 [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Herrera A.H., Li Y., Belani K.K. and Walcheck B. (2009) Regulation of mature ADAM17 by redox agents for L-selectin shedding. J. Immunol. 182, 2449–2457 10.4049/jimmunol.0802770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condon T.P., Flournoy S., Sawyer G.J., Baker B.F., Kishimoto T.K. and Bennett C.F. (2001) ADAM17 but not ADAM10 mediates tumor necrosis factor-α and L-selectin shedding from leukocyte membranes. Antisense Nucleic Acid Drug Dev. 11, 107–116 10.1089/108729001750171353 [DOI] [PubMed] [Google Scholar]
- 18.de Queiroz T.M., Lakkappa N. and Lazartigues E. (2020) ADAM17-mediated shedding of inflammatory cytokines in hypertension. Front. Pharmacol. 11, 1154 10.3389/fphar.2020.01154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni P., Yu M., Zhang R., He M., Wang H., Chen S.et al. (2020) Prognostic significance of ADAM17 for gastric cancer survival: a meta-analysis. Medicina 56, 322 10.3390/medicina56070322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canault M., Leroyer A.S., Peiretti F., Leseche G., Tedgui A., Bonardo B.et al. (2007) Microparticles of human atherosclerotic plaques enhance the shedding of the tumor necrosis factor-α converting enzyme/ADAM17 substrates, tumor necrosis factor and tumor necrosis factor receptor-1. Am. J. Pathol. 171, 1713–1723 10.2353/ajpath.2007.070021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shalaby L., Thounaojam M., Tawfik A., Li J., Hussein K., Jahng W.J.et al. (2020) Role of endothelial ADAM17 in early vascular changes associated with diabetic retinopathy. J. Clin. Med. 9, 400 10.3390/jcm9020400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maekawa M., Tadaki H., Tomimoto D., Okuma C., Sano R., Ishii Y.et al. (2019) A novel TNF-α converting enzyme (TACE) selective inhibitor JTP-96193 prevents insulin resistance in KK-Ay Type 2 diabetic mice and diabetic peripheral neuropathy in type 1 diabetic mice. Biol. Pharm. Bull. 42, 1906–1912 10.1248/bpb.b19-00526 [DOI] [PubMed] [Google Scholar]
- 23.Ishii S., Isozaki T., Furuya H., Takeuchi H., Tsubokura Y., Inagaki K.et al. (2018) ADAM-17 is expressed on rheumatoid arthritis fibroblast-like synoviocytes and regulates proinflammatory mediator expression and monocyte adhesion. Arthritis Res. Ther. 20, 159 10.1186/s13075-018-1657-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartl D., May P., Gu W., Mayhaus M., Pichler S., Spaniol C.et al. (2020) A rare loss-of-function variant of ADAM17 is associated with late-onset familial Alzheimer disease. Mol. Psychiatry 25, 629–639 10.1038/s41380-018-0091-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crowe P.D., Walter B.N., Mohler K.M., Otten-Evans C., Black R.A. and Ware C.F. (1995) A metalloprotease inhibitor blocks shedding of the 80-kD TNF receptor and TNF processing in T lymphocytes. J. Exped. Med. 181, 1205–1210 10.1084/jem.181.3.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canault M., Certel K., Schatzberg D., Wagner D.D. and Hynes R.O. (2010) The lack of ADAM17 activity during embryonic development causes hemorrhage and impairs vessel formation. PLoS ONE 5, 133–134 10.1371/journal.pone.0013433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiorentino L., Vivanti A., Cavalera M., Marzano V., Ronci M., Fabrizi M.et al. (2010) Increased tumor necrosis factor α–converting enzyme activity induces insulin resistance and hepatosteatosis in mice. Hepatology 51, 103–110 10.1002/hep.23250 [DOI] [PubMed] [Google Scholar]
- 28.Murthy A., Defamie V., Smookler D.S., Di Grappa M.A., Horiuchi K., Federici M.et al. (2010) Ectodomain shedding of EGFR ligands and TNFR1 dictates hepatocyte apoptosis during fulminant hepatitis in mice. J. Clin. Invest. 120, 2731–2744 10.1172/JCI42686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Kim K.A., Kim J.H. and Sul H.S. (2006) Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J. Nutr. 136, 2953–2956 10.1093/jn/136.12.2953 [DOI] [PubMed] [Google Scholar]
- 30.Menghini R., Fiorentino L., Casagrande V., Lauro R. and Federici M. (2013) The role of ADAM17 in metabolic inflammation. Atherosclerosis 228, 12–17 10.1016/j.atherosclerosis.2013.01.024 [DOI] [PubMed] [Google Scholar]
- 31.Gelling R.W., Yan W., Al-Noori S., Pardini A., Morton G.J., Ogimoto K.et al. (2008) Deficiency of TNFα converting enzyme (TACE/ADAM17) causes a lean, hypermetabolic phenotype in mice. Endocrinology 149, 6053–6064 10.1210/en.2008-0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews J.R., Herat L.Y., Magno A.L., Gorman S., Schlaich M.P. and Matthews V.B. (2020) Sglt2 inhibitor-induced sympathoexcitation in white adipose tissue: a novel mechanism for beiging. Biomedicines 8, 514 10.3390/biomedicines8110514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaneko H., Anzai T., Horiuchi K., Morimoto K., Anzai A., Nagai T.et al. (2011) Tumor necrosis factor-α converting enzyme inactivation ameliorates high-fat diet-induced insulin resistance and altered energy homeostasis. Circ. J. 75, 2482–2490 10.1253/circj.CJ-11-0182 [DOI] [PubMed] [Google Scholar]
- 34.Serino M., Menghini R., Fiorentino L., Amoruso R., Mauriello A., Lauro D.et al. (2007) Mice heterozygous for tumor necrosis factor-α converting enzyme are protected from obesity-induced insulin resistance and diabetes. Diabetes 56, 2541–2546 10.2337/db07-0360 [DOI] [PubMed] [Google Scholar]
- 35.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J.et al. (2020) Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 126, 1456–1474 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia H., Sriramula S., Chhabra K.H. and Lazartigues E. (2013) Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ. Res. 113, 1087–1096 10.1161/CIRCRESAHA.113.301811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J., Molinas A.J.R., Mukerjee S., Morgan D.A., Rahmouni K., Zsombok A.et al. (2020) Activation of ADAM17 (A Disintegrin and Metalloproteinase 17) on glutamatergic neurons selectively promotes sympathoexcitation. Hypertension 73, 1266–1274 10.1161/HYPERTENSIONAHA.119.12832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupte M., Boustany-Kari C.M., Bharadwaj K., Police S., Thatcher S., Gong M.C.et al. (2008) ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am. J. Physiol. Regul. Integr. Com. Physiol. 295, 781–788 10.1152/ajpregu.00183.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y. and Sul H.S. (2006) Ectodomain shedding of preadipocyte factor 1 (Pref-1) by tumor necrosis factor alpha converting enzyme (TACE) and inhibition of adipocyte differentiation. Mol. Cell. Biol. 26, 5421–5435 10.1128/MCB.02437-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt-Arras D. and Rose-John S. (2016) IL-6 pathway in the liver: from physiopathology to therapy. J. Hepatol. 64, 1403–1415 10.1016/j.jhep.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 41.Palau V., Pascual J., Soler M.J. and Riera M. (2019) Role of ADAM17 in kidney disease. Am. J. Physiol. Renal Physiol. 317, 333–342 10.1152/ajprenal.00625.2018 [DOI] [PubMed] [Google Scholar]
- 42.Kefaloyianni E., Muthu M.L., Kaeppler J., Sun X., Sabbisetti V., Chalaris A.et al. (2016) ADAM17 substrate release in proximal tubule drives kidney fibrosis. JCI Insight 1, e87023 10.1172/jci.insight.87023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R.et al. (2014) Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J. Mol. Cell Cardiol. 66, 167–176 10.1016/j.yjmcc.2013.11.017 [DOI] [PubMed] [Google Scholar]
- 44.Li R., Uttarwar L., Gao B., Charbonneau M., Shi Y., Chan J.S.et al. (2015) High glucose up-regulates ADAM17 through HIF-1α in mesangial cells. J. Biol. Chem. 290, 21603–21614 10.1074/jbc.M115.651604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wawro K., Wawro M., Strzelecka M., Czarnek M. and Bereta J. (2019) The role of NF-κB and Elk-1 in the regulation of mouse ADAM17 expression. Biol. Open 8, bio039420 10.1242/bio.039420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szalad A., Katakowski M., Zheng X., Jiang F. and Chopp M. (2009) Transcription factor Sp1 induces ADAM17 and contributes to tumor cell invasiveness under hypoxia. J. Exp. Clin. Cancer Res. 28, 10.1186/1756-9966-28-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawari F.I., Rouhani F.N., Cui X., Yu Z.X., Buckley C., Kaler M.et al. (2004) Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc. Natl. Acad. Sci. U.S.A. 101, 1297–1302 10.1073/pnas.0307981100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pennica D., Lam V.T., Mize N.K., Weber R.F., Lewis M., Fendly B.M.et al. (1992) Biochemical properties of the 75-kDa tumor necrosis factor receptor. Characterization of ligand binding, internalization, and receptor phosphorylation. J. Biol. Chem. 267, 21172–21178 10.1016/S0021-9258(19)36813-9 [DOI] [PubMed] [Google Scholar]
- 49.Düzgün N., Ayaşlioğlu E., Tutkak H. and Aydintuğ O.T. (2005) Cytokine inhibitors: soluble tumor necrosis factor receptor 1 and interleukin-1 receptor antagonist in Behçet’s disease. Rheumatol. Int. 25, 1–5 10.1007/s00296-003-0400-6 [DOI] [PubMed] [Google Scholar]
- 50.DeBerge M.P., Ely K.H., Wright P.F., Thorp E.B. and Enelow R.I. (2015) Shedding of TNF receptor 2 by effector CD8+ T cells by ADAM17 is important for regulating TNF-α availability during influenza infection. J. Leukoc. Biol. 98, 423–434 10.1189/jlb.3A0914-432RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chalaris A., Gewiese J., Paliga K., Fleig L., Schneede A., Krieger K.et al. (2010) ADAM17-mediated shedding of the IL6R induces cleavage of the membrane stub by γ-secretase. Biochim. Biophys. Acta 1803, 234–245 10.1016/j.bbamcr.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 52.Matthews V., Schuster B., Schütze S., Bussmeyer I., Ludwig A., Hundhausen C.et al. (2003) Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE). J. Biol. Chem. 278, 38829–38839 10.1074/jbc.M210584200 [DOI] [PubMed] [Google Scholar]
- 53.Kraakman M.J., Kammoun H.L., Allen T.L., Deswaerte V., Henstridge D.C., Estevez E.et al. (2015) Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metabol. 21, 403–416 10.1016/j.cmet.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 54.Stolarczyk M. and Scholte B.J. (2018) The EGFR-ADAM17 axis in chronic obstructive pulmonary disease and cystic fibrosis lung pathology. Mediators Inflamm. 2018, 1067134 10.1155/2018/1067134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahin U., Weskamp G., Kelly K., Zhou H.M., Higashiyama S., Peschon J.et al. (2004) Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 164, 769–779 10.1083/jcb.200307137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z., Li Y., Overstreet J., Chung S., Niu A., Fan X.et al. (2018) Inhibition of epidermal growth factor receptor activation is associated with improved diabetic nephropathy and insulin resistance in type 2 diabetes. Diabetes 67, 1847–1857 10.2337/db17-1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garton K.J., Gough P.J., Blobel C.P., Murphy G., Greaves D.R., Dempsey P.J.et al. (2001) Tumor necrosis factor-α-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J. Biol. Chem. 276, 37993–38001 10.1074/jbc.M106434200 [DOI] [PubMed] [Google Scholar]
- 58.Shah R., Hinkle C., Ferguson J., Mehta N., Li M., Qu L.et al. (2011) Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes 60, 1512–1518 10.2337/db10-0956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xueyao Y., Saifei Z., Dan Y., Qianqian P., Xuehong D., Jiaqiang Z.et al. (2014) Circulating fractalkine levels predict the development of the metabolic syndrome. Int. J. Endocrinol. 2014, Article ID 715148 10.1155/2014/715148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah R., O’Neill S., Hinkle C., Caughey J., Stephan S., Lynch E.et al. (2015) Metabolic effects of CX3CR1 deficiency in diet-induced obese mice. PLoS ONE 10, e0138317 10.1371/journal.pone.0138317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perrier S., Darakhshan F. and Hajduch E. (2006) IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde. FEBS Lett. 580, 6289–6294 10.1016/j.febslet.2006.10.061 [DOI] [PubMed] [Google Scholar]
- 62.Ballak D., Stienstra R., Tack C., Dinarello C. and van Diepen J. (2015) IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine 75, 280–290 10.1016/j.cyto.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez L., Garrie K. and Turner M. (2018) Type 2 diabetes – an autoinflammatory disease driven by metabolic stress. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 3805–3823 10.1016/j.bbadis.2018.08.034 [DOI] [PubMed] [Google Scholar]
- 64.Uchikawa S., Yoda M., Tohmonda T., Kanaji A., Matsumoto M., Toyama Y.et al. (2015) ADAM17 regulates IL-1 signaling by selectively releasing IL-1 receptor type 2 from the cell surface. Cytokine 71, 238–245 10.1016/j.cyto.2014.10.032 [DOI] [PubMed] [Google Scholar]
- 65.Düsterhöft S., Babendreyer A., Giese A.A., Flasshove C. and Ludwig A. (2019) Status update on iRhom and ADAM17: It's still complicated. Biochim. Biophys. Acta Mol. Cell. Res. 1866, 1567–1583 10.1016/j.bbamcr.2019.06.017 [DOI] [PubMed] [Google Scholar]
- 66.Dulloo I., Muliyil S. and Freeman M. (2019) The molecular, cellular and pathophysiological roles of iRhom pseudoproteases. Open Biol. 9, 190003 10.1098/rsob.190003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minxuan X., Chenxu G., Yuting Q., Deshuai L., Qiang L., Jing F.et al. (2019) iRhom2 serves as a facilitator in obesity by enhancing adipose inflammation and insulin resistance. bioRxiv 10.1101/600460 [DOI] [Google Scholar]
- 68.Hedemann N., Rogmans C., Sebens S., Wesch D., Reichert M., Schmidt-Arras D.et al. (2018) ADAM17 inhibition enhances platinum efficiency in ovarian cancer. Oncotarget 9, 16043–16058 10.18632/oncotarget.24682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharma A., Bender S., Zimmermann M., Riesterer O., Broggini-Tenzer A. and Pruschy M.N. (2016) Secretome signature identifies ADAM17 as novel target for radiosensitization of non–small cell lung cancer. Clin. Cancer Res. 22, 4428–4439 10.1158/1078-0432.CCR-15-2449 [DOI] [PubMed] [Google Scholar]
- 70.Lu H.Y., Zu Y.X., Jiang X.W., Sun X.T., Liu T.Y., Li R.L.et al. (2019) Novel ADAM-17 inhibitor ZLDI-8 inhibits the proliferation and metastasis of chemo-resistant non-small-cell lung cancer by reversing Notch and epithelial mesenchymal transition in vitro and in vivo. Pharmacol. Res. 148, 104406 10.1016/j.phrs.2019.104406 [DOI] [PubMed] [Google Scholar]
- 71.Xia C., Zhang D., Li Y., Chen J., Zhou H., Nie L.et al. (2019) Inhibition of hepatocellular carcinoma cell proliferation, migration, and invasion by a disintegrin and metalloproteinase-17 inhibitor TNF484. J. Res. Med. Sci. 24, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qian M., Bai S., Brogdon B., Wu J., Liu R., Covington M.et al. (2007) Pharmacokinetics and pharmacodynamics of DPC 333 ((2R)-2-((3R)-3-amino-3{4-[2-methyl-4-quinolinyl) methoxy] phenyl}-2-oxopyrrolidinyl)-N-hydroxy-4-methylpentanamide)), a potent and selective inhibitor of tumor necrosis factor alpha-converting enzyme in rodents, dogs, chimpanzees, and humans. Drug Metab. Dispos. 35, 1916–1925 10.1124/dmd.107.015933 [DOI] [PubMed] [Google Scholar]
- 73.Moss M. and Minond D. (2017) Recent advances in ADAM17 research: a promising target for cancer and inflammation. Mediators Inflamm. 2017, 9673537 10.1155/2017/9673537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li R., Uttarwar L., Gao B., Charbonneau M., Shi Y., Chan J.S.et al. (2015) High glucose up-regulates ADAM17 through HIF-1α in mesangial cells. J. Biol. Chem. 290, 21603–21614 10.1074/jbc.M115.651604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Araujo Silva T.G., Castorena-Gonzalez J., Restaino R.M., Foote C.A., Morales-Quinones M., Wheeler A.A.et al. (2019) ADAM17 cleaves the insulin receptor α-subunit on endothelial cells and induces vascular insulin resistance in type 2 diabetes. FASEB J. 33, 685–687 [Google Scholar]
- 76.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S.et al. (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 77.Herat L.Y., Magno A.L., Rudnicka C., Hricova J., Carnagarin R., Ward N.C.et al. (2020) SGLT2 inhibitor–induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC 5, 169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weerasekera L., Rudnicka C., Sang Q.X., Curran J.E., Johnson M.P., Moses E.K.et al. (2017) ADAM19: a novel target for metabolic syndrome in humans and mice. Mediators Inflamm. 2017, 7281986 10.1155/2017/7281986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jowett J.B., Okada Y., Leedman P.J., Curran J.E., Johnson M.P., Moses E.K.et al. (2012) ADAM28 is elevated in humans with the metabolic syndrome and is a novel sheddase of human tumour necrosis factor-α. Immunol. Cell Biol. 90, 966–973 10.1038/icb.2012.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herat L., Rudnicka C., Okada Y., Mochizuki S., Schlaich M. and Matthews V. (2017) The metalloproteinase ADAM28 promotes metabolic dysfunction in mice. Int. J. Mol. Sci. 18, 884 10.3390/ijms18040884 [DOI] [PMC free article] [PubMed] [Google Scholar]