Abstract
Background:
Identifying digital biomarkers of mobility is important for clinical trials in Parkinson’s disease (PD).
Objective:
To determine which digital outcome measures of mobility discriminate mobility in people with PD from healthy control (HC) subjects over a week of continuous monitoring.
Methods:
We recruited 29 people with PD, and 27 age-matched HC subjects. Subjects were asked to wear three inertial sensors (Opal by APDM) attached to both feet and to the lumbar region, and a subset of subjects also wore two wrist sensors, for a week of continuous monitoring. We derived 43 digital outcome measures of mobility grouped into five domains. An Area Under Curve (AUC) was calculated for each digital outcome measures of mobility, and logistic regression employing a ‘best subsets selection strategy’ was used to find combinations of measures that discriminated mobility in PD from HC.
Results:
Duration of recordings was 66±14 hours in the PD and 59±16 hours in the HC. Out of a total of 43 digital outcome measures of mobility, we found six digital outcome measures of mobility with AUC>0.80. Turn angle (AUC=0.89, 95% CI: 0.79–0.97) and swing time variability (AUC=0.87, 95% CI: 0.75–0.96) were the most discriminative individual measures. Turning measures were most consistently selected via the best subsets strategy to discriminate people with PD from HC, followed by gait variability measures.
Conclusion:
Clinical studies and clinical practice with digital biomarkers of daily life mobility in PD should include turning and variability measures.
Keywords: Parkinson’s disease, digital outcome measures of mobility, inertial sensors, biomarkers, continuous monitoring
INTRODUCTION
Digital biomarkers are characteristics that can be reliably collected, measured, and quantified by means of digital devices (such as wearable, inertial sensors) as indicators of a biological process [1]. The advantages of digital devices for ambulatory activity in daily life include: 1) frequent and unobtrusive measurement in passive monitoring conditions (i.e., measurements from activity of daily living) to objectively quantify and/or predict health-related outcomes with greater sensitivity [2, 3]; 2) surrogate endpoints for testing the efficacy of the new treatments [1]; 3) improved clinical decision making and help detecting presymptomatic disease [1]; 4) help in monitoring existing treatment [3], and 5) modernized clinical trial endpoints [3].
Identifying digital biomarkers is very important as objective endpoints to reduce the size, length, and overall cost of clinical trials focused on improving mobility. Comparing a digital biomarker with normative or baseline measurements may be used to predict, assist diagnosis, or monitor a disease state and effect of treatment or drug dosage. Currently, clinical trials in Parkinson’s disease (PD) depend primarily on the Unified Parkinson’s Disease Rating Scale (UPDRS) [4] or subjective patient-reported outcomes, such as diaries or questionnaires to determine a change in mobility [1]. In addition, the standard neurological examination of mobility in the clinic (e.g., PIGD subset of 4 items in the Motor Part III of the MDS-UPDRS) is subjective, coarse and depends upon the expertise of a movement disorder specialist. The key challenge in the clinic is that gait impairments may not be observable in a small examination room or during short walks when all attention is focused on walking [5]. Also, subjects may perform better in the clinic while being observed (white coat effect) than at home. Finally, the clinic observation at one-time point misses the daily fluctuations (variability) of mobility in response to medications, fatigue, and other contexts. Hence, there is an unmet need to investigate digital biomarkers that can objectively and continuously monitor mobility during daily life. Continuous, passive monitoring of mobility during daily life can be used to quantify mobility impairments and potentially be used to detect subtle changes that may not be apparent to a clinical observer [5].
Recently, the use of wearable sensors has made it possible to quantify mobility outside the clinic and during real-life for multiple days at a time [3, 6–14]. Various researchers have shown that wearable sensors can be used to augment the standard clinical assessment in PD based on active (such as performing prescribed/predefined tests at home) and passive monitoring [15]. The downside of active monitoring is that it puts an extra burden on patients to set up a reminder for the timing of each prescribed task and repeat the same specific task several times to re-evaluate the condition. To avoid the extra burden on subjects, in this study we derived digital outcome measures of mobility from passive monitoring from natural walking and turning during unrestricted daily activities.
Although there are many advantages of using wearable sensors for passive monitoring, a key challenge is that using wearable sensors to monitor mobility in daily life can result in hundreds of different measures with no consensus on the measures most sensitive: i) to PD, ii) to fluctuations due to medications, or iii) to specific interventions [11]. Therefore, determining which objective measures reflect most mobility impairments in PD compared to healthy control (HC) subjects could be of extreme importance to inform clinical trials for new interventions, track mobility changes over time, and more sensitively monitor the response to therapy [11, 16, 17].
Walking and turning impairments are among the most incapacitating symptoms of PD [18–20], and are very important contributors to falls [2, 21–24] and reduced quality of life [25]. However, quantification of quality of walking and turning results in many potential biomarkers for mobility in daily life.
The objective of the present study was to investigate which specific digital outcome measures of mobility discriminated PD from the HC group. Specifically, turning in PD is characterized by longer duration, larger number of steps to complete the turn, and slower speed compared to HC, as shown both in the laboratory [26, 27] and at home [10]. Gait variability quantifies the lack of regularity of stepping. Stride time variability has been shown to be associated with fall risk and PD [7, 28–30]. Arm swing measures captured during a 7-meter TUG test in the laboratory were found to be most sensitive in early PD compared to HC [31]. Thus, we hypothesized that turning, gait variability, and arm swing would discriminate PD from HC during daily life.
METHODS
Participants
Twenty-nine subjects with mild to moderate PD (age: 67.66±5.27 years, UPDRS part III total tested ON medication: 34.66±11.02) and 27 age-matched HC subjects (age: 64.44±7.52 years) participated in the study. Both groups had similar height (PD: 67.66±3.57, HC: 67.67±4.08; p = 0.99), weight (PD: 167.45±28.08, HC: 158.00±24.17; p = 0.18) and gender (PD: 17 M and 12 F, HC: 13 M and 14 F; p = 0.43). Inclusion criteria for PD were a diagnosis of idiopathic Parkinson’s disease treated with levodopa (Hoehn and Yahr stage I (1), II (26), III (1), and IV (1)) by a movement disorders specialist. Exclusion criteria for all the participants were dementia, other factors affecting gait such as hip replacement, musculoskeletal disorders, uncorrected vision or vestibular problems, or inability to stand or walk in the home without an assistive device. The experimental protocol used here was approved by the Ethical Committee of the Oregon Health & Science University. All the participants provided informed written consent.
Data collection
Subjects were asked to wear a minimum of three inertial sensors (Opals by APDM, Inc., Portland, OR, USA), one on each foot and one over the lumbar area, and optional sensors on each wrist, for a week of continuous monitoring for at least 8 hours/day. Each Opal sensor includes tri-axial accelerometer, gyroscope, and magnetometer and was configured to sample at a rate of 128Hz. The Opal is lightweight (22g), has a battery life of 16h, and includes 8 GB of storage, which can record over 30 days of data. Subjects removed the sensors at night and placed them in a charging station. Data were stored in the internal memory of the Opals. Subjects mailed back the sensors using a pre-paid box after completion of one week of data collection. Data were uploaded offline to a cloud service upon return of the devices and downloaded to a local computer for further processing.
Self-reported fall history based on the prior six months was collected for each of the subjects and categorized people with PD into non-faller (zero falls in the past six months) or faller (one or more falls in the past six months) groups.
Digital outcome measures of mobility
At the first stage of processing, our algorithm searches for possible bouts of walking using a time domain approach to inertial sensor data from the feet. This stage identifies sequences of stillness, movement, and stillness that correspond to the stance, swing, and stance phases of gait based on both the accelerometer and gyroscope magnitudes. Individual steps are combined into potential bouts of walking, as long as the duration from one step to the next step is no longer than 2.5 seconds. Each possible bout that contains at least three steps and is at least 3 seconds in duration is processed with the commercial gait analysis algorithms included in Mobility Lab (APDM, Inc., Portland, Oregon) [32–34]. Our gait analysis algorithm uses the Unscented Kalman Filter to fuse information from the accelerometers, gyroscopes, and magnetometers to precisely estimate the orientation and position trajectory of each foot between quiet stance periods [35, 36].
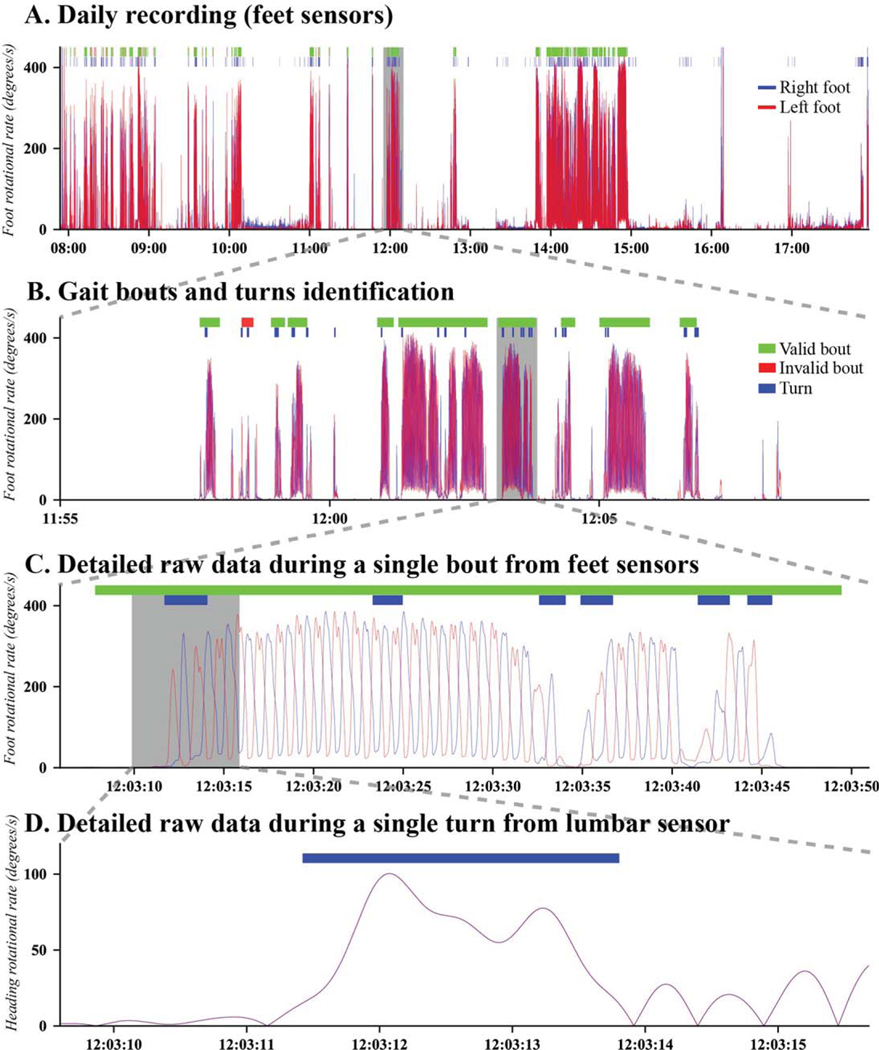
These algorithms use extensive validation criteria and many stages of processing to identify pairs of complete strides that are then used to calculate spatial-temporal gait metrics. For the results reported in this paper, we only included stride pairs during periods of straight walking, and we excluded walking during turns, that were characterized independently. For turning measures, we used the algorithm described in El-Gohary et al. 2014 [9] for detecting and characterizing each turn. Figure 1 shows an example of raw data from feet and lumbar gyroscopes, and the gait bouts and turns detected from the raw data using the above described algorithms. The detailed description of the definition of each mobility measure is given in Supplementary Table 1.
Fig. 1.
A representative example of a profile of the inertial sensor data from feet and lumbar over a day, and the zoomed-in version of identified gait bouts and turns.
The 43 digital outcome measures of mobility were grouped into five domains similar to previous factor analysis – Lower Body, Upper Body, Turning, Activity, and Variability [8, 31, 37]. Specifically, we have 10 digital outcome measures of mobility for Lower Body, 3 digital outcome measures of mobility for Upper Body, 7 digital outcome measures of mobility for Turning, 3 digital outcome measures of mobility in Activity, and 20 digital outcome measures of mobility incorporating variability of all four domains except the Activity domain (see Supplementary Table 1). Lower Body, Upper Body, and Turning have been previously shown to be relatively separate during clinical gait testing [31, 38]. In addition, Activity and Variability domains have been used by others [8, 11, 37, 39–41]. Each measure of lower body, upper body, turning and activity was calculated by taking mean over a week for each subject, and the variability measures were calculated from all the gait strides and turns over a week for each subject as the coefficient of variation (standard deviation divided by the mean, CV).
Note that instead of performing a factor analysis and using the factor scores for subsequent analysis, we opted to adopt previously identified domains due to the following reasons: Firstly, we felt that computing another set of factor scores based on modestly sized groups in the current study would likely resulted in unstable and/or non-reproducible factor scores. Secondly, we identified several larger studies of gait measures collected via wearable sensors that utilized factor analyses and/or principal components to characterize gait domains [8, 37, 40–42]. In addition, our team has conducted similar dimensionality reduction analyses based on in-clinic tests of 100 PD subjects with the same sensors as used in this investigation [38]. While domains across these studies were conceptually consistent, there were not always comprised of the same measures. Lastly, we were interested in ability of measures (individually and in combinations) to accurately classify PD vs. HC, and not necessarily, in how well factor scores classify patients.
Statistical analysis
To investigate how many measures of mobility within each domain differ between the PD compared to the HC group, we used Mann-Whitney U test.
To investigate which digital outcome measures of mobility discriminate mobility in PD from HC groups, we plotted Receiver Operating Characteristic (ROC) curves [43] and computed the ROC Area Under Curve (AUC) [44]. To validate the method, and check for the overfitting, we used a randomized 5-fold cross-validation. Five-fold cross-validation has been widely used as a validation method [13, 45]. In 5-fold cross-validation, the data are split in 5-parts such that 80% of randomly selected recordings are used for training, whereas the remaining 20% are used for validation.
We used logistic regression employing a best subset selection strategy since various combinations of digital outcome measures of mobility are possible with similar classification results [45]. The best subset selection strategy selects the best model from all possible subsets according to goodness-of-fit criteria. To assess the goodness-of-fit, we used the Bayesian Information Criteria (BIC) [45]. We selected the top 15 models based on BIC for two, three, and finally for four digital outcome measures of mobility (15*3=45 models total), and computed AUC, and 5-fold cross-validation AUC. All statistical analysis was performed using R Version 1.1.456 software.
Further, we explored if any of the top five individual digital outcome measures of mobility (Fig. 2) differentiate the PD faller (N= 20) from PD non-faller (N= 9) groups using a non-parametric (Wilcoxon) test. In addition, the discriminative ability of arm swing measures in PD from HC was investigated by computing AUC.
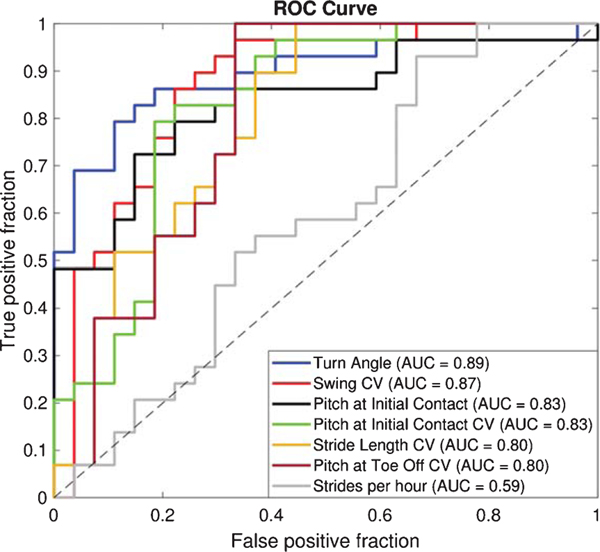
Fig. 2.
Receiver Operating Characteristic (ROC) curve showing the comparison of a typical activity measure (number of strides/hour) with the performance of the top digital outcome measures of mobility (AUC> 0.8) that differentiated PD from HC.
RESULTS
The weekly total duration of recordings for subjects with PD averaged 6.14±1.16 (mean±SD) days and 66.36±13.85 hours, and for HC averaged 5.81±1.21 days and 58.99±15.69 hours a week. Further, number of walking bouts/hour for HC was 22.50±7.93, and for PD was 20.70±6.86.
All aspects of mobility differed in PD from HC group, except the Upper Body. By investigating what percentage of the total measures in each domain were significantly different between PD and HC groups based on p-value with a threshold of 0.05, we found that 65% of gait variability measures, 60% measures of Lower Body, 57% measures of Turning, 33% measuresoftheActivityand0%measuresofUpperBody domain were significantly different between PD and HC.
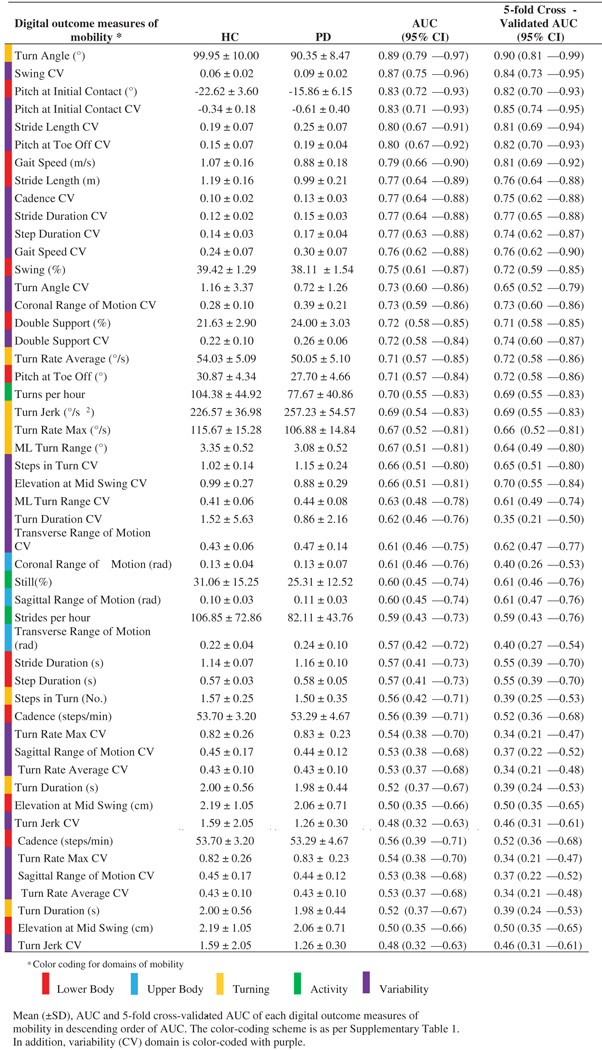
Out of the 43 total digital outcome measures of mobility, six individual digital outcome measures of mobility were most discriminative in differentiating mobility of the PD group from the HC group, with an AUC greater than 0.80 (Fig. 2). The ROC curve and AUC (with 5-fold cross-validation) results are shown in Fig. 2 and Table 1, respectively. Figure 2 shows that various gait measures (such as turn angle, terminal swing CV, swing CV, single limb support CV, the pitch at initial contact CV) performed better than the typical measure of activity – numbers of strides/hour).
Table 1.
Most discriminative to least discriminative individual digital outcome measures of mobility that separate mobility in HC from PD
 |
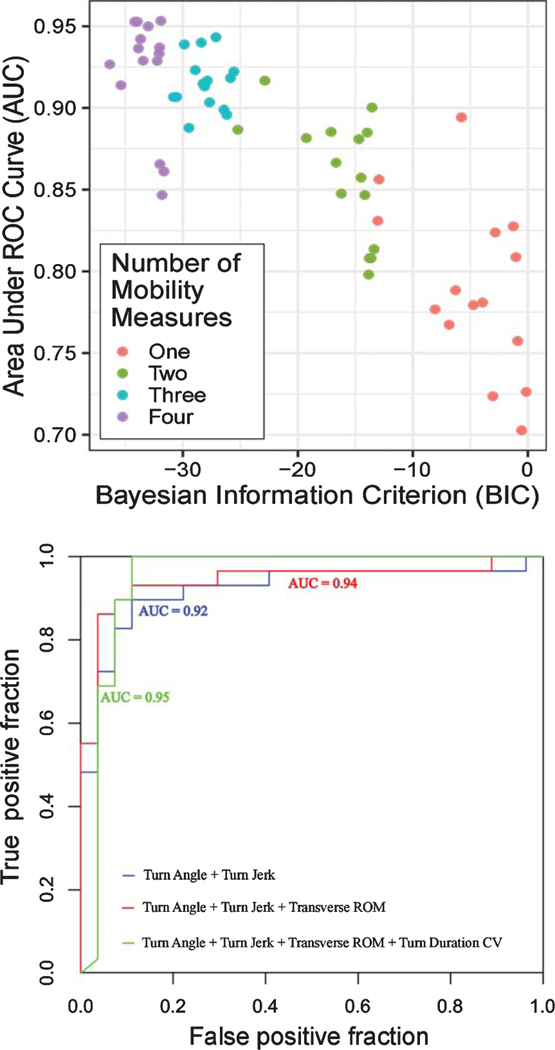
Results of logistic regression employing the best subset selection strategy show that combinations of digital outcome measures of mobility increased AUC, and hence discriminating ability (Fig. 3). In addition, we found various combinations of digital outcome measures of mobility resulted in similar 5-fold cross-validated AUC values as shown in Fig. 3.
Fig. 3.
A) 5-fold cross-validated AUC versus Bayesian Information Criterion (BIC) plot for various combinations of digital outcome measures of mobility. B) ROC curve for the top models with 2 to 4 digital outcome measures of mobility (see Table 2).
Most of the combinations of digital outcome measures of mobility separating the HC from the PD groups included turning measures indicating turning is more important than other domains of mobility (see Table 2). In addition to turning measures, some gait variability measures were consistently selected via the best subsets strategy (but only after turning was also included in the models).
Table 2.
Combination of digital outcome measures of mobility that best discriminate mobility in HC from PD
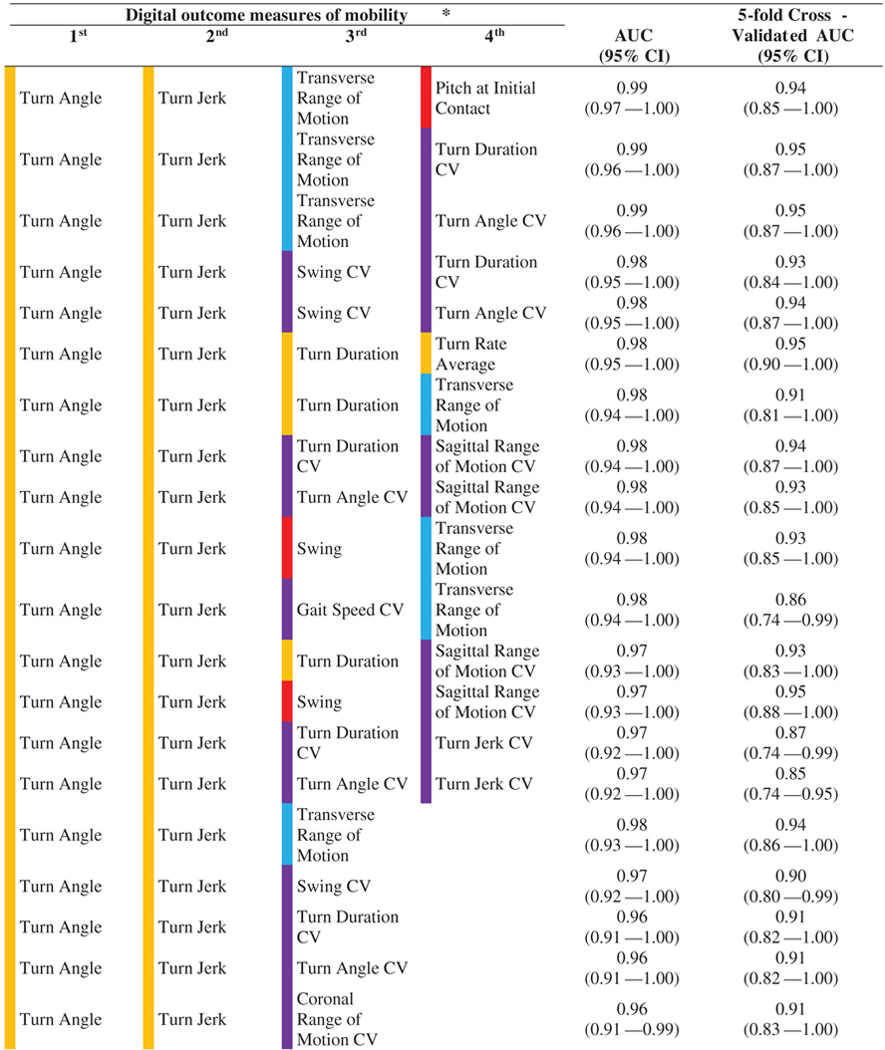 | |||||
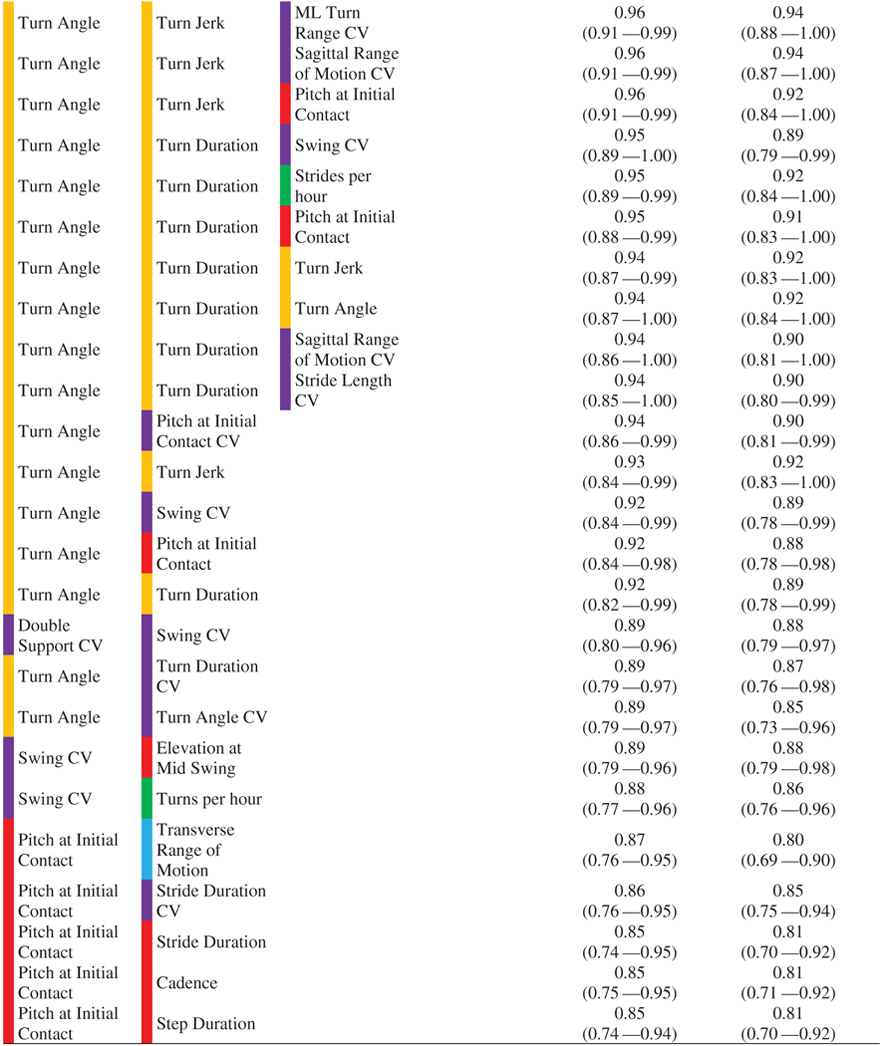 |
Color coding for domains of mobility is according to Table 1.
AUC and 5-fold CV AUC for digital outcome measures of mobility selected by the best subset selection method. The color-coding scheme is as per Supplementary Table 1. In addition, variability (CV) domain is color-coded with purple.
We also explored whether any of the top five individual digital outcome measures of mobility (Fig. 2) were statistically significant between the PD non-faller (N= 20) and PD faller (N= 9) groups. A non-parametric (Wilcoxon) test showed that out of the top five measures, three measures were statistically significant between the non-faller and faller groups. Specifically, turn angle was smaller in fallers (p= 0.040), pitch at initial contact angle was smaller in fallers (p= 0.028), and pitch at initial contact CV was higher in fallers (p= 0.017) compared to the non-fallers.
As wearing wrist sensors was optional, we had arm swing measures for 26 subjects with PD and 22 HC. We didn’t find a very good discriminating ability for arm swing measures. Specifically, AUC for arm range of motion was 0.63 (PD: 0.47±0.25, and HC: 0.52±0.21 rad), and AUC for maximum arm velocity was 0.46 (PD: 1.66±0.22, and HC: 1.71±0.19 rad/s).
DISCUSSION
Our findings suggested that turning is the most important domain of mobility compared to other domains as turning measures were most consistently selected via the best subsets strategy to discriminate PD from HC, followed by gait variability.
Various researchers have reported continuous monitoring of gait in people with PD [11, 15], but most of the larger studies have just used one sensor with an accelerometer at the lumbar region. A single lumbar sensor with only an accelerometer is not able to accurately measure turning or foot angle. To the best of our knowledge, this is the first study to compare various domains of gait including both walking and turning in daily life, and we found that turning was the most important domain of mobility.
Turning measures were most consistently selected via the best subsets strategy to discriminate PD from HC, turn angle was the most discriminative individual measure to separate PD from HC with a 5-fold cross-validated AUC of 0.90 (see Table 1). Specifically, the turn angle was smaller in subjects with PD than control subjects. This result agrees with studies on continuous monitoring of turning in PD and HC [9, 10], and a larger study of turning measures alone over three days in subjects with PD [39]. As turning is found to be associated with falls [23, 24], smaller turn angle in PD may reflect avoiding larger turns. However, the smaller turn angle in the PD group may also reflect longer duration of turning because of very slow, gradual increase and decrease in turning angular velocity as a turn starts and ends. In fact, an increased turn duration has been observed in older adults who have an elevated risk of falling during daily activity to protect against falls [46], and a simplified turning strategy was observed in older adults who subsequently experience multiple falls to assist in balance control [47].
After turning was included, gait variability was the second most consistently selected domain via the best subsets strategy to discriminate PD from HC. Specifically, swing time CV was the second most individual measure that discriminates mobility in PD from HC, with a 5-fold cross-validated AUC of 0.84 (see Table 1). Variability measures (such as stride-to-stride variability, and bout length variability) have previously been shown to be sensitive in discriminating between PD and HC, and also related to falls [7, 30]. In fact, we found a significant correlation between swing time CV and stride-to-stride variability (i.e. stride duration CV) with a Pearson correlation of r= 0.74, suggesting that swing time CV might be also sensitive to falls in PD in a larger sample with prospective falls.
The results of logistic regression employing best subsets selection strategy showed that some combinations of two mobility measure are stable and performed well in comparison to three digital outcome measures of mobility based on a 5-fold cross-validated AUC (see Table 2). For example, top models with two digital outcome measures of mobility combining turn angle and turn jerk (5-fold cross-validated AUC= 0.92, 95% CI: 0.83–1.00), and turn angle and Pitch at Initial Contact CV (5-fold cross-validated AUC= 0.90, 95% CI: 0.81–0.99) are not too much different from top models with three digital outcome measures of mobility combining turn angle, turn jerk, and transverse ROM (5-fold cross-validated AUC= 0.94, 95% CI: 0.86–1.00), and turn angle, turn jerk, and swing CV (5-fold cross-validated AUC= 0.90, 95% CI: 0.80–0.99). Further, more pronounce attenuation seen in models combining four digital outcome measures of mobility suggesting the overfitting. Thus, models with top two and three digital outcome measures of mobility sound promising as potential biomarkers.
To the best of our knowledge, this is the first study to show that the pitch of the foot at initial contact (both mean and CV) has a strong discriminative ability for PD when evaluated on its own (see Table 1). A small pitch angle of the foot at heel strike represents a lack of a heel-toe gait, and more shuffling, typical of parkinsonism. In fact, while combining variability of foot pitch with turning, we were able to achieve very good discriminative ability with an AUC of 0.94, and 5-fold cross-validated AUC of 0.90 (see Table 2). These findings suggest that a combination of turning and straight-ahead gait measures (with variability measures) will best capture parkinsonian mobility in natural daily life and will likely reflect fall risk (see below).
Further, considering the similarity in other statistical strategies such as regularized linear regression and ensemble techniques, we have also implemented the least absolute shrinkage and selection operator (LASSO) and random forest and found similar results. Specifically, the top four digital outcome measures of mobility using LASSO were swing CV, the pitch of the foot at initial contact, turn angle, and swing. Also, the top four digital outcome measures of mobility using random forest were turn angle, the pitch of foot at toe-off CV, Swing CV, and pitch of foot at initial contact CV.
We were surprised to find that arm swing measures were not different between PD and HC groups, as arm swing was found to be most sensitive to early untreated PD in laboratory testing [31]. With further investigation, we found that the average arm range of motion for PD group (26.92°) was slightly reduced compared to the laboratory setting (29.2°), while the average arm range of motion for HC group (29.79°) reduced drastically in daily living compared to laboratory setting (42.3°) [31]. We cannot know what people are doing with their arms while they walk (talk on the phone, carry purse, open doors, walk dog, etc.) during daily activities, all of which would decrease arm swing in control subjects.
Measures sensitive to falls in PD
We found that three of the five most discriminative digital outcome measures of mobility to PD, specifically, turn angle, foot pitch at initial contact and CV of foot pitch at initial contact, were also significantly different between the non-faller and faller groups. Although these findings are promising, further work is needed to validate the potential of these digital outcome measures of mobility to identify biomarkers sensitive to falls in larger cohorts.
Digital biomarkers
With the very large number of measures that can be derived from wearable sensor signals, identifying a few important digital outcome measures of mobility to use as digital biomarkers for clinical trials is important. We find it quite feasible for subjects to wear three inertial sensors all day for a week although it may be possible to detect turning quality from the foot sensors such that a lumbar sensor would not be needed. However, a single inertial sensor on the trunk would not detect some of these most sensitive measures to PD such as pitch angle of the foot and its variability during walking. Future studies will determine whether passive monitoring with body-worn sensors help detect significant changes with interventions sooner than single clinical/laboratory measures, and thus reduce the time required for detecting significant change. This might ultimately help in launching new treatments faster.
Limitations
There are several limitations of the current study. Firstly, the subjects were mostly H&Y stage 2. Further analysis is needed with larger cohorts to generalize potential biomarkers sensitivity to PD, and especially for earlier-stage PD. Secondly, future studies need to determine the test-retest reliability and sensitivity of the top mobility measures to disease progression in daily life to be useful as digital biomarkers for clinical trials. Thirdly, we ranked mobility measures characterizing mobility impairments in MS and PD based on AUC, but we cannot assume this ranking would be identical across all cohorts. Future work with larger cohorts is needed to investigate if these findings would generalize. Fourthly, other activity measures such as alpha, mean bout length [48] might be interesting to explore. Finally, we performed all analysis by taking the mean of each measure for all the strides over a week for each subject and thus giving equal weight to each stride. But in reality, gait is speed and other measures are different for gait bouts of different lengths [11]. Hence, future work will focus on analyzing the effect of bout length on each mobility measure and how bout length affects the discriminatory power of each mobility measure to distinguish PD from HC.
Conclusion
Our findings suggested that turning was the most important domain of mobility in discriminating PD from HC, followed by the variability domain. Hence, future clinical trial studies with digital biomarkers should include turning and variability measures of mobility.
Supplementary Material
ACKNOWLEDGMENTS
We thank our participants for generously donating their time to participate and Graham Harker for help with the data collection.
This study was supported by National Institutes of Health grants from National Institute on Aging (#R44AG055388 & #R43AG044863).
Footnotes
CONFLICT OF INTEREST
Drs. McNames, El Gohary, and Horak have a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology. Dr. Horak also consults with Biogen, Neuropore, Sanofi, and Takeda. This potential conflict has been reviewed and managed by OHSU.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-201914.
REFERENCES
- [1].Horak FB, Mancini M (2013) Objective biomarkers of balance and gait for Parkinson’s disease using body-worn sensors. Mov Disord 28, 1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tinetti M, Speechley M, Ginter S (1988) Risk factors for falls among elderly persons living in the community. N Engl J Med 319, 1701–1707. [DOI] [PubMed] [Google Scholar]
- [3].Lipsmeier F, Taylor KI, Kilchenmann T, Wolf D, Scotland A, Schjodt-Eriksen J, Cheng WY, Fernandez-Garcia I, Siebourg-Polster J, Jin L, Soto J, Verselis L, Boess F, Koller M, Grundman M, Monsch AU, Postuma RB, Ghosh A, Kremer T, Czech C, Gossens C, Lindemann M (2018) Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov Disord 33, 1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, Agarwal P, Athar S, Bordelan Y, Bronte-Stewart HM, Camicioli R, Chou K, Cole W, Dalvi A, Delgado H, Diamond A, Dick JP, Duda J, Elble RJ, Evans C, Evidente VG, Fernandez HH, Fox S, Friedman JH, Fross RD, Gallagher D, Goetz CG, Hall D, Hermanowicz N, Hinson V, Horn S, Hurtig H, Kang UJ, Kleiner-Fisman G, Klepitskaya O, Kompoliti K, Lai EC, Leehey ML, Leroi I, Lyons KE, McClain T, Metzer SW, Miyasaki J, Morgan JC, Nance M, Nemeth J, Pahwa R, Parashos SA, Schneider JSJS, Schrag A, Sethi K, Shulman LM, Siderow fA, Silverdale M, Simuni T, Stacy M, Stern MB, Stewart RM, Sullivan K, Swope DM, Wadia PM, Walker RW, Walker R, Weiner WJ, Wiener J, Wilkinson J, Wojcieszek JM, Wolfrath S, Wooten F, Wu A, Zesiewicz TA, Zweig RM (2008) Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 23, 2129–2170. [DOI] [PubMed] [Google Scholar]
- [5].Nonnekes J, Goselink RJM, Růzicka E, Fasano A, Nutt JG, Bloem BR (2018) Neurological disorders of gait, balance and posture: A sign-based approach. Nat Rev Neurol 14, 183–189. [DOI] [PubMed] [Google Scholar]
- [6].Hale LA, Pal J, Becker I (2008) Measuring free-living physical activity in adults with and without neurologic dysfunction with a triaxial accelerometer. Arch Phys Med Rehabil 89, 1765–1771. [DOI] [PubMed] [Google Scholar]
- [7].Weiss A, Sharifi S, Plotnik M, Van Vugt JPP, Giladi N, Hausdorff JM (2011) Toward automated, at-home assessment of mobility among patients with Parkinson disease, using a body-worn accelerometer. Neurorehabil Neural Repair 25, 810–818. [DOI] [PubMed] [Google Scholar]
- [8].Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L (2013) Independent domains of gait in older adults and associated motor and nonmotor attributes: Validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci 68, 820–827. [DOI] [PubMed] [Google Scholar]
- [9].El-Gohary M, Pearson S, McNames J, Mancini M, Horak F, Mellone S, Chiari L (2014) Continuous monitoring of turning in patients with movement disability. Sensors (Switzerland) 14, 356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mancini M, El-Gohary M, Pearson S, Mcnames J, Schlueter H, Nutt JG, King LA, Horak FB (2015) Continuous monitoring of turning in Parkinson’s disease: Rehabilitation potential. Neurorehabilitation 37, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Del Din S, Godfrey A, Galna B, Lord S, Rochester L (2016) Free-living gait characteristics in ageing and Parkinson’s disease: Impact of environment and ambulatory bout length. J Neuroeng Rehabil 13, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].De Lima ALS, Hahn T, Evers LJW, De Vries NM, Cohen E, Afek M, Bataille L, Daeschler M, Claes K, Boroojerdi B, Terricabras D, Little MA, Baldus H, Bloem BR, Faber MJ (2017) Feasibility of large-scale deployment of multiple wear able sensors in Parkinson’s disease. PLoS One 12,1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arora S, Baig F, Lo C, Barber TR, Lawton MA, Zhan A, Rolinski M, Ruffmann C, Klein JC, Rumbold J, Louvel A, Zaiwalla Z, Lennox G, Quinnell T, Dennis G, Wade-Martins R, Ben-Shlomo Y, Little MA, Hu MT (2018) Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD. Neurology 91, e1528-e1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhan A, Mohan S, Tarolli C, Schneider RB, Adams JL, Sharma S, Elson MJ, Spear KL, Glidden AM, Little MA, Terzis A, Ray Dorsey E, Saria S (2018) Using smartphones and machine learning to quantify Parkinson disease severity the mobile Parkinson disease score. JAMA Neurol 75, 876880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Del Din S, Godfrey A, Mazzá C, Lord S, Rochester L (2016) Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov Disord 31, 1293–1313. [DOI] [PubMed] [Google Scholar]
- [16].Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A (2015) Digital health revolution: Is it time for affordable remote monitoring for Parkinson’s disease? Front Neurol 6, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Espay AJ, Hausdorff JM, Sanchez-Ferro A, Klucken J, Merola A, Bonato P, Paul SS, Horak FB, Vizcarra JA, Mestre TA, Reilmann R, Nieuwboer A, Dorsey RE, Rochester L, Bloem BR, Maetzler W, Movement Disorder Society Task Forceon Technology (2019) A roadmap for implementation of patient-centered digital outcome measures in Parkinson’s disease obtained using mobile health technologies. Mov Disord 34, 657–663. d [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Morris ME, Iansek R, Matyas TA, Summers JJ (1994) The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain 117, 1169–1181. [DOI] [PubMed] [Google Scholar]
- [19].Boonstra TA, van der Kooij H, Munneke M, Bloem BR (2009) Gait disorders and balance disturbances in Parkinson’s disease: Clinical update and pathophysiology. Curr Opin Neurol 24, 461–471. [DOI] [PubMed] [Google Scholar]
- [20].Mirelman A, Bonato P, Camicioli R, Ellis TD, Giladi N, Hamilton JL, Hass CJ, Hausdorff JM, Pelosin E, Almeida QJ (2019) Gait impairments in Parkinson’s disease. Lancet Neurol 4422, 1–12. [DOI] [PubMed] [Google Scholar]
- [21].Bloem B, Grimbergen Y, Cramer M, Willemsen M, Zwinderman A (2001) Prospective assessment of falls in Parkinson’s disease. J Neurol 248, 950–958. [DOI] [PubMed] [Google Scholar]
- [22].Weiss A, Brozgol M, Dorfman M, Herman T, Shema S, Giladi N, Hausdorff JM (2013) Does the evaluation of gait quality during daily life provide insight into fall risk? A novel approach using 3-Day accelerometer recordings. Neurorehabil Neural Repair 27, 742–752. [DOI] [PubMed] [Google Scholar]
- [23].Mancini M, Schlueter H, El-Gohary M, Mattek N, Duncan C, Kaye J, Horak FB (2016) Continuous monitoring of turning mobility and its association to falls and cognitive function: A pilot study. J Gerontol A Biol Sci Med Sci 71, 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Leach JM, Mellone S, Palumbo P, Bandinelli S, Chiari L (2018) Natural turn measures predict recurrent falls in community-dwelling older adults: A longitudinal cohort study. Sci Rep 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shibley R, Griffin HJ, Quinn NP, Jahanshahi M (2008) Quality of life in Parkinson’s disease: The relative importance of the symptoms. Mov Disord 23, 1428–1434. [DOI] [PubMed] [Google Scholar]
- [26].Stack E, Ashburn A (2008) Dysfunctional turning in Parkinson’s disease. Disabil Rehabil 30, 1222–1229. [DOI] [PubMed] [Google Scholar]
- [27].Huxham F, Baker R, Morris ME, Iansek R (2008) Footstep adjustments used to turn during walking in Parkinson’s disease. Mov Disord 23, 817–823. [DOI] [PubMed] [Google Scholar]
- [28].Hausdorff JM (2005) Gait variability: Methods, modeling and meaning. J Neuroeng Rehabil 6, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hausdorff JM (2009) Gait dynamics in Parkinson’s disease: Common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos 19, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lord S, Godfrey A, Galna B, Mhiripiri D, Burn D, Rochester L (2013) Ambulatory activity in incident Parkinson’s: More than meets the eye? J Neurol 260, 2964–2972. [DOI] [PubMed] [Google Scholar]
- [31].Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB (2010) The instrumented timed up and go test: Potential outcome measure for disease modifying therapies in Parkinson’s disease. J Neurol Neurosurg Psychiatry 81, 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mancini M, King L, Salarian A, Holmstrom L, James M, Horak FB (2011) Mobility lab to assess balance and gait with synchronized body-worn sensors. J Bioeng Biomed Sci (Suppl 1), 007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Washabaugh EP, Kalyanaraman T, Adamczyk PG, Claflin ES, Krishnan C (2017) Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 55, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Morris R, Stuart S, Mcbarron G, Fino PC, Mancini M, Curtze C (2019) Validity of mobility lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiol Meas 40, 095003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wan EA, Van Der Merwe R (2000) The unscented Kalman filterfornonlinearestimation.ProceedingsoftheIEEE2000 Adaptive Systems for Signal Processing, Communications, and Control Symposium (Cat. No.00EX373), Lake Louise, Alberta, Canada, pp. 153–158. [Google Scholar]
- [36].Van Der Merwe R (2004) Sigma-point Kalman filters for probabilistic inference in dynamic state-space models (thesis). Wan EA, supervisor, Oregon Health & Science University. [Google Scholar]
- [37].Morris R, Hickey A, Del Din S, Godfrey A, Lord S, Rochester L (2017) A model of free-living gait: A factor analysis in Parkinson’s disease. Gait Posture 52, 68–71. [DOI] [PubMed] [Google Scholar]
- [38].Horak FB, Mancini M, Carlson-kuhta P, Nutt JG, Salarian A (2016) Balance and gait represent independent domains of mobility in Parkinson disease. Phys Ther 96, 1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mancini M, Weiss A, Herman T, Hausdorff JM (2018) Turn around freezing: Community-living turning behavior in people with Parkinson’s disease. Front Neurol 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hollman JH, Mcdade EM, Petersen RC (2011) Normative spatiotemporal gait parameters in older adults. Gait Posture 34, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Arcolin I, Corna S, Giardini M, Giordano A, Nardone A, Godi M (2019) Proposal of a new conceptual gait model for patients with Parkinson’s disease based on factor analysis. Biomed Eng Online 18, 70. Erratum in: Biomed Eng Online 2019;18(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lord S, Galna B, Rochester L (2013) Moving forward on gait measurement: Toward a more refined approach. Mov Disord 28, 1534–1543. [DOI] [PubMed] [Google Scholar]
- [43].Fawcett T (2006) An introduction to ROC analysis. Pattern Recognit Lett 27, 861–874. [Google Scholar]
- [44].Turck N, Vutskits L, Sanchez-Pena P, Robin X, Hainard A, Gex-Fabry M, Fouda C, Bassem H, Mueller M, Lisacek F, Puybasset L, Sanchez J-C (2011) pROC: An open-source package for R and S+to analyze and compare ROC curves. BMC Bioinformatics 8, 12–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hastie T, Tibshirani R, Friedman J (2009) The Elements of Statistical Learning: Data Mining, Inference, and Prediction, Second Edition. Springer Science+Business Media, LLC. [Google Scholar]
- [46].Deshpande N, Metter EJ, Bandinelli S, Lauretani F, Windham BG, Ferrucci L (2008) Psychological, physical, and sensory correlates of fear of falling and consequent activity restriction in the elderly: The InCHIANTI study. Am J Phys Med Rehabil 87, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wright RL, Peters DM, Robinson PD, Sitch AJ, Watt TN, Hollands MA (2012) Differences in axial segment reorientation during standing turns predict multiple falls in older adults. Gait Posture 36, 541–545. [DOI] [PubMed] [Google Scholar]
- [48].Del Din S, Galna B, Godfrey A, Bekkers EMJ, Pelosin E, Nieuwhof F, Mirelman A, Hausdorff JM, Rochester L (2017) Analysis of free-living gait in older adults with and without Parkinson’s disease and with and without a history of falls: Identifying generic and disease-specific characteristics. J Gerontol A Biol Sci Med Sci 74, 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.