Abstract
Hypomethylating agents (HMA) with venetoclax is a new standard for older/unfit patients with acute myeloid leukemia (AML). However, it is unknown how HMA with venetoclax compare to intensive chemotherapy (IC) in patients who are ‘fit’ or ‘unfit’ for IC. We compared outcomes of older patients with newly diagnosed AML receiving 10-day decitabine with venetoclax (DEC10-VEN) versus IC. DEC10-VEN consisted of daily venetoclax with decitabine 20 mg/m2 for 10-days for induction and decitabine for 5-days as consolidation. The IC cohort received regimens containing cytarabine ≥1 g/m2/d. A validated treatment-related mortality score (TRMS) was used to classify patients at high- or low-risk for TRM with IC. Propensity scores were used to match patients to minimize bias. Median age of the DEC10-VEN cohort (n=85) was 72 years (range 63–89) and 28% patients were at high-risk of TRM with IC. The comparator IC group (n=85) matched closely in terms of baseline characteristics. DEC10-VEN was associated with significantly higher CR/CRi compared to IC (81% vs 52%, p<.001), and lower rate of relapse (34% vs 56%, p=.01), 30-day mortality (1% vs 24%, p<.01), and longer overall survival (OS; 12.4 vs 4.5 months, HR=0.48, 95%CI 0.29–0.79, p<.01). In patients at both at high- and low-risk of TRM, DEC10-VEN showed significantly higher CR/CRi, lower 30-day mortality, and longer OS compared to IC. Patients at both high- and low-risk of TRM had comparable outcomes with DEC10-VEN. DEC10-VEN offers superior outcomes compared to IC in older patients with AML, particularly in patients at high-risk of TRM.
Keywords: treatment-related mortality, venetoclax, decitabine, intensive chemotherapy, acute myeloid leukemia
INTRODUCTION
Acute myeloid leukemia (AML) commonly presents in older patients, many of whom are at high risk of treatment-related mortality (TRM) with intensive chemotherapy.1,2 Consequently, lower-intensity regimens of venetoclax with hypomethylating agents (HMA) are attractive options for such patients.3,4 Venetoclax with azacitidine was recently shown to be superior compared to azacitidine alone and has emerged as a new standard for older or unfit patients.5,6 We conducted a phase 2 study of 10-day decitabine with venetoclax (DEC10-VEN) which showed excellent outcomes and low TRM in older patients with AML.7
There has been ongoing debate about the true utility of venetoclax and HMA regimens in AML.8,9 These issues include questions about the proportion of truly ‘unfit’ patients in the clinical trials of venetoclax-based lower-intensity regimens and the appropriateness of the accelerated U.S. Food and Drug Administration approval based on the earlier venetoclax trials.10,11 Alternatively, if an older patient is not truly ‘unfit’, could they benefit more from intensive chemotherapy instead, and if venetoclax-based regimens improve outcomes in patients who are truly unfit for intensive chemotherapy.
In addition, there is lack of consensus regarding methods for establishing “fitness” for intensive chemotherapy, and in practice, this distinction is often subjective. Several objective models have been developed to identify patients with AML ‘unfit’ for intensive treatment or predict the risk of TRM.12–15 These models have varying degrees of accuracy and ease of implementation in the clinic, but have the potential to match patients to appropriate therapies and improve outcomes in patients with AML.
Given these questions about patient ‘fitness’ for AML therapy in the context of venetoclax trials and real benefit of venetoclax and HMA regimens for patients who are truly ‘unfit’, we sought to determine outcomes with DEC10-VEN in ‘fit’ and ‘unfit’ patients with newly diagnosed AML based on validated predictive models, and compare those outcomes with a closely matched historical cohort of newly diagnosed patients treated with intensive chemotherapy.
METHODS
We conducted a retrospective study to compare outcomes with DEC10-VEN and intensive chemotherapy. We incorporated two validated models for determining ‘fitness’ for intensive chemotherapy to classify patients according to their risk of TRM from intensive chemotherapy. We used propensity score matching to balance important effect modifiers and minimize bias between the two intervention groups.16,17
Treatment regimens
Patients receiving DEC10-VEN were treated on a prospective phase 2 trial. The regimen comprised of daily venetoclax with decitabine 20 mg/m2 IV for 10 days for “induction”, followed by decitabine for 5-days as consolidation.18 Venetoclax dose was 400 mg daily or equivalent with concomitant azoles. Venetoclax was held on cycle 1 day 21 if bone marrow evaluation showed response or aplasia. Further reduction in venetoclax duration was allowed in cases of myelosuppression. The full protocol including eligibility criteria have been published previously.7
The comparison cohort was selected from patients treated with intensive chemotherapy containing at least moderate dose of cytarabine ≥1 g/m2/d, either as standard of care or on clinical trials. Other agents administered in combination included idarubicin, liposomal daunorubicin, clofarabine, fludarabine, and others (Table S1). Eligible patients in either group could proceed to stem-cell transplantation after achievement of a response. Supportive care including prophylactic antibiotics during neutropenia and treatment in laminar air-flow room, were similar in both groups.
Risk of treatment-related mortality with intensive chemotherapy
Risk of TRM with intensive chemotherapy was determined using the TRM score15 and findings in the overall population were confirmed using a separate MD Anderson Cancer Center (MDACC) score (Table S2).12 The TRM score combines age, Eastern Co-operative Oncology Group performance status (ECOG PS), AML subtype, and laboratory values and predicts the risk of 30-day mortality after intensive chemotherapy. The validated TRM score cut-off of 13.1 clinically used for treatment assignment was used to classify patients at high or low risk for TRM with high accuracy (receiver operator characteristics curve AUC 0.82).15 The MDACC model incorporated age, ECOG PS, clinical and laboratory values to predict risk of 8-week mortality with intensive chemotherapy.12
Response evaluation and endpoints
Responses were determined per the modified IWG criteria for AML.19 Overall survival (OS) was determined from start of therapy until death, or censored at last follow-up. Event-free survival (EFS) was determined from start of therapy until date of refractory disease, relapse, death, or censored at last follow-up.
Propensity score matching and statistical analysis
Logistic regression was used to calculate propensity scores from baseline characteristics of age, ECOG PS, European LeukemiaNet 2017 risk group, and previously validated TRM score (TRMS >13.1 vs. ≤13).15,20 Propensity score matching with the nearest neighbor method was used to match patients treated with DEC10-VEN to those treated with intensive chemotherapy using methods described previously.21,22 The absolute standardized mean difference of the propensity scores of selected variables was evaluated before and after match and a value <0.25 would suggest a substantial reduction of bias between the two intervention groups.23
A 1:1 matching was used for the overall comparison (85 patients in each group) and low TRM risk group (61 patients in each group). A 1:2 matching was used to match 24 high TRM risk patients in DEC10-VEN cohort to 48 patients treated with intensive chemotherapy to improve statistical power of the comparison. A separate matching was performed using the MDACC score in place of the TRM score to validate findings in the overall group (85 patients in both groups). A schema showing populations and factors used for propensity score matching is shown in Fig S1.
Chi square test, t-test and Kaplan-Meier method with log-rank test were used to compare categorical, continuous and time-to-event variables. Stratified logistic regression and Cox models were used to evaluate the treatment effects. Multivariable logistic regression models were fit to assess the association between patient characteristics and outcomes. Characteristics significant in the univariate models at level 0.10 were included in the multivariate model. Prism v7.0 (GraphPad Software, San Diego, CA), SAS v9.4 (SAS Institute, Cary, NC), R v3.3.3 (R Core Team, Vienna, Austria) and the R package MatchIt were used for statistical analyses.
RESULTS
All 85 of newly diagnosed patients treated with DEC10-VEN were matched to 85 out of 405 newly diagnosed patients treated with intensive chemotherapy. The patients in DEC10-VEN cohort were treated between January 10, 2018 and December 10, 2019. The intensive chemotherapy recipients were treated between May 4, 2000 and July 11, 2018 (median year of treatment 2003). Among patients treated with DEC10-VEN, 74% were older than 70 years, 35% patients had ECOG PS ≥2, and 65% patients had ELN adverse risk disease. The historic intensive chemotherapy cohort matched closely with the DEC10-VEN cohort in terms of baseline characteristics and propensity scores (Table 1, S3, S4).
Table 1.
Baseline characteristics of propensity score matched patients who received 10-day decitabine with venetoclax, and intensive chemotherapy
| Patient characteristics | 10-day decitabine and venetoclax (N=85) |
Intensive chemotherapy (N=85) |
p |
|---|---|---|---|
| Age, years | 72 [69–78] | 73 [67–76] | .24 |
| Age ≥ 70 years | 63 (74) | 55 (65) | .18 |
| Age ≥ 80 years | 15 (18) | 10 (12) | .28 |
| Male sex | 45 (53) | 48 (56) | .64 |
| ECOG Performance Status | |||
| 0–1 | 55 (65) | 58 (68) | .63 |
| ≥2 | 30 (35) | 27 (32) | |
| Bone marrow blasts, % | 45 [22–62] | 65 [38–84] | <.01 |
| Diagnosis | |||
| De novo AML | 55 (65) | 59 (69) | |
| Secondary AML with AHD | 15 (18) | 9 (11) | .38 |
| Therapy-related AML | 16 (19) | 19 (22) | |
| Prior therapies | 0 (0) | 0 (0) | NA |
| ELN 2017 cytogenetic risk group | |||
| Favorable | 0 (0) | 0 (0) | |
| Intermediate | 44 (52) | 33 (39) | .08 |
| Adverse | 40 (47) | 52 (61) | |
| FLT3-ITD/TKD | 14 (16) | 22 (24) | .09 |
| ELN 2017 risk group | |||
| Favorable | 19 (22) | 14 (16) | |
| Intermediate | 11 (13) | 17 (20) | .36 |
| Adverse | 55 (65) | 54 (64) | |
| Stem-cell transplantation after response | 12 (14) | 7 (8) | .23 |
| Treatment-related mortality (TRM) risk | |||
| High (TRM score >13.1) | 24 (28) | 24 (28) | 1.00 |
| Low (TRM score ≤13.1) | 61 (72) | 61 (72) | |
| Expected TRM rate, %, mean ± SD1 | 12 ± 12 | 14 ± 14 | .27 |
Results reported as n (%), or median [interquartile range]. ECOG = Eastern Co-operative Oncology Group, AHD = antecedent hematological disorder, ELN = European LeukemiaNet.
Expected mortality with intensive chemotherapy using the TRM model.
In the DEC10-VEN cohort, there was no difference in 30-day mortality (0% vs 2%) but higher CR rate (69% vs 42%, p=.02) in high TRM risk patients compared to low-risk patients (Table S5). In the matched population, DEC10-VEN was associated with significantly higher rate of CR compared to intensive chemotherapy ( 62% vs 42%, p=.01 Table 2), lower rates of relapse (34% vs 56%, p=.01), 30-day mortality (1% vs 24%, p<.01). Causes of 30-day mortality in recipients of intensive chemotherapy are shown in Table S6. For patients deemed at high-risk of TRM, DEC10-VEN compared to intensive chemotherapy offered comparable rates of CR but lower 30-day mortality (0% vs 33%, p=N/A). Among patients at low-risk of TRM, DEC10-VEN produced significantly higher rate of CR (70% vs 43%, p<.01), and lower rates of refractory disease (10% vs 25%, p=.03) and 30-day mortality (2% vs 16%, p=.03).
Table 2.
Comparison of outcomes with 10-day decitabine and venetoclax (DEC10-VEN) versus intensive chemotherapy (IC) using a propensity score-matched analysis stratified by treatment related-mortality score (TRMS).
| Outcomes by TRM risk | DEC10-VEN | Intensive Chemotherapy | Odds Ratio (95% CI) |
stratified p |
|---|---|---|---|---|
| Overall population | N=85 | N=85 | ||
| CR | 52 (62) | 36 (42) | 2.21 (1.18, 4.16) | .01 |
| CR/CRi | 69 (81) | 44 (52) | 3.78 (1.81, 7.88) | <.001 |
| Refractory | 11 (13) | 19 (22) | 0.52 (0.23, 1.17) | .12 |
| Relapse | 25/74 (34) | 25/45 (56) | 0.41 (0.19, 0.87) | .01 |
| 30-day mortality | 1 (1) | 20 (24) | 0.04 (0.01, 0.30) | <.01 |
| 60-day mortality | 6 (7) | 25 (29) | 0.18 (0.07, 0.47) | <.001 |
| Median OS, months | 12.4 | 5.0 | HR 0.48 (0.29, 0.79) | <.01 |
| High risk of TRM (TRMS >13.1) | N=24 | N=48 | ||
| CR | 10 (42) | 16 (33) | 1.43 (0.52, 3.92) | .47 |
| CR/CRi | 17 (71) | 17 (35) | 4.00 (1.40, 11.44) | <.01 |
| Refractory | 5 (21) | 13 (27) | 0.71 (0.22, 2.29) | .54 |
| Relapse | 6/19 (32) | 9/17 (53) | 0.41 (0.11, 1.59) | .99 |
| 30-day mortality | 0 (0) | 16 (33) | NA | NA |
| 60-day mortality | 4 (17) | 21 (44) | 0.26 (0.08, 0.87) | 0.03 |
| Median OS, months | 9.1 | 2.4 | HR 0.30 (0.13, 0.69) | <.01 |
| Low risk of TRM (TRMS ≤13.1) | N=61 | N=61 | ||
| CR | 42 (70) | 26 (43) | 2.89 (1.35, 6.17) | <.01 |
| CR/CRi | 52 (85) | 33 (54) | 5.75 (1.99, 16.63) | .001 |
| Refractory | 6 (10) | 15 (25) | 0.34 (0.12, 0.93) | .03 |
| Relapse | 19/55 (35) | 20/34 (59) | 0.37 (0.15, 0.63) | .12 |
| 30-day mortality | 1 (2) | 10 (16) | 0.10 (0.01, 0.78) | .03 |
| 60-day mortality | 2 (3) | 14 (23) | 0.11 (0.02, 0.53) | <.01 |
| Median OS, months | 15.2 | 6.8 | HR 0.48 (0.26, 0.90) | .02 |
Results reported as n (%), or n/N (%). Total responding patients were used as the denominator for relapse, this included patients who achieved a morphologic leukemia-free state, in addition to complete remission (CR) and CR with incomplete hematologic recovery. HR = hazard ratio, NA = not applicable as not enough events to calculate stratified p value.
At a median follow-up of 12.4 months in the DEC10-VEN cohort and 81.2 months in the intensive chemotherapy cohort, OS was significantly longer compared to intensive chemotherapy in the overall comparison (12.4 vs 5.0 months, hazard ratio [HR] = 0.48, 95% confidence interval [CI] 0.29, 0.79, p<.01, Fig. 1a) and among patients with high-risk of TRM (9.1 vs 2.4 months, HR=0.30, 95% CI 0.13, 0.69, p<.01, Fig. 1b) as well as low-risk of TRM (15.2 vs 6.8 months, HR=0.48, 95% CI 0.26, 0.90, p=.02 Fig. 1c). The sample size was limited to detect a difference in OS with DEC10-VEN in high vs low TRM risk group (9.1 vs 15.2 months, HR 1.44, 95% CI 0.72, 2.88, p=.27, Fig. 1d) but was significantly different for high TRM vs low TRM risk patients receiving intensive chemotherapy (2.4 vs 15.2 months, HR 4.35, 95% CI 2.61, 7.24, p<.001, Fig. S2). Post-transplant OS was comparable among DEC10-VEN and intensive chemotherapy recipients (HR 0.68, 95% CI 0.10, 4.51, p=.67).
Fig 1.
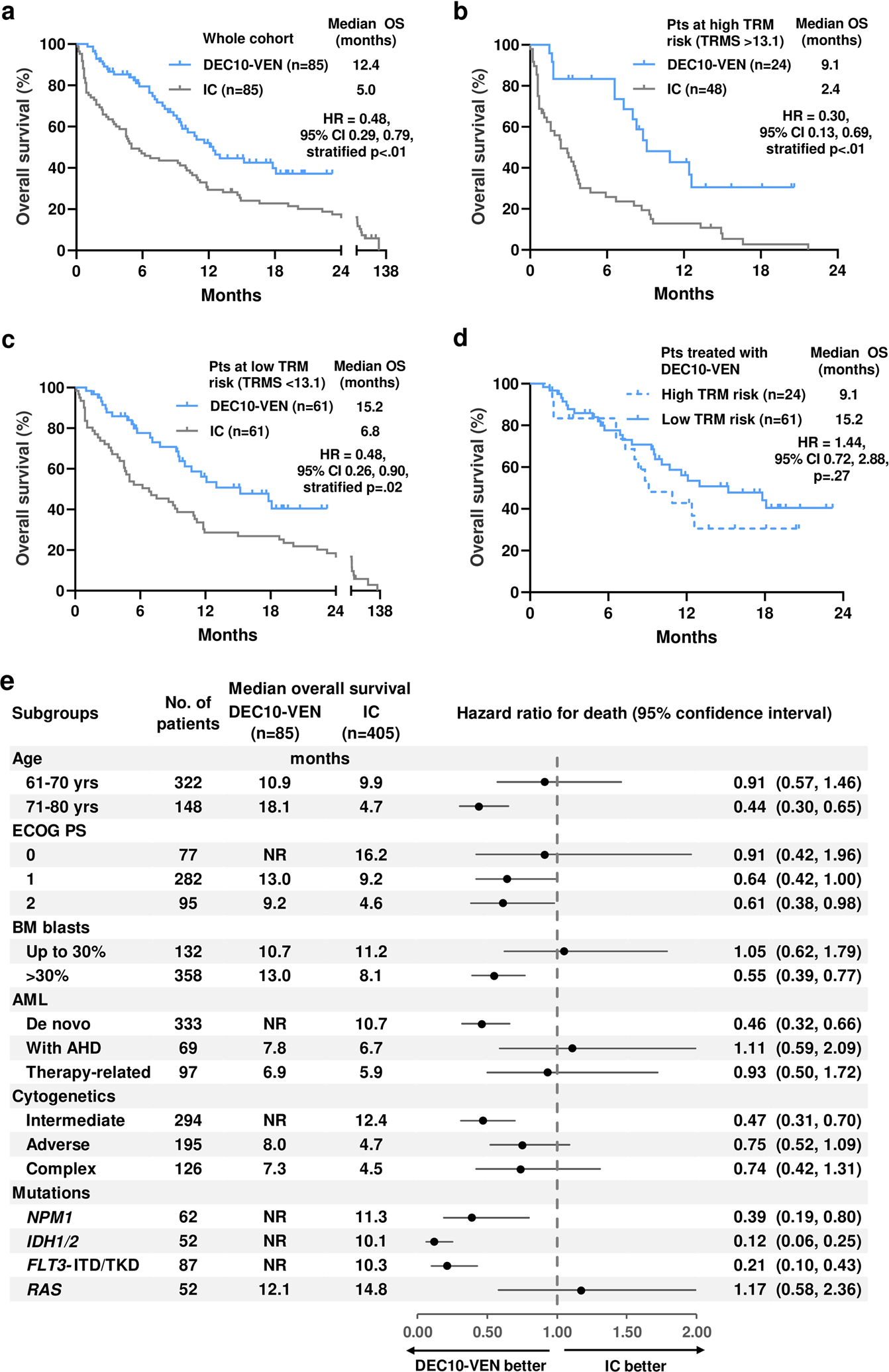
a. Overall survival (OS) in patients who received 10-day decitabine with venetoclax (DEC10-VEN) and intensive chemotherapy (IC), b. OS in patients with high treatment-related mortality risk score (TRMS >13.1), c. OS in patients with low risk of TRM (TRMS ≤13.1), d. OS with DEC10-VEN in patients at high and low risk of TRM, e. forest plot of exploratory subgroup analyses. FLT3 inhibitors were administered in 10 out of 14 FLT3mut patients in the DEC10-VEN cohort and 15 out of 73 patients in IC cohort. ECOG PS = Eastern Co-operative Oncology Group Performance Status, BM = bone marrow, AHD = antecedent hematological disorder.
Exploratory subgroup analyses for OS in unmatched cohorts favored DEC10-VEN in most subgroups, with significant benefit in patients with NPM1mut, IDH1/2mut and FLT3mut AML (Fig. 1e). Analysis for EFS showed similar degree of benefit with DEC10-VEN over intensive chemotherapy in the overall cohort, patients at high risk of TRM and those at low risk of TRM (Fig. 2a–c). Comparison of EFS between patients at high vs low risk of TRM treated with DEC10-VEN had limited power to detect a difference (Fig. 2d).
Fig 2.
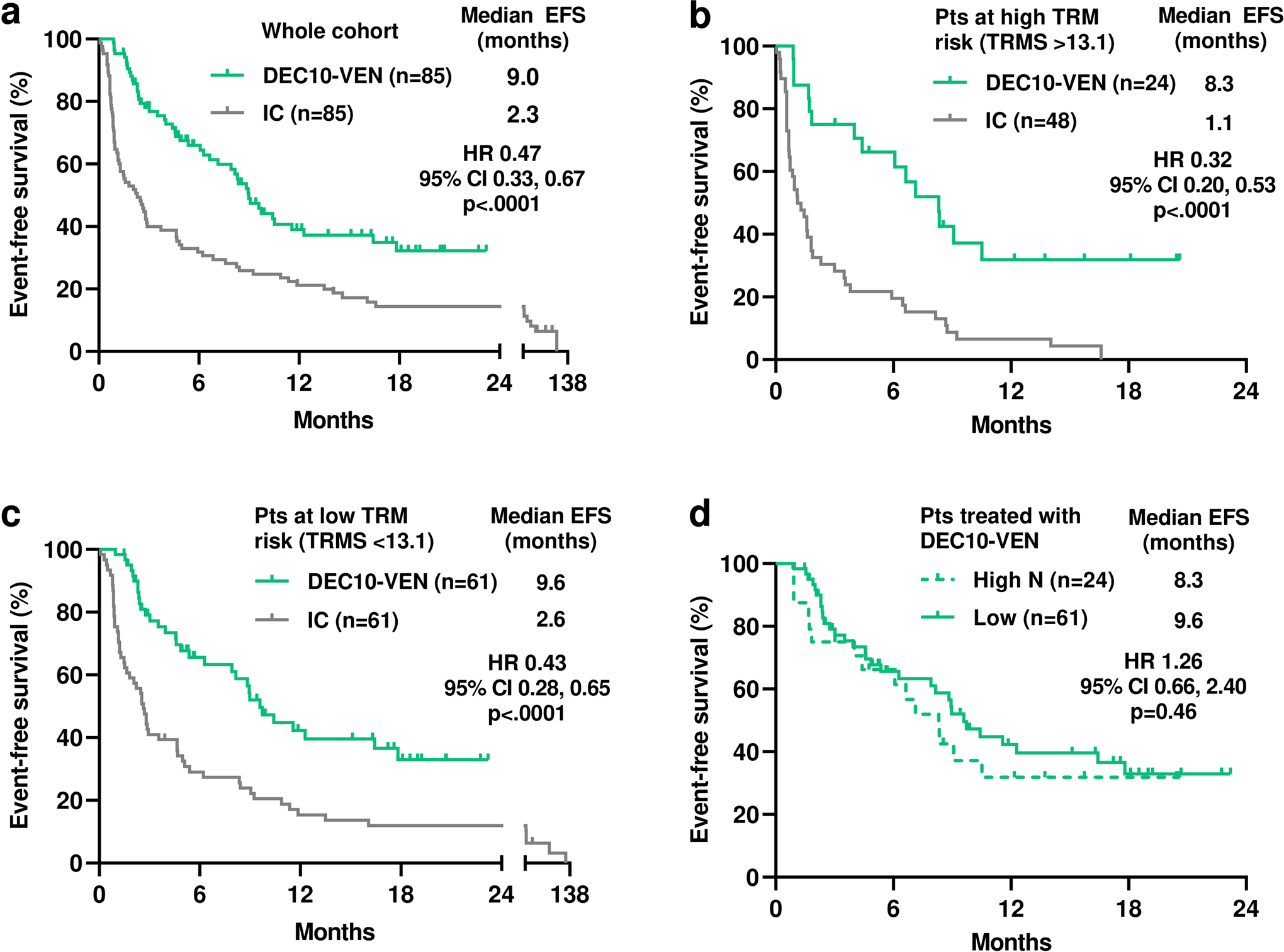
a. Event-free survival (EFS) in patients who received 10-day decitabine with venetoclax (DEC10-VEN) and intensive chemotherapy (IC), b. EFS in patients with high treatment-related mortality risk score (TRMS >13.1), c. EFS in patients with low risk of TRM (TRMS ≤13.1), d. EFS with DEC10-VEN in patients at high and low risk of TRM.
Multivariable analysis for non-matched variables in the whole cohort showed DEC10-VEN was associated with significantly higher odds of CR (odds ratio [OR] 2.66, 95%CI 1.12, 6.28, p=.03, Table S7), and lower odds of relapse (OR 0.20, 95%CI 0.05, 0.82, p=.03), 30-day mortality (OR 0.05, 95%CI 0.01, 0.42, p=.01) and death (hazard ratio [HR] 0.40, 95%CI 0.21, 0.76, p=.01). Compared to patients with de novo AML, those with sAML with AHD had lower odds of CR (OR 0.07, 95%CI 0.01, 0.68, p=.02) and higher risk of death (HR 7.78, 95%CI 1.58, 38.35, p=.01) irrespective of treatment and therapy-related AML was associated with higher risk of death (HR 4.56, 95%CI 1.44, 14.49, p=.01).
A confirmatory analysis was conducted using the MDACC which can predict 8-week morality with intensive chemotherapy. The standardized mean differences after matching were mostly favorable (Table S8) and majority baseline characteristics were well balanced between the two groups (Table S9). In this analysis, there were more patients with lower ECOG PS of 0–1 in the intensive chemotherapy cohort compared to DEC10-VEN (82% vs 65%). However, this analysis still confirmed nearly identical benefit with DEC10-VEN and significantly better compared to intensive chemotherapy for all outcomes. In 85 patients receiving DEC10-VEN, there were higher rates of CR (62% vs 44%, p=.019), CR/CRi (81% vs 53%, p<.001), lower rates of relapse (34% vs 58%, p<.01), 30-day mortality (1% vs 21%, p<.001) and 60-day mortality (7% vs 28%, p=.002, Table S10) compared to 85 patients treated with intensive chemotherapy. In these patients matched using the MDACC score, the median OS with DEC10-VEN was 12.4 months vs 5.0 months with intensive chemotherapy (HR 0.42, 95% CI 0.25, 0.70, p<.001, Fig. S3). Of note, in the primary analysis using TRM score for propensity score matching, the observed 30-day mortality with intensive chemotherapy was higher than predicted by using the TRM score.15 However, the 8-week mortality with intensive chemotherapy matched closely with predictions per the MDACC score (Table S11).12
DISCUSSION
While venetoclax with HMA has been shown to be superior to HMA, it is unknown how such regimens impact patients based on their ‘fitness’ for intensive therapy and how the outcomes compare to intensive regimens. Our retrospective analysis was designed to answer these questions which are relevant for patients and clinicians. The approach of propensity score matching helped to find a closely matched comparator group treated with intensive chemotherapy to minimize selection bias inherent in retrospective comparisons.17,21,24
We showed that older patients with newly diagnosed AML, and particularly those at high-risk of TRM with intensive therapy, experience favorable outcomes in terms of rates of response, relapse, early mortality, and survival with DEC10-VEN compared to intensive chemotherapy containing at least moderate dose of cytarabine. Even patients at high-risk of TRM with intensive chemotherapy based on validated models did not experience increased early mortality with DEC10-VEN. While the sample size for comparing high and low TRM risk group treated with DEC10-VEN limited the power to compare OS, the difference in level of ‘fitness’ between these two groups, as determined by the TRM score, likely explain the numerically lower OS in the high TRM risk group. The nearly superimposed EFS curves of patients at high vs low TRM risk treated with DEC10-VEN further support our hypothesis that DEC10-VEN offers comparable long-term outcomes in both groups. Patients in the DEC10-VEN and IC cohorts were treated during different era which need to be taken into consideration while interpreting these results. While supportive care has evolved over the 18-years during which the control group was treated, we have been uniformly using antimicrobial prophylaxis, laminar air-flow rooms, etc. at our institution during that period.12 The EFS analysis was helpful to remove any potential confounding effect which may have creeped in due to the improvement in supportive care and salvage therapies over this 20-year time period.25 The nearly identical benefit noted in both the OS and EFS analysis further strengthen our findings.
This study had a retrospective design which has inherent limitations. Limited number of older patients in our curated intensive chemotherapy database restricted matching to 1:1 and led to inclusion of some younger patients in the high TRM risk group who received intensive chemotherapy. The shorter median follow-up for DEC10-VEN compared to intensive chemotherapy was one potential limitation. However, even with longer follow-up, the difference in median OS and EFS in favor of DEC10-VEN is unlikely to change because only a small number of patients were censored prior to the median OS and EFS point. While propensity-score matching can balance important factors, unmeasured confounders could have influenced these findings.
Observed TRM with intensive chemotherapy was higher than expected in the overall and low TRM risk groups, which possibly contributed to the OS benefit observed with DEC10-VEN. However, baseline characteristics in these two groups were similar and nearly identical results in the overall comparison with two different models for early mortality further strengthen the validity of our findings. In addition, the discrepancy between predicted early mortality with the different models reflect the uncertainty with implementing such models in practice and highlight the need for periodic recalibration with incorporation of advancements in therapeutics and supportive care.26 While other models exist for predicting early mortality in AML, those require additional prospective data which were outside the scope of our analysis.13,27
Our analysis focused on the DEC10-VEN regimen and inclusion of patients treated with venetoclax and azacitidine or 5-day decitabine were outside the scope of this analysis. Other chemotherapy regimens like ‘3+7’ are less frequently used at our institution and newer regimens like CPX-351 are reserved for secondary AML patients which explains why these regimens were not included in our comparator group. These aspects may limit generalizability of our findings. Evolution of measurable residual disease (MRD) assays over the last 20 years also precluded comparison of MRD negativity rates with DEC10-VEN versus intensive chemotherapy.
Overall, this retrospective study showed that DEC10-VEN offers better outcomes compared to intensive chemotherapy in older patients with newly diagnosed AML. Patients deemed at either high or low risk of early mortality with intensive chemotherapy appear to benefit from DEC10-VEN. Clinical trials evaluating venetoclax with HMA in “fitter” and younger patients are ongoing. These results can help in designing future randomized trials of venetoclax with HMA versus intensive chemotherapy.
Supplementary Material
Acknowledgements:
We thank the patients and their caregivers; co-investigators, collaborators, and members of the study teams involved in these trials. We appreciate the comments and feedback from Dr. Elihu Estey, MD, and Dr. Ronald Walter, Seattle Cancer Care Alliance, during the initial conceptualization of this project and assistance with the treatment-related mortality score model.
Funding:
This study was supported in part by the MD Anderson Cancer Center Support Grant CA016672 from the National Cancer Institute, and Research Project Grant Program (R01CA235622) from the National Institutes of Health.
Conflict of Interests
AM: Research funding from Celgene Corporation
CRR: None
NP: Consulting/honorarium: Celgene; Stemline; Incyte; Novartis; MustangBio; Roche Diagnostics, LFB; Research funding/clinical trials support: Stemline; Novartis; Abbvie; Samus; Cellectis; Plexxikon; Daiichi-Sankyo; Affymetrix; Grants/funding: Affymetrix, SagerStrong Foundation
NGD: Sunesis Pharmaceuticals, Inc.: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Immunogen: Research Funding; Pfizer Inc.: Consultancy, Research Funding; Incyte Corporation: Honoraria, Research Funding; Bristol-Myers Squibb Company: Consultancy, Research Funding; Daiichi-Sankyo: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Otsuka America Pharmaceutical, Inc.: Consultancy; Kiromic: Research Funding; Jazz: Consultancy.
FR: Research funding from Amgen, Bristol-Myers Squibb, Merck, Seattle Genetics, Sunesis Pharmaceuticals, Honoraria from Amgen, Pfizer, Seattle Genetics, Sunesis Pharmaceuticals; Consulting or advisory role for Amgen, Seattle Genetics, Sunesis Pharmaceuticals.
GGM: None
GB: AbbVie: Research Funding; Incyte: Research Funding; Janssen: Research Funding; GSK: Research Funding; Cyclacel: Research Funding; BioLine Rx: Consultancy and Research Funding; NKarta: Consultancy; PTC Therapeutics: Consultancy; Oncoceutics, Inc.: Research Funding.
KN: None
MO: None
NJS: Takeda Oncology: Consultancy and Research Funding; AstraZeneca: Consultancy: Amgen: Honoraria
YA: Jazz Pharmaceuticals: Research Funding; Abbott: Honoraria
TMK: None
KT: Consultancy Symbio Pharmaceuticals.
NJ: AbbVie: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria and Membership on an entity’s Board of Directors or advisory committees; Genentech: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; BMS: Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Incyte: Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Servier: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Cellectis: Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding
KS: Otsuka: Honoraria; Pfizer: Consultancy
MA: Daiichi Sankyo, Inc. Consultancy, Patents & Royalties: Patents licensed, royalty bearing and Research Funding Jazz Pharmaceuticals Consultancy; Celgene Consultancy; Amgen Consultancy; AstaZeneca Consultancy; 6 Dimensions Capital Consultancy; Reata Equity Ownership; Aptose Equity Ownership; Eutropics Equity Ownership; Senti Bio Equity Ownership and Membership on an entity’s Board of Directors or advisory committees; Oncoceutics Equity Ownership; Oncolyze Equity Ownership; Breast Cancer Research Foundation Research Funding; CPRIT Research Funding; NIH/NCI Research Funding; Center for Drug Research & Development Membership on an entity’s Board of Directors or advisory committees; Cancer UK Membership on an entity’s Board of Directors or advisory committees; NCI-CTEP Membership on an entity’s Board of Directors or advisory committees; German Research Council Membership on an entity’s Board of Directors or advisory committees; Leukemia Lymphoma Society Membership on an entity’s Board of Directors or advisory committees; NCI-RDCRN (Rare Disease Cliln Network) Membership on an entity’s Board of Directors or advisory committees; CLL Foundation Membership on an entity’s Board of Directors or advisory committees; BiolineRx Membership on an entity’s Board of Directors or advisory committees;
PB: Incyte Corporation Consultancy, Research Funding and Speakers Bureau; Celgene Corporation Consultancy and Research Funding; Blueprint Medicines Corporation Consultancy and Research Funding; Kartos Therapeutics Consultancy and Research Funding; Constellation Pharmaceuticals Research Funding; Pfizer Research Funding; Astellas Pharmaceuticals Research Funding; NS Pharma Research Funding; Promedior Research Funding; CTI BioPharma Consultancy and Research Funding;
AF: None
GCI: None
EJJ: Consultancy Research funding from Takeda, BMS, Adaptive, Amgen, AbbVie, Pfizer, Cyclacel LTD
LM: None
PAT: None
SAP: None
JN: None
WQ: None
JSW: ArcherDx: Honoraria. Rigel Pharmaceuticals: Honoraria. Jansen Pharmaceuticals: Research Funding. Notable Laboratories: Research Funding.
CDD: AbbVie: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding, Calithera Biosciences – Research Funding, Jazz Pharmaceuticals – Honoraria, Notable Laboratories – Honoraria, Scientific Advisory Board Member
HMK: Ariad Research Funding; Astex Research Funding; BMS Research Funding; Cyclacel Research Funding; Daiichi-Sankyo Research Funding; Pfizer Honoraria and Research Funding; Immunogen Honoraria and Research Funding; Jazz Research Funding; Actinium Honoraria; Novartis Research Funding; Takeda Honoraria.
MYK: Calithera Research Funding; Stemline Therapeutics Consultancy, Honoraria and Research Funding; Forty-Seven Consultancy and Honoraria; Eli Lilly Research Funding; AbbVie Consultancy, Honoraria and Research Funding; Cellectis Research Funding; Amgen Consultancy and Honoraria; F. Hoffman La-Roche Consultancy, Honoraria and Research Funding; Genentech Honoraria and Research Funding; Ascentage Research Funding; Kisoji Consultancy and Honoraria; Reata Pharmaceuticals Equity Ownership and Patents & Royalties; Ablynx Research Funding; Astra Zeneca Research Funding; Agios Research Funding.
Footnotes
Clinical trial registration information: NCT03404193, NCT01019317, NCT02115295, NCT01289457, NCT00422591, NCT01802333, NCT00542971, NCT02464657, MD Anderson protocol # DM01-093, DM99-071.
Prior Presentation: These results were presented in an abstract form at the European Hematology Association Virtual Meeting, 2020.
Data Availability Statement: We will not be able to share individual patient level data outside of our institution.
Supplementary Material: Supplementary information is available on the journal website
References
- 1.Kantarjian H, Ravandi F, O’Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 2010;116(22):4422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin MG, Abboud CN. Induction therapy for elderly patients with acute myeloid leukemia. Blood Rev 2008;22(6):311–20. [DOI] [PubMed] [Google Scholar]
- 3.Webster JA, Pratz KW. Acute myeloid leukemia in the elderly: therapeutic options and choice. Leuk Lymphoma 2018;59(2):274–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeida AM, Ramos F. Acute myeloid leukemia in the older adults. Leuk Res Rep 2016;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med 2020;383(7):617–29. [DOI] [PubMed] [Google Scholar]
- 6.Pollyea DA, Pratz K, Letai A, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: Long term follow-up from a phase 1b study. Am J Hematol [Internet] [cited 2020 Nov 20];n/a(n/a). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/ajh.26039 [DOI] [PubMed] [Google Scholar]
- 7.DiNardo CD. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estey E, Karp JE, Emadi A, Othus M, Gale RP. Recent drug approvals for newly diagnosed acute myeloid leukemia: gifts or a Trojan horse? Leukemia 2020;34(3):671–81. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara F Venetoclax plus hypomethylating agents or low-dose cytarabine in acute myeloid leukemia: all that glitters is gold? Blood Cancer J 2020;10(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019;133(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei AH, Strickland SA, Hou J-Z, et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J Clin Oncol 2019;JCO.18.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantarjian H, O’brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer 2006;106(5):1090–8. [DOI] [PubMed] [Google Scholar]
- 13.Krug U, Röllig C, Koschmieder A, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet 2010;376(9757):2000–8. [DOI] [PubMed] [Google Scholar]
- 14.Wheatley K, Brookes CL, Howman AJ, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol 2009;145(5):598–605. [DOI] [PubMed] [Google Scholar]
- 15.Walter RB, Othus M, Borthakur G, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol Off J Am Soc Clin Oncol 2011;29(33):4417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haukoos JS, Lewis RJ. The Propensity Score. JAMA 2015;314(15):1637–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan CJ. Reducing bias using propensity score matching. J Nucl Cardiol 2018;25(2):404–6. [DOI] [PubMed] [Google Scholar]
- 18.Maiti A, DiNardo CD, Rausch CR, et al. Ten-Day Decitabine with Venetoclax (DEC10-VEN) in Acute Myeloid Leukemia: Updated Results of a Phase II Trial. Blood 2019;134(Supplement_1):2637–2637. [Google Scholar]
- 19.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003;21(24):4642–9. [DOI] [PubMed] [Google Scholar]
- 20.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129(4):424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas L, Li F, Pencina M. Using Propensity Score Methods to Create Target Populations in Observational Clinical Research. JAMA 2020;323(5):466–7. [DOI] [PubMed] [Google Scholar]
- 22.Thoemmes F Propensity score matching in SPSS. 2012. [cited 2020 Sep 4];Available from: https://arxiv.org/abs/1201.6385v1
- 23.Rubin DB. Using Propensity Scores to Help Design Observational Studies: Application to the Tobacco Litigation. Health Serv Outcomes Res Methodol 2001;2(3):169–88. [Google Scholar]
- 24.Jupiter DC. Propensity Score Matching: Retrospective Randomization? J Foot Ankle Surg 2017;56(2):417–20. [DOI] [PubMed] [Google Scholar]
- 25.Maiti A, Kantarjian HM, Popat V, et al. Clinical value of event-free survival in acute myeloid leukemia. Blood Adv 2020;4(8):1690–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter RB, Estey EH. Selection of initial therapy for newly-diagnosed adult acute myeloid leukemia: Limitations of predictive models. Blood Rev 2020;100679. [DOI] [PubMed] [Google Scholar]
- 27.Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol 2007;136(4):624–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


