Abstract
High body mass index (BMI) and obesity have been implicated as risk factors for lumbar degenerative disc disease and low back pain. Despite this, there is limited in vivo data quantifying how obesity influences the mechanical function of intervertebral discs (IVD) in response to activities of daily living. Recently, our lab has developed methodologies to non-invasively measure in vivo IVD deformation resulting from activities of daily living using magnetic resonance (MR) imaging and solid modeling techniques. This pilot study expands on these methodologies to assess how BMI influences IVD deformation following treadmill walking in eight asymptomatic individuals. Ordinary least squares regression analyses revealed a statistically significant relationship between BMI and compressive deformation (strain (%)) in the L5-S1 IVD (R2 = 0.61, p < 0.05). This relationship was weaker in the L3-L4 (R2 = 0.28, p > 0.05) and L4-L5 IVDs (R2 = 0.28, p > 0.05). Importantly, no relationship between pre-exercise disc height and BMI was identified (p > 0.05). Therefore, the results of this study suggest that BMI may alter the mechanical response of lumbar spine IVDs, particularly at the L5-S1 level. Furthermore, the observed relationship between increased BMI and IVD compressive deformation, in the absence of a detected relationship between pre-exercise disc height and BMI, suggests that changes in IVD mechanical function may be more sensitive to alterations in disc health than static clinical imaging alone. This finding highlights the importance of quantifying disc mechanical function when examining the relationship between BMI and IVD degeneration.
Keywords: obesity, MRI, in vivo, spine, strain, gait, imaging
Introduction
Despite a number of studies identifying relationships between elevated body mass index (BMI; overweight > 25 kg/m2: obesity > 30 kg/m2), and the prevalence of low back pain and lumbar degenerative disc disease (Akhavanfar et al., 2018; Nilsen et al., 2011; Raastad et al., 2015; Shiri et al., 2010; WHO, 2000), there is relatively limited evidence exploring the etiological mechanisms underlying these relationships. From a diagnostic perspective, static imaging of the spine has been inconsistent in identifying those at risk of disc degeneration or the development of low back pain (Beattie et al., 2000; Goode et al., 2013; Steffens et al., 2014; Taylor et al., 2014; Tonosu et al., 2017). Moreover, while several studies have attributed the increased risk of lumbar pathology to elevated body mass (Liuke et al., 2005; Rodriguez-Martinez et al., 2016; Samartzis et al., 2011; Takatalo et al., 2013), there is a lack of in vivo data to evaluate the effects of increased BMI and obesity on intervertebral disc (IVD) mechanical function.
Recently, our lab has developed methods that allow for non-invasive measurement of in vivo IVD function in response to daily activity. These methods leverage magnetic resonance (MR) imaging and solid modeling techniques to measure changes in disc height, which arise due to fluid flow out of the disc with loading (Martin et al., 2018). Expanding on this framework, the objective of this pilot study was to evaluate the effect of BMI on IVD mechanical function following 30 minutes of treadmill walking by examining exercised-induced IVD compressive deformation (strain (%)). As changes in disc health may affect the mechanical function of the disc, we hypothesized that compressive IVD deformation resulting from a treadmill walking stress test would increase with increasing BMI.
Methods
Subject Recruitment:
In this pilot study, we recruited eight asymptomatic participants (5 males and 3 females; mean age 32 years [range 20–63]; mean BMI 26.6 kg/m2 [range 22.0–34.2 kg/m2]) with no history of pain, injury, or surgery in the lower back. Institutional review board (IRB) approval was granted prior to the start of all study activities, and participants provided written informed consent prior to their participation.
Data Collection:
Subjects were instructed not to perform any strenuous activity the day prior to and the morning of the study visit. Upon arrival, participants’ height, weight, and demographic information were collected. Height and weight data were used in the calculation of BMI. Participants remained supine for 45 minutes prior to the baseline MRI scan (Martin et al., 2018). Imaging began at 8AM to minimize the effects of diurnal changes in disc height (Martin et al., 2018). To avoid weight-bearing, subjects were transported to the MRI scanner on a stretcher. MRIs were obtained using a 3.0-T scanner (Tim Trio, Siemens) and an 8-channel body matrix coil. Sagittal-plane images of the subject’s lumbar spine were generated using a 3D T2-weighted SPACE sequence (TE: 223 ms; TR: 2500 ms; in-plane resolution, 0.9 mm × 0.9 mm; slice thickness, 0.9 mm; matrix, 320×320 pixels2). Additionally, a 3D FLASH sequence (TE: 3.7 ms; TR: 9.0 ms; flip angle, 20°; in-plane resolution, 1 mm × 1 mm; slice thickness, 1 mm; matrix, 256×224 pixels2) was obtained for use in segmentation of IVD geometry.
IVD Stress Test
Following the baseline scan, subjects walked on a treadmill for 30 minutes at a constant speed normalized to their limb length using a Froude number (Fr) equal to 0.25 (Alexander and Jayes, 1983; Collins et al., 2018; Paranjape et al., 2019). Limb length was defined as the vertical distance from the ground to the greater trochanter of the femur. Immediately following exercise, subjects were transported back to the scanner to complete post-exercise MRI scans using the same sequences described above.
Model Segmentation
Deidentified MRIs were imported into solid modeling software (Rhinoceros Robert McNeel and Associates, Seattle, WA), where a single investigator manually segmented the outer surfaces of the L3-L4, L4-L5 and L5-S1 IVDs for both the pre and post-exercise scans (Figure 1A). Segmentations were reviewed by a musculoskeletal radiologist with 30 years of experience. Due to sequence availability, IVD models for two subjects were segmented using the FLASH sequence while the remaining six were segmented using the SPACE sequence The segmentations were then exported to MATLAB (Mathworks, Natick, MA) as point clouds for further data processing.
Figure 1: IVD segmentation processing.
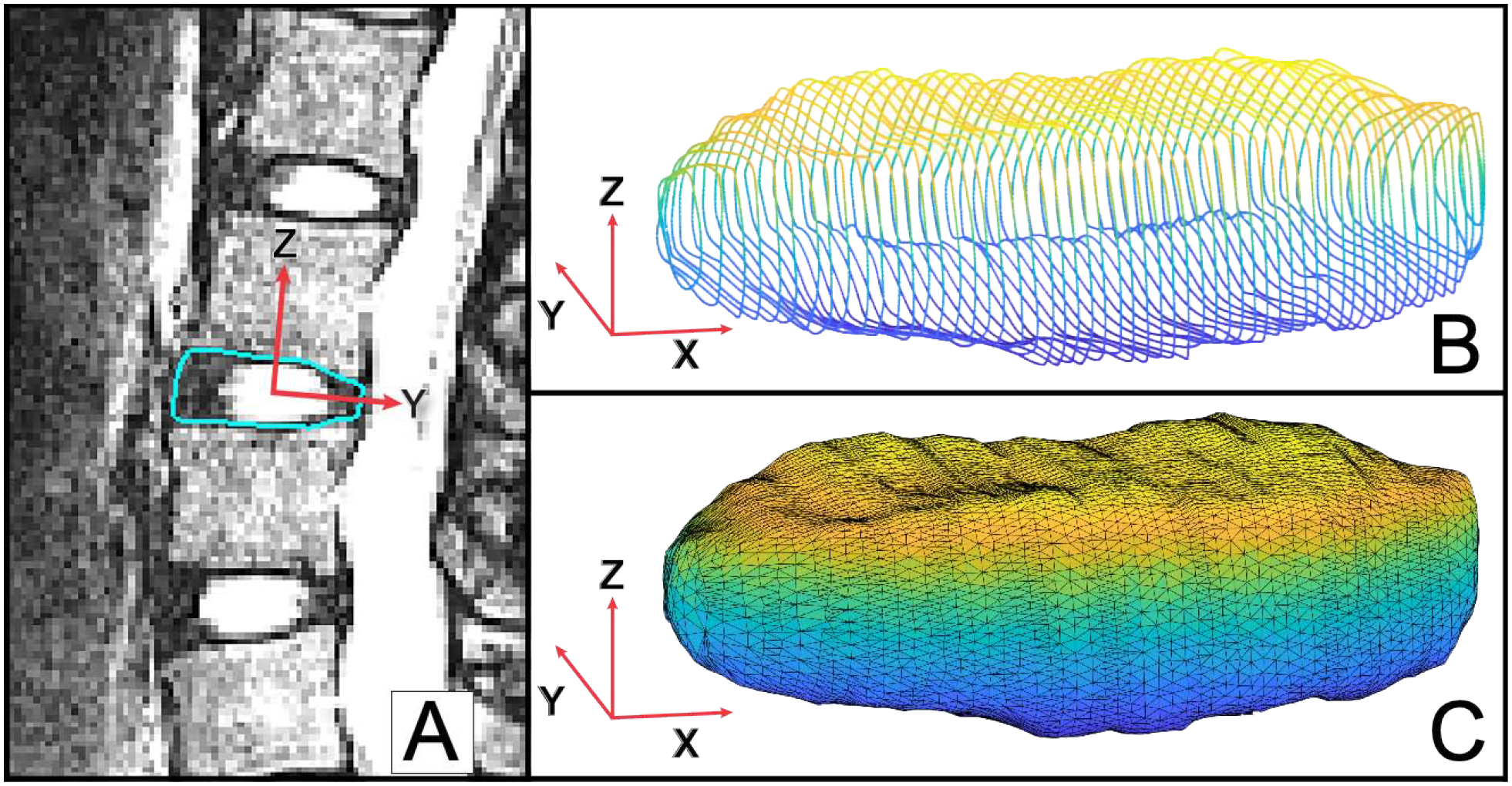
Visualization of the directions of the 1st, 2nd and 3rd principal component vectors are denoted by the X, Y and Z axes, respectively. The Z-axis demonstrates the axis along which height is calculated. (A) The outer contours of each IVD were segmented on each slice of the MR images. (B) Segmentation yielded a 3D wireframe model of each IVD. (C) Following alignment of the 3rd principal component vector with the z-axis of the cartesian coordinate system, a 3D triangulation mesh of the disc was created for subsequent analyses.
Segmentation Repeatability
A repeatability analysis was performed in order to investigate the reliability of the SPACE and FLASH sequences for IVD segmentation. To this end, the L5-S1 disc of one normal and one high BMI subject were traced in triplicate for both FLASH and SPACE sequences (n = 12 segmentations). Repeatability was assessed by evaluating the root mean-squared error (RMSE) of mean disc height between segmentations.
Model Processing
The three principal axes of each disc segmentation were identified via eigenvalue decomposition of the disc point clouds. All disc models were then aligned such that the third principal axis of each disc, which corresponds to the height of the disc, was coincident with the z-axis of the cartesian coordinate system (Figure 1B). Following alignment, 3D triangulated meshes of the discs were created using Geomagic Studio 11 (3D Systems, Cary, NC) and reimported into MATLAB (Figure 1C). The superior and inferior surfaces (Figure 2A) of the disc were then identified using a custom perimeter detection algorithm. Each surface was determined to lie within the boundary points when the absolute value of the angle between the z-axis and the mesh-element (Figure 1C) face normal vector fell below 45°. Mesh vertices within the perimeter were then uniformly sampled (Figure 2A).
Figure 2: IVD surface generation, height calculation and regional disc divisions.
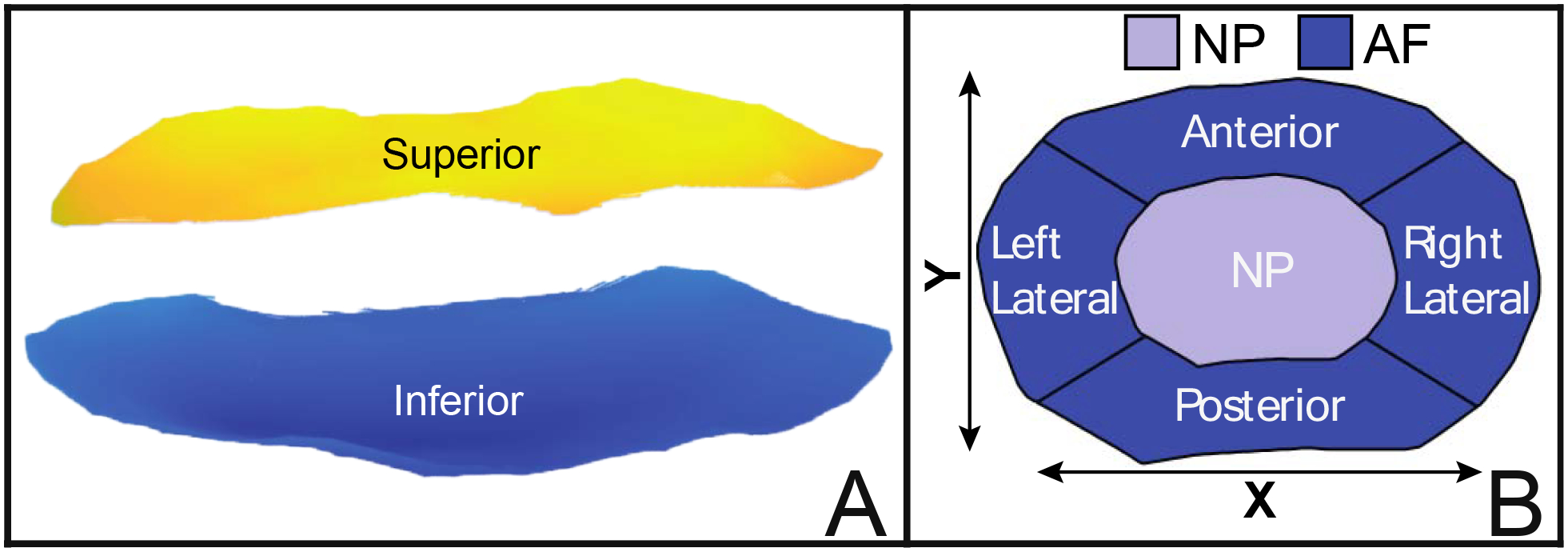
(A) An automatic perimeter detection algorithm was applied to the triangulated disc meshes (Figure 1C) and the mesh elements within the perimeter were then used to create uniformly sampled superior (yellow) and inferior (blue) surfaces. (B) Each disc was subsequently divided into five regions to obtain measures of disc height: Nucleus Pulposus (NP), Anterior Annulus Fibrosus (AF), Posterior AF, Left Lateral AF, and Right Lateral AF. Starting at the centroid of the disc, the NP Region was defined by all points within [0–50%] of an individual disc’s semi-axes length (i.e., X/Y-axis), while the AF was defined by all points within [50–100%] of disc semi-axes length. Lateral divisions of the AF form an arc of 60°, while anterior-posterior divisions form an arc of 120°. Disc height within each region was then calculated as the mean difference between the points in the superior disc region minus the points in the corresponding inferior region.
Disc surfaces were subsequently divided into 5 regions (Figure 2B): the Nucleus Pulposus (NP), Anterior Annulus Fibrosus (AF), Posterior AF, Left Lateral AF, and Right Lateral AF. Starting at the centroid of the disc, the NP was defined as the region comprised of all data points within [0–50%] of disc semi-axes length, while Annulus Fibrosus regions were defined on the bounds [50–100%] of disc semi-axes length. Semi-axis lengths were defined as half of the maximum distance across the disc, corresponding to the directions of the first (lateral diameter) and second (anterior-posterior diameter) principal component vectors (X-Axis, Y-Axis, Figure 2B).
Outcome Variables
Disc height within each region (Figure 2B) was defined as the mean difference between the points in the superior disc region and the points in the corresponding inferior region. Accordingly, IVD strain was defined as the percent change in disc height from the pre (L0) to the post (L) MRI scan . Mean strain and height values were calculated as the unweighted averages of the outcome variables across all regions within the level of interest. Figure 3 depicts a visualization of the mean pre/post-disc height and the corresponding strain values for each disc level evaluated in this study.
Figure 3: Subject IVD maps.
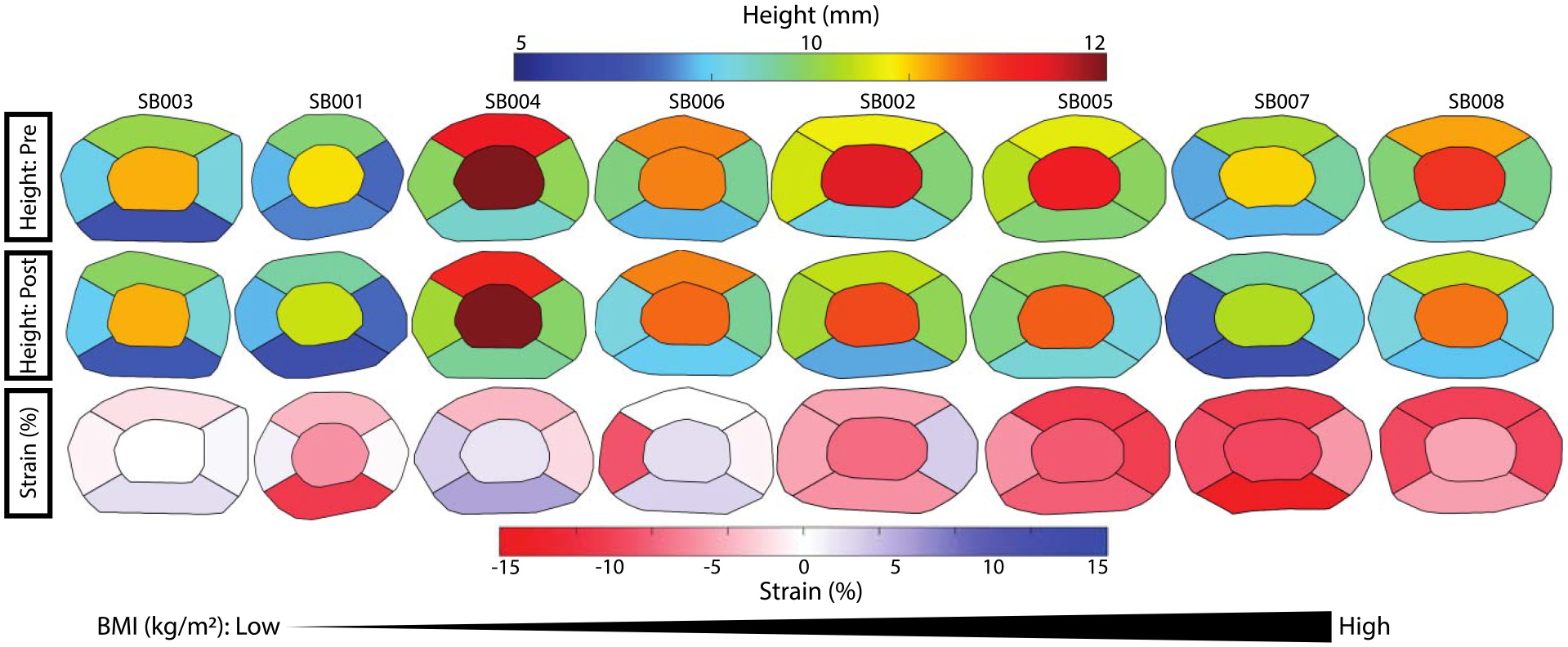
Subject-level IVD maps showing mean disc height (all levels) and regional strain measurements. In rows one (pre) and two (post) warmer colors are indicative of greater disc thickness, whereas cooler colors indicate smaller disc thicknesses. In row three compressive strain (%) is shown in red. Subjects are organized in order of increasing BMI from left to right across the page.
Statistical Analysis:
The relationship between mean BMI and pre-exercise disc height with mean IVD strain at each level was evaluated using separate ordinary least squares (OLS) regression analyses. Age was examined as a potential confounding variable. However, no substantial change in the effect size was noted between bivariate and age-controlled models. As such, here we present our primary results from the bivariate models. In the case of the L5-S1 level, one subject’s (SB002) disc was not included in the final analyses due to difficulties visualizing the disc. In order to preserve sample size across all models, an imputation of SB002s L5-S1 data was made. The imputed values were based upon the mean value of all other subjects’ L5-S1 discs. Additionally, we conducted a simple sensitivity analysis comparing the regression models with and without imputation. We found that imputation did not affect the statistical significance of the regression analyses. Therefore, results utilizing the imputed data are presented here. A p-value of less than 0.05 was used to indicate statistical significance; additionally the measure of effect-size (R2) and model accuracy (RMSE) were obtained via the OLS regressions.
Results
Repeatability results yielded a mean regional height standard deviation of 87 μm in segmentations performed using the SPACE MRI sequence and 114 μm in those utilizing FLASH, across triplicate segmentations, demonstrating comparable results to previously reported repeatability studies using FLASH (Martin et al., 2018). Here, the results indicated negligible differences between segmentations obtained using FLASH and SPACE.
Statistically, no significant relationship (p > 0.05) between BMI and strain was detected in either the L3-L4 or L4-L5 IVDs. However, the OLS regressions revealed a significant relationship between BMI and IVD strain (p < 0.05; R2 = 0.61; RMSE: 3.95, Figure 4C) in the L5-S1 IVD. Additionally, no significant relationship (p > 0.05, Figure 5) was detected between BMI and disc height prior to exercise at any level.
Figure 4: OLS regression results:
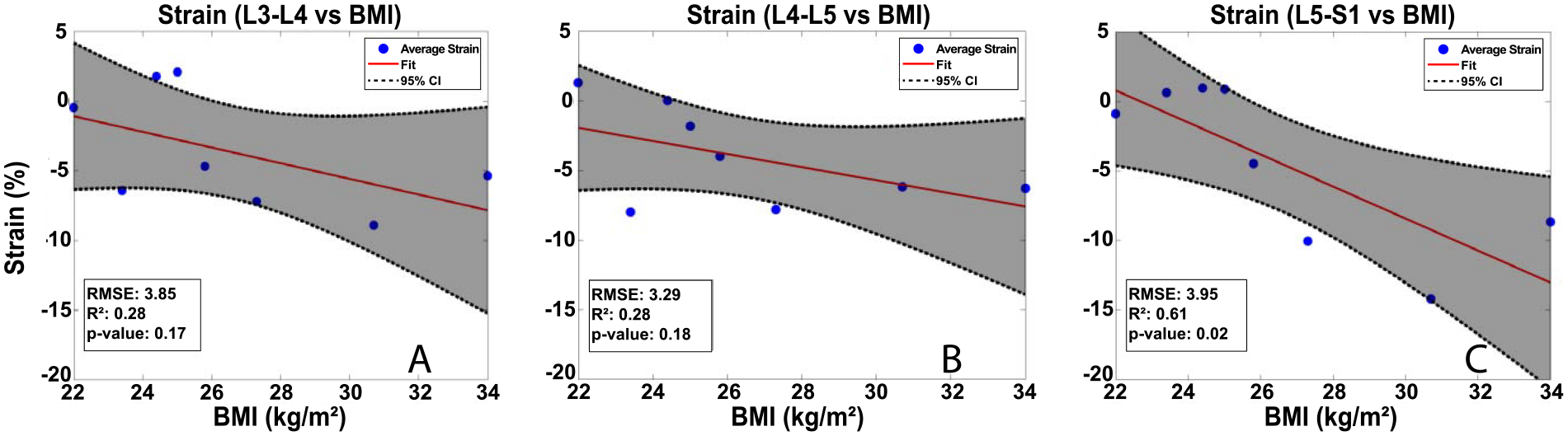
IVD strain increased with increasing BMI at the L3-L4 (A) and L4-L5 (B) levels, although these relationships were not statistically significant at p<0.05. (C) A strong statistically significant (p<0.05) relationship between BMI and IVD strain was detected in the L5-S1 level.
Figure 5: OLS regression results:
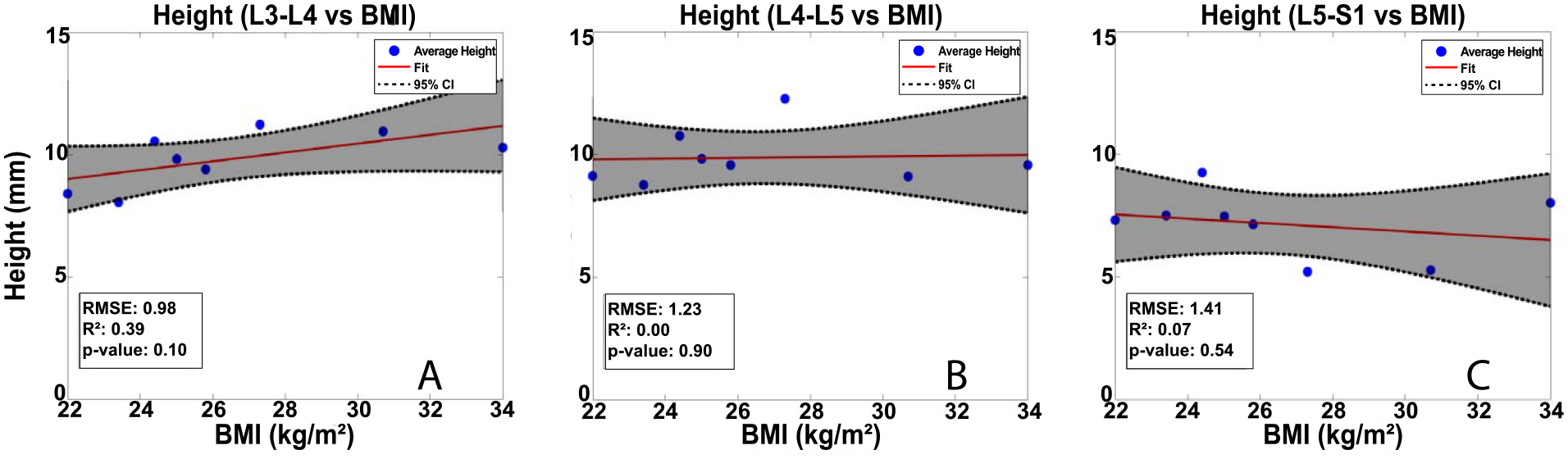
Mean pre-exercise disc height was not related to BMI at any level. Baseline disc height for each level and subject was defined as the unweighted average of disc heights in the 5 regions described in Figure 2.
Discussion
Obesity has been implicated as a risk factor for lumbar IVD degenerative disc disease and low back pain (Akhavanfar et al., 2018; Raastad et al., 2015; Shiri et al., 2010). However, the mechanisms by which obesity increases the risk of IVD degeneration and low back pain is poorly understood (Nilsen et al., 2011; Rodriguez-Martinez et al., 2016). Furthermore, there is conflicting evidence regarding the efficacy of static clinical imaging techniques in identifying degenerative lumbar spine structures that are related to the development of low back pain (Beattie et al., 2000; Goode et al., 2013; Steffens et al., 2014; Taylor et al., 2014; Tonosu et al., 2017). Therefore, in the present study we aimed to improve understanding of the relationship between increased BMI and the mechanical function of the IVD by measuring the influence of BMI on IVD mechanical response to exercise. The results of this study indicate that lumbar IVD deformation following treadmill walking increases with increasing BMI, particularly at the L5-S1 level. Moreover, no statistically significant relationship between pre-exercise disc height and BMI was observed, suggesting that pre-exercise disc height did not affect the observed relationship between BMI and IVD strain.
Several reviews have highlighted the difficulty clinicians and researchers face in finding reliable predictors of low back pain from static clinical images (Beattie et al., 2000; Steffens et al., 2014; Taylor et al., 2014; Tonosu et al., 2017). In the present study, there was likewise no discernable relationship between baseline disc height (Griffith et al., 2007; Luoma et al., 2000) and BMI. Meanwhile, we observed that BMI was strongly correlated to IVD deformation following treadmill walking. This finding underscores the importance of utilizing dynamic measures of lumbar spine mechanics to better assess the health of the IVDs. Indeed, functional measures of lumbar disc mechanics may be able to identify those at risk for disc degeneration prior to disc height collapse, which may provide insight into the etiology of low back pain (De Schepper et al., 2010; Luoma et al., 2000).
Importantly, although we sought to minimize the time between exercise cessation and the post-exercise MR acquisition [mean: 4 min 17 sec, range: 3–6 min], the IVD strains measured in this study may be an underestimate of the strains occurring immediately after walking due to recovery of disc height prior to imaging. In addition, although the findings of this pilot study provide important information regarding the relationship between BMI and IVD mechanics, questions remain regarding the mechanisms that underlie this relationship. Therefore, future work will investigate the potential influences of quantitative MRI (qMR) metrics of disc composition, such as T2 and T1rho map (Battié et al., 2009; Belavý et al., 2017; Iriondo et al., 2020; Paul et al., 2018; Yoon et al., 2016), on the relationship between BMI and IVD strain due to walking.
To this point, there remain conflicting theories surrounding the role of loading in IVD health. Recent evidence suggests that individuals who incur greater magnitudes of physiologic loading (i.e. runners and obese individuals) may have beneficially altered IVD composition and mechanical function as indicated by qMR metrics (Battié et al., 2009; Belavý et al., 2017; Mitchell et al., 2020; Videman et al., 2010). Yet, others hypothesize that increased loading due to high BMI may produce cumulative injury to the discs, ultimately leading to low back pain or IVD degeneration (Adams et al., 2015; Leboeuf-Yde et al., 2008; Liuke et al., 2005; MacEdo and Battié, 2019). Accordingly, biomechanical modeling studies have shown that increased BMI substantially increases loads in the lumbar IVDs (Ghezelbash et al., 2017; Hajihosseinali et al., 2015; Han et al., 2013). Thus, it remains unclear whether the observed relationship between BMI and increased compressive IVD strain following walking can be attributed to increased loading alone or to differences in the mechanical function of the IVDs, such as stiffness (Iriondo et al., 2020; Pandit et al., 2016; Yoon et al., 2016).
Moving forward, we aim to expand upon these whole-disc measurements by investigating more localized measures of IVD mechanical function. Future work may also investigate how additional variables, such as age, sex and exercise modality affect IVD mechanical response. Such data may provide additional insight into the contribution of altered kinematics to the localized changes in IVD disc deformations.
In summary, we implemented an MR imaging protocol to obtain a measure of IVD mechanics in response to treadmill walking. Using this protocol, we identified a significant relationship between increasing BMI and IVD strain in the L5-S1 disc in the absence of a relationship between pre-exercise disc height and BMI. Therefore, this study suggests that measures of in vivo IVD function may improve insight into IVD health, particularly in the context of understanding the increased risk of IVD disease with obesity and high BMI.
Acknowledgements
This work was supported by NIH grants R01AR074800, R01AR065527, R01AR075399, and R01071440. We would like to thank Jean Shaffer and Raven Boykin at the Duke Center for Advanced Magnetic Resonance Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflicts of interests.
References
- Adams MA, Lama P, Zehra U, Dolan P, 2015. Why do some intervertebral discs degenerate, when others (in the same spine) do not? Clin. Anat 10.1002/ca.22404 [DOI] [PubMed] [Google Scholar]
- Akhavanfar MH, Kazemi H, Eskandari AH, Arjmand N, 2018. Obesity and spinal loads; a combined MR imaging and subject-specific modeling investigation. J. Biomech 10.1016/j.jbiomech.2017.08.009 [DOI] [PubMed] [Google Scholar]
- Alexander RMN, Jayes AS, 1983. A dynamic similarity hypothesis for the gaits of quadrupedal mammals. J. Zool 10.1111/j.1469-7998.1983.tb04266.x [DOI] [Google Scholar]
- Battié MC, Videman T, Kaprio J, Gibbons LE, Gill K, Manninen H, Saarela J, Peltonen L, 2009. The Twin Spine Study: Contributions to a changing view of disc degeneration†. Spine J. 10.1016/j.spinee.2008.11.011 [DOI] [PubMed] [Google Scholar]
- Beattie PF, Meyers SP, Stratford P, Millard RW, Hollenberg GM, 2000. Associations between patient report of symptoms and anatomic impairment visible on lumbar magnetic resonance imaging. Spine (Phila. Pa. 1976). 10.1097/00007632-200004010-00010 [DOI] [PubMed] [Google Scholar]
- Belavý DL, Quittner MJ, Ridgers N, Ling Y, Connell D, Rantalainen T, 2017. Running exercise strengthens the intervertebral disc. Sci. Rep 10.1038/srep45975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AT, Kulvaranon ML, Cutcliffe HC, Utturkar GM, Smith WAR, Spritzer CE, Guilak F, Defrate LE, 2018. Obesity alters the in vivo mechanical response and biochemical properties of cartilage as measured by MRI. Arthritis Res. Ther 10.1186/s13075-018-1727-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schepper EIT, Damen J, Van Meurs JBJ, Ginai AZ, Popham M, Hofman A, Koes BW, Bierma-Zeinstra SM, 2010. The association between lumbar disc degeneration and low back pain: The influence of age, gender, and individual radiographic features. Spine (Phila. Pa. 1976). 10.1097/BRS.0b013e3181aa5b33 [DOI] [PubMed] [Google Scholar]
- Ghezelbash F, Shirazi-Adl A, Plamondon A, Arjmand N, Parnianpour M, 2017. Obesity and Obesity Shape Markedly Influence Spine Biomechanics: A Subject-Specific Risk Assessment Model. Ann. Biomed. Eng 10.1007/s10439-017-1868-7 [DOI] [PubMed] [Google Scholar]
- Goode AP, Carey TS, Jordan JM, 2013. Low back pain and lumbar spine osteoarthritis: How are they related? Curr. Rheumatol. Rep 10.1007/s11926-012-0305-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JF, Wang YXJ, Antonio GE, Choi KC, Yu A, Ahuja AT, Leung PC, 2007. Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine (Phila. Pa. 1976). 10.1097/BRS.0b013e31815a59a0 [DOI] [PubMed] [Google Scholar]
- Hajihosseinali M, Arjmand N, Shirazi-Adl A, 2015. Effect of body weight on spinal loads in various activities: A personalized biomechanical modeling approach. J. Biomech 10.1016/j.jbiomech.2014.11.033 [DOI] [PubMed] [Google Scholar]
- Han KS, Rohlmann A, Zander T, Taylor WR, 2013. Lumbar spinal loads vary with body height and weight. Med. Eng. Phys 10.1016/j.medengphy.2012.09.009 [DOI] [PubMed] [Google Scholar]
- Iriondo C, Pedoia V, Majumdar S, 2020. Lumbar intervertebral disc characterization through quantitative MRI analysis: An automatic voxel-based relaxometry approach. Magn. Reson. Med 10.1002/mrm.28210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboeuf-Yde C, Kjær P, Bendix T, Manniche C, 2008. Self-reported hard physical work combined with heavy smoking or overweight may result in so-called Modic changes. BMC Musculoskelet. Disord 10.1186/1471-2474-9-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuke M, Solovieva S, Lamminen A, Luoma K, Leino-Arjas P, Luukkonen R, Riihimäki H, 2005. Disc degeneration of the lumbar spine in relation to overweight. Int. J. Obes 10.1038/sj.ijo.0802974 [DOI] [PubMed] [Google Scholar]
- Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A, 2000. Low back pain in relation to lumbar disc degeneration. Spine (Phila. Pa. 1976). 10.1097/00007632-200002150-00016 [DOI] [PubMed] [Google Scholar]
- MacEdo LG, Battié MC, 2019. The association between occupational loading and spine degeneration on imaging- A systematic review and meta-analysis. BMC Musculoskelet. Disord 10.1186/s12891-019-2835-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JT, Oldweiler AB, Spritzer CE, Soher BJ, Erickson MM, Goode AP, DeFrate LE, 2018. A magnetic resonance imaging framework for quantifying intervertebral disc deformation in vivo: Reliability and application to diurnal variations in lumbar disc shape. J. Biomech 10.1016/j.jbiomech.2018.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell UH, Bowden JA, Larson RE, Belavy DL, Owen PJ, 2020. Long-term running in middle-aged men and intervertebral disc health, a cross-sectional pilot study. PLoS One. 10.1371/journal.pone.0229457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen TIL, Holtermann A, Mork PJ, 2011. Physical exercise, body mass index, and risk of chronic pain in the low back and neck/shoulders: Longitudinal data from the nord-trøndelag health study. Am. J. Epidemiol 174, 267–273. 10.1093/aje/kwr087 [DOI] [PubMed] [Google Scholar]
- Pandit P, Talbott JF, Pedoia V, Dillon W, Majumdar S, 2016. T1ρ and T2-based characterization of regional variations in intervertebral discs to detect early degenerative changes. J. Orthop. Res 10.1002/jor.23311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape CS, Cutcliffe HC, Grambow SC, Utturkar GM, Collins AT, Garrett WE, Spritzer CE, DeFrate LE, 2019. A New Stress Test for Knee Joint Cartilage. Sci. Rep 10.1038/s41598-018-38104-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul CPL, Smit TH, de Graaf M, Holewijn RM, Bisschop A, van de Ven PM, Mullender MG, Helder MN, Strijkers GJ, 2018. Quantitative MRI in early intervertebral disc degeneration: T1rho correlates better than T2 and ADC with biomechanics, histology and matrix content. PLoS One. 10.1371/journal.pone.0191442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raastad J, Reiman M, Coeytaux R, Ledbetter L, Goode AP, 2015. The association between lumbar spine radiographic features and low back pain: A systematic review and meta-analysis. Semin. Arthritis Rheum 10.1016/j.semarthrit.2014.10.006 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martinez NG, Perez-Orribo L, Kalb S, Reyes PM, Newcomb AGUS, Hughes J, Theodore N, Crawford NR, 2016. The role of obesity in the biomechanics and radiological changes of the spine: An in vitro study. J. Neurosurg. Spine 10.3171/2015.7.SPINE141306 [DOI] [PubMed] [Google Scholar]
- Samartzis D, Karppinen J, Mok F, Fong DYT, Luk KDK, Cheung KMC, 2011. A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J. Bone Jt. Surg. - Ser. A 10.2106/JBJS.I.01568 [DOI] [PubMed] [Google Scholar]
- Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E, 2010. The association between obesity and low back pain: A meta-analysis. Am. J. Epidemiol 10.1093/aje/kwp356 [DOI] [PubMed] [Google Scholar]
- Steffens D, Hancock MJ, Maher CG, Williams C, Jensen TS, Latimer J, 2014. Does magnetic resonance imaging predict future low back pain? A systematic review. Eur. J. Pain (United Kingdom) 10.1002/j.1532-2149.2013.00427.x [DOI] [PubMed] [Google Scholar]
- Takatalo J, Karppinen J, Taimela S, Niinimäki J, Laitinen J, Sequeiros RB, Samartzis D, Korpelainen R, Näyhä S, Remes J, Tervonen O, 2013. Association of Abdominal Obesity with Lumbar Disc Degeneration - A Magnetic Resonance Imaging Study. PLoS One. 10.1371/journal.pone.0056244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JB, Goode AP, George SZ, Cook CE, 2014. Incidence and risk factors for first-time incident low back pain: A systematic review and meta-analysis. Spine J. 10.1016/j.spinee.2014.01.026 [DOI] [PubMed] [Google Scholar]
- Tonosu J, Oka H, Higashikawa A, Okazaki H, Tanaka S, Matsudaira K, 2017. The associations between magnetic resonance imaging findings and low back pain: A 10-year longitudinal analysis. PLoS One. 10.1371/journal.pone.0188057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videman T, Gibbons LE, Kaprio J, Battié MC, 2010. Challenging the cumulative injury model: positive effects of greater body mass on disc degeneration. Spine J. 10.1016/j.spinee.2009.10.005 [DOI] [PubMed] [Google Scholar]
- WHO, 2000. World Health Organization. Obesity Preventing and Managing the Global Epidemic, Report of a WHO Consultation. [PubMed] [Google Scholar]
- Yoon MA, Hong SJ, Kang CH, Ahn KS, Kim BH, 2016. T1rho and T2 mapping of lumbar intervertebral disc: Correlation with degeneration and morphologic changes in different disc regions. Magn. Reson. Imaging 10.1016/j.mri.2016.04.024 [DOI] [PubMed] [Google Scholar]


