Abstract
Clinical outcomes vary for individuals at clinical high risk (CHR) for psychosis, ranging from conversion to a psychotic disorder to full remission from the risk syndrome. Given that the majority of CHR individuals do not convert to psychosis, recent research efforts have turned toward identifying specific predictors of CHR remission, a task that is conceptually and empirically dissociable from identification of predictors of conversion to psychosis, and one that may reveal specific biological characteristics that confer resilience to psychosis and provide further insights into the mechanisms associated with the pathogenesis of schizophrenia and those underlying a transient CHR syndrome. Such biomarkers may ultimately facilitate the development of novel early interventions and support the optimization of individualized care. In this review, we focus on two event-related brain potential measures, mismatch negativity (MMN) and P300, that have attracted interest as predictors of future psychosis among CHR individuals. We describe several recent studies examining whether MMN and P300 predict subsequent CHR remission and suggest that intact MMN and P300 may reflect the integrity of specific neurocognitive processes that confer resilience against the persistence of the CHR syndrome and its associated risk for future transition to psychosis. We also highlight several major methodological concerns associated with these studies that apply to the broader literature examining predictors of CHR remission. Among them is the concern that studies that predict dichotomous remission vs. non-remission and/or dichotomous conversion vs. non-conversion outcomes potentially confound remission and conversion effects, a phenomenon we demonstrate with a data simulation.
Keywords: electroencephalography, mismatch negativity, P300, clinical high risk for psychosis, remission, schizophrenia
Observations that a shorter duration of untreated psychosis predicts better clinical outcomes (1) has led to international efforts to understand the early phases of psychotic disorders. In support of these efforts, clinical criteria have been developed for identifying individuals who are at “clinical high risk” (CHR) for developing psychosis, also known as the “psychosis risk syndrome” or “at-risk mental state” (2–5). These CHR criteria generally include the presence of attenuated positive symptoms, brief intermittent full psychosis symptoms, and/or recent deterioration in functioning with genetic risk for psychosis (2–5). Using these criteria, researchers can prospectively identify and follow individuals considered to be at risk with the goal of clarifying pathogenic mechanisms associated with psychosis onset.
While algorithms using clinical and cognitive data to predict future conversion to psychosis among CHR individuals have now been developed and validated (6,7), they are not yet sufficiently accurate to support major treatment decisions in the clinic. Accordingly, recent research has focused on identifying biomarkers that precede psychosis onset, and major advances have been made in identifying electrophysiological (see 8,9,10), neuroimaging (see 10,11), and other biological markers (12,13) that are associated with future conversion to psychosis among CHR individuals.
Although the validity of CHR criteria for predicting future risk for psychosis has been demonstrated (14), the 40–50% psychosis conversion rate initially reported when the CHR paradigm was developed (15) has since declined substantially (14), with recent estimates indicating a rate of 22–36% within two to three years of ascertainment (6,14,16–19). According to meta-analysis, 73% of CHR individuals do not convert to psychosis within two years, and approximately 43% fully remit from the CHR syndrome (20). Given the range of clinical outcomes among CHR individuals and the fact that most do not transition to psychosis, recent efforts have turned toward identifying specific predictors of CHR remission in addition to markers of future psychosis conversion.
Indeed, there are several advantages to focusing on predictors of remission among CHR individuals. Importantly, the identification of biomarkers that can reliably distinguish future CHR remitters from nonremitters may reveal specific characteristics that confer resilience to psychosis and provide further insights into the mechanisms associated with both the pathogenesis of schizophrenia as well as those underlying transient attenuated psychosis symptoms. Such biomarkers may, in turn, also help to identify more precise, mechanistically informed treatment targets, facilitating the development of novel early interventions. In addition, the ability to distinguish future remitters from those whose symptoms are likely to persist would benefit future clinical trials; enriching trial samples for psychosis risk by screening out CHR individuals most likely to remit would minimize the likelihood of inflated placebo effects or even false positive results (21). Furthermore, biomarkers that predict clinical remission may ultimately support efforts to develop staged treatment algorithms, ultimately providing an opportunity for the optimization of individualized care.
While current efforts to identify predictors of CHR remission are examining a range of biomarker, neurocognitive, and/or clinical measures, the goal of the current article is to review studies that have specifically assessed electroencephalography (EEG)-based event-related potential (ERP) measures. ERP components, which reflect voltage fluctuations in EEG scalp-recordings time-locked to specific task stimuli, have several attractive features as potential predictive biomarkers of CHR outcomes, including their low-cost, high temporal resolution, and translational links with homologous electrophysiological signals recorded in animal models. Here, we specifically focus on two ERP measures, mismatch negativity (MMN) and P300, that have been the major focus of EEG-based CHR remission studies to date, likely because they were already shown to predict conversion to psychosis (e.g., 22,23–26), and because of the substantial prior literature documenting their deficient amplitudes in schizophrenia (e.g., 27,28–30).
In addition to reviewing the MMN and P300 CHR remission studies published to date, we highlight methodological issues illustrated by these studies, many of which are applicable to the broader literature and inform our recommendations for best practices for future studies. One of these issues, the confounding of prediction of remission with prediction of conversion effects, is identified as a major concern that arises in most studies examining a wide range of predictors of CHR remission. We also provide a data simulation to demonstrate how these confounds arise when CHR individuals are dichotomized as remitters or non-remitters based on their clinical outcomes while including converters among the non-remitter group.
MMN and P300 as Predictors of CHR Remission
Mismatch Negativity
Auditory MMN is a negative voltage, frontocentrally-distributed, ERP component elicited automatically between 100–250ms following infrequent deviant sounds interspersed among frequent “standard” sounds (31–33). Deviance in any number of sound features (e.g., pitch, duration, intensity) can elicit MMN (32). MMN generators have been localized to regions of auditory and frontal cortex (33,34) and it is considered to be at least partially mediated by glutamatergic neurotransmission at N-methyl-D-aspartate receptors (NMDARs) (35–37). MMN reflects automatic feature analysis involving a form of sensory echoic memory (31,38) and also reflects longer-term neural plasticity (39) and auditory predictive coding (40–42). MMN is largely unaffected by top-down processes (31,43,44), allowing the examination of auditory processing without the confounding influence of attention and motivation (45). Deficient MMN has been well-documented in schizophrenia (27,28) and is sensitive to cognition (46–49) and functional outcomes (50,51). Several studies have also reported that reduced MMN amplitudes are associated with greater future risk for psychosis conversion (22,52–55) and shorter time to conversion (22,23) among CHR individuals.
Two studies have examined whether MMN predicts future CHR remission. In 48 CHR individuals and 47 healthy controls (HCs), Kim and colleagues (56) examined whether duration-deviant MMN predicts prognosis among CHR individuals followed clinically for up to 6 years by comparing baseline MMN amplitudes across CHR remitter (n=17), non-remitter (n=31), and HC groups. CHR remission was defined according to the Scale of Psychosis Risk Symptoms (SOPS) criteria (2); that is, receiving a rating of “mild” or lower on all positive symptom subscale items, as well as a score 60 or higher on the Global Assessment of Functioning (GAF) scale (57). Non-remitters included 7 individuals who converted to psychosis and 24 individuals who continued to meet CHR criteria during the study follow-up period. CHR remitters and HCs did not differ in baseline MMN amplitude, and both groups had larger MMN amplitudes than the CHR non-remitters. Moreover, larger MMN amplitudes predicted a greater likelihood of being a remitter in a logistic regression analysis. Greater MMN amplitude also predicted functional recovery and was associated with improved SOPS positive symptom ratings after adjusting for antipsychotic medication dosage and years of education.
Fujioka et al. (58) examined whether duration-deviant and pitch-deviant MMN predicts CHR remission and cognitive function. Twenty-four CHR participants were followed for at least 6 months, at which point clinical status and neurocognitive function were assessed. CHR remission was defined by both symptomatic and functional improvement, indicated by SOPS positive symptom subscale scores and the GAF score (≥61). The non-remitter group (n=18) comprised individuals who continued to meet CHR criteria (n=15) and those who converted to psychosis (n=3). The amplitude of duration-deviant MMN, but not pitch-deviant MMN, was greater in the remitter (n=6) than the non-remitter group, while only pitch-deviant MMN together with the SOPS positive subscale score predicted scores on a measure of attention (59) at follow-up.
P300
P300 is a positive voltage ERP component typically elicited during auditory or visual oddball target detection tasks by behaviorally relevant or salient infrequent stimuli interspersed among “standard” stimuli (60). P300 amplitude is posited to reflect attentional resource allocation (60–62), contextual updating of working memory (63,64), and stimulus salience processing (65,66). There are two subcomponents of P300 that are elicited under specific task conditions. P3b, which is maximal over parietal scalp electrodes, is elicited by infrequent target stimuli that participants are asked to detect and that require a response (e.g., button press, count). P3a, which occurs approximately 50ms earlier than P3b and is maximal over frontocentral electrodes, is elicited by infrequent novel or otherwise salient non-target distractor stimuli that require no response (60). P3b reflects effortful top-down attentional allocation, whereas P3a reflects automatic, bottom-up orienting of attention (60,67). P300 generators have been primarily localized to prefrontal cortex (P3a) and temporal-parietal junction (P3b) (68). Like MMN, P300 depends on NMDAR neurotransmission (69), but noradrenergic (70), dopaminergic (60), GABAergic (71), serotonin 5-HT2A (72), cholinergic muscarinic (73) and cannabinoid receptors (74,75) may also be involved. Both target P3b and novelty P3a amplitude reductions are well-established in schizophrenia, especially during auditory tasks (29,30,76). Some studies have also shown that P300 amplitude fluctuates with clinical state (77,78) and that abnormalities worsen with longer illness duration (79,80).
A number of studies have consistently reported that auditory and visual target P3b is associated with future conversion to psychosis among CHR individuals (24–26,81), and that smaller P3b amplitudes predict the imminence of conversion (24–26). In contrast, CHR studies of novelty P3a have yielded mixed results, with some (82), but not others (25,26,83) reporting deficient baseline P3a amplitudes in future CHR converters relative to non-converters. P3a has not been shown to predict time-to-conversion (25,26).
Several studies have asked whether P300 amplitudes are associated with CHR remission. Kim et al. (84) examined whether baseline auditory target P3b, measured during a two-stimulus (targets, standards) auditory oddball paradigm, predicted remission from the CHR syndrome in 45 CHR individuals followed clinically for at least two years. Based on clinical outcomes, CHR individuals were classified as having achieved full symptom remission (n=19) or having persistent attenuated psychotic symptoms without converting to psychosis (n=26). Remission was defined by SOPS positive symptom scores and GAF score (≥60). There were no differences in baseline target P3b between CHR remitters and CHR individuals with persistent symptoms but larger baseline P3b amplitude predicted subsequent improvement in SOPS negative and general symptoms.
In another study, approximately 130 CHR and 69 HC participants completed two auditory oddball tasks that involved counting target tones (82). P3b amplitudes to target tones were measured during a two-stimulus (targets, standards) paradigm and P3a amplitudes to novel stimuli were measured during a three-stimulus (targets, novel sounds, standards) paradigm. After one year of clinical follow-up, CHR individuals were categorized into psychosis converters, individuals who continued to meet CHR criteria, and those who met SOPS positive symptom subscale remission criteria. Novelty P3a amplitudes of the CHR remitter group (n=68) at baseline were similar to the HC group (n=69) and were greater than CHR converters (n=23) and CHR individuals whose syndrome persisted (n=40). Target P3b amplitude was also reduced in CHR converters (n=19) relative to remitters (n=53).
Finally, an analysis of P300 data from the large North American Prodrome Longitudinal Study (NAPLS-2) examined whether baseline target P3b and novelty P3a amplitudes are associated with future conversion and remission outcomes among CHR individuals who were followed for at least two years (25). CHR (n=552) and HC (n=236) participants completed a three-stimulus (targets, novels, standards) auditory oddball task in which participants pressed a button in response to target tones. Baseline P300 amplitudes were compared between CHR converters (n=73) and CHR non-converters, defined as having been followed for 24 months and either continuing to meet CHR criteria (n=135) or meeting SOPS remission criteria (n=90).
While target P3b amplitude was reduced among converters relative to the whole non-converter group, further analysis demonstrated that CHR remitters had baseline target P3b amplitudes that were equivalent to HC and were greater than both the CHR individuals with a persistent CHR syndrome and the CHR converters to psychosis. In contrast, novelty P3a amplitude at baseline did not differentiate future CHR remitters from persistently CHR individuals or from psychosis converters.
Summary
Although most prior CHR studies that have followed individuals longitudinally have examined whether baseline neurophysiological abnormalities predict future psychosis, this review highlights recently emerging evidence from several independent studies suggesting that both MMN and P300, which are known to be abnormal in schizophrenia, not only predict conversion to full psychosis but are also associated with future remission among CHR individuals. Specifically, these studies provide initial evidence that relatively normal amplitudes of MMN and P300 at the time of CHR diagnosis may be associated with future CHR remission and/or improvements in functional status. Together, these initial studies implicate MMN and P300 as possible neurophysiological manifestations of pre-attentive and attention-mediated neurocognitive functions or neuroreceptor processes that confer resilience against persistence of the CHR syndrome and its associated risk for psychosis onset. Interestingly, both MMN and P300 rely on contextually-derived expectancies and stimulus probabilities, such that both show greater amplitudes to improbable events when the deviant stimulus is preceded by longer sequences of standard stimuli (85,86). Relatively normal MMN and P300 amplitudes among remitters relative to CHR individuals with a persistent syndrome suggest that both intact predictive coding and top-down attentional control processes may support future CHR remission and may even promote improvement in CHR symptoms. Moreover, given the dependence of MMN on glutamate NMDAR neurotransmission, as well as the involvement of NMDARs in P300 generation (69), intact MMN and P300 among future CHR remitters may reflect relatively normal NMDAR function that confers resilience against persistence of CHR symptoms or progression to full psychosis (e.g., see 87,88–90).
Methodological Issues Regarding ERP Studies Predicting CHR Remission
The small literature suggesting a role for MMN and P300 as predictors of CHR remission is associated with several methodological limitations. First, given the relatively small samples of CHR remitters examined in most of the studies to date, more large-scale studies are needed to definitively determine the predictive value of these measures. Second, the paradigms used to assess MMN and P300 differed across studies; differences in task instructions and the type and probability of stimuli used complicate direct comparison of results. Broad adoption of standard paradigms for eliciting MMN and P300 can be achieved in large multi-site studies but reaching convergence of paradigms across independent labs is far more challenging. Third, studies differed in the scalp electrode sites analyzed, the EEG preprocessing and data cleaning pipelines implemented, and the methods used to extract the signals of interest. Efforts to standardize electrode montages, data preprocessing pipelines, and ERP component measurement approaches would further facilitate comparisons across studies. At the same time, it may still be premature to foreclose on the possibility that novel and more sophisticated EEG processing pipelines and measurement approaches will yield measures that more accurately predict CHR remission.
General Methodological Issues Associated with Studies Predicting CHR Outcomes
Importantly, the ERP literature reviewed here also highlights several critical methodological issues that apply across all studies aimed at prediction of CHR remission, irrespective of the type of predictor examined.
Variable definitions of remission and the need for a standard definition
Criteria and measures used to define CHR remission have varied across studies, limiting direct comparisons of their results. Current CHR diagnostic systems have paid relatively little attention to classification at follow-up for outcomes other than psychosis conversion (91). While some CHR studies define remission as an attenuated positive symptom score (e.g., SOPS score) below the CHR threshold, others have additionally required functional remission. Whether to incorporate functional status in CHR remission criteria is an important consideration, as a previous study found that 56% of CHR participants were classified as remitters based on symptomatic improvement after one to two years of follow-up, but that the proportion of remitters in the sample declined to 40% when functional improvement was also required (92). In addition, while some have defined functional remission based on a single cutoff score, other studies, including NAPLS (91), have defined functional improvement in relation to an individual’s premorbid level of functioning.
Documenting remission with greater temporal granularity
While many studies have utilized time-to-event analyses such as Cox regression to examine whether baseline clinical variables predict the imminence of psychosis onset, no studies have attempted to predict time-to-remission. In order to evaluate predictors of time-to-remission in a manner similar to predictors of time-to-conversion, CHR outcomes need to be assessed with greater temporal granularity. While conversions to psychosis that occur between longitudinal assessments are often reported to the research team as they occur, or are straightforward to retrospectively date (e.g., hospitalization date), CHR remissions do not similarly come to the attention of the research team outside of scheduled assessments. Accordingly, time-to-remission analyses require assessment of clinical status at more frequent intervals than is typical in longitudinal CHR studies. Moreover, while conversion to psychosis typically ends a CHR individual’s clinical tracking, transition to a remitted state does not preclude subsequent recurrence of the CHR syndrome. Therefore, ongoing clinical follow-up is needed after CHR remission to confirm its durability.
It is important to note that analyses of time-to-remission using statistical methods like Cox Proportional Hazard Models must account for both the CHR individuals who convert to psychosis and those who drop out of the study prematurely. A common statistical error in these situations is to binarize the data focused on one outcome at a time, with censoring applied in a similar fashion to participants who are lost to follow-up and participants who develop the competing outcome. More appropriate proportional hazard models have been developed to estimate hazard or survival functions in groups where time to competing clinical outcomes are tracked and distinguished from study discontinuation (93,94).
Heterogeneity of non-remitter (and non-converter) groups with respect to future outcomes
To date, most studies attempting to identify predictors of CHR remission have tended to treat remission as a binary clinical outcome, comparing remitters with non-remitters, and have included among the non-remitters those CHR individuals who progressed to full psychosis. Indeed, both MMN studies described above (56,58) included converters in the non-remitter group, while the P300 studies either excluded converters from their analysis (84) or considered converters separately from non-remitters with a persistent CHR syndrome (25,82). The dichotomization of remission outcomes creates the potential for predictors of remission to be confounded by prediction of conversion effects. The result is that analyses aimed at identifying measures that predict remission are not independent of the prediction of conversion afforded by these same measures, leading to the potentially spurious observation that the same variable that predicts conversion to psychosis based on values from one tail of the variable’s distribution also predicts CHR remission based on values from the opposite tail of the distribution. While this can reflect the true state of nature with respect to a biomarker’s predictive relationships with CHR outcomes, the degree to which the confounding influence of conversion effects can contaminate and inflate estimates of a variable’s accuracy in predicting remission cannot be disentangled unless CHR converters are excluded from the CHR non-remitter group, leaving CHR individuals with a persistent CHR syndrome (CHR-Persistent) as the group to be distinguished from future CHR remitters.
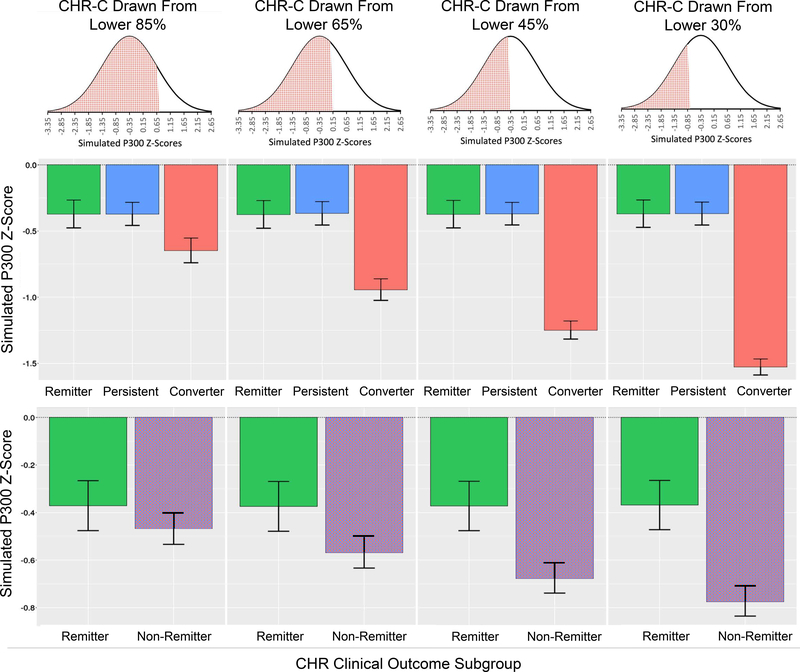
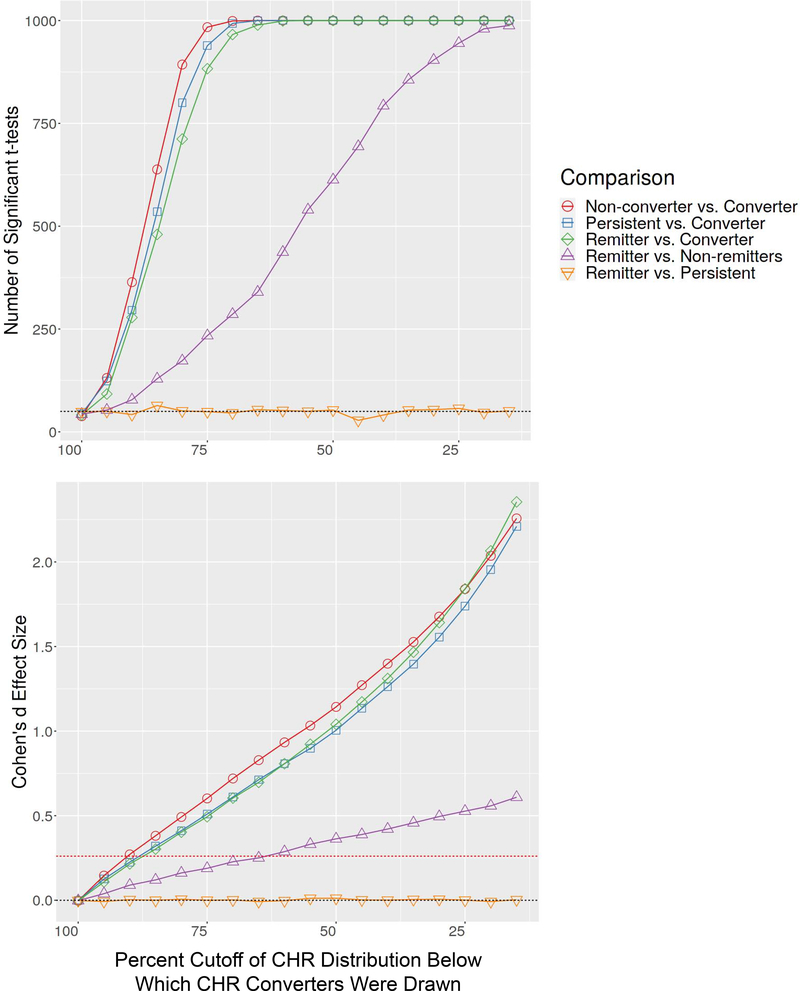
We illustrate this point with a simulation based on features from our prior study showing auditory P300 to predict CHR remission distinct from its prediction of conversion (25). The simulation proceeded by generating 1000 normally distributed samples of 500 CHR individuals with a mean equal to the mean target P300 amplitude z-score value (standardized with respect to the HC mean) from our prior study of z=−.35 (25), and a standard deviation of 1 (a simplifying assumption). For each of the 1000 samples, three subgroups were randomly drawn with sizes matched exactly to the CHR-Remitter (n=90), CHR-Persistent (n=135), and CHR-Converter (n=73) subgroups analyzed in Hamilton et al. (25). Our simulation involved a hypothetical scenario in which the baseline biomarker (e.g., P300 z-score) does not actually differ between the CHR-Remitter and CHR-Persistent subgroups but shows a significant ability to predict future CHR-Converter status. In order to demonstrate how this conversion effect alone can drive a spurious prediction of a remission effect when remission is defined by the CHR-Remitter versus CHR-Non-remitter comparison, we systematically varied the strength of the prediction of conversion effect. This was done by drawing the CHR-Converter subgroup from the bottom 85%, 65%, 45%, and 30% of the normally distributed CHR simulation samples, each time without replacement, reflecting progressively larger CHR-Converter versus CHR-Non-converter effect sizes. Because the CHR-Remitter and CHR-Persistent subgroups do not differ, each of these groups was drawn randomly without replacement from the full CHR n=500 samples. The set of three subgroups was drawn once from each of 1000 randomly generated full CHR samples, and mean estimates over these 1000 samples were obtained for each subgroup’s mean and standard deviation. Figure 1 shows these hypothetical subgroup means and standard deviations, illustrating the lack of difference between the CHR-Remitter and CHR-Persistent subgroups and the progressively greater deficits in the CHR-Converter subgroup. Based on the same data, Figure 1 also shows the collapse of CHR-Persistent and CHR-Converter groups into a single CHR-Non-remitter subgroup, demonstrating the progressively greater CHR-Remitter versus CHR-Non-remitter effect size that is driven entirely by the increasing deficit in the CHR-Converters. That is, the biomarker appears to increasingly predict CHR remission despite its complete insensitivity to remission when defined by comparison of the CHR-Remitter and CHR-Persistent subgroups. Based on the same simulation approach, but with generation of subgroups across a finer-grained range of CHR-Converter versus CHR-Non-converter effect sizes, Figure 2 shows the number of significant independent group t-tests when testing pairwise subgroup differences for each of the 1000 randomly drawn sets of three subgroups. As can be seen, the CHR-Remitter versus CHR-Non-remitter comparison yields an increasing number of significant t-tests as a function of sampling CHR-Converters from increasingly extreme sections of the leftward tail of the parent CHR sample distribution. Figure 2 also shows the same results expressed as Cohen’s d effect sizes, illustrating how the CHR-Remitter versus CHR-Non-remitter effect size grows solely as a function of the increasing CHR-Converter versus CHR-Non-converter effect size.
Figure 1. Simulated Clinical High Risk P300 Z-score Data Across Clinical Outcome Sub-Groups.
Simulated data presented here involve a hypothetical scenario in which the baseline biomarker (e.g., P300 z-score) does not differ between CHR-Remitter and CHR-Persistent subgroups but can predict future CHR-Converter status. Plots in the top row show hypothetical subgroup means and standard deviations when the CHR-Converter subgroup is drawn from the bottom 85%, 65%, 45%, and 30% of the normally distributed CHR simulation samples. Plots in the bottom row collapse the CHR-Persistent and CHR-Converter groups into a single CHR-Non-remitter group, showing that the progressively greater CHR-Remitter versus CHR-Non-remitter effect size is driven entirely by the increasing biomarker deficit in the CHR-Converters.
Figure 2. Significant t-tests and Effect Sizes of Simulated Clinical High Risk P300 Z-score Data.
The plot on the top shows the number of significant independent samples t-tests when testing pairwise subgroup differences for each of the randomly drawn sets of three subgroups. The CHR-Remitter versus CHR-Non-remitter comparison yields an increasing number of significant t-tests as a function of sampling CHR-Converters from increasingly extreme sections of the leftward tail of the parent CHR sample distribution. The plot on the bottom expresses these results as Cohen’s d effect sizes, illustrating how the CHR-Remitter versus CHR-Non-remitter effect size rises as a function the increasing CHR-Converter versus CHR-Non-Converter effect size. The dotted red line indicates the CHR-Converter vs. CHR-Non-Converter effect size observed for auditory target P300 amplitude z-scores in our prior report (25).
The above simulation highlights the importance of comparing CHR-Remitters to CHR with a persistent CHR syndrome when attempting to identify variables that predict remission independently of the variable’s ability to predict psychosis conversion. One implication of this is that similar concerns and potential confounds arise when predictors of psychosis conversion are derived from comparisons of CHR-Converters with CHR-Non-converters. To the extent that the CHR-Non-converter group includes a subgroup of individuals who subsequently achieve clinical remission, a variable’s ability to predict remission can contaminate and inflate the variable’s apparent effect size for predicting conversion. As most prior studies predicting conversion in CHR individuals have dichotomized the conversion outcome without regard to the presence of remitters in the non-converter group, this is not just a theoretical concern. In our previous report from the NAPLS2 sample, we initially reported that baseline auditory P3b amplitude was significantly reduced in CHR-Converters relative to CHR-Non-converters with a Cohen’s d of 0.26 (25). Most biomarker studies predicting CHR conversion do not further classify CHR-Non-converters into CHR-Remitters and CHR-Persistent subgroups, but when we implemented this additional classification in our previous report, two findings emerged. First, intact target P3b amplitude at baseline significantly differentiated future CHR-Remitters from the CHR-Persistent subgroup, a true prediction of remission effect. Second, the significant CHR-Converter versus CHR-Non-converter effect we reported (d=0.26, p=.048), when re-defined as a comparison of the CHR-Converter and CHR-Persistent subgroups, was reduced in magnitude (d=.02) and was no longer statistically significant (p=.55). These data showed that the apparent ability of target P3b amplitude deficits to predict future conversion to psychosis was substantially inflated by a stronger, yet initially obscured, prediction of future CHR remission by intact P3b amplitude at baseline.
Conclusions
While our review documents early evidence that intact MMN and P300 may have clinical utility as predictors of remission from the CHR syndrome, this work is part of a broader and growing literature identifying predictors of CHR remission, including 1) intact cortical surface area assessed with structural MRI (95–97) (see (98) in current issue), 2) intact cognitive function (99), and 3) clinical trajectories showing symptom and functional improvements (100,101). Thus, there is some convergence across measurement domains showing more intact or neurotypical assessments to predict remission. Attempts to combine clinical, neurocognitive, and biomarker measures, including ERP measures, to predict CHR conversion outcomes have been limited (e.g., 102,103), and no published studies to date have attempted to apply such a multivariate approach to the prediction of CHR remission. Moreover, whether ERP and other biomarkers may not only serve as prognostic predictors of outcomes but also moderate the influence of other factors (e.g., stress, other protective factors) that contribute to CHR outcomes has not yet been studied.
Irrespective of the predictor considered in studies aimed at predicting CHR remission, several general methodological concerns arise that lead us to propose recommendations for future work. Specifically, future CHR research should:
Distinguish CHR-Remitter from both CHR-Persistent and CHR-Converter groups in analyses predicting remission, and, conversely, distinguish CHR-Converter from both CHR-Persistent and CHR-Remitter groups when predicting conversion. To estimate relatively uncontaminated remission and conversion effects, each should be calculated with reference to the CHR-Persistent group.
Increase the frequency of longitudinal clinical assessments in order to prospectively detect CHR remission and time-to-remission in a manner that approaches the temporal precision with which time-to-conversion is typically assessed.
Simultaneously consider conversion and remission outcomes in the same statistical models and include both as competing outcomes in time-to-conversion models.
Report metrics regarding the accuracy of individual prediction (e.g., area under the receiver operating characteristic curve) of CHR remission and conversion to extend findings beyond group mean effects, setting the stage for evaluating the potential role of predictors in guiding prognosis and treatment in individual patients.
Establish consensus definitions of CHR remission for the field.
Standardize optimized biomarker assessments and preprocessing protocols to render results more comparable across studies.
While prediction and prevention of psychosis conversion remains the primary focus of most CHR research, broadening the focus to include CHR remission and other clinical outcomes (e.g., social/occupational functioning) will likely yield novel insights that distinctly inform clinical practice. From a clinical perspective, biomarkers that successfully predict future CHR remission would have utility for assessing prognosis and staged treatment planning, possibly involving intermittent clinical monitoring of those likely to remit while reserving more time intensive (e.g., weekly psychotherapy) and invasive (e.g., psychotropic medication) intervention for those CHR at greatest risk of conversion to full psychosis. Furthermore, relative to conversion, CHR remission may provide a more feasible and meaningful primary endpoint in clinical trials aiming to develop novel treatments for the CHR syndrome. ERPs are one of many biomarker domains that warrant further study in current efforts to more accurately forecast CHR remission.
Acknowledgments
This work was supported by Career Development Award 1K2CX001878 from the United States Department of Veterans Affairs Clinical Sciences Research and Development Service to Dr. Hamilton and by National Institute of Mental Health grant MH076989 to Dr. Mathalon. Drs. Hamilton and Mathalon are employees of the United States government. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the United States government, or the National Institutes of Health.
Footnotes
Disclosures
Dr. Hamilton and Mr. Roach report no biomedical financial interests or potential conflicts of interest. Dr. Mathalon is a consultant for Boehringer Ingelheim and Cadent Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perkins DO, Gu H, Boteva K, Lieberman JA (2005): Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 162:1785–1804. [DOI] [PubMed] [Google Scholar]
- 2.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. (2003): Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 29:703–715. [DOI] [PubMed] [Google Scholar]
- 3.McGlashan TH, Walsh BC, Woods SW (2010): The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-up. New York: Oxford University Press. [Google Scholar]
- 4.Phillips LJ, Yung AR, McGorry PD (2000): Identification of young people at risk of psychosis: validation of Personal Assessment and Crisis Evaluation Clinic intake criteria. Aust N Z J Psychiatry. 34 Suppl:S164–169. [DOI] [PubMed] [Google Scholar]
- 5.Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, et al. (2005): Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 39:964–971. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, et al. (2016): An Individualized Risk Calculator for Research in Prodromal Psychosis. Am J Psychiatry. 173:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fusar-Poli P, Rutigliano G, Stahl D, Davies C, Bonoldi I, Reilly T, et al. (2017): Development and Validation of a Clinically Based Risk Calculator for the Transdiagnostic Prediction of Psychosis. JAMA Psychiatry. 74:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton HK, Boos AK, Mathalon DH (2020): Electroencephalography and Event-Related Potential Biomarkers in Individuals at Clinical High Risk for Psychosis. Biol Psychiatry. 88:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepock JR, Mizrahi R, Korostil M, Bagby RM, Pang EW, Kiang M (2018): Event-Related Potentials in the Clinical High-Risk (CHR) State for Psychosis: A Systematic Review. Clin EEG Neurosci. 49:215–225. [DOI] [PubMed] [Google Scholar]
- 10.Niznikiewicz MA (2019): Neurobiological approaches to the study of clinical and genetic high risk for developing psychosis. Psychiatry Res. 277:17–22. [DOI] [PubMed] [Google Scholar]
- 11.Bois C, Whalley HC, McIntosh AM, Lawrie SM (2015): Structural magnetic resonance imaging markers of susceptibility and transition to schizophrenia: a review of familial and clinical high risk population studies. J Psychopharmacol. 29:144–154. [DOI] [PubMed] [Google Scholar]
- 12.Perkins DO, Jeffries CD, Addington J, Bearden CE, Cadenhead KS, Cannon TD, et al. (2015): Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr Bull. 41:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, et al. (2013): Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatry. 74:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. (2012): Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 69:220–229. [DOI] [PubMed] [Google Scholar]
- 15.Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, et al. (2002): Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 159:863–865. [DOI] [PubMed] [Google Scholar]
- 16.Fusar-Poli P, Bechdolf A, Taylor MJ, Bonoldi I, Carpenter WT, Yung AR, et al. (2013): At risk for schizophrenic or affective psychoses? A meta-analysis of DSM/ICD diagnostic outcomes in individuals at high clinical risk. Schizophr Bull. 39:923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeVylder JE, Muchomba FM, Gill KE, Ben-David S, Walder DJ, Malaspina D, et al. (2014): Symptom trajectories and psychosis onset in a clinical high-risk cohort: the relevance of subthreshold thought disorder. Schizophr Res. 159:278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsura M, Ohmuro N, Obara C, Kikuchi T, Ito F, Miyakoshi T, et al. (2014): A naturalistic longitudinal study of at-risk mental state with a 2.4 year follow-up at a specialized clinic setting in Japan. Schizophr Res. 158:32–38. [DOI] [PubMed] [Google Scholar]
- 19.Nelson B, Yuen HP, Wood SJ, Lin A, Spiliotacopoulos D, Bruxner A, et al. (2013): Long-term follow-up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry. 70:793–802. [DOI] [PubMed] [Google Scholar]
- 20.Simon AE, Borgwardt S, Riecher-Rossler A, Velthorst E, de Haan L, Fusar-Poli P (2013): Moving beyond transition outcomes: meta-analysis of remission rates in individuals at high clinical risk for psychosis. Psychiatry Res. 209:266–272. [DOI] [PubMed] [Google Scholar]
- 21.Ferrarelli F, Mathalon D (2020): The prodromal phase: Time to broaden the scope beyond transition to psychosis? Schizophr Res. 216:5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH, et al. (2014): Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry. 75:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathalon DH, Hamilton HK, Bachman PM, Belger A, Carrion RE, Duncan E, et al. (2016): Mismatch negativity and repetition positivity predict transition to psychosis in clinical high risk individuals. International Journal of Psychophysiology.37. [Google Scholar]
- 24.van Tricht MJ, Nieman DH, Koelman JH, van der Meer JN, Bour LJ, de Haan L, et al. (2010): Reduced parietal P300 amplitude is associated with an increased risk for a first psychotic episode. Biol Psychiatry. 68:642–648. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton HK, Roach BJ, Bachman PM, Belger A, Carrion RE, Duncan E, et al. (2019): Association Between P300 Responses to Auditory Oddball Stimuli and Clinical Outcomes in the Psychosis Risk Syndrome. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton HK, Woods SW, Roach BJ, Llerena K, McGlashan TH, Srihari VH, et al. (2019): Auditory and Visual Oddball Stimulus Processing Deficits in Schizophrenia and the Psychosis Risk Syndrome: Forecasting Psychosis Risk With P300. Schizophr Bull. 45:1068–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson MA, Ruffle A, Gold JM (2016): A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biological Psychiatry. 79:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umbricht D, Krljes S (2005): Mismatch negativity in schizophrenia: a meta-analysis. Schizophrenia Research. 76:1–23. [DOI] [PubMed] [Google Scholar]
- 29.Jeon YW, Polich J (2003): Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 40:684–701. [DOI] [PubMed] [Google Scholar]
- 30.Ford JM (1999): Schizophrenia: the broken P300 and beyond. Psychophysiology. 36:667–682. [PubMed] [Google Scholar]
- 31.Näätänen R, Teder W, Alho K, Lavikainen J (1992): Auditory attention and selective input modulation: A topographical ERP study. NeuroReport. 3:493. [DOI] [PubMed] [Google Scholar]
- 32.Näätänen R, Pakarinen S, Rinne T, Takegata R (2004): The mismatch negativity (MMN): towards the optimal paradigm. Clinical Neurophysiology. 115:140–144. [DOI] [PubMed] [Google Scholar]
- 33.Alho K (1995): Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 16:38–51. [DOI] [PubMed] [Google Scholar]
- 34.Molholm S, Martinez A, Ritter W, Javitt DC, Foxe JJ (2005): The neural circuitry of pre-attentive auditory change-detection: an fMRI study of pitch and duration mismatch negativity generators. Cereb Cortex. 15:545–551. [DOI] [PubMed] [Google Scholar]
- 35.Siegel SJ, Talpos JC, Geyer MA (2013): Animal models and measures of perceptual processing in schizophrenia. Neurosci Biobehav Rev. 37:2092–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lakatos P, O’Connell MN, Barczak A, McGinnis T, Neymotin S, Schroeder CE, et al. (2019): The Thalamocortical Circuit of Auditory Mismatch Negativity. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosburg T, Kreitschmann-Andermahr I (2016): The effects of ketamine on the mismatch negativity (MMN) in humans - A meta-analysis. Clin Neurophysiol. 127:1387–1394. [DOI] [PubMed] [Google Scholar]
- 38.Näätänen R, Jacobsen T, Winkler I (2005): Memory‐based or afferent processes in mismatch negativity (MMN): A review of the evidence. Psychophysiology. 42:25–32. [DOI] [PubMed] [Google Scholar]
- 39.Stephan KE, Baldeweg T, Friston KJ (2006): Synaptic plasticity and dysconnection in schizophrenia. Biological Psychiatry. 59:929–939. [DOI] [PubMed] [Google Scholar]
- 40.Garrido MI, Kilner JM, Stephan KE, Friston KJ (2009): The mismatch negativity: A review of underlying mechanisms. Clinical Neurophysiology. 120:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friston K (2005): A theory of cortical responses. Philosophical Transactions of the Royal Society B: Biological Sciences. 360:815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Todd J, Michie PT, Schall U, Ward PB, Catts SV (2012): Mismatch negativity (MMN) reduction in schizophrenia—Impaired prediction-error generation, estimation or salience? EEG Coherence. 83:222–231. [DOI] [PubMed] [Google Scholar]
- 43.Sussman E, Sheridan K, Kreuzer J, Winkler I (2003): Representation of the standard: stimulus context effects on the process generating the mismatch negativity component of event-related brain potentials. Psychophysiology. 40:465–471. [DOI] [PubMed] [Google Scholar]
- 44.Rinne T, Antila S, Winkler I (2001): Mismatch negativity is unaffected by top-down predictive information. Neuroreport. 12:2209–2213. [DOI] [PubMed] [Google Scholar]
- 45.Mathalon DH, Ford JM (2008): Divergent Approaches Converge on Frontal Lobe Dysfunction in Schizophrenia. American Journal of Psychiatry. 165:944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baldeweg T, Klugman A, Gruzelier J, Hirsch SR (2004): Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophrenia Research. 69:203–217. [DOI] [PubMed] [Google Scholar]
- 47.Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB (2010): Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 34:822–829. [DOI] [PubMed] [Google Scholar]
- 48.Kiang M, Light GA, Prugh J, Coulson S, Braff DL, Kutas M (2007): Cognitive, neurophysiological, and functional correlates of proverb interpretation abnormalities in schizophrenia. J Int Neuropsychol Soc. 13:653–663. [DOI] [PubMed] [Google Scholar]
- 49.Thomas ML, Green MF, Hellemann G, Sugar CA, Tarasenko M, Calkins ME, et al. (2017): Modeling Deficits From Early Auditory Information Processing to Psychosocial Functioning in Schizophrenia. JAMA Psychiatry. 74:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Light GA, Braff DL (2005): Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Archives of General Psychiatry. 62:127. [DOI] [PubMed] [Google Scholar]
- 51.Hamilton HK, Perez VB, Ford JM, Roach BJ, Jaeger J, Mathalon DH (2018): Mismatch Negativity But Not P300 Is Associated With Functional Disability in Schizophrenia. Schizophr Bull. 44:492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bodatsch M, Ruhrmann S, Wagner M, Muller R, Schultze-Lutter F, Frommann I, et al. (2011): Prediction of psychosis by mismatch negativity. Biol Psychiatry. 69:959–966. [DOI] [PubMed] [Google Scholar]
- 53.Shaikh M, Valmaggia L, Broome MR, Dutt A, Lappin J, Day F, et al. (2012): Reduced mismatch negativity predates the onset of psychosis. Schizophr Res. 134:42–48. [DOI] [PubMed] [Google Scholar]
- 54.Higuchi Y, Sumiyoshi T, Seo T, Miyanishi T, Kawasaki Y, Suzuki M (2013): Mismatch negativity and cognitive performance for the prediction of psychosis in subjects with at-risk mental state. PLoS One. 8:e54080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atkinson RJ, Michie PT, Schall U (2012): Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol Psychiatry. 71:98–104. [DOI] [PubMed] [Google Scholar]
- 56.Kim M, Lee TH, Yoon YB, Lee TY, Kwon JS (2018): Predicting Remission in Subjects at Clinical High Risk for Psychosis Using Mismatch Negativity. Schizophr Bull. 44:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Association AP (2002): Multiaxial Assessment. In: Association AP, editor. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text revision ed. Washington, DC. [Google Scholar]
- 58.Fujioka M, Kirihara K, Koshiyama D, Tada M, Nagai T, Usui K, et al. (2020): Mismatch negativity predicts remission and neurocognitive function in individuals at ultra-high risk for psychosis. Frontiers in Psychiatry. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L (2004): The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 68:283–297. [DOI] [PubMed] [Google Scholar]
- 60.Polich J (2007): Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 118:2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isreal JB, Wickens CD, Donchin E (1980): The dynamics of P300 during dual-task performance. Prog Brain Res. 54:416–421. [DOI] [PubMed] [Google Scholar]
- 62.Kramer AF, Strayer DL (1988): Assessing the development of automatic processing: an application of dual-task and event-related brain potential methodologies. Biol Psychol. 26:231–267. [DOI] [PubMed] [Google Scholar]
- 63.Donchin E, Coles M (1988): Is the P300 component a manifestation of context updating? (Commentary on Verleger’s critique of the context updating model). Behavioral and Brain Science. 11:357–374. [Google Scholar]
- 64.Johnson R Jr. (1986): A triarchic model of P300 amplitude. Psychophysiology. 23:367–384. [DOI] [PubMed] [Google Scholar]
- 65.Sutton S, Braren M, Zubin J, John ER (1965): Evoked potential correlates of stimulus uncertainty. Science. 150:1187–1188. [DOI] [PubMed] [Google Scholar]
- 66.Sutton S, Tueting P, Zubin J, John ER (1967): Information delivery and the sensory evoked potential. Science. 155:1436–1439. [DOI] [PubMed] [Google Scholar]
- 67.Goldstein A, Spencer KM, Donchin E (2002): The influence of stimulus deviance and novelty on the P300 and novelty P3. Psychophysiology. 39:781–790. [PubMed] [Google Scholar]
- 68.Halgren E, Marinkovic K, Chauvel P (1998): Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalography and Clinical Neurophysiology. 106:156–164. [DOI] [PubMed] [Google Scholar]
- 69.Schwertner A, Zortea M, Torres FV, Caumo W (2018): Effects of Subanesthetic Ketamine Administration on Visual and Auditory Event-Related Potentials (ERP) in Humans: A Systematic Review. Front Behav Neurosci. 12:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nieuwenhuis S, Aston-Jones G, Cohen JD (2005): Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull. 131:510–532. [DOI] [PubMed] [Google Scholar]
- 71.Watson TD, Petrakis IL, Edgecombe J, Perrino A, Krystal JH, Mathalon DH (2009): Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. Int J Neuropsychopharmacol. 12:357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Umbricht D, Vollenweider FX, Schmid L, Grubel C, Skrabo A, Huber T, et al. (2003): Effects of the 5-HT2A agonist psilocybin on mismatch negativity generation and AX-continuous performance task: implications for the neuropharmacology of cognitive deficits in schizophrenia. Neuropsychopharmacology. 28:170–181. [DOI] [PubMed] [Google Scholar]
- 73.Brown SB, van der Wee NJ, van Noorden MS, Giltay EJ, Nieuwenhuis S (2015): Noradrenergic and cholinergic modulation of late ERP responses to deviant stimuli. Psychophysiology. 52:1620–1631. [DOI] [PubMed] [Google Scholar]
- 74.D’Souza DC, Fridberg DJ, Skosnik PD, Williams A, Roach B, Singh N, et al. (2012): Dose-related modulation of event-related potentials to novel and target stimuli by intravenous Delta(9)-THC in humans. Neuropsychopharmacology. 37:1632–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roser P, Juckel G, Rentzsch J, Nadulski T, Gallinat J, Stadelmann AM (2008): Effects of acute oral Delta9-tetrahydrocannabinol and standardized cannabis extract on the auditory P300 event-related potential in healthy volunteers. Eur Neuropsychopharmacol. 18:569–577. [DOI] [PubMed] [Google Scholar]
- 76.Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S (2004): Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 70:315–329. [DOI] [PubMed] [Google Scholar]
- 77.Mathalon DH, Ford JM, Pfefferbaum A (2000): Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biol Psychiatry. 47:434–449. [DOI] [PubMed] [Google Scholar]
- 78.Ford JM, Mathalon DH, Marsh L, Faustman WO, Harris D, Hoff AL, et al. (1999): P300 amplitude is related to clinical state in severely and moderately ill patients with schizophrenia. Biol Psychiatry. 46:94–101. [DOI] [PubMed] [Google Scholar]
- 79.O’Donnell BF, Faux SF, McCarley RW, Kimble MO, Salisbury DF, Nestor PG, et al. (1995): Increased rate of P300 latency prolongation with age in schizophrenia: Electrophysiological evidence for a neurodegenerative process. Archives of General Psychiatry. 52:544–549. [DOI] [PubMed] [Google Scholar]
- 80.Mathalon DH, Ford JM, Rosenbloom M, Pfefferbaum A (2000): P300 reduction and prolongation with illness duration in schizophrenia. Biol Psychiatry. 47:413–427. [DOI] [PubMed] [Google Scholar]
- 81.van Tricht MJ, Nieman DH, Koelman JH, Bour LJ, van der Meer JN, van Amelsvoort TA, et al. (2011): Auditory ERP components before and after transition to a first psychotic episode. Biol Psychol. 87:350–357. [DOI] [PubMed] [Google Scholar]
- 82.Tang Y, Wang J, Zhang T, Xu L, Qian Z, Cui H, et al. (2019): P300 as an index of transition to psychosis and of remission: Data from a clinical high risk for psychosis study and review of literature. Schizophr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Atkinson RJ, Fulham WR, Michie PT, Ward PB, Todd J, Stain H, et al. (2017): Electrophysiological, cognitive and clinical profiles of at-risk mental state: The longitudinal Minds in Transition (MinT) study. PLoS One. 12:e0171657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim M, Lee TY, Lee S, Kim SN, Kwon JS (2015): Auditory P300 as a predictor of short-term prognosis in subjects at clinical high risk for psychosis. Schizophr Res. 165:138–144. [DOI] [PubMed] [Google Scholar]
- 85.Baldeweg T (2006): Repetition effects to sounds: evidence for predictive coding in the auditory system. Trends Cogn Sci. 10:93–94. [DOI] [PubMed] [Google Scholar]
- 86.Squires KC, Wickens C, Squires NK, Donchin E (1976): The effect of stimulus sequence on the waveform of the cortical event-related potential. Science. 193:1142–1146. [DOI] [PubMed] [Google Scholar]
- 87.Krystal JH, Anand A, Moghaddam B (2002): Effects of NMDA Receptor Antagonists: Implications for the Pathophysiology of Schizophrenia. Archives of General Psychiatry. 59:663–664. [DOI] [PubMed] [Google Scholar]
- 88.Moghaddam B, Javitt D (2012): From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 37:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D (2012): Has an Angel Shown the Way? Etiological and Therapeutic Implications of the PCP/NMDA Model of Schizophrenia. Schizophrenia Bulletin. 38:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coyle JT, Tsai G, Goff D (2003): Converging Evidence of NMDA Receptor Hypofunction in the Pathophysiology of Schizophrenia. Annals of the New York Academy of Sciences: Blackwell Publishing Ltd, pp 318–327. [DOI] [PubMed] [Google Scholar]
- 91.Woods SW, Walsh BC, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, et al. (2014): Current status specifiers for patients at clinical high risk for psychosis. Schizophr Res. 158:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee TY, Kim SN, Correll CU, Byun MS, Kim E, Jang JH, et al. (2014): Symptomatic and functional remission of subjects at clinical high risk for psychosis: a 2-year naturalistic observational study. Schizophr Res. 156:266–271. [DOI] [PubMed] [Google Scholar]
- 93.Fine JP, Gray RJ (1999): A prioprtional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 94:496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scrucca L, Santucci A, Aversa F (2007): Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 40:381–387. [DOI] [PubMed] [Google Scholar]
- 95.Chung Y, Allswede D, Addington J, Bearden CE, Cadenhead K, Cornblatt B, et al. (2019): Cortical abnormalities in youth at clinical high-risk for psychosis: Findings from the NAPLS2 cohort. Neuroimage Clin. 23:101862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Wit S, Wierenga LM, Oranje B, Ziermans TB, Schothorst PF, van Engeland H, et al. (2016): Brain development in adolescents at ultra-high risk for psychosis: Longitudinal changes related to resilience. Neuroimage Clin. 12:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wierenga LM, Langen M, Oranje B, Durston S (2014): Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 87:120–126. [DOI] [PubMed] [Google Scholar]
- 98.Vargas T, Damme KSF, Ered A, Capizzi R, Frosch I, Ellman LM, et al. (2020): Neuroimaging Markers of Resiliency in Youth at Clinical High Risk for Psychosis: A Qualitative Review. Biol Psychiatry Cogn Neurosci Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee TY, Shin YS, Shin NY, Kim SN, Jang JH, Kang DH, et al. (2014): Neurocognitive function as a possible marker for remission from clinical high risk for psychosis. Schizophr Res. 153:48–53. [DOI] [PubMed] [Google Scholar]
- 100.Addington J, Stowkowy J, Liu L, Cadenhead KS, Cannon TD, Cornblatt BA, et al. (2019): Clinical and functional characteristics of youth at clinical high-risk for psychosis who do not transition to psychosis. Psychol Med. 49:1670–1677. [DOI] [PubMed] [Google Scholar]
- 101.Allswede DM, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, Mathalon DH, et al. (2020): Characterizing Covariant Trajectories of Individuals at Clinical High Risk for Psychosis Across Symptomatic and Functional Domains. Am J Psychiatry. 177:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nieman DH, Ruhrmann S, Dragt S, Soen F, van Tricht MJ, Koelman JH, et al. (2014): Psychosis prediction: stratification of risk estimation with information-processing and premorbid functioning variables. Schizophr Bull. 40:1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruhrmann S, Schultze-Lutter F, Schmidt SJ, Kaiser N, Klosterkotter J (2014): Prediction and prevention of psychosis: current progress and future tasks. Eur Arch Psychiatry Clin Neurosci. 264 Suppl 1:S9–16. [DOI] [PubMed] [Google Scholar]