Abstract
Elastase is a proteolytic enzyme belonging to the family of hydrolases produced by human neutrophils, monocytes, macrophages, and endothelial cells. Human neutrophil elastase is known to play multiple roles in the human body, but an increase in its activity may cause a variety of diseases. Elastase inhibitors may prevent the development of psoriasis, chronic kidney disease, respiratory disorders (including COVID-19), immune disorders, and even cancers. Among polyphenolic compounds, some flavonoids and their derivatives, which are mostly found in herbal plants, have been revealed to influence elastase release and its action on human cells. This review focuses on elastase inhibitors that have been discovered from natural sources and are biochemically characterised as flavonoids. The inhibitory activity on elastase is a characteristic of flavonoid aglycones and their glycoside and methylated, acetylated and hydroxylated derivatives. The presented analysis of structure–activity relationship (SAR) enables the determination of the chemical groups responsible for evoking an inhibitory effect on elastase. Further study especially of the in vivo efficacy and safety of the described natural compounds is of interest in order to gain better understanding of their health-promoting potential.
Keywords: Flavonoids, elastase, inhibition, structure–activity relationship
Introduction
The inhibition of human enzyme activity is an interesting strategy for treating global diseases and may be an attractive target for pursuing new drug discoveries1. Regulation of enzyme activity by elastase inhibitors is a promising endeavour for treating rheumatoid arthritis, glomerulonephritis, emphysema, pulmonary diseases, psoriasis, and cancers2,3.
Neutrophils are critical for the innate immune response; thus, they are involved in fighting infections. Neutrophil activation and degranulation lead to the release of serine proteases (elastase, proteinase 3, cathepsin G) into the extracellular space as proteolytically active enzymes that are capable of degrading a broad spectrum of extracellular matrix (ECM) proteins, such as fibronectin, elastin, or collagen, which provide physical support and stability to tissues4–7. Neutrophil-derived proteases, including elastase, have the ability to control the action of inflammatory cytokines by developing the immune response. However, human neutrophil elastase (HNE) is also able to intensify the emergence of other diseases8–10. HNE belongs to the chymotrypsin superfamily of serine proteases and is involved in the nonoxidative pathway of intracellular and extracellular pathogen destruction. Elastase is produced by human neutrophils, monocytes, macrophages, and endothelial cells and stored mainly in azurophilic granules and the nuclear envelope11,12. Under physiological conditions, HNE is counteracted by natural serine protease inhibitors, including elafin, α1-antitrypsin, and secretory leukocyte protease inhibitor (SLIP)5. Nevertheless, the protective role of endogenous inhibitors can be inactivated by the adhesion of neutrophils to the ECM, oxidants, and proteases produced by other leukocytes and by strongly linking HNE to receptors on the cell membrane, thus inhibiting the binding to accessible endogenous inhibitors13. Overall, the fluctuation in the quantity of HNE and its inhibitors plays a critical role in inducing a number of human diseases.
Although synthetic inhibitors are available, the identification of naturally derived drugs is a valuable research field for identifying inhibitors with a lack of unpleasant side effects. Among polyphenolic compounds, some flavonoids and their derivatives, which are mostly found in herbal plants, are potential inhibitors of elastase with few side effects. This review focuses on the diverse effects and efficacy of flavonoids and their derivatives in the development of elastase inhibitors.
Methodology/search strategy
The search strategy helps to clarify the adequate search string and find the relevant subject databases to accurately identify appropriate scientific research. The search databases for this review were Taylor & Francis Online, Google Scholar, EBSCO Discovery Service (EDS), REAXYS Database, SCOPUS, PubMed/MEDLINE, Web of Science (SCI-EXPANDED), Wiley Online Library, and Science Direct/ELSEVIER. For the review method, the above databases were searched using different combinations of the following keywords: elastase, neutrophil, biological functions, elastase activity, serine protease, infection, inhibitor, flavonoids, human disorders, enzyme, biological activity, and immune response.
Biological functions of human neutrophil elastase
In adult mammalian organisms, neutrophils are produced in the bone marrow and released into blood and tissues under certain physiological conditions. The human body makes over 1 billion neutrophils per day/kg body weight. Nevertheless, during various autoimmune and inflammatory diseases, their number can expand to 10 billion. In an inflammatory environment, neutrophils can survive for seven days, which may be connected with cytokine-activated endothelial cell action14. They are the first line of defence against bacterial and fungal infections and help combat parasites and viruses. Their diverse functions include protection against reactive oxygen species (ROS) and hydrolytic enzymes and elimination of pathogens, thus making them an important part of the overall immune and inflammatory response (phagocytosis, degranulation, and NETosis). On the other hand, this type of leucocyte is capable of contributing to tissue damage during various autoimmune and inflammatory diseases and plays important roles in various pathologies15.
One of the neutrophil functions is to produce and release serine proteases (elastase, proteinase 3, and cathepsin G). HNE is known to play multiple roles in the human body. Elastase is a cytotoxic 29-kDa protease, and sequence analysis has demonstrated that it consists of polypeptides with single chains and 218 amino acids with four intramolecular disulphide bonds linking eight half-cystine residues16,17.
Enzymes are released to defend against invading pathogens via their ability to control apoptosis18,19. The mechanism of action of neutrophil elastase (NE) is based on cleaving bacterial virulence factors and their outer membrane proteins and binding to the bacterial membrane10. Furthermore, HNE is involved in the inflammatory response by inducing interleukin 8 (IL-8) through Toll-like receptor 4 (TLR4) activation and the release of other proinflammatory cytokines20–22. This enzyme may also cause degradation of elastic fibres and induce proliferation of keratinocytes23,24. Other biological functions of HNE are given in Table 1.
Table 1.
Biological functions of HNE.
| Elastase functions | Model of the study | References |
|---|---|---|
| Bactericidal ability | The respiratory tract cells | 18 |
| Control of apoptosis and participation in phagocytosis | ||
| Role in mucin production | ||
| Bioactivity and ability to control some inflammatory cytokines | Membrane-bound human leukocyte elastase | 4,19,25 |
| Cleaves immunoglobulins, complement components, complement receptor type 1 on neutrophils | Human neutrophils | 26,27 |
| Participates in cell differentiation, migration, and angiogenesis | Extracellular matrix | 28 |
| Induces IL-8 expression by activating TLR4 and degrading components of the lung matrix | Bronchial epithelium | 20 |
| Cleaves receptors and lung surfactant protein | Animal models | 11 |
| Increases PAR2 expression and mucin5ac protein release in mucus hypersecretions | Epithelial cells | 29 |
| Regulates lung endothelial cell barrier integrity through proteinase-activated receptor (PAR1) | Endothelial cells | 30,31 |
| Stimulates airway submucosal gland secretion | In vitro and in vivo mice model | 32 |
| Promotes the neutrophil-mediated activation of platelets | Platelets | 33 |
| Induces proliferation of keratinocytes in tissue repair | In vitro on murine keratinocyte cell line; in vivo on mice skin | 24 |
| Degenerates elastic fibres in tissue repair | In vivo on mice skin | 23 |
Role of HNE in infections
The main goal of the innate immune response is to locate and destroy pathogens that have entered the human body. One of the first immune system components that reaches the site of infection is neutrophils. They fight pathogens through non-specific immune mechanisms with the help of ROS and enzymes that are involved in oxidative and nonoxidative defence pathways. HNE is one of the critical factors in the innate immune system with antimicrobial activity. Neutrophil elastase can be activated by cathepsin-C, and then the enzyme is involved in many nonoxidative immune responses34.
Phagocytosis is a defence mechanism against pathogens. It is an intracellular process initiated by the binding and recognition of pathogens through cell membrane receptors that are subsequently absorbed into structures called phagosomes35. Afterwards, the granules are attached to the absorbed phagosome and shed its contents, and the resulting phagolysosome starts the degradation of the absorbed pathogen. NE kills microbes, e.g. Escherichia coli, by degrading the outer membrane protein A (OmpA), which disrupts the cell membrane integrity and leads to subsequent death36. Moreover, the simultaneous action of serine proteases led to the death of gram-positive Streptococcus pneumoniae during phagocytosis in vivo37.
Another way of fighting microbes that requires elastase is via degranulation. Unlike phagocytosis, this process shows activity in the ECM. The stimulation of neutrophils by cytokines leads to the transfer of granularity to the cell periphery, where the granules are fused with the cell membrane and their content is poured out of the cell11. The primary granule content is targeted at the pathogen killing process. However, they are released due to their high toxicity and simultaneously there is a high possibility of damage to surrounding tissues38,39. Extracellular HNE shows a cleavage effect on many bacterial proteins, e.g. leukotoxins, which is a factor leading to the lysis of leukocytes40,41.
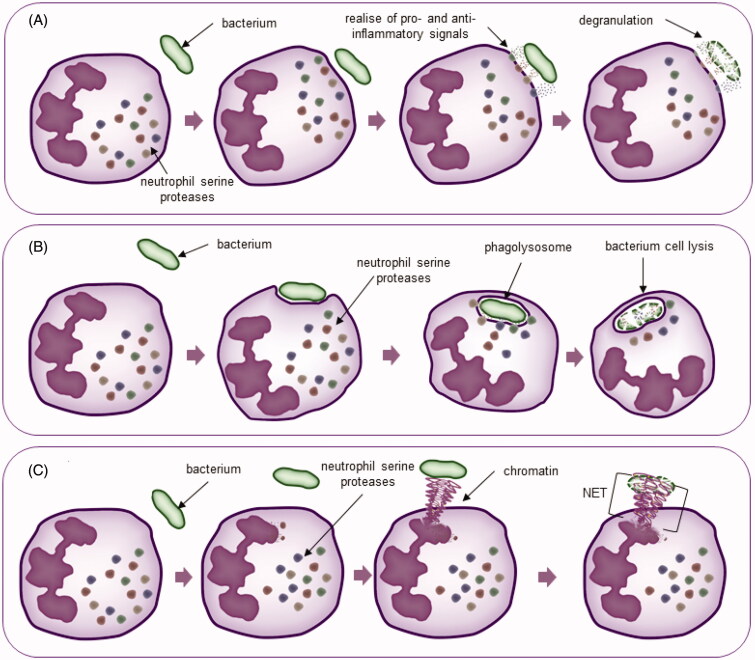
Moreover, HNE demonstrates its activity in NETosis, a mechanism used by neutrophils to tackle pathogens. NETosis is a complex of decondensed and unfolded DNA with histones and cytoplasmic granule proteins42. Induction by IL-8 and lipopolysaccharide (LPS) leads to the activation of neutrophils, which contain proteolytic enzymes. Thus, in this process, NETs are involved in fighting the infection because NE is one of the factors affecting the release of DNA from its condensed form. Elastase is transported to the nucleus, and its enzymatic activity is a determinant of the degradation of histones, which promotes the release of DNA43. Additionally, elastase presence in NETs is a destructive factor for yeasts, hyphal forms of fungi, e.g. Candida albicans, and bacteria, e.g. Shigella flexneri42,44 (Figure 1).
Figure 1.
Neutrophil mechanisms of action. (A) Degranulation; (B) phagocytosis; (C) NETosis.
Role of neutrophil elastase inhibitors in human diseases
Extended increases in the activity of HNE may cause tissue destruction that is linked with infections and inflammation. Thus, HNE functions are involved in a variety of severe chronic diseases, particularly respiratory, urinary, integumentary, digestive, reproductive, nervous, and skeletal pathologies (Table 2).
Table 2.
HNE in human disorders
| System | Type of disorder | Model used in the study | References |
|---|---|---|---|
| Respiratory | Acute lung injury (ALI) | In vitro and in vivo studies, both clinical and animals models | 22,45 |
| Severe pneumonia | Clinical features in adult patients | 46 | |
| Acute respiratory distress syndrome (ARDS) | In vitro and in vivo studies | 4 | |
| Asthmatic exacerbations | |||
| Pulmonary fibrosis | |||
| Adult respiratory distress syndrome | Epithelial cells in the respiratory system | 47 | |
| Chronic bronchitis | |||
| Viral- or pollution-triggered asthma | |||
| Chronic obstructive pulmonary disease (COPD) | Clinical and pre-clinical trials | 26,48 | |
| Smoke-induced pulmonary emphysema | Mice | 49 | |
| Chronic obstructive airways disease (COAD) | Clinical trials | 50 | |
| Ventilator-induced lung disease | Mutant neonatal mice | 51 | |
| Metastasis formation of lung cancer | Immunodeficiency mice | 52 | |
| Bronchiolitis obliterans syndrome | In vitro and in vivo studies | 53 | |
| Urinary | End-stage renal disease (ESRD) | In vitro and in vivo studies, clinical trials | 54 |
| Chronic kidney disease | |||
| Glomerulonephritis | 50 | ||
| Integumentary | Chronic skin ulceration | Skin cells | 3 |
| Bullous pemphigoid | Mice | 55 | |
| Papillon-Lefèvre syndrome | In vitro and in vivo studies, clinical trials | 11 | |
| Psoriasis | In vitro and in vivo studies | 53 | |
| Digestive | Inflammatory bowel disease | Mice | 56 |
| Reproductive | Metastasis formation of human breast cancer | Immunodeficient mice | 53 |
| Prostate cancer | In vitro and in vivo studies | ||
| Skeletal | Rheumatoid arthritis | In vitro and in vivo studies | |
| Immunity | Graft-versus-host disease | Pre-clinical trials | 57 |
For example, alvelestat (MPH-966), an oral NE inhibitor, has adverse effects on 5-FU-induced intestinal mucositis in patients with colorectal cancer by controlling aberrant inflammatory responses, intestinal barrier dysfunction, and gut microbiota imbalance58. It is worth mentioning that sivelestat (ONO-5046), another HNE inhibitor, might be useful as a potent drug for the treatment of acute lung injury, acute respiratory distress syndrome or coagulopathy in patients with COVID-1917,59. Moreover, this selective NE inhibitor could be considered for its role in suppressing excessive inflammation post-myocardial infarction and apoptosis and preventing left ventricular remodelling in a mouse model60. ONO-5046 also limited the incidence of collagen-induced arthritis in rat and mouse models61 and prevented bleomycin-induced pulmonary fibrosis in mice62. It has been reported that after the administration of other elastase inhibitors, such as ZD-0892 and M249314 (peptidyl trifluoromethyl ketones), pulmonary artery pressure and muscularisation were reduced when used in clinical trials63.
Furthermore, elastase is able to damage the integrity of the ECM barrier, which can directly cause cancer expansion7.
Inhibitory effect of flavonoids on elastase activity
Phenolic compounds represent a large percentage of the secondary metabolites of diverse plants. Thus, flavonoid aglycones and glycosides remain one of the most extensive groups of polyphenols in the plant kingdom. Flavonoids consist of two benzene rings and one heterocyclic pyran ring, which can be divided into subgroups depending on the point of attachment of the B-carbon ring to the C-carbon ring and the degree of its oxidation and according to their chemical substitutions64,65. Due to the significant role of NE in the healing process and the development of rheumatoid arthritis, glomerulonephritis, emphysema, pulmonary diseases, psoriasis, and even cancers, several studies have reported the identification of elastase inhibitors from natural sources. Plants producing secondary metabolites and phytochemicals have great potential to act as therapeutics2,3,66. The elastase inhibitory activity of many plant extracts and compounds has been investigated to identify new sources of anti-elastase drugs. A wide range of flavonoid compounds, including aglycones and their O- and C-glycosides, were investigated for their potential elastase inhibitory activity (Table 3).
Table 3.
Flavonoids measured for anti-elastase activity and their respective IC50 values.
| Tested compound | IC50 value | References |
|---|---|---|
| Luteolin | >300 µM | 67 |
| 12 µM | 68 | |
| 8.06 ± 2.73 μM | 69 | |
| 12.7 ± 0.5 μM | 70 | |
| 6.91 μM | 71 | |
| 36.01 ± 1.15 μM | 72 | |
| 7.65 ± 0.77 μM | 73 | |
| Luteolin 4′-O-β-d-glucoside | 13.72 ± 5.26 μM | |
| Luteolin 4′-methylether | 4.13 ± 0.47 μM | 74 |
| Luteolin 7-O-β-d-glucoside | No significant inhibitory activity | 73,75 |
| Luteolin 8-C-glucoside | 146.1 ± 38.8 μM | 76 |
| Apigenin | 27.6 ± 1.0 µg/mL | |
| 46.1 ± 0.9 µM | 70 | |
| 37.94 ± 2.06 µM | 72 | |
| 13.35 ± 0.37 μM | 73 | |
| Apigenin 4′-O-β-d-glucoside | No significant inhibitory activity | 77 |
| >23.13 μM | 73 | |
| Apigenin 7-O-β-d-glucoside | No significant inhibitory activity | |
| Apigenin 7-O-rhamnoglucoside | >10 µM | 78 |
| Apigenin 8-C-glucoside | 120.95 ± 10.6 μM | 76 |
| Apigenin 6-C-glucoside | 4.34 ± 0.58 µM | 79 |
| Baicalein | 2.2 µM | 68 |
| 3.53 µM | 80 | |
| 25 µM | 67 | |
| No significant inhibitory activity | 81 | |
| Baicalein 6,7-di-O-methyl | >10 µM | 82 |
| Baicalein 7-O-methylether | ||
| 6-Hydroxy-5,7-dimethoxyflavon | ||
| Diosmetin 7-O-rutinoside | >16.43 μM | 73 |
| Chrysin | 2.44–0.09 µM | 82 |
| 6.7 µM | 68 | |
| No significant inhibitory activity | 61 | |
| Norartocarpetin | >300 µM | 83 |
| Cupressuflavone | 8.09 ± 0.92 µM | 84 |
| Amentoflavone | 1.27 ± 0.16 µM | |
| 0.75 ± 0.18 µM | 85 | |
| Robustaflavone | 1.33 ± 0.21 µM | 84 |
| 0.45 ± 0.11 µM | 85 | |
| Rhusflavanone | 19.54 ± 2.4 μM | 76 |
| Mesuaferrone B | 19.06 ± 2.4 μM | |
| Tricin | 17.69 ± 1.71 µM | 86 |
| 4′-O-Geranyltricin | 12.80 ± 6.84 µM | |
| 3′-O-Geranylpolloin | 17.34 ± 3.81 µM | |
| Velutin | 4.26 ± 0.12 µM | |
| Afrormosin | No significant inhibitory activity | 87 |
| Boeravinone T | 88 | |
| Boeravinone B | ||
| Boeravinone U | ||
| Boeravinone J | ||
| Boeravinone X | ||
| Hypolaetin 7-O-β-xyloside | >100 µM | 84 |
| 6,8-Diprenylorobol | 1.3 ± 0.3 µM | 89 |
| 5,7,3′,4′-Tetrahydroxy-2′,5′-di(3-methylbut-2-enyl)isoflavon | 213.1 ± 1.9 µM | |
| Flemiphilippinin A | 8.3 ± 0.4 µM | |
| 5,7,3′-Trihydroxy-2′-(3-methylbut-2-enyl)-4′,5′-(3,3-dimethylpyrano)isoflavone | 22.4 ± 0.7 µM | |
| 8-γ,γ-Dimethylallylwighteone | 6.0 ± 0.3 µM | |
| Osajin | 26.0 ± 0.6 µM | |
| Flemingsin | 12.0 ± 0.4 µM | |
| Flemichin D | 5.3 ± 0.5 µM | |
| Lupinifolin | 13.3 ± 0.1 µM | |
| Khonklonginol H | 110.2 ± 0.8 µM | |
| Auriculasin | 3.1 ± 0.2 µM | 11 |
| Orobol 7,3′-di-O-methyl ether | >10 µM | 85 |
| Genistein | 25.87 ± 5.99 μM | 73,82 |
| 51.4 ± 0.5 µM | 89 | |
| 63 µM | 90 | |
| 42.15 ± 2.88 µM | 79 | |
| Daidzein | 4.29 ± 0.49 µM | |
| Vigvexin A | 17.27 ± 4.19 µM | |
| Vigvexin B | 12.62 ± 7.17 µM | |
| 5,7,4′-Trihydroxy-3′-methoxy isoflavone | 19.37 ± 4.16 µM | |
| Quercetin | 5.51 ± 1.07 µM | |
| 14.3 ± 0.2 µM | 70 | |
| 2.6 µM | 68 | |
| 1.5 µM | 91 | |
| 334.18 ± 3.3 μM | 92 | |
| 20 µM | 67 | |
| 2.65 μM | 92,93 | |
| Quercetin 7-O-methylether | 18.3 µM | 68 |
| Quercetin 3-O-rhamnoside | 113.29 ± 1.9 μM | 76 |
| 36.98 ± 9.1 μM | 81 | |
| Quercetin 3-methylether | 19 µM | 94 |
| Quercetin 3,3′-dimethylethe | 129 µM | |
| Quercetin 3-O-rutinoside | 6.9 µM | 91 |
| 9.8 µM | 68 | |
| Quercetin 3-O-galactoside | 0.3 µM | |
| 0.32 μM | 93 | |
| 1.94 μM | 95 | |
| Quercitrin | 11.1 µM | 68 |
| >100 µM | 84 | |
| Isoquercitrin | ||
| 1.4 µM | 68 | |
| 1.5 μM | 93,95 | |
| Quercetagetin 3,6-dimethylether | 115 µM | 94 |
| Fisetin | 16 µM | 67 |
| Myricetin | 4 µM | |
| 21.1 µM | 68 | |
| Myricetin 3-O-rhamnoside | No significant inhibitory activity | 84 |
| Morin 3-O-α-rhamnoside | 8.52 ± 0.18 μM | 96 |
| Morin | 4.5 µM | 67 |
| 11.6 µM | 68 | |
| Naringenin | 84 µM | |
| Vitexicarpin | >10 µM | 78 |
| Ugonin M | 1.6 ± 0.33 µM | 97 |
| Ugonin O | 3.4 ± 0.50 µM | |
| Ugonin Q | 0.49 ± 0.27 µM | |
| Ugonin R | 4.56 ± 0.32 µM | |
| Ugonin S | 1.9 ± 0.52 µM | |
| Ugonin T | 1.2 ± 0.13 µM | |
| Ugonin K | >10 µM | |
| Ugonin L | 3.8 ± 0.08 µM | |
| Kaempferol | 5000 µM | 67 |
| Kaempferol 6-hydroxy-3,6-dimethylether | 194 µM | 94 |
| Kaempferol 3,7-dimethylether | 61 µM | |
| 6,8-Diprenylkaempferol | 29.3 ± 0.3 µM | 89 |
| Kaempferol 3-O-α-rhamnoside | >100 µM | 84 |
| 154.71 ± 6.48 μM | 76 | |
| 38.09 ± 12.19 μM | 96 | |
| Kaempferol 3-O-α-glucoside | 19.20 ± 3.08 μM | |
| 142.28 ± 6.24 μM | 76 | |
| Kaempferol 3-O-rutinoside | >100 µM | 91 |
| Formononetin 7-O-glucoside | >232 μM | 98 |
| Sativanone 7-O-glucoside | >215 μM | |
| Eriodictyol 7-O-rutinoside | >400 µM | 68 |
| 2-(3,4-Dihydroxy-2-[(2,6,6-trimethylcyclohex-2-enyl)-methyl]phenyl)-3,5,7-trihydroxy-4H-chromen-4-one | 0.98 ± 0.15 µM | 99 |
| 2-(3,4-Dihydroxyphenyl)-6-((2,2-dimethyl-6-methylenecyclohexyl)-methyl)-5,7-dihydroxy-chroman-4-one | >10 µM | |
| 4″a,5″,6″,7″,8″,8″a-Hexahydro-5,3′,4′-trihydroxy-5″,5″,8″a-trimethyl-4H-chromeno[2″,3″:7,8]flavone | 2.50 ± 0.37 µM | |
| 4″a,5″,6″,7″,8″,8″a-Hexahydro-5,3′,4′- trihydroxy-5″,5″,8″a-trimethyl-4H-chromeno[2″,3″:7,6]flavone | >10 µM | |
| 7-Hydroxy-6-methoxy-2-(2-phenylethyl)chromone | 3.91 ± 0.87 µM | 86 |
| 5-Hydroxy-7,3′,4′-trimethoxyflavon | 9.32 ± 1.37 µM | |
| 6,7-Dimethoxy-2-(2-phenylethyl)chromone | 10.48 ± 1.35 µM | |
| (2R, 3R)-6-methyl-3′-geranyl-2,3-trans-5,7,4′-trihydroxy-flavonol | 17.9 ± 1.5 µM | 100 |
| (E)-3-(3-(3,7-dimethylocta-2,6-dienyl)-2,4-dihydroxyphenyl)-3,5,7-trihydroxy-chroman-4-one | 8.4 ± 0.8 µM | |
| 3′-Geranyl-5,7,2′,4′ tetrahydroxyisoflavanone | 30.8 ± 1.3 µM |
It has been reported that the 3-O-β-d-glucuronides of myricetin, mearsetin, quercetin, isorhamnetin, kaempferide, and kaempferol, the 3-O-β-2″-O-acetyl-β-d-glucuronides of kaempferol, isorhamnetin, and the 3-O-β-3″-O-acetyl-β-d-glucuronides of quercetin and kaempferol significantly decrease the release of elastase by neutrophils at a concentration of 1 μM101. In another chemical and biological study, extracts from aerial parts of Hedysarum coronarium L. with a high concentration of quercetin and tannins revealed dose-dependent inhibitory properties102.
Breviscapine, a flavonoid obtained from Erigeron breviscapus reduces NE levels associated with pulmonary inflammatory response and lung function in children undergoing open-heart surgery. A positive effect was observed in patients taking 1 mg/kg or 0.5 mg/kg breviscapine103. Compounds isolated from the ethyl acetate extract of Scorzonera latifolia were also selected for further investigation of their inhibitory effect. Quercetin 3-O-β-apiofuranosyl-(1‴→2″)-β-d-glucoside and 7-methylisoorientin display anti-elastase activities of 30.16% and 28.60%, respectively104. Phloretin obtained from Malus doumeri var. formosana has been shown to inhibit elastase in a concentration-dependent manner. At concentrations of 36.5–366 µM, 51.8–77.3% enzyme inhibition was observed105,106. The flavonone sakuranetin at a concentration of 100 µM reduces the release of elastase by 60%107. In a different study, sakuranetin was applied in an in vivo mouse model and did not show adverse clinical effects in preventing elastase-induced emphysema108. A 7-O-methylaromadendrin isolated from Inula viscosa decreased elastase production by 50% at 100 µM107. Glycitin was also evaluated for its NE release inhibitory properties, and the results revealed that a compound at 10 µM lowered enzyme activity109. 5-O-demethylnobiletin, a polymethoxyflavone isolated from Sideritis tragoriganum, inhibited elastase release by 48% at 10 µM. It is worth mentioning that the described flavonoids did not affect the activity of this enzyme110. The results of the elastase assays showed that at a concentration of 100 µM, naringenin, liquiritigenin, quercetin, apigenin, and sulfuretin possess inhibitory activities of 39%, 52%, 65%, 57%, and 38%, respectively111. The elastase inhibitory activities of the isolated compounds from the EtOAc (ethyl acetate) subextract of Epilobium angustifolium were also evaluated. Hyperoside, kaempferol, kaempferol 3-O-α-l-rhamnoside, quercetin 3-O-α-l-rhamnoside, and quercetin 3-O-α-l-arabinoside at a concentration of 100 µg/mL revealed inhibitory potentials of 19.87%, 15.33%, 9.76%, 8.92%, and 7.08%, respectively112.
The inhibitory effect of water–ethanol extract obtained from Cecropia pachystachya leaves, which has a total flavonoid content of 72.71 µg QE/mg DE, began at 0.8 µg/mL (15.79% elastase inhibition) and notably increased at 4 µg/mL (41.44%), 8 µg/mL (55.45%), and 16 µg/mL (50.99%)113. The anti-elastase activity of aqueous extracts from the leaves of Ligustrum vulgare L. was determined based on the contents of the flavonoids aglycones and glycosides (luteolin glucoside, quercetin rutinoside, and ligustroflavone). The aqueous extract at concentrations ranging from 5 µg/mL to 50 µg/mL inhibited HNE release by 23.9–34.1%114. It is worth mentioning that an ethanol extract of Aceriphyllum rossii leaves, which has a total flavonoid content of 206.3 mg/g, exhibits 99.2% inhibition at 10 mg/mL in vitro115. Fermenting red ginseng (FRG) was investigated as a novel skin-care antiaging ingredient based on its elastase inhibition potency. FRGs consist of 133.2 µg/mL flavonoid compounds, which may be connected with the IC50 value (117.07 µg/mL) for elastase inhibitory activity116. The leaf hydroalcoholic extract (EDE) from Eugenia dysenterica was characterised to determine its quercetin and other phenolic contents, and EDE was capable of inhibiting elastase in a dose-dependent manner at 25–100 µg/mL, with 45% activity observed at a concentration of 100 µg/mL117.
Many authors have identified anti-elastase activity based on EC50 values. Meum athamanticum, Centella asiatica, and Aegopodium podagraria water–glycerin extracts are described by a high amount of flavonoid compounds and demonstrate EC50 (%) values of inhibitory activity at 0.92, 0.52, and 1.03, respectively118.
The extracts obtained by subcritical water extraction from the stems, leaves, and berries of Aronia melanocarpa also reveal anti-elastase potential. At this stage, researchers determined both the total phenolic and total flavonoid contents. The leaves had the highest total phenolic and flavonoid contents, followed by the stems and berries, with 131.53 mg CAE/g extract, 49.96 mg CAE/g extract, and 13.88 mg CAE/g extract for phenolics, respectively, and 88.64 mg RE/g extract, 25.10 mg RE/g extract, and 10.00 mg RE/g extract for flavonoids, respectively. Moreover, flavonoids constitute over 70% of all phenolic compounds in aronia berries. All A. melanocarpa extracts expressed elastase inhibitory activity, with the highest potential observed in berry extracts (3.549 ± 0.113 mmol CAE/g extract)119. According to the LC–MS analysis, Libidibia ferrea bark and pod extracts are the sources of rutin, quercetin, kaempferol, apigenin, isorhamnetin, and taxifolin, and the samples showed approximately 36% elastase inhibition at 250 µg/mL for bark extract and 20% for pod extract120. Three flavonoids were isolated from the ethyl acetate fraction of the Alchornea cordifolia leaves: quercetin, myricetin 3-glucoside, and myricetin 3-rhamnoside. The anti-elastase activity was evaluated for aqueous and ethyl acetate extracts in cell-free and cellular models. In an acellular system, the IC50 values reached 4.7 and 2.2 mg/L for aqueous and ethyl acetate extracts, respectively. In a cellular model, polymorphonuclear neutrophils were stimulated by PMA (4β-phorbol-12-myristate-13-acetate), CaI (calcium ionophore), and fMLP (N-formyl-methionyl-leucine-phenylalanine). The IC50 values in the stimulated cellular experiment were in the range of 5.9–8.6 mg/L in the ethyl acetate extract and 7.3–12.1 mg/L in the aqueous extract. Among the ethyl acetate and aqueous extracts, the more active extract was the ethyl acetate, which may be connected with its higher content of flavonoids121.
Flavonoid structure–activity relationship (SAR)
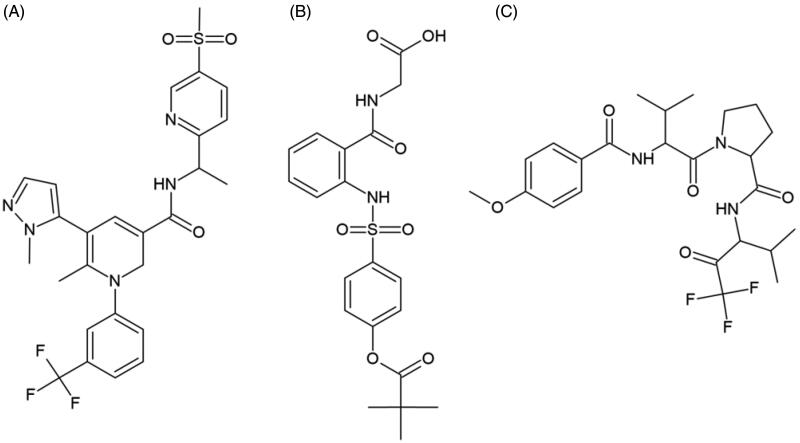
Flavonoid SAR analyses enable the determination of the chemical groups responsible for evoking a target biological effect in the organism. The SAR can be used to explain the effect of the structural characteristics of molecules on their activity (Figure 2) and is essential for determining the mechanism underlying drug action122–124 (Figure 3).
Figure 2.
Chemical structures of clinical HNE inhibitors. (A) MPH-966, (B) ONO-5046, and (C) ZD-0892.
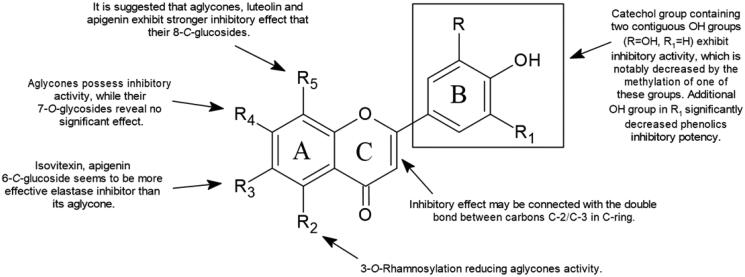
Figure 3.
Chemical groups responsible for flavonoid activity (SAR).
Special attention was paid to the number, O-methylation, O-glycosylation of free hydroxyl groups as well as the C-glycosylation in position C-6 and C-8 in A-ring. Natural compounds bearing a catechol group containing two contiguous phenolic OH groups (3′,4′-dihydroxy) exhibit inhibitory activity, which is notably decreased by the methylation of one of these groups. Compounds with a lack of catechol groups possess a weak inhibitory effect on elastase action125. Among the four investigated flavonoids, quercetin, myricetin, kaempferol, and galangin, the leading inhibitory potency possesses quercetin, followed by myricetin. It is worth mentioning that the additional OH group in the myricetin molecule at the B-ring (C5′) significantly decreased the phenolic inhibitory potency. The kaempferol and galangin without catechol groups did not exhibit significant inhibitory activity. Moreover, it seems that O-methylation in B-ring leads to an increase in this activity. Luteolin 4′-methyl ether (IC50 4.13 µM) possess higher inhibitory potential than luteolin (IC50 6.91–36.01 µM). Moreover, it seems that O-methylation in B-ring leads to increase inhibitory activity. Luteolin 4′-methyl ether (IC50 4.13 µM) possess higher inhibitory potential than luteolin (IC50 6.91–36.01 µM)71,74.
The significance of O-glycosylation at the A-ring (C7) and C-ring (C3) positions can be observed by comparing the inhibitory levels of apigenin and luteolin and its 7-O-glucosides cosmosiin, and cynaroside, respectively. Based on the IC50 values, aglycones possess stronger activity while their 7-O-glucosides reveal no significant inhibitory effect. It is worth mentioning that 3-O-rhamnosylation of quercetin and kaempferol also reduced their activity. The values presented in Table 3 suggest that glucosylation or rhamnosylation at positions C-7 or C-3 presumably produce steric hindrances that prevent molecules from binding to enzymes126. In addition, a comparison of an anti-elastase potential of apigenin and apigenin 4′-O-β-d-glucoside leads to the conclusion that glucosylation of the hydroxyl group in B-ring also reduces its activity70,77.
C-glycosylation of the A-ring occurs at the C6 and C8 positions, which are the most typical locations for glycosyl radicals in the flavonoid skeleton. It seems that the aglycones luteolin and apigenin exhibit stronger inhibitory effects than their 8-C-glucosides. On the other hand, isovitexin and apigenin 6-C-glucoside are more effective elastase inhibitors than their aglycones (see Table 3).
The inhibitory effect may also be connected with the double bond between carbons C-2 and C-3 in the C-ring of flavonoids125,127. It is suggested that double bonds in the C-ring allow for the maintenance of a spatial and practically planar flavonoid skeleton. The saturation of the double bond may result in the presence of an obtuse angle in the flavonoid structure. Previous findings assumed that the almost flat structure of flavonoids is an important factor in enzyme inhibition activity126. These conclusions explain the significantly stronger inhibitory activity of apigenin than naringenin (see Table 3).
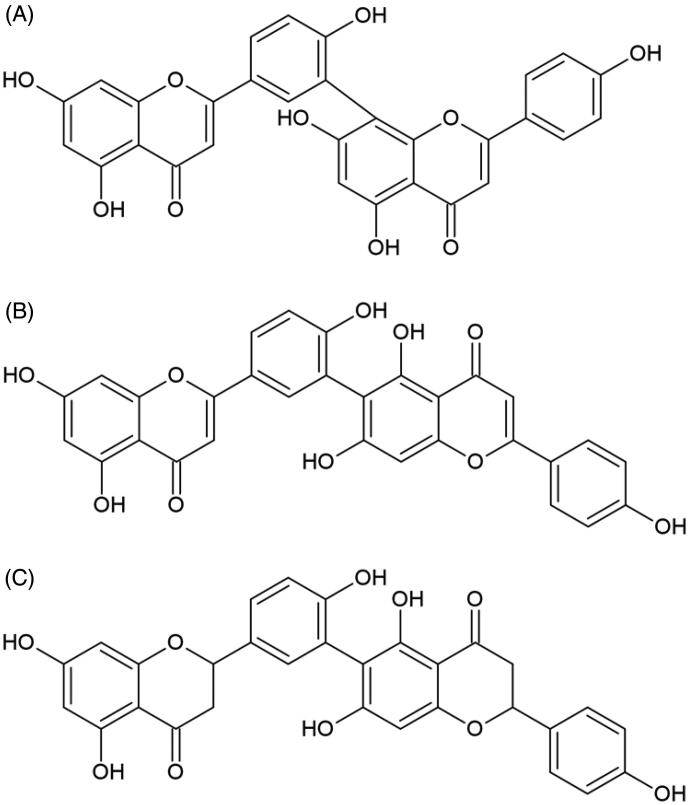
In the group of biflavonoids, anti-elastase activities were examined for cupressuflavone, amentoflavone, robustaflavone, and rhusflavanone (Figure 4)76,84,85. The amentoflavone and robustaflavone differ in the chromene ring substituent, C-8 and C-6, respectively. The authors observed that their high inhibitory activity might be connected with the optimum number of free hydroxyl moieties. It is worth mentioning that the distinction between chromene ring position does not influence biflavonoids biological effect. The difference in two structures of robustaflavone and rhusflavanone is connected with saturation on double bond in C-ring. Rhusflavanone with a lack of double bond between C-2 and C-3 exhibit much lower inhibitory potential than robustaflavone. Results from this assay were well correlated with those from studies using apigenin and naringenin.
Figure 4.
Chemical structures of biflavonoids with anti-elastase potential. (A) Amentoflavone, (B) robustaflavone, and (C) rhusflavonone.
The position of the B-ring in the C-ring allows us to compare flavone and isoflavone activity. Genistein with a 3-B-ring and one hydroxyl group at the A-ring shows similar activity to apigenin, while daidzein with two hydroxyl groups at the A-ring exhibits a stronger effect than flavone. However, the values presented in Table 3 are not sufficient to identify the SAR for this class of compounds.
Summarising, comparison of IC50 values allowed pointing out characteristics of flavonoids structures that facilitate their elastase inhibition: catechol structure for B-ring, double bond between C2–C3 at C-ring, O-methylation and C-glycosylation at A-, B-, and C-ring. The level of plant derivative activity on HNE has been reported to be also connected with the hydrophobicity and molar refractivity of these derivatives, with a bilinear correlation representing the most important relationship128. Nevertheless, to correlate the SAR with flavonoid inhibitory effects, additional experiments in different cellular and enzymatic systems must be performed.
Discussion and conclusions
Significant progress has been made towards discovering natural products as enzyme inhibitors. The high potential of natural compounds lies in their role as lead structures that can be optimised in terms of bioavailability and biological activity. Nevertheless, our knowledge regarding the SAR among the various flavonoid compounds and their impact on elastase action and release is still incomplete. Emerging reports on the activity of various groups of compounds provide information about new elastase inhibitors. On the other hand, the available results yield conflicting information about the level of their inhibitory activity.
To clarify, the authors of this review verified the method criteria for establishing IC50 values for compounds presented in Table 3. For example, the difference between IC50 values in studies describing luteolin activity may be connected with experiments involving blocking elastase release from neutrophils as well as inhibition of already freed enzymes. It was noted that an IC50>300 µM for luteolin activity was established in the test, and it represented the change in absorbance measured after adding enzyme to substrates followed by the incubation process. In comparison, elastase release was measured by degranulation of azurophilic granules and activation of human neutrophils with fMLP. The results are expressed in a fMLP/CB (cytochalasin B)-activated, drug-free control system. In this case, the IC50 for luteolin activity reached 6.91 µM. Correspondingly, another flavone commonly found in the plant kingdom, namely, apigenin, has been tested as an elastase inhibitor, and its IC50 ranges from 13.35 µM to 46.1 µM. Potent inhibition of HNE release occurs by apigenin after stimulation of cells with fMLP. These results are compatible with data obtained with the use of luteolin as an inhibitor. It was deduced that experiments involving fMLP/CB-stimulated neutrophils showed that apigenin and luteolin were effective. Similar conclusions can also be drawn from the analysis of chrysin IC50 values. Superior chrysin activity in human neutrophils was assessed as inhibition of fMLP/CB-induced elastase release (IC50=2.44 µM).
Quercetin has been used in many studies as a reference compound with a proven inhibitory effect on elastase. Based on these results, it appears that the value that adequately describes the IC50 for quercetin is in the range of 2.6–2.65 µM. However, some researchers established a positive control for this compound at over 300 µM (0.101 mg/mL). In this situation, the distinction between the obtained values seems to be connected with the substrate concentrations (N-succinyl-Ala-Ala-Ala-p-nitroanilide, elastase), pH scale, incubation time, and temperature and the volumes of elastase, inhibitor, and medium solutions.
Moreover, the 5,6,7-trihydroxyflavone baicalein binds not only to the active site but also to the allosteric sites of pancreatic elastase and exhibits a competitive and non-competitive inhibition model, which indicates that the inhibitor molecule may link to either the enzyme–substrate complex or the enzyme alone80. According to available data, baicalein exhibits a significant anti-elastase effect (IC50=3.53 µM), although research has also indicated a lack of relevant inhibitory activity. The distinction between those extreme results values can be related to different conditions of the conducted experiment. In summary, to specify the ability to inhibit either elastase activity or its release from cells, a wide range of necessary experimental conditions (including substrates, pH level, incubation time, wavelength, volumes, concentrations, and inhibition of enzyme release or free enzyme activity) should be taken into consideration.
The data presented above highlight the diversity of natural phenolic-based structures as elastase inhibitors, thus indicating that novel synthetic inhibitors can be designed and developed based on the structure of phenolic compounds. In practice, a considerable part of every therapy is the selectivity the drug has for its target. On the other hand, compounds may also reveal off-target outcomes due to their toxic and side effects. Anti-target effects follow a narrow level between efficacy and toxicity doses that initiate problems with drug candidate compounds' development. A protein–ligand interaction assessment can be built with in silico virtual screening and docking. The available theoretical techniques provide essential information on the compounds and show methods to calculate their binding affinities for the HNE129,130. Structure-shape virtual screening may be practical to identify selective flavonoid inhibitors from databases. Molecular docking is a tool allowing for predicting the potential inhibitory activity and provides a better indication of how a flavonoid can influence its enzyme target131. Theoretical methods and computational programmes, including virtual screening, analysis of structure-base and pharmacophore, as well as molecular docking, can be used to pick compounds that target an enzyme and to determine expected targets for well-known and newly discovered phytochemicals132. Thus, in silico studies can improve successive stages for decreasing off-target effects, activity profiling, and further analysis of natural compounds. It is recommended to establish the efficacy and safety of the described inhibitors using in vivo and in vitro models, including docking, especially when using such compounds in products to promote health.
Disclosure statement
The authors report no conflict of interest.
References
- 1.Copeland RA, Harpel MR, Tummino PJ.. Targeting enzyme inhibitors in drug discovery. Expert Opin Ther Targets 2007;11:967–78. [DOI] [PubMed] [Google Scholar]
- 2.Reboud-Ravaux M. Les inhibiteurs d’élastases. J Biosoc Sci 2001;195:143–50. [PubMed] [Google Scholar]
- 3.Tundis R, Loizzo MR, Bonesi M, Menichini F.. Potential role of natural compounds against skin aging. Curr Med Chem 2015;22:1515–38. [DOI] [PubMed] [Google Scholar]
- 4.Fitch PM, Roghanian A, Howie SEM, Sallenave JM.. Human neutrophil elastase inhibitors in innate and adaptive immunity. Biochem Soc Trans 2006;34:279–82. [DOI] [PubMed] [Google Scholar]
- 5.Pham CTN. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 2006;6:541–50. [DOI] [PubMed] [Google Scholar]
- 6.Korkmaz B, Moreau T, Gauthier F.. Neutrophil elastase, proteinase 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie 2008;90:227–42. [DOI] [PubMed] [Google Scholar]
- 7.Sun Z, Yang P.. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol 2004;5:182–90. [DOI] [PubMed] [Google Scholar]
- 8.Burg ND, Pillinger MH.. The neutrophil: function and regulation in innate and humoral immunity. Clin Immunol 2001;99:7–17. [DOI] [PubMed] [Google Scholar]
- 9.Wright HL, Moots RJ, Bucknall RC, Edwards SW.. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 2010;49:1618–31. [DOI] [PubMed] [Google Scholar]
- 10.Amulic B, Cazalet C, Hayes GL, et al. . Neutrophil function: from mechanisms to disease. Annu Rev Immunol 2012;30:459–89. [DOI] [PubMed] [Google Scholar]
- 11.Korkmaz B, Horwitz MS, Jenne DE, Gauthier F.. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev 2010;62:726–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen G, An W, Chen J, Maguire EM, et al. . Genetic and pharmacologic inhibition of the neutrophil elastase inhibits experimental atherosclerosis. J Am Heart Assoc 2018;7:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siedle B, Hrenn A, Merfort I.. Natural compounds as inhibitors of human neutrophil elastase. Planta Med 2007;73:401–20. [DOI] [PubMed] [Google Scholar]
- 14.Németh T, Sperandio M, Mócsai A.. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov 2020;19:253–75. [DOI] [PubMed] [Google Scholar]
- 15.Ley K, Hoffman HM, Kubes P, et al. . Neutrophils: new insights and open questions. Sci Immunol 2018;3:1–14. [DOI] [PubMed] [Google Scholar]
- 16.Sinha S, Watorek W, Karr S, et al. . Primary structure of human neutrophil elastase. Proc Natl Acad Sci U S A 1987;84:2228–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sjö P. Neutrophil elastase inhibitors: recent advances in the development of mechanism-based and nonelectrophilic inhibitors. Future Med Chem 2012;4:651–60. [DOI] [PubMed] [Google Scholar]
- 18.Taggart CC, Greene CM, Carroll TP, et al. . Elastolytic proteases: inflammation resolution and dysregulation in chronic infective lung disease. Am J Respir Crit Care Med 2005;171:1070–6. [DOI] [PubMed] [Google Scholar]
- 19.Bank U, Ansorge S.. More than destructive: neutrophil-derived serine proteases in cytokine bioactivity control. J Leukoc Biol 2001;61:197–206. [PubMed] [Google Scholar]
- 20.Devaney JM, Greene CM, Taggart CC, et al. . Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Lett 2003;544:129–32. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi F, Means TK, Luster AD.. Toll-like receptors stimulate human neutrophil function. Blood 2003;102:2660–9. [DOI] [PubMed] [Google Scholar]
- 22.Kawabata K, Hagio T, Matsuoka S.. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol 2002;451:1–10. [DOI] [PubMed] [Google Scholar]
- 23.Tsuji N, Moriwaki S, Suzuki Y, et al. . The role of elastases secreted by fibroblasts in wrinkle formation: implication through selective inhibition of elastase activity. Photochem Photobiol 2001;74:283. [DOI] [PubMed] [Google Scholar]
- 24.Rogalski C, Meyer-Hoffert U, Proksch E, Wiedow O.. Human leukocyte elastase induces keratinocyte proliferation in vitro and in vivo. J Invest Dermatol 2002;118:49–54. [DOI] [PubMed] [Google Scholar]
- 25.Owen CA, Campbell MA, Boukedes SS, Campbell EJ.. Cytokines regulate membrane-bound leukocyte elastase on neutrophils: a novel mechanism for effector activity. Am J Physiol Lung Cell Mol Physiol 1997;272:385–93. [DOI] [PubMed] [Google Scholar]
- 26.Döring G. The role of neutrophil elastase in chronic inflammation. Am J Respir Crit Care Med 1994;150:S114–S7. [DOI] [PubMed] [Google Scholar]
- 27.Savill JS, Wyllie AH, Henson JE, et al. . Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest 1989;83:865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egeblad M, Werb Z.. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161–74. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Perelman JM, Kolosov VP, Xiangdong Z.. Neutrophil elastase induces MUC5AC secretion via protease-activated receptor 2. Mol Cell Biochem 2013;377:75–85. [DOI] [PubMed] [Google Scholar]
- 30.Tsai Y, Hwang T.. Neutrophil elastase inhibitors: a patent review and potential applications for inflammatory lung diseases (2010–2014). Expert Opin Ther Pat 2015;25:1145–58. [DOI] [PubMed] [Google Scholar]
- 31.Mihara K, Ramachandran R, Renaux B, et al. . Neutrophil elastase and proteinase-3 trigger G protein-biased signaling through proteinase-activated receptor-1 (PAR1). J Biol Chem 2013;288:32979–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadel JA. Role of enzymes from inflammatory cells on airway submucosal gland secretion. Respir Int J Thorac Med 1991;58:3–5. [DOI] [PubMed] [Google Scholar]
- 33.Renesto P, Chignard M.. Enhancement of cathepsin G-induced platelet activation by leukocyte elastase: consequence for the neutrophil-mediated platelet activation. Blood 1993;82:139–44. [PubMed] [Google Scholar]
- 34.Adkison AM, Raptis SZ, Kelley DG, Pham CTN.. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest 2002;109:363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flannagan RS, Jaumouillé V, Grinstein S.. The cell biology of phagocytosis. Annu Rev Pathol Mech Dis 2012;7:61–98. [DOI] [PubMed] [Google Scholar]
- 36.Belaaouaj AA, Kim KS, Shapiro SD.. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science 2000;289:1185–7. [DOI] [PubMed] [Google Scholar]
- 37.Hahn I, Klaus A, Janze AK, et al. . Cathepsin G and neutrophil elastase play critical and nonredundant roles in lung-protective immunity against Streptococcus pneumoniae in mice. Infect Immun 2011;79:4893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol 2006;2:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosales C. Neutrophils at the crossroads of innate and adaptive immunity. J Leukoc Biol 2020;108:377–96. [DOI] [PubMed] [Google Scholar]
- 40.Johansson A, Claesson R, Hänström L, et al. . Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans. J Periodontal Res 2000;35:85–92. [DOI] [PubMed] [Google Scholar]
- 41.López-Boado YS, Espinola M, Bahr S, Belaaouaj A.. Neutrophil serine proteinases cleave bacterial flagellin, abrogating its host response-inducing activity. J Immunol 2004;172:509–15. [DOI] [PubMed] [Google Scholar]
- 42.Brinkmann V, Reichard U, Goosmann C, et al. . Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–5. [DOI] [PubMed] [Google Scholar]
- 43.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A.. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 2010;191:677–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urban CF, Reichard U, Brinkmann V, Zychlinsky A.. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol 2006;8:668–76. [DOI] [PubMed] [Google Scholar]
- 45.Polverino E, Rosales-Mayor E, Dale GE, et al. . The role of neutrophil elastase inhibitors in lung diseases. Chest 2017;152:249–62. [DOI] [PubMed] [Google Scholar]
- 46.Matsuse H, Yanagihara K, Mukae H, et al. . Association of plasma neutrophil elastase levels with other inflammatory mediators and clinical features in adult patients with moderate and severe pneumonia. Respir Med 2007;101:1521–8. [DOI] [PubMed] [Google Scholar]
- 47.Voynow JA, Young LR, Wang Y, et al. . Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am J Physiol 1999;276:L835–43. [DOI] [PubMed] [Google Scholar]
- 48.Lucas SD, Costa E, Guedes RC, Rui M.. Targeting COPD: advances on low-molecular-weight inhibitors of human neutrophil elastase. Med Res Rev 2013;33:E73–E101. [DOI] [PubMed] [Google Scholar]
- 49.Shapiro SD, Goldstein NM, Houghton AMG, et al. . Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol 2003;163:2329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henriksen PA. The potential of neutrophil elastase inhibitors as anti-inflammatory therapies. Curr Opin Hematol 2014;21:23–8. [DOI] [PubMed] [Google Scholar]
- 51.Hilgendorff A, Parai K, Ertsey R, et al. . Neonatal mice genetically modified to express the elastase inhibitor elafin are protected against the adverse effects of mechanical ventilation on lung growth. Am J Physiol Lung Cell Mol Physiol 2012;303:L215–L27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato T, Takahashi S, Mizumoto T, et al. . Neutrophil elastase and cancer. Surg Oncol 2006;15:217–22. [DOI] [PubMed] [Google Scholar]
- 53.Crocetti L, Quinn MT, Schepetkin IA, Giovannoni MP.. A patenting perspective on human neutrophil elastase (HNE) inhibitors (2014–2018) and their therapeutic applications. Expert Opin Ther Pat 2019;29:555–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bronze-Da-Rocha E, Santos-Silva A.. Neutrophil elastase inhibitors and chronic kidney disease. Int J Biol Sci 2018;14:1343–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z, Shapiro SD, Zhou X, et al. . A critical role for neutrophil elastase in experimental bullous pemphigoid. J Clin Invest 2000;105:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Motta JP, Bermúdez-Humarán LG, Deraison C, et al. . Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci Transl Med 2012;4:1–14. [DOI] [PubMed] [Google Scholar]
- 57.Magenau JM, Goldstein SC, Peltier D, et al. . α1-antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease. Blood 2018;131:1372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen KJ, Chen YL, Ueng SH, et al. . Neutrophil elastase inhibitor (MPH-966) improves intestinal mucosal damage and gut microbiota in a mouse model of 5-fluorouracil-induced intestinal mucositis. Biomed Pharmacother 2021;134:111152. [DOI] [PubMed] [Google Scholar]
- 59.Sahebnasagh A, Saghafi F, Safdari M, et al. . Neutrophil elastase inhibitor (sivelestat) may be a promising therapeutic option for management of acute lung injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19. J Clin Pharm Ther 2020;45:1515–9. [DOI] [PubMed] [Google Scholar]
- 60.Ogura Y, Tajiri K, Murakoshi N, et al. . Neutrophil elastase deficiency ameliorates myocardial injury post myocardial infarction in mice. Int J Mol Sci 2021;22:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kakimoto K, Matsukawa A, Yoshinaga M, Nakamura H.. Suppressive effect of a neutrophil elastase inhibitor on the development of collagen-induced arthritis. Cell Immunol 1995;165:26–32. [DOI] [PubMed] [Google Scholar]
- 62.Takemasa A, Ishii Y, Fukuda T.. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur Respir J 2012;40:1475–82. [DOI] [PubMed] [Google Scholar]
- 63.Cowan K, Heilbut A, Humpl T, et al. . Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med 2000;6:698–702. [DOI] [PubMed] [Google Scholar]
- 64.Jakimiuk K, Wink M, Tomczyk M.. Flavonoids of the Caryophyllaceae. Phytochem Rev 2021;20:1–41. [Google Scholar]
- 65.Wink M. Modes of action of herbal medicines and plant secondary metabolites. Medicines (Basel) 2015;2:251–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, et al. . Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv 2015;33:1582–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sartor L, Pezzato E, Dell'Aica I, et al. . Inhibition of matrix-proteases by polyphenols: chemical insights for anti-inflammatory and anti-invasion drug design. Biochem Pharmacol 2002;64:229–37. [DOI] [PubMed] [Google Scholar]
- 68.Melzig M, Loser B, Ciesielski S.. Inhibition of neutrophil elastase activity by phenolic compounds from plants. Pharmazie 2001;56:967–70. [PubMed] [Google Scholar]
- 69.Yang SC, Chen PJ, Chang SH, et al. . Luteolin attenuates neutrophilic oxidative stress and inflammatory arthritis by inhibiting Raf1 activity. Biochem Pharmacol 2018;154:384–396. [DOI] [PubMed] [Google Scholar]
- 70.Ryu HW, Park YJ, Lee SU, et al. . Potential anti-inflammatory effects of the fruits of Paulownia tomentosa. J Nat Prod 2017;80:2659–65. [DOI] [PubMed] [Google Scholar]
- 71.Tóth B, Chang FR, Hwang TL, et al. . Screening of Luzula species native to the Carpathian Basin for anti-inflammatory activity and bioactivity-guided isolation of compounds from Luzula luzuloides (Lam.) Dandy & Wilmott. Fitoterapia 2017;116:131–8. [DOI] [PubMed] [Google Scholar]
- 72.Lee SM, Song YH, Uddin Z, et al. . Prenylated flavonoids from Epimedium koreanum Nakai and their human neutrophil elastase inhibitory effects. Rec Nat Prod 2017;11:514–20. [Google Scholar]
- 73.Liou JR, El-Shazly M, Du YC, et al. . 1,5-Diphenylpent-3-en-1-ynes and methyl naphthalene carboxylates from Lawsonia inermis and their anti-inflammatory activity. Phytochemistry 2013;88:67–73. [DOI] [PubMed] [Google Scholar]
- 74.Lin AS, Lin CR, Du YC, Lübken T, et al. . Acasiane A and B and farnesirane A and B, diterpene derivatives from the roots of Acacia farnesiana. Planta Med 2009;75:256–61. [DOI] [PubMed] [Google Scholar]
- 75.Süntar I, Akkol EK, Keles H, et al. . Efficacy of Daphne oleoides subsp. kurdica used for wound healing: identification of active compounds through bioassay guided isolation technique. J Ethnopharmacol 2012;141:1058–70. [DOI] [PubMed] [Google Scholar]
- 76.Wynn MK, Kido T, Kusakari K, et al. . Rhusflavanone and mesuaferrone B: tyrosinase and elastase inhibitory biflavonoids extracted from the stamens of Mesua ferrea L. Nat Prod Res 2019;35:1–5. [DOI] [PubMed] [Google Scholar]
- 77.Süntar I, Akkol EK, Keles H, et al. . Exploration of the wound healing potential of Helichrysum graveolens (Bieb.) Sweet: isolation of apigenin as an active component. J Ethnopharmacol 2013;149:103–10. [DOI] [PubMed] [Google Scholar]
- 78.Kuo PC, Liao YR, Hung HY, et al. . Anti-inflammatory and neuroprotective constituents from the peels of Citrus grandis. Molecules 2017;22:967–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leu YL, Hwang TL, Kuo PC, et al. . Constituents from Vigna vexillata and their anti-inflammatory activity. Int J Mol Sci 2012;13:9754–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghosh D, Bansode S, Joshi R, et al. . Molecular elucidation of pancreatic elastase inhibition by baicalein. J Biomol Struct Dyn 2021;15:1–10. [DOI] [PubMed] [Google Scholar]
- 81.Han J, Ji Y, Youn K, et al. . Baicalein as a potential inhibitor against BACE1 and AChE: mechanistic comprehension through in vitro and computational approaches. Nutrients 2019;11:2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsu YM, Wu TY, Du YC, et al. . 3-Methyl-4,5-dihydro-oxepine, polyoxygenated seco-cyclohexenes and cyclohexenes from Uvaria flexuosa and their anti-inflammatory activity. Phytochemistry 2016;122:184–92. [DOI] [PubMed] [Google Scholar]
- 83.Ban YJ, Baiseitova A, Nafiah MA, et al. . Human neutrophil elastase inhibitory dihydrobenzoxanthones and alkylated flavones from the Artocarpus elasticus root barks. Appl Biol Chem 2020;63:8. [Google Scholar]
- 84.Xu GH, Ryoo IJ, Kim YH, et al. . Free radical scavenging and antielastase activities of flavonoids from the fruits of Thuja orientalis. Arch Pharm Res 2009;32:275–82. [DOI] [PubMed] [Google Scholar]
- 85.Ayoub IM, Korinek M, Hwang TL, et al. . Probing the antiallergic and anti-inflammatory activity of biflavonoids and dihydroflavonols from Dietes bicolor. J Nat Prod 2018;81:243–53. [DOI] [PubMed] [Google Scholar]
- 86.Wang SL, Hwang TL, Chung MI, et al. . New flavones, a 2-(2-phenylethyl)-4H-chromen-4-one derivative, and anti-inflammatory constituents from the stem barks of Aquilaria sinensis. Molecules 2015;20:20912–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Araújo Lopes A, Magalhães TR, de Andrade Uchôa DE, et al. . Afrormosin, an isoflavonoid from Amburana cearensis A. C. Smith, modulates the inflammatory response of stimulated human neutrophils. Basic Clin Pharmacol Toxicol 2013;113:363–9. [DOI] [PubMed] [Google Scholar]
- 88.Yang EJ, Lee T, Song KS.. β-Secretase inhibition by C-methylisoflavones from Abronia nana. Nat Prod Res 2019;33:1705–12. [DOI] [PubMed] [Google Scholar]
- 89.Kim YJ, Wang Y, Uddin Z, et al. . Competitive neutrophil elastase inhibitory isoflavones from the roots of Flemingia philippinensis. Bioorg Chem 2018;78:249–57. [DOI] [PubMed] [Google Scholar]
- 90.Rotondo S, Krauze-Brzósko B, Manarini S, et al. . Inhibition by soya isoflavones of human polymorphonuclear leukocyte function: possible relevance for the beneficial effects of soya intake. Br J Nutr 2008;99:240–7. [DOI] [PubMed] [Google Scholar]
- 91.Xu GH, Kim YH, Choo SJ, et al. . Chemical constituents from the leaves of Ilex paraguariensis inhibit human neutrophil elastase. Arch Pharm Res 2009;32:1215–20. [DOI] [PubMed] [Google Scholar]
- 92.Prasad Pandey B, Pradhan SP, Adhikari K.. LC-ESI-QTOF-MS for the profiling of the metabolites and in vitro enzymes inhibition activity of Bryophyllum pinnatum and Oxalis corniculata collected from Ramechhap district of Nepal. Chem Biodivers 2020;17:e2000155. [DOI] [PubMed] [Google Scholar]
- 93.Melzig MF, Pertz HH, Krenn L.. Anti-inflammatory and spasmolytic activity of extracts from Droserae herba. Phytomedicine 2001;8:225–9. [DOI] [PubMed] [Google Scholar]
- 94.Krenn L, Wollenweber E, Steyrleuthner K, et al. . Contribution of methylated exudate flavonoids to the anti-inflammatory activity of Grindelia robusta. Fitoterapia 2009;80:267–9. [DOI] [PubMed] [Google Scholar]
- 95.Krenn L, Beyer G, Pertz HH, et al. . In vitro antispasmodic and anti-inflammatory effects of Drosera rotundifolia. Arzneimittel-Forschung/Drug Res 2004;54:402–5. [DOI] [PubMed] [Google Scholar]
- 96.Yen CT, Hsieh PW, Hwang TL, et al. . Flavonol glycosides from Muehlenbeckia platyclada and their anti-inflammatory activity. Chem Pharm Bull (Tokyo) 2009;57:280–2. [DOI] [PubMed] [Google Scholar]
- 97.Huang YC, Hwang TL, Chang CS, et al. . Anti-inflammatory flavonoids from the rhizomes of Helminthostachys zeylanica. J Nat Prod 2009;72:1273–8. [DOI] [PubMed] [Google Scholar]
- 98.Öz BE, İşcan GS, Akkol EK, et al. . Isoflavonoids as wound healing agents from Ononidis radix. J Ethnopharmacol 2018;211:384–93. [DOI] [PubMed] [Google Scholar]
- 99.Huang YC, Hwang TL, Yang YL, et al. . Acetogenin and prenylated flavonoids from Helminthostachys zeylanica with inhibitory activity on superoxide generation and elastase release by neutrophils. Planta Med 2010;76:447–53. [DOI] [PubMed] [Google Scholar]
- 100.Tan XF, Kim DW, Song YH, et al. . Human neutrophil elastase inhibitory potential of flavonoids from Campylotropis hirtella and their kinetics. J Enzyme Inhib Med Chem 2016;31:16–22. [DOI] [PubMed] [Google Scholar]
- 101.Granica S, Czerwińska ME, Żyżyńska-Granica B, Kiss AK.. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia 2013;91:180–8. [DOI] [PubMed] [Google Scholar]
- 102.Burlando B, Pastorino G, Salis A, et al. . The bioactivity of Hedysarum coronarium extracts on skin enzymes and cells correlates with phenolic content. Pharm Biol 2017;55:1984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen J, Zhao YH, Liu XL, et al. . Effects of breviscapine on pulmonary inflammatory response and lung injury in children undergoing open heart surgery. J Asian Nat Prod Res 2012;14:270–5. [DOI] [PubMed] [Google Scholar]
- 104.Akkol EK, Šmejkal K, Kurtul E, et al. . Inhibitory activity of Scorzonera latifolia and its components on enzymes connected with healing process. J Ethnopharmacol 2019;245:1–7. [DOI] [PubMed] [Google Scholar]
- 105.Leu SJ, Lin YP, Lin RD, et al. . Phenolic constituents of Malus doumeri var. formosana in the field of skin care. Biol Pharm Bull 2006;29:740–5. [DOI] [PubMed] [Google Scholar]
- 106.Casarini TPA, Frank LA, Pohlmann AR, Guterres SS.. Dermatological applications of the flavonoid phloretin. Eur J Pharmacol 2020;889:1–9. [DOI] [PubMed] [Google Scholar]
- 107.Hernández V, Recio MC, Máñez S, et al. . Effects of naturally occurring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci 2007;81:480–8. [DOI] [PubMed] [Google Scholar]
- 108.Taguchi L, Pinheiro NM, Olivo CR, et al. . A flavanone from Baccharis retusa (Asteraceae) prevents elastase-induced emphysema in mice by regulating NF-κB, oxidative stress and metalloproteinases. Respir Res 2015;16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim YM, Huh JS, Lim Y, Cho M.. Soy isoflavone glycitin (4′-hydroxy-6-methoxyisoflavone-7-d-glucoside) promotes human dermal fibroblast cell proliferation and migration via TGF-β signaling. Phytother Res 2015;29:757–69. [DOI] [PubMed] [Google Scholar]
- 110.Bas E, Recio MC, Giner RM, et al. . Anti-inflammatory activity of 5-O-demethylnobiletin, a polymethoxyflavone isolated from Sideritis tragoriganum. Planta Med 2006;72:136–42. [DOI] [PubMed] [Google Scholar]
- 111.Son NT, Suenaga M, Matsunaga Y, et al. . Serine protease inhibitors and activators from Dalbergia tonkinensis species. J Nat Med 2020;74:257–63. [DOI] [PubMed] [Google Scholar]
- 112.Karakaya S, Süntar I, Yakinci OF, et al. . In vivo bioactivity assessment on Epilobium species: a particular focus on Epilobium angustifolium and its components on enzymes connected with the healing process. J Ethnopharmacol 2020;262:113207. [DOI] [PubMed] [Google Scholar]
- 113.Fernandes MF, Conegundes JLM, Pinto NDCC, et al. . Cecropia pachystachya leaves present potential to be used as new ingredient for antiaging dermocosmetics. Evid Based Complement Alternat Med 2019;2019:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Czerwińska ME, Granica S, Kiss AK.. Effects of an aqueous extract from leaves of Ligustrum vulgare on mediators of inflammation in a human neutrophils model. Planta Med 2013;79:924–32. [DOI] [PubMed] [Google Scholar]
- 115.Ha BG, Park MA, Lee CM, Kim YC.. Antioxidant activity and anti-wrinkle effects of Aceriphyllum rossii leaf ethanol extract. Toxicol Res 2015;31:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee HS, Kim MR, Park Y, et al. . Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: in vitro and animal study. J Med Food 2012;15:1015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moreira LC, de Ávila RI, Veloso DFMC, et al. . In vitro safety and efficacy evaluations of a complex botanical mixture of Eugenia dysenterica DC. (Myrtaceae): prospects for developing a new dermocosmetic product. Toxicol In Vitro 2017;45:397–408. [DOI] [PubMed] [Google Scholar]
- 118.Nizioł-Łukaszewska Z, Zagórska-Dziok M, Ziemlewska A, Bujak T.. Comparison of the antiaging and protective properties of plants from the Apiaceae family. Oxid Med Cell Longev 2020;2020:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cvetanović A, Zengin G, Zeković Z, et al. . Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia melanocarpa's extracts obtained by subcritical water extraction. Food Chem Toxicol 2018;121:458–66. [DOI] [PubMed] [Google Scholar]
- 120.Pedrosa TN, Barros AO, Nogueira JR, et al. . Anti-wrinkle and anti-whitening effects of jucá (Libidibia ferrea Mart.) extracts. Arch Dermatol Res 2016;308:643–54. [DOI] [PubMed] [Google Scholar]
- 121.Kouakou-Siransy G, Sahpaz S, Nguessan GI, et al. . Effects of Alchornea cordifolia on elastase and superoxide anion produced by human neutrophils. Pharm Biol 2010;48:128–33. [DOI] [PubMed] [Google Scholar]
- 122.Cos P, Ying L, Calomme M, et al. . Structure–activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod 1998;61:71–6. [DOI] [PubMed] [Google Scholar]
- 123.Martinez-Gonzalez AI, Díaz-Sánchez G, de la Rosa LA, et al. . Inhibition of α-amylase by flavonoids: structure activity relationship (SAR). Spectrochim Acta A Mol Biomol Spectrosc 2019;206:437–47. [DOI] [PubMed] [Google Scholar]
- 124.Vaya J, Hagai T, Soliman K.. Structure–activity relationship of flavonoids. Curr Org Chem 2011;15:2641–57. [Google Scholar]
- 125.Kanashiro A, Souza JG, Kabeya LM, et al. . Elastase release by stimulated neutrophils inhibited by flavonoids: importance of the catechol group. Z Naturforsch C J Biosci 2007;62:357–61. [DOI] [PubMed] [Google Scholar]
- 126.Guerrero L, Castillo J, Quiñones M, et al. . Inhibition of angiotensin-converting enzyme activity by flavonoids: structure–activity relationship studies. PLoS One 2012;7:e49493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bombardelli E, Morazzoni P.. The flavonoids: new perspectives in biological activities and therapeutics. Chim Oggi 1993;11:25–8. [Google Scholar]
- 128.Verma RP, Hansch C.. An approach towards the quantitative structure–activity relationships of caffeic acid and its derivatives. ChemBioChem 2004;5:1188–95. [DOI] [PubMed] [Google Scholar]
- 129.Steinbrecher T, Case D, Labahn A.. A multistep approach to structure-based drug design: studying ligand binding at the human neutrophil elastase. J Med Chem 2006;49:1837–44. [DOI] [PubMed] [Google Scholar]
- 130.Glisic S, Sencanski M, Perovic V, et al. . Arginase flavonoid anti-leishmanial in silico inhibitors flagged against anti-targets. Molecules 2016;21:589–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen H, Yao K, Nadas J, et al. . Prediction of molecular targets of cancer preventing flavonoid compounds using computational methods. PLoS One 2012;7:e38261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.García-Sosa AT, Maran U.. Improving the use of ranking in virtual screening against HIV-1 integrase with triangular numbers and including ligand profiling with antitargets. J Chem Inf Model 2014;54:3172–85. [DOI] [PubMed] [Google Scholar]