ABSTRACT
Ferroptosis is a cell death mechanism triggered by lipid peroxidation. Our recent study linked cyst(e)ine availability with glutathione peroxidase 4 (GPX4) protein synthesis and ferroptosis mitigation via a Rag-mechanistic target of rapamycin complex 1 (mTORC1) axis, and proposed that co-targeting mTORC1 and ferroptosis is a promising strategy for cancer therapy.
KEYWORDS: ferroptosis, mTORC1, lipid peroxidation, SLC7A11, GPX4, cysteine, cystine, cancer therapy
The mechanistic target of rapamycin (mTOR), a key serine-threonine kinase in cell signaling, exists in at least two functionally and structurally distinct complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2).1 mTORC1, comprising mTOR, regulatory protein associated with mTOR (Raptor), and other subunits, senses a diverse array of nutritional and environmental cues (such as energy status, growth factors, and amino acids) and modulates multiple cellular processes.1 One critical function of mTORC1 is to coordinate amino acid availability with protein synthesis. mTORC1 is activated by diverse amino acids in a Rag GTPase-dependent manner, and then phosphorylates its downstream effectors, such as p70S6 kinase and eukaryotic initiation factor 4E (eIF4E)-binding proteins (4EBPs), to promote protein synthesis.1 Amino acid deficiency inactivates mTORC1 and suppresses protein synthesis.1 mTORC1 hyperactivation has an important role in tumor development, and targeting mTORC1 is a promising strategy in cancer therapy.1 However, the efficacy of mTOR inhibition monotherapy is moderate with limited cytotoxic effects, calling for the development of better combination strategies to induce more potent cytotoxic effects and to improve the efficacy of mTOR inhibition.
Ferroptosis is a recently identified regulated cell death triggered by the aberrant accumulation of lipid peroxides on cellular membranes, and is morphologically and biochemically distinct from other forms of regulated cell death.2 Ferroptosis onset reflects a cellular state wherein iron-dependent peroxidation of polyunsaturated-fatty-acid–containing phospholipids (PUFA-PLs) on cellular membranes overwhelms the buffering capacity of lipid peroxidation detoxification systems.2 The solute carrier family 7 member 11 (SLC7A11)-cyst(e)ine-glutathione (GSH)-glutathione peroxidase 4 (GPX4) axis represents the most powerful lipid peroxidation detoxification system,2 wherein SLC7A11 functions as an amino acid transporter to take up extracellular cystine, which is subsequently reduced to intracellular cysteine for GSH biosynthesis;3 GPX4, a phospholipid hydroperoxidase, then uses GSH as its cofactor to detoxify lipid hydroperoxides to lipid alcohols, thereby repressing ferroptosis2 (Figure 1). Ferroptosis has recently been characterized as a mechanism underlying tumor suppression and mediating the efficacy of multiple cancer therapies.2,4,5 Several classes of ferroptosis inducers (FINs) have been established with promising effectiveness in killing cancer cells or tumors, including class 1 FINs targeting SLC7A11 and class 2 FINs inhibiting GPX42 (Figure 1).
Figure 1.
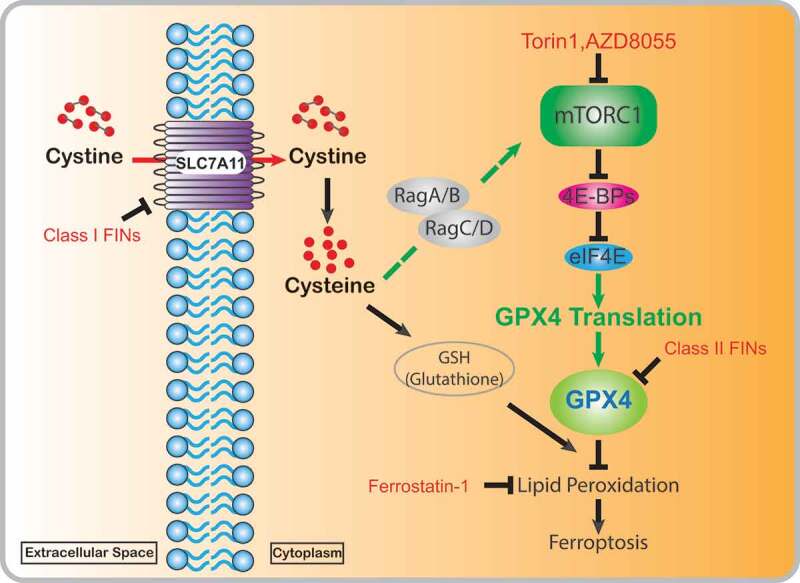
Cyst(e)ine partially suppresses ferroptosis by promoting GPX4 protein synthesis. Extracellular cystine is imported into cells through solute carrier family 7 member 11 (SLC7A11) and reduced to cysteine. Cyst(e)ine (cystine and/or cysteine) activates mechanistic target of rapamycin complex 1 (mTORC1) through Rag GTPases. Activated mTORC1 promotes glutathione peroxidase 4 (GPX4) protein synthesis by relieving the inhibitory effect of eukaryotic initiation factor 4E (eIF4E)-binding proteins (4E-BPs) on eIF4E, and increased GPX4 protein levels help strengthen cells’ defense systems to detoxify lipid peroxidation and to prevent ferroptosis. mTORC1 inactivation by mTOR inhibitors (Torin1 and AZD8055) suppresses GPX4 protein synthesis, reduces GPX4 protein levels, and sensitizes cancer cells or tumors to ferroptosis inducer (FIN)-induced ferroptosis
Cyst(e)ine has been proposed to suppress ferroptosis mainly by promoting the synthesis of GSH, which operates upstream of GPX42; therefore, it is supposed that cyst(e)ine deprivation or GSH depletion would not affect cellular sensitivity to class 2 FINs. However, it was observed that class 1 FINs or cyst(e)ine starvation generally induces much more potent ferroptosis than GSH depletion and could sensitize cells to class 2 FINs,6 suggesting that cyst(e)ine may suppress ferroptosis through other GSH-independent mechanisms. Our recent study revealed that cyst(e)ine regulates GPX4 protein levels and that the modulation of class 2 FIN sensitivity by cyst(e)ine is at least partially attributed to alterations in GPX4 protein levels.7 Specifically, SLC7A11 inhibition or cyst(e)ine starvation reduces GPX4 protein levels but neither decreases its mRNA levels nor increases proteasome- or autophagy-mediated GPX4 protein degradation, whereas SLC7A11 overexpression promotes GPX4 protein synthesis through enhancing cystine uptake.7 Further analyses showed that GSH levels are not involved in cyst(e)ine regulation of GPX4 protein synthesis and sensitivity of class 2 FINs,7 suggesting a model that SLC7A11-mediated cystine uptake promotes GPX4 protein synthesis via a GSH-independent mechanism .
What is the mechanism underlying cyst(e)ine regulation of GPX4 protein synthesis? mTORC1 is the major nutrient sensor that coordinates amino acid availability with protein synthesis, and activation of mTORC1 by amino acids is mainly mediated by Rags.1 We showed that cyst(e)ine stimulation activates mTORC1 and promotes its localization on lysosomes, resulting in increased GPX4 protein synthesis.7 Mechanically, cyst(e)ine-induced mTORC1 activation involves Rag GTPases, since RagA/B abrogation almost completely abolished mTORC1 activation and GPX4 protein level increase upon cyst(e)ine stimulation.7 Notably, GPX4 protein synthesis was repressed by selective ATP-competitive mTOR inhibitors, such as Torin1 and AZD8055, that repress both p70S6 kinase and 4EBPs, but not by the allosteric mTORC1 inhibitor rapamycin that only represses p70S6 kinase, suggesting that it is 4EBPs downstream of mTORC1 that regulate GPX4 protein synthesis.7 Consistent with this, overexpression of a non-phosphorylatable 4EBP1 mutant significantly decreased GPX4 protein levels, whereas deletion of 4EBP1/2 blocked the suppressive effect of Torin1 on GPX4 protein levels.7 These data compellingly support the model that cyst(e)ine regulates GPX4 protein synthesis through the Rag-mTORC1-4EBP axis (Figure 1).
Further, we demonstrated that Torin1 or AZD8055, but not rapamycin, potentiated FIN-induced lipid peroxidation and sensitized cancer cells to ferroptosis, whereas 4EBP1/2 deletion inhibited such sensitization effects.7 Importantly, the combination of mTORC1 inhibitor and SLC7A11 inhibitor was more effective than monotherapy in reducing 4EBP1 phosphorylation and GPX4 levels, increasing lipid peroxidation levels, and suppressing tumor growth in patient-derived xenograft models, providing a rationale for co-targeting mTORC1 and ferroptosis in cancer treatment.7
Overall, our study identifies a previously unappreciated mechanism for cyst(e)ine regulation of ferroptosis via mTORC1-mediated GPX4 protein synthesis (Figure 1) and provides new insights into co-targeting mTORC1 and ferroptosis in cancer therapy. It should be noted that other mechanisms connecting mTORC1 to ferroptosis also exist. Two other recent studies revealed that mTORC1 can suppress ferroptosis at least partly through inhibiting autophagy-dependent ferroptosis or upregulating sterol responsive element-binding protein (SREBP)-stearoyl coenzyme A desaturase 1 (SCD1)-axis–mediated monounsaturated fatty acid synthesis.8,9 Interestingly, yet another recent study reported that mTOR inhibition could even suppress ferroptosis.10 Future studies are required to investigate this seemingly context-dependent role of mTORC1 in governing ferroptosis.
Funding Statement
This work was supported by R01CA181196, R01CA244144, and R01CA247992 from the National Institutes of Health (to B.G.), and National Institutes of Health Cancer Center Support Grant [P30CA016672] from National Cancer Institute (to The University of Texas MD Anderson Cancer Center).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Saxton RA, Sabatini DM.. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:1–3. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockwell BR, Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koppula P, Zhuang L, Gan B.. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2020:1–22. doi: 10.1007/s13238-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, Sirohi K, Li X, Wei Y, Lee H, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20(10):1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H, et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30(2):146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris IS, Endress JE, Coloff JL, Selfors LM, McBrayer SK, Rosenbluth JM, Takahashi N, Dhakal S, Koduri V, Oser MG, et al. Deubiquitinases maintain protein homeostasis and survival of cancer cells upon glutathione depletion. Cell Metab. 2019;29(5):1166–1181. e6. doi: 10.1016/j.cmet.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Swanda RV, Nie L, Liu X, Wang C, Lee H, Lei G, Mao C, Koppula P, Cheng W, et al. mTORC1 couples cyst (e) ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat Commun. 2021;12(1):1589. doi: 10.1038/s41467-021-21841-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Wang Y, Liu J, Kang R, Tang D. Interplay between MTOR and GPX4 signaling modulates autophagy-dependent ferroptotic cancer cell death. Cancer Gene Ther. 2021;28(1–2):55–63. doi: 10.1038/s41417-020-0182-y. [DOI] [PubMed] [Google Scholar]
- 9.Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc National Acad Sci. 2020;117(49):31189–31197. doi: 10.1073/pnas.2017152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conlon M, Poltorack CD, Forcina GC, Armenta DA, Mallais M, Perez MA, Wells A, Kahanu A, Magtanong L, Watts JL, et al. A compendium of kinetic modulatory profiles identifies ferroptosis regulators. Nat Chem Biol. 2021;1–10. doi: 10.1038/s41589-021-00751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


