ABSTRACT
In a recent report, we have revealed a new interaction between the BRCA2 DNA repair associated protein (BRCA2) and the DEAD-box helicase 5 (DDX5) at DNA breaks that promotes unwinding DNA-RNA hybrids within transcribed chromatin and favors repair. Interestingly, BRCA2–DDX5 interaction is impaired in cells expressing the BRCA2T207A missense variant found in breast cancer patients.
KEYWORDS: BRCA2, breast cancer, DDX5, DNA-RNA hybrid, double-strand break, homologous recombination, DSB repair
Cancer is a genetic disease linked to the appearance of alterations in the genetic information. As a consequence, the maintenance of genetic stability is crucial to prevent the process of tumorigenesis and its development. The stability of the DNA is threatened by multiple sources of damage ranging from exogenous to endogenous agents that can cause different sort of lesions such as DNA breaks. In particular, the breakage of both DNA strands (DNA double-strand break, DSB) is a very cytotoxic lesion. To ensure its accurate repair, two major DSB repair pathways have evolved: Non-Homologous End Joining, which re-ligates the broken DNA ends after more or less processing, and Homologous Recombination (HR), which uses a homologous template to restore the genetic information at the break. Not surprisingly, the inheritance of mutations in genes that encode factors involved in HR are strongly linked to cancer susceptibility. This is the case of the breast cancer susceptibility genes 1 and 2 (BRCA1 and BRCA2) which are involved in important steps of HR.1 Hence, individuals who inherit one of the multiple pathogenic mutations described for these genes show an enhanced predisposition to develop breast and ovarian cancers.
BRCA2 codes for a large protein of 3418 amino acids and 385-kDa molecular weight. In addition to its main role in loading the DNA repair protein RAD51 homolog 1 (RAD51) on single-stranded DNA, an essential step for DSB repair by HR, and its reported critical function in preventing aberrant degradation of stalled replication forks, BRCA2 DNA repair associated protein (BRCA2) has emerging functions through a growing list of interactors.1 We have uncovered a new interaction between BRCA2 and the DEAD-box helicase 5 (DDX5) (also known as p68),2 a known regulator of transcription. These findings reveal a new layer of complexity in the role of BRCA2 (and of DDX5) in HR within transcribed chromatin. It is known that DSBs within transcriptionally active genes are channeled to HR, mainly due to the chromatin context in which the DSB occurred.3 In addition, there is an emergent interest in the impact of both the nascent RNA as well as potential new RNA species induced at the break that promote DNA damage signaling. Interestingly, a growing body of evidence indicates that DNA breakage can promote hybridization of the nascent RNA with its DNA template.4 Supporting a model in which the thus formed DNA-RNA hybrids need to be removed to allow efficient DSB repair by HR, we have observed that BRCA2 retains the DDX5 helicase at DSBs, boosting its ability to unwind DNA-RNA hybrids (Figure 1).2,5,6
Figure 1.
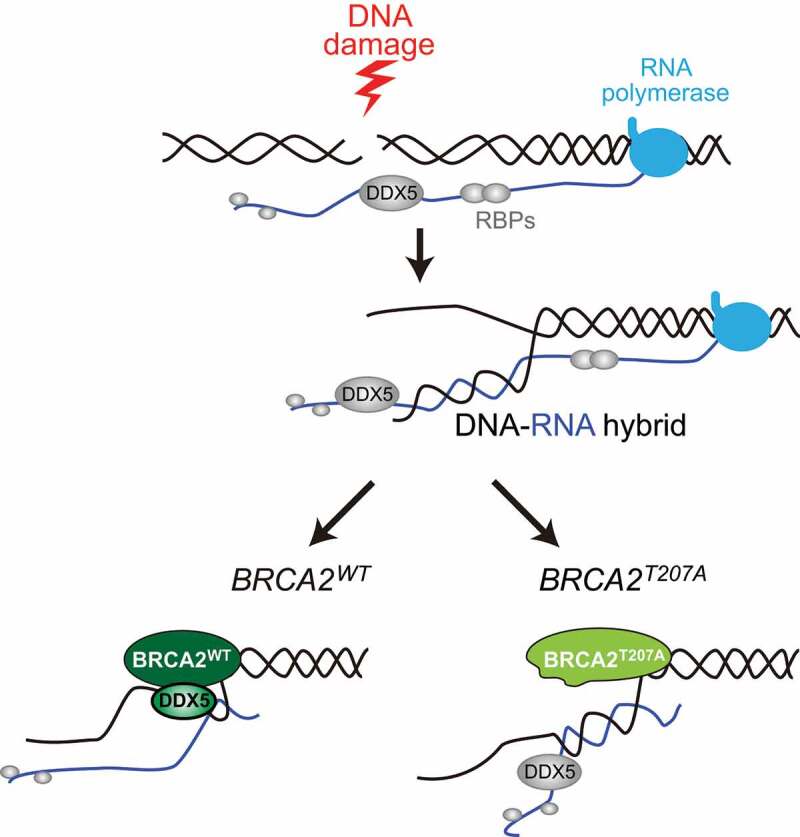
Fate of DNA breaking during transcription in BRCA2WT- versus BRCA2T207A-bearing cells. In wild-type cells, RNA binding proteins (RBPs) prevent harmful DNA-RNA hybrid accumulation. In particular, when the DNA is broken, the interaction of the BRCA2 DNA repair associated protein (BRCA2) with the DEAD-box helicase 5 (DDX5) boosts its ability to unwind the occasional formation of DNA-RNA hybrids at the break site thus enabling further repair. In BRCA2T207A-bearing cells, BRCA2 interaction with DDX5 is impaired. Hence, DNA-RNA hybrids accumulate at the break
In addition to clearly pathogenic mutations, such as those resulting in truncation or inactivation of the BRCA proteins, genetic screenings performed in cancer patients with a family history of breast and ovarian cancers have led to the identification of different mutations of unknown clinical relevance, which encode variants of uncertain significance. We have recently found that one of such variants, BRCA2T207A (c.619A>G), impairs the interaction between BRCA2 and DDX5 (Figure 1).2 Notably, cells expressing this mutation show increased levels of DNA-RNA hybrids and a delay in RAD51 loading, supporting that DNA-RNA hybrid removal promotes subsequent steps in the HR reaction.2
Therefore, BRCA2 appears to take advantage of the preexistence of DDX5 in transcribed chromatin to overcome the obstacle that incidental DNA-RNA hybrids may constitute for HR. Other DNA-RNA hybrid removal pathways may be also affected by BRCA2 or by other DSB repair factors to promote hybrid clearance at DSBs. Interestingly, BRCA2 has also been shown to interact with ribonuclease H2 at DSBs to regulate DNA-RNA hybrid degradation.7 Thus, we envision that a cohort of factors could act redundantly at DSBs to promote the removal of incidental DNA-RNA hybrids to ensure proper repair by HR and that some of them, as it is the case for DDX5, might be stimulated by DNA damage repair factors such as BRCA2.
General DNA-RNA hybrid accumulation at non-DSB sites was also observed in cells bearing the BRCA2T207A mutation and was previously reported in cells depleted of BRCA2 or DDX5.2,5,8 Thus, in addition to contributing to the removal of DNA-RNA hybrids at DSBs, BRCA2 and DDX5 seem important in the prevention or removal of harmful DNA-RNA hybrids formed spontaneously throughout the genome. Interestingly, whereas BRCA2 tumor-associated mutations are mostly associated with loss of function, DDX5 amplification can be detected in multiple cancer types. Indeed, DDX5 amplification is required for cell proliferation in breast cancer cells, and this was proposed to be due to the role of DDX5 in the expression of DNA replication genes.9 It is also possible that DDX5 amplification contributes to survival in BRCA2-deficient breast cancer by counteracting the DNA-RNA hybrid accumulation caused by BRCA2 loss. In agreement with this hypothesis, DDX5 overexpression can rescue the DNA-RNA hybrid accumulation in BRCA2-deficient human cells.2
In summary, our recent study2 adds a new phenotype to the BRCA2T207A variant of uncertain clinical significance found in oncogenic patients that consists in a defect in the removal of incidental DNA-RNA hybrids at DSBs occurring within transcribed chromatin and a less efficient repair of such breaks. This mutation also imposes a defect in the alignment of chromosomes resulting in faulty chromosome segregation and aneuploidy.10 These phenotypes could account for the putative contribution of the BRCA2T207A mutation to oncogenesis and exemplify how studying these variants can help us understand the physiological relevance of this large and multitasking protein.
Funding Statement
B.G.-G. is supported by the Spanish Association Against Cancer (AECC), G. S. is supported by the Fondation ARC (French Cancer Research Association). Research in A.C. lab is funded by La Ligue Contre le Cancer, Fondation ARC and the French Breast Cancer Association “Ruban Rose”. Research in A.A.’s lab is funded by the European Research Council, the Spanish Ministry of Economy and Competitiveness, and the European Union (FEDER).
References
- 1.Martinez JS, Baldeyron C, Molding CA.. BRCA2 function through its interacting partners. Cell Cycle. 2015;14:1–2. doi: 10.1080/15384101.2015.1093702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sessa G, Gómez-González B, Silva S, Perez-Calero C, Beaurepere R, Martineau S, Martin C, Å E, Martinez JS, Lombard B, et al. BRCA2 promotes R‐loop resolution by DDX5 helicase at DNA breaks to facilitate their repair by homologous recombination. Embo J. 2021:e106018. doi: 10.15252/embj.2020106018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clouaire T, Legube G.. DNA double strand break repair pathway choice: a chromatin based decision? Nucleus. 2015;6:107–113. doi: 10.1080/19491034.2015.1010946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilera A, Gómez-González B. DNA-RNA hybrids: the risks of DNA breakage during transcription. Nat Struct Mol Biol. 2017;24:439–443. doi: 10.1038/nsmb.3395. [DOI] [PubMed] [Google Scholar]
- 5.Mersaoui SY, Yu Z, Coulombe Y, Karam M, Busatto FF, Masson JY, Richard S. Arginine methylation of the DDX5 helicase RGG/RG motif by PRMT5 regulates resolution of RNA:DNA hybrids. Embo J. 2019;38:1–20. doi: 10.15252/embj.2018100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Z, Mersaoui SY, Guitton-Sert L, Coulombe Y, Song J, Masson JY, Richard S. DDX5 resolves R-loops at DNA double-strand breaks to promote DNA repair and avoid chromosomal deletions. NAR Cancer. 2020;2:1–19. doi: 10.1093/narcan/zcaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Alessandro G, Whelan DR, Howard SM, Vitelli V, Renaudin X, Adamowicz M, Iannelli F, Jones-Weinert CW, Lee M, Matti V, et al. BRCA2 controls DNA:RNA hybrid level at DSBs by mediating RNase H2 recruitment. Nat Commun. 2018;9:5376. doi: 10.1038/s41467-018-07799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia V, Barroso SI, García-Rubio ML, Tumini E, Herrera-Moyano E, Aguilera A. BRCA2 prevents R-loop accumulation and associates with TREX-2 mRNA export factor PCID2. Nature. 2014;511:362–365. doi: 10.1038/nature13374. [DOI] [PubMed] [Google Scholar]
- 9.Mazurek A, Luo W, Krasnitz A, Hicks J, Powers RS, Stillman B. DDX5 regulates DNA replication and is required for cell proliferation in a subset of breast cancer cells. Cancer Discov. 2012. doi: 10.1158/2159-8290.CD-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Å E, Martin C, Miron S, Julien M, Theillet FX, Ropars V, Sessa G, Beaurepere R, Boucherit V, Duchambon P, et al. Proper chromosome alignment depends on BRCA2 phosphorylation by PLK1. Nat Commun. 2020;11:1819. doi: 10.1038/s41467-020-15689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


