Abstract
Background
Interleukin‐6 (IL‐6) is an inflammatory factor that increases rapidly in response to infectious diseases including sepsis. The aim of this study is to develop a quantum dot (QD)‐based fluorescence lateral flow immunoassay (LFIA) strip that can rapidly and accurately detect IL‐6 levels.
Methods
QD‐based LFIA strips were fabricated by conjugating CdSe/ZnS QDs to the IL‐6 antibody. Performance verification and clinical sample analysis were carried out to evaluate the newly developed strip.
Results
QD‐based LFIA strips were successfully fabricated. The test strip's linear range was 10–4000 pg/ml, with a linear correlation coefficient of R 2 ≥ .959. The sensitivity of the test strip was 1.995 pg/ml. The recovery rate was 95.72%–102.63%, indicating satisfying accuracy. The coefficient of variation (CV) of the intra‐assay was 2.148%–3.903%, while the inter‐assay was 2.412%–5.293%, verifying the strip's high precision. The cross‐reaction rates with various interleukins (IL‐1α, IL‐1β, IL‐2, IL‐4, and IL‐8) and interferon‐γ (IFN‐γ) were all <0.1%. When the strip was placed in a 50°C oven for 1, 2, 3, and 4 weeks, the test results were not significantly altered compared to storage at room temperature. Furthermore, 200 clinical serum samples were analyzed to compare the strip with the Beckman chemiluminescence immunoassay (CLIA) kit, which revealed a high correlation (n = 200, R 2 = .9971) for the detection of IL‐6.
Conclusions
The QD‐based test strip can rapidly and quantitatively detect IL‐6 levels, thus meeting the requirement of point‐of‐care test (POCT) and showing excellent clinical prospects.
Keywords: fluorescence lateral flow immunoassay, interleukin‐6, performance verification, point‐of‐care test, quantum dots
A novel quantum dot‐based lateral flow immunoassay strip has been developed to detect IL‐6 levels. This strip can rapidly and quantitatively detect IL‐6 with high sensitivity of 1.99 pg/ml and a broad linear range of 10–4000 pg/ml. This IL‐6 detection strip also has satisfactory specificity, precision, accuracy, and stability.

1. INTRODUCTION
Interleukin‐6 (IL‐6) is a multifunctional protein belonging to the glycoprotein‐130 (GP130) cytokine family. 1 The human IL‐6 protein is encoded by the IL‐6 gene located on chromosome 7p21, consisting of 212 amino acids. It is primarily produced by macrophages, monocytes, fibroblasts, and T lymphocytes, 2 , 3 , 4 and plays important roles in immune regulation, hematopoiesis, inflammation, and tumorigenesis. 5 IL‐6 elevation mainly results from infectious diseases, in which sepsis is the most severe life‐threatening condition. Sepsis is caused by dysfunctional systemic responses to certain infections, often leading to multiple organ failure. 6 During the onset of sepsis, innate immune cells produce large amounts of IL‐6 by recognizing microbial pathogens. 7 IL‐6 begins to increase shortly after an infection, peaking around 3 h post‐infection. High IL‐6 levels are associated with increased infection severity, which is closely related to the prognosis of sepsis. Besides, IL‐6 has shown the highest value in the diagnosis of sepsis compared with procalcitonin (PCT) and c‐reactive protein (CRP), which are also important biomarkers of infection. 8
Sepsis has a high mortality rate, and each hour of delayed antibiotic administration can decrease an average survival rate by 7.6%. 9 Hence, it is critical to diagnose and treat patients with sepsis as early as possible. IL‐6 concentrations are normally below 7 pg/ml in the blood of healthy people; however, blood IL‐6 concentrations of 7–150 pg/ml, 150–250 pg/ml, or more than 250 pg/ml are associated with mild inflammation or infections, general bacterial infections, systemic inflammatory responses, and sepsis, respectively. 10 , 11 As IL‐6 is such an important biomarker for early detection of sepsis, rapid and accurate detection of IL‐6 by various diagnostic methods is also important.
Traditional IL‐6 detection methods include chemiluminescence immunoassays (CLIA), electrochemiluminescence immunoassays (ECLIA), and enzyme‐linked immunosorbent assays (ELISA). 12 Besides, newer technologies have been developed, including photochemical immunoassays, 13 magnetic colorimetric immunoassays, 14 and combined electrochemiluminescent and electrochemical immunoassays. 15 However, the traditional CLIA or ECLIA requires large instruments, expensive reagents, and professional technicians to operate. In addition, the ELISA‐based detection of IL‐6 requires tedious operations and long detection time. 16 , 17 , 18 Similarly, most of the newly developed detection technologies are immature, unstable, and require complex instruments, and the specific differences are shown in Table 1. Therefore, improved technologies are required for the detection of IL‐6. Recently, the point‐of‐care test (POCT), known for its easy portability of instruments or absence of instruments, and its rapid, economical, sensitive, accurate, and user‐friendly characteristics, 19 have been popularized and applied in clinic worldwide. Hence, POCT is an ideal method for rapidly detecting IL‐6 levels and assisting in the early diagnosis of sepsis. The most commonly used technique in POCT is colloidal gold‐based lateral flow immunoassays (LFIA). Although other labeling materials including carbon nanoparticles, 20 fluorescent dyes, 21 liposomes, 22 and magnetic nanoparticles 23 are developed in the LFIA, colloidal gold is still the most widely used label as it is easily manufactured, cost‐effective, and visually distinguishable. 24 However, the sensitivity of colloidal gold‐based LFIA is often limited, 25 and the test results can be hindered by false negatives, false positives, or background interference. 26 In addition, Yan et al. reported that the minimum visible limit of IL‐6 detected by colloidal gold‐based LFIA strips was 62.5 ng/ml, 27 which may not well distinguish mild infections or inflammation when the IL‐6 concentrations are 7–150 pg/ml. A recent study reported on time‐resolved LFIA based on europium nanoparticles showed its applicability for IL‐6, yet the limited detection range (2–500 pg/ml) limited its clinical utility. 12 Hence, a novel material‐based LFIA for IL‐6 detection needs to be developed.
TABLE 1.
| Methodology | CLIA/ECLIA | ELISA | LFIA* |
|---|---|---|---|
| Data characteristic | QUANTITATIVE | Quantitative | Semi‐quantitative |
| Detection range (pg/ml) | 0.5–10,000 | 9.5–2500 | >62.5 |
| Testing time (min) | 18 | 240 | 10 |
| Degree of automation | fully automatic | semi‐automatic | manual |
LFIA was based on colloidal gold.
Quantum dots (QDs) have emerged as a new type of labeling material. These nanoscale semiconductor particles are often constructed from cadmium selenide (CdSe), indium arsenide (InAs), and cadmium telluride (CdTe). Due to quantum constraints, different sizes of QDs can emit different colors of light. 28 QDs are estimated to be 20‐times brighter and 100‐times more stable than conventional fluorescent materials. 29 In addition, QD‐based LFIA has been successfully developed to rapidly detect various blood indexes. For example, CRP, PCT, and antigens of rotavirus or hepatitis B virus, usually result in better sensitivity than colloidal gold‐based LFIA. 30 , 31 , 32 Currently, there is limited information about the use of QD‐based LFIA for IL‐6 measurements. Hence, the aim of this study is to develop QD‐based LFIA to rapidly, accurately, and cost‐effectively detect IL‐6 for the early diagnosis of sepsis and other diseases involving IL‐6 dysregulation, accomplished by labeling IL‐6 antibodies with cadmium selenide/zinc sulfide (CdSe/ZnS) QDs.
2. MATERIALS AND METHODS
2.1. Materials and instruments
CdSe/ZnS (core/shell) QDs, mercaptosuccinic acid (MES), N‐hydroxysuccinimide (NHS), 1‐ethyl‐3‐(3‐(dimethylamino) propyl) carbodiimide (EDC), mercaptopolyethylene glycol monomethyl ether (mPEG‐SH) were purchased from Sigma‐Aldrich. IL‐6 mouse monoclonal antibodies (IL‐6 mAb1, IL‐6 mAb2) were purchased from MEDIX Biochemica. Antigens including interleukins (IL‐1α, IL‐1β, IL‐2, IL‐4, and IL‐8) and interferon‐γ (IFN‐γ) were obtained from Abcam. The strip made of nitrocellulose (NC) membranes, semi‐rigid polyvinylchloride (PVC) sheets, and polyester fiber were purchased from Shanghai JieYi Biotechnology company. The IL‐6 CLIA kits were purchased from Beckman Coulter.
The fluorescence intensity of the test (T) line and the control (C) line on the strip were detected using the self‐designed KF‐Q001‐A fluorescence immunoassay analyzer (Kingfocus Biomedical Engineering Co., Ltd). The QDs and antibodies were dispensed onto the strip using a piece of three‐dimensional gold‐spraying equipment. IL‐6 CLIA detection was performed on the DXI800 instrument (Beckman Coulter).
2.2. Preparation of water‐soluble QDs and QD‐antibody conjugates
The hydrophobic CdSe/ZnS QDs were dissolved in hexane, while mPEG‐SH was dissolved in water and dispersed by ultrasound until completely dissolved. Next, the two solutions were mixed and stirred at RT temperature and pH 6.5 for 30 min. When the organic solvent was completely volatilized, the water‐soluble QDs were obtained. Next, a total of 30 μl water‐soluble QDs were washed with 1 ml MES. The supernatant was discarded after centrifugation at 8000 g for 20 min, and the precipitate water‐soluble QDs were repeatedly washed once and re‐dissolved with MES buffer. Next, the QDs in MES were activated by 20 mg/10 mg of EDC/NHS for 30 min, and the pellet was washed twice by centrifugation at 8000 g for 20 min. Finally, 200 μg of IL‐6 mAb1 was added to the QD pellet, incubated at room temperature for 3 h. Finally, the pellet of QD‐IL‐6 mAb1 conjugates was washed by centrifugation, blocked with 5% BSA for 30 min, washed again, and preserved in 30 μl PBS (PH7.4.2 ~ 8°C). To test the binding nature of the QD‐IL‐6 mAb1 conjugates, the fluorescence emission spectra and quantum yields of both the QD‐antibody conjugates and the hydrophobic CdSe/ZnS QDs were acquired with the ultraviolet‐visible (UV‐Vis) spectrophotometer (Shimadzu 2450, Shimadzu) or the fluorescence spectrometer (Ls55, PerkinElmer).
2.3. Fabrication of QD‐based LFIA strips
First, 10 μl of the labeling antibody QD‐IL‐6 mAb1 conjugates was diluted into appropriate concentrations according to the concentration of the IL‐6 mAb1 antibody (5.6 mg/ml), and dispensed onto the polyester fiber membrane (conjugate pads) using the gold‐spraying equipment at the speed of 5 μl/cm. The samples were allowed to dry under 25% relative humidity at 37°C for 4 h. Next, the capture antibody IL‐6 mAb2 was dispensed onto the test line (T line) area on the NC membrane, and rabbit anti‐mouse IgG was sprayed onto the quality control line (C line) area on the NC membrane. Third, the test strip was orderly assembled with the sample pad, conjugate pad, NC membrane, and wick pad onto the PVC membrane, and was cut into a width of 3 mm. Lastly, the strip was inserted into a shell, and 100 μl serum sample was added to the sample pad vertically for IL‐6 detection. After 18 min, the fluorescence signals at the T and C lines were read by a self‐designed fluorescence analyzer at 610 nm.
2.4. Standard curve plotting for linearity and sensitivity
The IL‐6 standards were diluted with IL‐6 free calf serum into different concentrations (0, 10, 50, 120, 250, 400, 800, 1600, 3000, 4000 pg/ml). Each sample was detected for the fluorescence signal ratio of T/C ten times by the fluorescence analyzer, and the mean values were recorded. The standard curve was plotted according to the mean value of each concentration. Considering that non‐linear interval may occur at high concentrations, a quasi‐curve was plotted using the multiple concentration points. The linear range was determined according to the results, and the standard curve was saved in the fluorescence analyzer for the quantification of other testing samples in the following experiments. The sensitivity was calculated according to the international union of pure and applied chemistry (IUPAC), which is the limit of detection (LOD) = K*Sb/Sl; where K is recommended to be 3, Sb is the standard deviation of blank measurement (measuring 0 pg/ml standard substance) for 20 times, and Sl is the slope of the standard curve.
2.5. Recovery test for accuracy
Different amounts of the IL‐6 standards were added into the IL‐6‐free calf serum to obtain three expected dilutions of IL‐6 standards (50, 2500, 5000 pg/ml). Each sample was measured six times using the strip, and the percentage ratio (measured concentration/expected concentration) was defined as the recovery rate.
2.6. Precision assay
Diluted samples of the IL‐6 standards from low to high concentrations (0, 50, 250, 800, 4000 pg/ml) were tested. The intra‐assay precision of the strip was determined using the same batch of test strips on the same day, and each sample was tested ten times repeatedly. The inter‐assay precision was determined in ten consecutive days, with two replicates. The coefficient variance (CV) values at each concentration were calculated as the percentage of mean and standard deviation (SD), and a CV below 15% was considered satisfactory.
2.7. Specificity assay
Interleukins (IL‐1α, IL‐1β, IL‐2, IL‐4, and IL‐8) and IFN‐γ, which were common competitors of IL‐6, were added in the serum at a concentration of 10,000 pg/ml. Each sample was detected six times with the strip. The antibody cross‐reaction rate was calculated according to Abraham's formula 32 : cross‐reaction rate (%) = (S/Z) × 100%; where S represents the actual measured concentration of the antigens and Z represents the added concentration of the antigens.
2.8. Stability study
LFIA strips were kept at room temperature in a dry and cool environment. According to the conventional stability study of the colloidal gold strip, 32 the strips were placed in an oven at 50°C for up to one month for an accelerated study of its stability. If the strip was stable, it was assumed that the strip could be preserved at room temperature for up to 1 year. In this study, the test strips were placed at room temperature (0 weeks) or 50°C for 1, 2, 3, and 4 weeks, and the strips were tested at various concentrations of standard IL‐6 solutions (0, 50, 250, 800, and 4000 pg/ml). Each experiment was performed in triplicate, and mean values were recorded.
2.9. Clinical sample assay
A total of 200 human serum samples were obtained from Dalang Hospital in Dongguan City (China). The IL‐6 concentrations of these samples ranged from 0 to 580.42 ng/ml. The samples were detected using our strip and the Beckman IL‐6 CLIA kit within 2 h of each other. This study was approved by the Clinical Research Ethics Committee of Dalang Hospital in Dongguan, China.
2.10. Statistical analysis
The standard curve was plotted by Origin 9 (OriginLab), and the mean value and standard deviation (SD) were calculated using Microsoft Excel 2016 (Microsoft Corporation). Pearson linear regression was performed to test the linearity of the testing strip. The correlation coefficient R 2 was calculated to evaluate the correlation between the QD‐based LFIA strip and the Beckman CLIA kit in detecting IL‐6.
3. RESULTS
3.1. Characterization of the QD‐IL6 mAb conjugates
Fluorescence emission spectra before and after QD modification are shown in Figure 1. There was a slight redshift of the fluorescence emission peak of the QDs after they were modified by antibodies. The maximum fluorescence wavelength emitted was 610 nm for the modified QDs, with the corresponding peak width of 30 nm. The fluorescence emission peaks were narrow and nearly symmetrical. This revealed a satisfactory binding nature of the QD‐antibody conjugates.
FIGURE 1.
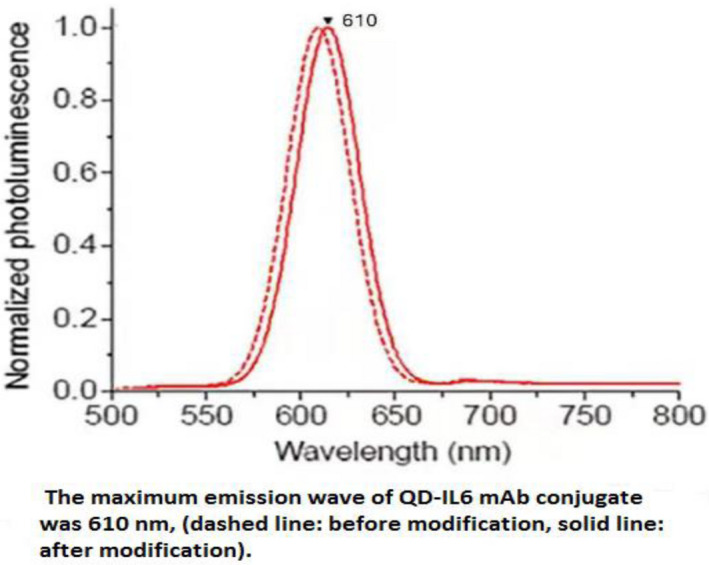
The maximum emission wave of QD‐IL6 mAb conjugates was 610 nm
3.2. Principle of the assay
Based on the traditional double antibody sandwich immunoassays in LFIA, CdSe/ZnS QDs were used as the label for the LFIA instead of colloidal gold. As shown in Figure 2, the test strip is composed of the sample pad, conjugate pad, NC membrane, wick pad, and PVC pad. The capture antibody IL‐6 mAb2 is distributed in the test line (T line) area, and the rabbit anti‐mouse IgG is distributed in the quality control line (C line) area on the NC membrane. When the sample containing IL‐6 is loaded to the sample well, the IL‐6 antigen will bind to the labeling antibody QD‐IL‐6 mAb1 conjugates to form a complex. When the complex flows to the T line, it will be captured and fixed by the capture antibody IL‐6 mAb2 on the T line and the double antibody sandwich QD‐IL‐6 mAb1‐IL‐6 antigen‐IL‐6 mAb2 is formed, and the fluorescence signal is detected at this line. When the IL‐6 antigen is absent in the samples, there will be no double antibody sandwich fixed on the T line. However, the QD‐IL‐6 mAb1 conjugates will continue to flow forward to the C line and bind to the rabbit anti‐mouse secondary antibody on the C line. Hence, the fluorescence signal will be detected at the C line despite the absence of IL‐6 antigen, thereby making it the quality control. The fluorescence intensity ratio of the T line and C line is presented as T/C. Based on this principle, the concentration of IL‐6 in the specimen is directly proportional to the T/C in the linear range. The standard curve is shown in Figure 3.
FIGURE 2.
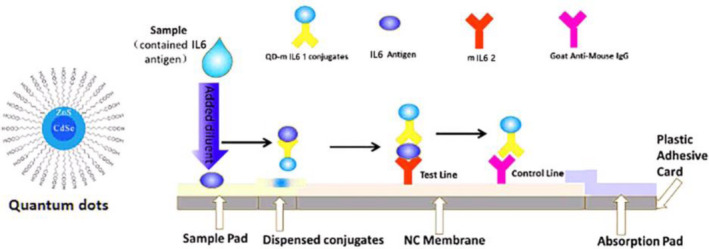
Illustration of the QD‐based LFIA strip and fluorescence signals when detecting different concentrations of IL‐6
FIGURE 3.
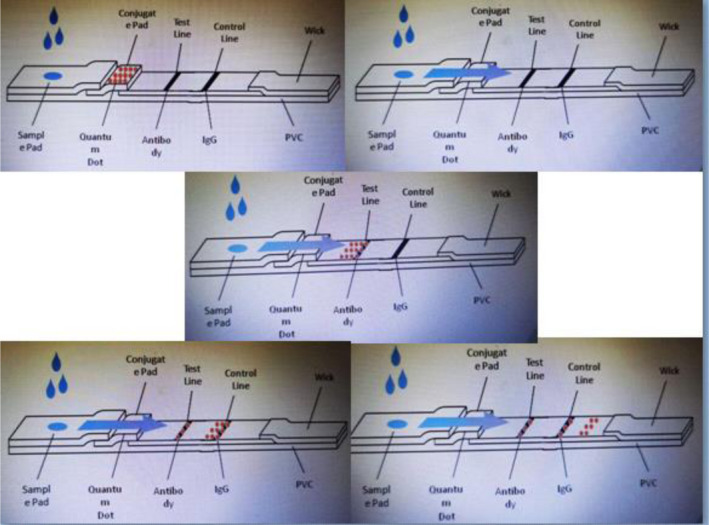
Schematic diagram of the test strip
3.3. The standard curve, linearity, and sensitivity of the strip
The standard curve was plotted according to the measurement of a series of IL‐6 standards dilutions (0, 10, 50, 120, 250, 400, 800, 1600, 3000, and 4000 pg/ml). The results are shown in Table 2 and Figure 4. The linear regression analysis equation of the standard curve was y = 0.0013x + 1.1648, R 2 = .9587, indicating good linearity between the fluorescence intensity and IL‐6 concentration. Furthermore, when the sample concentration was 10 pg/ml, the T/C approached the value measured at 0 pg/ml and still fit the linear curve. However, when the sample concentration was 4500 pg/ml, the T/C was 6.3248, which no longer satisfied the linear equation. Therefore, the linear range of the QD‐based fluorescence LFIA was 10–4000 pg/ml. According to the calculation formula recommended by IUPAC, the sensitivity of this strip was 1.995 pg/ml. The results showed that the newly developed QD‐based strip has excellent linearity and sensitivity.
TABLE 2.
Standard curve plotting of the IL‐6 QD‐based LFIA strips (n = 10)
| IL−6 (pg/ml) | 0 | 10 | 50 | 120 | 250 | 400 | 800 | 1600 | 3000 | 4000 |
|---|---|---|---|---|---|---|---|---|---|---|
| T/C mean value | 0.832 | 0.864 | 0.913 | 1.164 | 1.577 | 2.025 | 2.587 | 3.863 | 5.128 | 5.824 |
FIGURE 4.
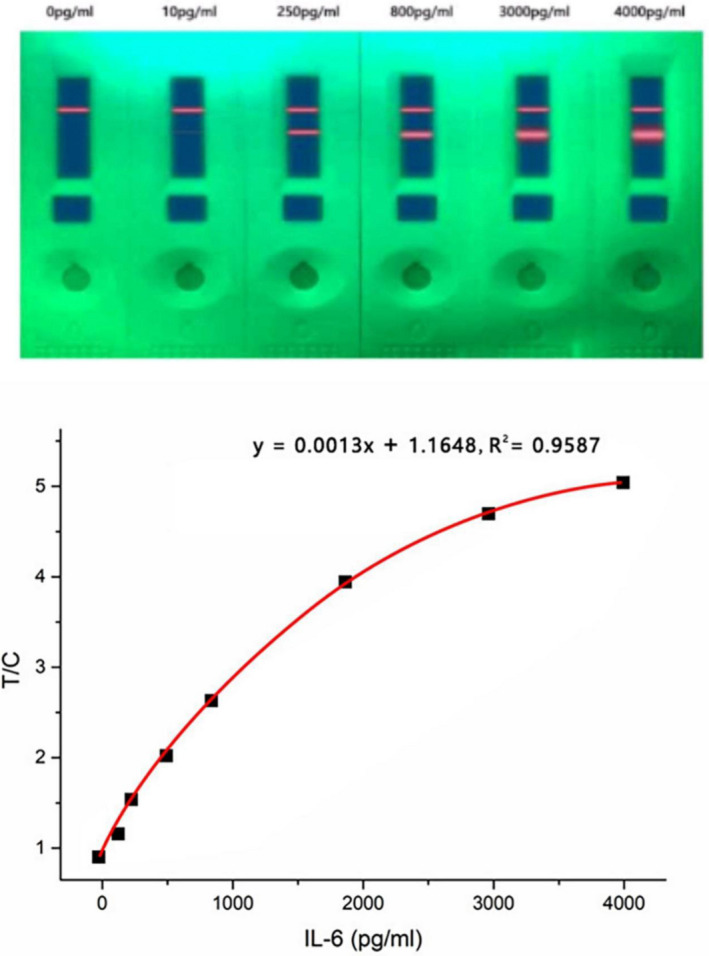
Standard curve and linearity of the QD‐based LFIA strip (n = 10)
3.4. Accuracy of the strip
The accuracy of the strip was assessed by a recovery test. A recovery rate closer to 100% indicates higher accuracy. As shown in Table 3, the average recovery rate of the diluted IL‐6 standards was 95.72% at a low concentration (50 pg/ml), 102.63% at a median concentration (2500 pg/ml), and 101.14% at a high concentration (5000 pg/ml). All three results are close to 100%, indicating the satisfactory accuracy of the QD‐based fluorescence LFIA strip.
TABLE 3.
Recovery test for accuracy of the strips (n = 6)
| Concentration | Low | Median | High |
|---|---|---|---|
| Expected IL‐6 (pg/ml) | 50 | 2500 | 5000 |
| Measured IL‐6 (pg/ml) | 47.842 ± 4.103 | 2567.964 ± 179.419 | 5055.970 ± 240.633 |
| Recovery rate (%) | 95.72 ± 9.01 | 102.63 ± 7.94 | 101.14 ± 5.27 |
Data are presented as mean ± SD.
3.5. Precision of the strip
IL‐6 standards dilutions (0, 50, 250, 800, and 4000 pg/ml) were detected by the same batch of IL‐6 QD‐based LFIA strips to evaluate intra‐assay or inter‐assay precision, respectively. As shown in Table 4, the CV of the intra‐assay precision study ranged from 2.148% to 3.903%, while the CV of the intra‐assay precision study was 2.412%–5.293%. They were all less than 15%, indicating the high precision and acceptable reproducibility of the newly developed strip.
TABLE 4.
Intra‐assay precision of the QD‐based LFIA strips (n = 10) and inter‐assay precision of strips (n = 20)
| IL−6 (pg/ml) | Intra‐assay precision | Inter‐assay precision | ||||
|---|---|---|---|---|---|---|
| Mean (T/C) | SD | CV (%) |
Mean (T/C) |
SD | CV (%) | |
| 0 | 0.932 | 0.031 | 3.382 | 0.934 | 0.031 | 3.241 |
| 50 | 0.936 | 0.033 | 2.668 | 0.942 | 0.021 | 2.412 |
| 250 | 1.266 | 0.041 | 3.438 | 1.257 | 0.071 | 5.293 |
| 800 | 1.988 | 0.082 | 3.903 | 1.957 | 0.101 | 4.911 |
| 4000 | 5.224 | 0.112 | 2.148 | 5.232 | 0.158 | 3.071 |
Abbreviations: CV, coefficient variance; SD, standard deviation.
3.6. Specificity of the strip
The interfering substances of IL‐6 were detected using the strip, with an added concentration of 10000 pg/ml. The cross‐reaction rates of the IL‐6 competitors were <0.1%, as shown in Table 5, indicating that there is minimal cross‐reactivity between IL‐6 and other substances in the samples detected by the strip. Hence, the strip shows good specificity.
TABLE 5.
Specificity of the QD‐based LFIA strips (n = 6)
| Interferent | Added (pg/ml) | Measured (pg/ml) | CRR (%) |
|---|---|---|---|
| IL−1α | 10,000 | 5.563 | 0.056 |
| IL−1β | 10,000 | 3.109 | 0.031 |
| IL−2 | 10,000 | 5.637 | 0.056 |
| IL−4 | 10,000 | 0 | 0 |
| IL−8 | 10,000 | 4.213 | 0.042 |
| IFN‐γ | 10,000 | 0 | 0 |
Abbreviation: CRR, cross‐reaction rate; measured values are presented as the mean.
3.7. Stability of the strip
The stability of the QD‐based LFIA strip was evaluated by a heat‐accelerated stability test. As shown in Table 6, there were no significant changes in T/C values of the IL‐6 diluted solutions at different concentrations (0, 50, 250, 800, and 4000 pg/ml) when placed at 50°C for a month, indicating that the newly developed strip has satisfactory stability and can be preserved at room temperature for up to 1 year.
TABLE 6.
Stability of the QD‐based LFIA strips (n = 3)
| IL−6 (pg/ml) | T/C (mean value) at consecutive weeks | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| 0 | 0.933 | 0.937 | 0.934 | 0.929 | 0.941 |
| 50 | 0.943 | 0.946 | 0.943 | 0.938 | 0.934 |
| 250 | 1.272 | 1.264 | 1.264 | 1.263 | 1.258 |
| 800 | 1.992 | 1.964 | 1.963 | 1.957 | 1.961 |
| 4000 | 5.224 | 5.222 | 5.223 | 5.214 | 5.212 |
3.8. Clinical sample analysis
IL‐6 was detected in 200 human serum samples using the QD‐based fluorescence LFIA strip and Beckman IL‐6 CLIA kit. The results are presented in Figure 5. There was a high correlation between the strip and CLIA kit measurements, with a linear regression equation of y = 0.9894 + 2.9985x, R 2 = .9971. As the correlation coefficient R 2 is above .95, the IL‐6 QD‐based fluorescence LFIA strip could perform as well as the CLIA method in the clinical laboratory test.
FIGURE 5.
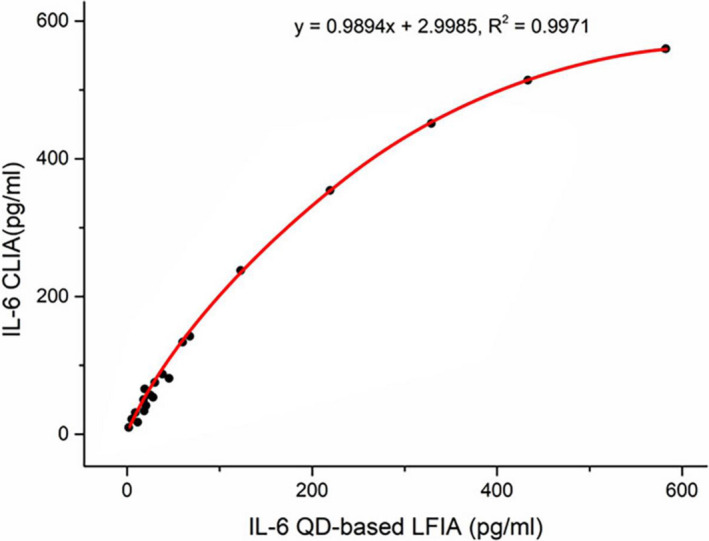
Correlation between measurement by the QD‐based LFIA strip and the IL‐6 CLIA kit (n = 200)
4. DISCUSSION
In recent years, POCT has been widely applied in the clinic due to its rapidness, convenience, and economic characteristics. Colloidal gold‐based LFIA is one of the most common POCT technologies. 19 In this study, a QD‐based LFIA strip was successfully developed for the rapid detection of IL‐6 in human serum by labeling IL‐6 antibodies with CdSe/ZnS QDs instead of colloidal gold. The detection strip has a wide linear range (10–4000 pg/ml), high sensitivity (LOD = 1.995 pg/ml), short detection time (18 min), and simple portability of fluorescent instruments, with a high correlation (R 2 = .997) to the traditional method in detecting human serum IL‐6.
In a previous report, Dezhi et al. showed that the linear range of an IL‐6 LFIA strip based on the time‐resolved europium nanoparticles was 2–500 pg/ml, 12 while the linear range of the strip we have developed was 10–4000 pg/ml. As the serum IL‐6 concentrations in patients with sepsis often increase to levels higher than 500 pg/ml, 33 our test could be used to help detect sepsis. In addition, the best cutoff value of IL‐6 in amniotic fluid in pregnant women was 1000 pg/ml for predicting microbial invasion of the amniotic cavity. 34 Hence, our QD‐based LFIA strip is more suitable for detecting high concentrations of IL‐6 than the time‐resolved europium nanoparticle‐based LFIA strip. In another study, Yan et al. reported that the minimum visible limit of IL‐6 detected by colloidal gold‐based LFIA strips was 62.5 ng/ml, 27 while the lowest limit of detection for IL‐6 using our strip was 1.99 pg/ml. Since the cutoff value of serum IL‐6 for diagnosing gram‐negative bacterial infection is 57 pg/ml, 35 and the serum IL‐6 concentration in healthy individuals is about 6 pg/ml, 36 it can be concluded that our strip can better meet the requirement of the minimum detection limit for IL‐6 in infectious diseases.
The sensitivity of our strip is lower than other detection technologies. For example, Liu et al. reported that the sensitivity of combined electrochemiluminescent and electrochemical immunoassay for IL‐6 was 0.32 fg/ml, 15 while Juan et al. demonstrated that the sensitivity of a magnetic colorimetric immunoassay for IL‐6 was 0.04 pg/ml. 14 Despite the sensitivity, our strip has several other advantages. First, the strip does not need complex instruments required by other immunoassays. Our technique only requires a small machine with an ultraviolet lamp. Secondly, compared with the magnetic colorimetric immunoassay, the procedure is simple and less time‐consuming. Besides, our strip remains stable at room temperature for up to 1 year and shows excellent intra‐assay or inter‐assay precision, with high specificity and a CV < 15%. In addition, there is no interference by other kinds of interleukins (IL‐1, IL‐2, IL‐4, IL‐8) or IFN‐γ Lastly, there is a high correlation between the detection results of IL‐6 concentrations using our strip and the Beckman IL‐6 CLIA kit, with R 2 = .997, indicating that it is promising to apply this technology in the bedside assessment of IL‐6.
However, compared to the colloidal gold, QDs have disadvantages as well. They cannot be observed by the naked eyes, the materials are more expensive than colloidal gold, and the portable fluorescence instrument has not been popularized. Hence, the value of the QD‐based LFIA method is easily underestimated. With a higher sensitivity, broader linear range, and more stable properties, QD‐based LFIA needs more promotion. Besides, the sizes of QDs can easily be altered to emit different wavelengths of fluorescence, making it possible to develop QDs for the simultaneous detection of multiple indicators, for example, the IL‐6/PCT/CRP combined detection. In addition, it would be possible to simply distinguish the indicators based on the different colors of fluorescence emitted by the QDs.
In conclusion, we successfully established a QD‐based LFIA method for rapid and accurate detection of human serum IL‐6, providing a more simple, robust, and economic POCT technology for IL‐6 quantification, which is of great value for the early detection and treatment of sepsis and related disorders.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENTS
The authors are grateful for financial support from the Key Social Development Project of Dongguan City (No. 201950715040199).
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Liu X, Teichtahl AJ, Wicks IP. Interleukin‐6 in rheumatoid arthritis ‐ from the laboratory to the bedside. Curr Pharm Des. 2015;21(17):2187‐2197. [DOI] [PubMed] [Google Scholar]
- 2. Liu C, Wang F, Cui L, Zhou J, Xu Z. Diagnostic value of serum neutrophil gelatinase‐associated lipocalin, interleukin‐6 and anti‐citrullinated alpha‐enolase peptide 1 for lower respiratory tract infections. Clin Biochem. 2020;75:30–34. [DOI] [PubMed] [Google Scholar]
- 3. Iwahasi S, Rui F, Morine Y, et al. Hepatic stellate cells contribute to the tumor malignancy of hepatocellular carcinoma through the IL‐6 pathway. Anticancer Res. 2020;40(2):743‐749. [DOI] [PubMed] [Google Scholar]
- 4. Zhang S, Li J, Fan J, Wu X. Bisphenol A triggers the malignancy of acute myeloid leukemia cells via regulation of IL‐4 and IL‐6. J Biochem Mol Toxicol. 2020;34(1):e22412. [DOI] [PubMed] [Google Scholar]
- 5. Kishimoto T. IL‐6: from its discovery to clinical applications. Int Immunol. 2010;22(5):347‐352. [DOI] [PubMed] [Google Scholar]
- 6. Napolitano LM. Sepsis 2018: definitions and guideline changes. Surg Infect (Larchmt). 2018;19(2):117‐125. [DOI] [PubMed] [Google Scholar]
- 7. Iwase S, Nakada TA, Hattori N, et al. Interleukin‐6 as a diagnostic marker for infection in critically ill patients: a systematic review and meta‐analysis. Am J Emerg Med. 2019;37(2):260‐265. [DOI] [PubMed] [Google Scholar]
- 8. Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605‐1608. [DOI] [PubMed] [Google Scholar]
- 9. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589‐1596. [DOI] [PubMed] [Google Scholar]
- 10. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864‐874. [PubMed] [Google Scholar]
- 11. Bender L, Thaarup J, Varming K, Krarup H, Ellermann‐Eriksen S, Ebbesen F. Early and late markers for the detection of early‐onset neonatal sepsis. Dan Med Bull. 2008;55(4):219‐223. [PubMed] [Google Scholar]
- 12. Huang D, Ying H, Jiang D, et al. Rapid and sensitive detection of interleukin‐6 in serum via time‐resolved lateral flow immunoassay. Anal Biochem. 2020;588:113468. [DOI] [PubMed] [Google Scholar]
- 13. Fan GC, Ren XL, Zhu C, Zhang JR, Zhu JJ. A new signal amplification strategy of photoelectrochemical immunoassay for highly sensitive interleukin‐6 detection based on TiO2/CdS/CdSe dual co‐sensitized structure. Biosens Bioelectron. 2014;59:45–53. [DOI] [PubMed] [Google Scholar]
- 14. Peng J, Guan J, Yao H, Jin X. Magnetic colorimetric immunoassay for human interleukin‐6 based on the oxidase activity of ceria spheres. Anal Biochem. 2016;492:63–68. [DOI] [PubMed] [Google Scholar]
- 15. Liu N, Yi H, Lin Y, et al. Combined electrochemiluminescent and electrochemical immunoassay for interleukin 6 based on the use of TiO(2) mesocrystal nanoarchitectures. Mikrochim Acta. 2018;185(5):277. [DOI] [PubMed] [Google Scholar]
- 16. Liu C, Li W, Liu G, Yang Y, Gong P, Hou Y. Detection of procalcitonin based on fluorescence immune chromatography. Sheng Wu Gong Cheng Xue Bao. 2018;34(3):440‐448. [DOI] [PubMed] [Google Scholar]
- 17. Macrea MM, Owens RL, Martin T, Smith D, Oursler KK, Malhotra A. The effect of isolated nocturnal oxygen desaturations on serum hs‐CRP and IL‐6 in patients with chronic obstructive pulmonary disease. Clin Respir J. 2019;13(2):120‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan HH, Hsiao YP, Chen PJ, et al. Epithelial growth factor receptor tyrosine kinase inhibitors alleviate house dust mite allergen Der p2‐induced IL‐6 and IL‐8. Environ Toxicol. 2019;34(4):476‐485. [DOI] [PubMed] [Google Scholar]
- 19. St John A, Price CP. Existing and emerging technologies for point‐of‐care testing. Clin Biochem Rev. 2014;35(3):155‐167. [PMC free article] [PubMed] [Google Scholar]
- 20. Bogdanovic J, Koets M, Sander I, et al. Rapid detection of fungal alpha‐amylase in the work environment with a lateral flow immunoassay. J Allergy Clin Immunol. 2006;118(5):1157‐1163. [DOI] [PubMed] [Google Scholar]
- 21. Chen C, Wu J. A fast and sensitive quantitative lateral flow immunoassay for Cry1Ab based on a novel signal amplification conjugate. Sensors (Basel). 2012;12(9):11684‐11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ho JA, Wauchope RD. A strip liposome immunoassay for aflatoxin B1. Anal Chem. 2002;74(7):1493‐1496. [DOI] [PubMed] [Google Scholar]
- 23. Serrate D, De Teresa JM, Marquina C, et al. Quantitative biomolecular sensing station based on magnetoresistive patterned arrays. Biosens Bioelectron. 2012;35(1):206‐212. [DOI] [PubMed] [Google Scholar]
- 24. O’Farrell B. Lateral flow technology for field‐based applications‐basics and advanced developments. Top Companion Anim Med. 2015;30(4):139‐147. [DOI] [PubMed] [Google Scholar]
- 25. Posthuma‐Trumpie GA, Korf J, van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393(2):569‐582. [DOI] [PubMed] [Google Scholar]
- 26. Huang X, Aguilar ZP, Xu H, Lai W, Xiong Y. Membrane‐based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: a review. Biosens Bioelectron; 2016;75:166‐180. [DOI] [PubMed] [Google Scholar]
- 27. Man Y, Lv X, Iqbal J, et al. Microchip based and immunochromatographic strip assays for the visual detection of interleukin‐6 and of tumor necrosis factor α using gold nanoparticles as labels. Microchim Acta. 2015;182(3–4):597‐604. [Google Scholar]
- 28. Li H, Vaughan JC. Switchable fluorophores for single‐molecule localization microscopy. Chem Rev. 2018;118(18):9412‐9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Michalet X, Pinaud FF, Bentolila LA, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307(5709):538‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu G, Zhang YX, Wei JT, Liu QQ, Chen HL. A method for detection of rotavirus based on quantum dot immunochromatography. Chinese J Biochem Mol Biol. 2020;11:1367‐1373. [Google Scholar]
- 31. Wu R, Zhou S, Chen T, et al. Quantitative and rapid detection of C‐reactive protein using quantum dot‐based lateral flow test strip. Anal Chim Acta. 2018;1008:1–7. [DOI] [PubMed] [Google Scholar]
- 32. Shen J, Zhou Y, Fu F, et al. Immunochromatographic assay for quantitative and sensitive detection of hepatitis B virus surface antigen using highly luminescent quantum dot‐beads. Talanta. 2015;142:145–149. [DOI] [PubMed] [Google Scholar]
- 33. Russell C, Ward AC, Vezza V, et al. Development of a needle shaped microelectrode for electrochemical detection of the sepsis biomarker interleukin‐6 (IL‐6) in real time. Biosens Bioelectron. 2019;126:806–814. [DOI] [PubMed] [Google Scholar]
- 34. Kacerovsky M, Musilova I, Hornychova H, et al. Bedside assessment of amniotic fluid interleukin‐6 in preterm prelabor rupture of membranes. Am J Obstet Gynecol. 2014;211(4):385.e381‐389. [DOI] [PubMed] [Google Scholar]
- 35. Celik IH, Demirel G, Uras N, Oguz ES, Erdeve O, Dilmen U. The role of serum interleukin‐6 and C‐reactive protein levels for differentiating aetiology of neonatal sepsis. Arch Argent Pediatr. 2015;113(6):534‐537. [DOI] [PubMed] [Google Scholar]
- 36. Chen X, Dong T, Wei X, et al. Electrochemical methods for detection of biomarkers of Chronic Obstructive Pulmonary Disease in serum and saliva. Biosens Bioelectron. 2019;142:111453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


