Abstract
Background
Chronic inflammation is a hallmark of colorectal mucinous adenocarcinoma (CMA). Albumin‐to‐fibrinogen ratio (AFR) and fibrinogen‐to‐pre‐albumin ratio (FPR) were independent prognostic factors for many kinds of solid malignancies. However, the association between the inflammatory scores and progression of metastatic CMA remains unknown.
Methods
Peripheral blood neutrophil count and circulating fibrinogen, albumin, and pre‐albumin levels were detected, and neutrophil‐to‐albumin ratio (NAR), neutrophil‐to‐pre‐albumin ratio(NPAR), AFR, and FPR were calculated in 42 metastatic MCA patients. Kaplan‐Meier curve, Cox regression, time‐dependent receiver operating characteristic curve (tdROC) were selected to investigate the prognostic utility of them in the patients.
Results
Metastatic CMA patients commonly occurred in middle‐younger patients (80.95%). NPAR (adjusted hazard ratio (HR)=2.405, 95% confidence interval (CI)=1.195–4.842) and FPR (p log‐rank=0.007, adjusted HR=2.364, 95% CI=1.203–4.645) were significantly associated with poor progression‐free survival in these patients. The prognostic prediction area under tdROC (AUROC) of FPR was significantly higher than that of NPAR(0.703 versus 0.537). Moreover, the patients with a high CA19‐9‐FPR score showed worse outcomes than those with the low score (p log‐rank<0.001, adjusted HR=7.273, 95% CI=2.721–19.435 for the score 1 versus 0). The prediction AUROC, sensitivity, and specificity of the score were 0.892 (0.788–0.996), 76.32%, and 100.00%, respectively, and its predicted efficacy was better than that of the single biomarkers.
Conclusion
The combined CA19‐9‐FPR score is an economical, simple, effective, and independent prognostic factor for metastatic MCA.
Keywords: fibrinogen‐to‐pre‐albumin ratio, inflammation, mucinous colorectal carcinoma, prognosis
The metastatic CMA patients commonly occurred in middle‐younger patients (80.95%). NPAR (adjusted HR = 2.405, 95% CI = 1.195–4.842) and FPR(p log‐rank = 0.007, adjusted HR = 2.364, 95% CI = 1.203–4.645) were significantly associated with poor progression‐free survival in these patients. The prognostic predicted area under tdROC (AUROC) of FPR were significantly higher than NPAR (0.703 versus 0.537). Moreover, the patients with high CA199‐FPR score showed the worse outcome than the low scores in the metastatic cohort (p log‐rank < 0.001, adjusted HR = 7.273, 95% CI = 2.721–19.435 for the score 1 versus 0). The predicted AUROC, sensitivity, and specificity of the score were 0.892 (0.788–0.996), 76.32%, and 100.00%, respectively, and its predicted efficacy was better than the single biomarkers.
![]()
1. INTRODUCTION
Colorectal mucinous adenocarcinoma (CMA) is a rare histological subset of colorectal cancer (CRC), accounting for 1.6%‐25.4% of all CRC. 1 , 2 CMAs commonly contain >50% extracellular mucin in the tumor volume. Due to the aggressive biological behavior and distinct genetic background of the disease, clinical chemotherapy response and prognosis are unsatisfactory in patients with CMA. 3 , 4 As such, accurate prediction of recurrence and progression in patients with localized and metastatic disease is important for appropriate, targeted treatment.
It is well‐established that chronic inflammation is a hallmark of cancer, including CMA. 5 It contributes to alternations of oncogenes and tumor‐suppression genes, which in turn leads to carcinogenesis. 6 Cancer‐elicited inflammation also can help to form a cancer cell‐protected “niche” for distal metastasis, resulting in progression and poor prognosis in these patients. 7 Accumulating evidence suggests that inflammatory cells and specific factors can reflect the degree of chronic inflammation. 8 , 9 Inflammatory biomarkers, such as neutrophil‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio, prognostic nutritional index, and lymphocyte‐to‐monocyte ratio are associated with response to clinical neoadjuvant chemoradiotherapy response and survival of patients with CMA. 10 , 11 The most recent studies have also reported circulating albumin‐to‐fibrinogen ratio (AFR), fibrinogen‐to‐pre‐albumin ratio (FPR), and neutrophil‐to‐albumin ratio (NAR), as independent prognostic indicators for many types of malignancies. 12 , 13 , 14 , 15 , 16 , 17 However, to our knowledge, no study has investigated the prognostic utility of AFR, FPR, NAR, and neutrophil‐to‐pre‐albumin ratio (NPAR) in patients with metastatic CMA.
Accordingly, we determined pre‐treatment circulating neutrophil count, and albumin, pre‐albumin, and fibrinogen levels and calculated the four inflammatory ratios to investigate their utility in predicting the clinical prognosis of 42 patients with metastatic CMA.
2. MATERIALS AND METHODS
Patients with metastatic CMA patients were initially recruited to screen and identify those eligible to participate in the present study. All the included patients were diagnosed and confirmed according to imaging and pathological analysis between January 2011 and December 2017 at the Second Affiliated Hospital of Nanchang University (Nanchang, China). None of the eligible patients had other malignancies and did not undergo any emergent or neoadjuvant chemoradiotherapy. Patients who recently experience diarrhea, infection, hereditary polyposis, ulcerative colitis, autoimmune or chronic kidney disease, hematopathy, hepatopathy, or cardiovascular and cerebrovascular disease, those taking non‐steroidal anti‐inflammatory drugs and intravenous albumin supplements and individuals without clinical data, contact information, samples, or lost to follow‐up within three months, were excluded from the study. Written informed consent was obtained from each enrolled patient, and the study was approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University.
Baseline characteristics, contact information, and pathological results were obtained from all patients. Peripheral blood samples, plasma, and serum samples were collected from each eligible patient one or two days before clinical treatment. Peripheral neutrophil count was detected by SYSMEX HST‐302 machine (Sysmex, Tokyo, Japan). Serum albumin, pre‐albumin, and plasma fibrinogen levels were determined by bromocresol green staining method, immunoturbidimetry, and Clauss method using OLYMPUS AU5400 (Beckman Coulter, Tokyo, Japan), and SYSMEX CA‐7000 machine (Sysmex, Tokyo, Japan), respectively. Circulating carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19‐9) were determined by chemiluminescence method using SIEMENS ADVIA Centaur XP machine (Siemens, Erlangen, Germany). The intra‐ and inter‐assay coefficients of variation of the above detections were less than 10%. NAR, NPAR, AFR, and FPR were calculated according to the formulae listed in Table 1. In addition, the combined CA199‐FPR score was calculated. Patients with both low CA19‐9 and FPR scored 0, and those with a single or two high biomarkers of them scored 1.
TABLE 1.
The optimal cut‐off values and definitions of four inflammatory ratios in present study.
| Inflammatory ratios | Cut‐off value | Definition of the score | Score |
|---|---|---|---|
| AFR (albumin‐to‐fibrinogen ratio) | 9.30 | ≤9.30 | 0 |
| >9.30 | 1 | ||
| FPR (fibrinogen‐to‐pre‐albumin ratio ×1000) | 26.20 | ≤26.20 | 0 |
| >26.20 | 1 | ||
| NAR (neutrophil‐to‐albumin ratio×100) | 12.10 | ≤12.10 | 0 |
| >12.10 | 1 | ||
| NPAR (neutrophil‐to‐pre‐albumin ratio ×1000) | 23.40 | ≤23.40 | 0 |
| >23.40 | 1 |
the optimal cut‐off values of the included ratios are calculated using X‐tile software according to progression‐free survival.
Progression‐free survival (PFS) was the primary survival endpoint in this study, and it was defined as the time from the clinical diagnosis to disease progression or death with any reason. Three‐year follow‐up was performed at a frequency of three months in the first two years and six months in the third year, with a deadline of December 1, 2020. In each follow‐up, a physical examination, common tumor biomarkers (ie, CEA and CA19‐9), and abdominal computed tomography scan or magnetic resonance imaging detection were performed during the follow‐up investigations.
X‐tile software (Yale University, New Haven, CT, USA) was used to calculate the NAR, NPAR, AFR, and FPR cut‐off values according to PFS. Continuous and binary variables are expressed as median and interquartile range, and number and frequency, respectively. The Kolmogorov‐Smirnov, Mann‐Whitney U test, chi‐squared, and Fisher's exact tests were used to analyze differences in the comparisons between the continuous and binary variables, as appropriate Kaplan‐Meier curves with a log‐rank test and univariable and multivariable Cox regression (LR method, Backward) were used to examine the survival differences in the different groups. Time‐dependent receiver operating characteristic (tdROC) curves were used to discriminate and to compare the prediction efficacies of these ratios. All statistical analyses were performed using SPSS version22.0 (IBM Corp, Armonk, NY, USA), R 3.5.1 (Institute for Statistics and Mathematics, Vienna, Austria) with the “survivalROC” package. All analyses were two‐sided, and differences with p < 0.05 were considered to be statically significant.
3. RESULTS
According to the inclusion and exclusion criteria, 42 patients with metastatic CMA were included in the present study. Baseline characteristics and sample values are summarized in Table 2. The majority of included patients were <60 years of age (80.95%), 52.38% of the patients were observed in right‐tumor location. 52.38% underwent palliative resection, and 97.62% received adjuvant chemoradiotherapy. The progression rates were 90.48% within three years of follow‐up, and the median PFS was 9 months (interquartile range, 5–16.25 months).
TABLE 2.
The baseline and clinicopathological characteristics of patients with metastatic CMA.
| Parameter | Number | Percentage (%) |
|---|---|---|
| Gender (male) | 22 | 52.38 |
| Age (>60 year) | 8 | 19.05 |
| Smoking (yes) | 5 | 11.90 |
| Drinking(yes) | 3 | 7.14 |
| Diabetes (yes) | 4 | 9.52 |
| Hypertension (yes) | 2 | 4.76 |
| T stage (T3‐4) | 31 | 73.81 |
| LN status (N1‐2) | 18 | 42.86 |
| Differentiation (G1‐2) | 16 | 38.10 |
| Cancer bulk (>5 cm) | 13 | 30.95 |
| Primary location (right) | 22 | 52.38 |
| Palliative surgery (yes) | 22 | 52.38 |
| Chemotherapy(yes) | 41 | 97.62 |
| Radiotherapy (yes) | 6 | 14.29 |
| Targeted therapy (yes) | 8 | 19.05 |
| CEA (>5 ng/mL) | 22 | 52.38 |
| CA19‐9 (>37 U/mL) | 24 | 57.14 |
| NAR (score=1) | 10 | 23.81 |
| NPAR (score=1) | 14 | 33.33 |
| AFR (score=1) | 32 | 76.19 |
| FPR (score=1) | 16 | 38.10 |
| Progression rate | 38 | 90.48 |
LN: lymph node; NAR: neutrophil‐to‐albumin×100; NPAR: neutrophil‐to ‐pre‐albumin ratio×1000; AFR: albumin‐to‐fibrinogen ratio; FPR: fibrinogen‐to‐ pre‐albumin ratio ×1000; right location means the caecum, ascending colon, and transverse colon, the others were considered as left tumor location.
The optimal cut‐off values for NAR, NPAR, AAPR, AGR, AFR, and FPR were 12.10, 23.40, 9.30, and 26.20 in present study(Table 1). High CA19‐9 (plog‐rank < 0.001, crude hazard ratio (HR)=4.196, 95% confidence interval (CI) =1.896–9.286) and FPR (plog‐rank = 0.007, crude HR=2.521, 95%CI=1.282–4.960) were significantly associated with poor PFS according to Kaplan‐Meier curve analysis and univariable Cox regression. However, the other factors were not associated with PFS among the included patients. Moreover, adjusted according to the common baseline and pathological and treatment variables, PFS in the patients with high CA199 (p=0.001, adjusted HR=3.855, 95%CI=1.746–8.509), NPAR (p = 0.014, adjusted HR=2.405, 95%CI=1.195–4.842), and FPR (p = 0.013, adjusted HR=2.364, 95%CI=1.203–4.645) was still significantly inferior to those with low CA199, NPAR, and FPR, respectively (Figure 1 and Table 3).
FIGURE 1.
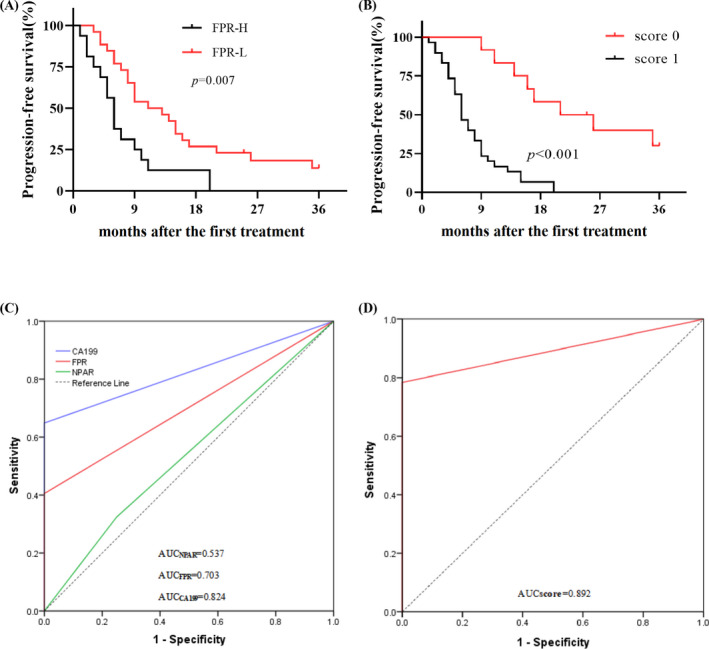
Prognostic roles of FPR and CA19‐9‐FPR combined score in 42 metastatic CMA patients. A: Kaplan‐Meier (K‐M) curve of FPR; B: K‐M curve of CA19‐9‐FPR combined score; C: time‐dependent receiver operating characteristic curve (tdROC) of CA19‐9, NPAR, FPR; D: tdROC of CA19‐9‐FPR combined score.
TABLE 3.
Kaplan‐Meier curve and Cox regression of the clinical characteristics and the four inflammatory ratios in patients with metastatic CMA.
| Parameter | P‐value | Crude HR(95%CI) | Adjusted HR(95%CI) | |
|---|---|---|---|---|
| Gender (male) | 0.128 | 1.660(0.864–3.189) | 1.195(0.544–2.629) | |
| Age (>60 year) | 0.070 | 2.116(0.941–4.761) | 2.020(0.898–4.542) | |
| Smoking (yes) | 0.306 | 1.651(0.632–4.309) | 2.085(0.377–11.528) | |
| Drinking (yes) | 0.473 | 1.549(0.469–5.113) | 0.552(0.119–2.567) | |
| Diabetes (yes) | 0.304 | 1.729(0.608–4.917) | 1.375(0.424–4.460) | |
| Hypertension (yes) | 0.827 | 1.173(0.279–4.943) | 0.542(0.108–2.733) | |
| T stage (T3‐4) | 0.920 | 3.211(0.623–6.214) | 3.655(0.320–6.230) | |
| LN status (N1‐2) | 0.095 | 1.949(0.891–4.267) | 2.521(0.983–5.871) | |
| Differentiation (G1‐2) | 0.912 | 1.053(0.425–2.607) | 1.021(0.410–2.599) | |
| Cancer bulk (>5 cm) | 0.833 | 1.095(0.470–2.553) | 1.015(0.479–2.563) | |
| Primary location (right) | 0.079 | 0.551(0.283–1.071) | 0.441(0.216–0.901) | |
| Palliative operation (yes) | 0.300 | 0.692(0.345–1.388) | 0.666(0.324–1.370) | |
| Radio‐chemotherapy (yes) | 0.227 | 0.557(0.215–1.441) | 0.551(0.209–1.456) | |
| Targeted therapy (yes) | 0.906 | 0.954(0.434–2.095) | 1.462(0.535–3.998) | |
| CEA (>5 ng/mL) | 0.135 | 1.694(0.849–3.383) | 1.545(0.764–3.126) | |
| CA19‐9 (>37 U/mL) | <0.001 | 4.196(1.896–9.286) | 3.855(1.746–8.509) | |
| NAR (score=1) | 0.529 | 1.272(0.600–2.696) | 1.902(0.885–4.088) | |
| NPAR (score=1) | 0.133 | 1.678(0.853–3.300) | 2.405(1.195–4.842) | |
| AFR (score=1) | 0.156 | 0.585(0.279–1.227) | 0.863(0.283–2.364) | |
| FPR (score=1) | 0.007 | 2.521(1.282–4.960) | 2.364(1.203–4.645) |
LN: lymph node; NAR: neutrophil‐to‐albumin ×100; NPAR: neutrophil‐ to‐pre‐albumin ratio×1000; AFR: albumin‐to‐fibrinogen ratio; FPR: fibrinogen‐to ‐pre‐albumin ratio ×1000; p‐value: the value of Kaplan‐Meier curve with log‐rank test; HR: hazard ratio; CI: confidence interval; right location means the caecum, ascending colon and transverse colon, the others were considered as left tumor location; multivariable Cox regression is adjusted by gender, age, tobacco, alcohol, diabetes, hypertension, treatment, T and N status, differentiation, cancer size, primary location.
The area under the time‐dependent ROC (AUROC) was used to evaluate the prediction efficacy of CA19‐9 and FPR in patients with metastatic CMA. The prediction AUCs for FPR, NPAR, and CA19‐9 were 0.703(0.499–0.906), 0.537 (0.358–0.744), and 0.824(0.683–0.966), respectively, and their prediction sensitivity and specificity were 42.11% and 100.00%, 34.21% and 75.00%, and 64.86% and 100.00%, respectively (Figure 1 and Table 4).
TABLE 4.
The performance discriminative ability between FPR, CA199, and the combined score in metastatic CMA patients.
| Biomarkers | Progression‐free survival | |||
|---|---|---|---|---|
| AUROC(95%CI) | Sensitivity | Specificity | ||
| FPR | 0.703(0.499–0.906) | 42.11% | 100.00% | |
| CA19‐9 | 0.824(0.683–0.966) | 64.86% | 100.00% | |
| CA19‐9‐FPR score | 0.892(0.788–0.996) | 76.32% | 100.00% | |
FPR: fibrinogen‐to‐pre‐albumin ratio ×1000; AUROC: area under time‐dependent receiver operating characteristic curve; CI: confidence interval.
In this study, we calculated and investigated the role of the combined CA19‐9 and FPR score in predicting clinical outcomes in the study. Twelve (28.57%) and 30 (71.43%) patients had scores of 0, 1, respectively, using the combined CA19‐9‐FPR score. The PFS of the patients who scored 1 was significantly worse than that of one case (p log‐rank<0.001, adjusted HR=7.273, 95%CI=2.721–19.435) (Figure 1). The predicted AUC was higher than that for CA19‐9 (0.892 versus 0.824, p = 0.034) and FPR (0.892 versus. 0.703, p < 0.01), respectively, and the prediction sensitivity and specificity of the combined score were 76.32% and 100.00%, respectively (Table 4).
4. DISCUSSION
Prediction of disease progression is an important metric that can influence clinical treatment decision aimed at improving survival in patients with metastatic CMA. 11 In this study, we found that patients with metastatic CMA exhibiting a high FPR demonstrated extremely poor PFS compared to those with low‐FPR. Moreover, patients with a high combined CA19‐9‐FPR score experienced worse outcomes than those with low score, and its prediction efficacy was high up to 0.892, which was significantly better than the single biomarkers.
CMA exhibits the distinct clinical and histological characteristics. 18 Previous studies have shown that the disease occurs primarily in females and younger populations. 19 Our study showed that males accounted for 52.38% of the total patients, and 80.95% of the eligible cases were <60 years of age. Moreover, 38.10% of the metastatic patients exhibited a high FPR. Accumulating evidence indicates that high FPR implies high‐grade inflammation. 15 Severe cancer‐elicited inflammation attenuates sensitivity to radio‐chemotherapy and can even lead resistance to the treatment, 12 resulting in poor survival in CRC patients. 12 , 20 Hence, 90.48% of the included patients were found to exhibit disease progression during the follow‐up period.
TNM stage, venous and lymphoid invasion, microsatellite instability (MSI) status, CEA, and CA19‐9 are common factors used to evaluate the prognosis of patients with metastatic CMA. In this study, we found that only lymph node status and CA19‐9 were significantly associated with the poor outcomes in these patients. Recent studies have reported that chronic inflammatory biomarkers, such as FPR, lymphocyte‐to‐monocyte ratio (LMR), and neutrophil‐to‐lymphocyte ratio (NLR) were effective in predicting survival in patients with CRC. 12 , 20 , 21 , 22 Our study also showed that circulating FPR was superior, in terms of prognostic ability, to the other inflammatory biomarkers as a useful recurrence indicator in stage II‐III surgical CRC patients. 23 In this study, we found that only NPAR and FPR were also associated with poor PFS adjusted by the other common confounders, suggesting that the two factors may be an independent prognostic metric to predict the progression of metastatic CMA. However, the prediction AUROC for FPR was significantly higher than that for NPAR, indicating that NPAR was inferior to FPR in predicting survival. Furthermore, the combined CA19‐9‐FPR score harbored high efficacy and predicted sensitivity, which were high up to 76.32% and 100.00%, respectively. Our findings indicated that the combined CA19‐9 and FPR score was superior to that of single factors, and the score was a practical, simple, and effective biomarker for predicting disease progression.
To our knowledge, this study is the first time to investigate the role of these inflammatory ratios in metastatic CMA patients. Although we obtained interesting findings, the following limitations should be addressed. This was not a prospective study, and, because it was retrospective study, selection bias of eligible cases may have affected the findings. Only 42 eligible patients were included in this study, and the small sample size may have affected the statistical power and the cut‐off values of each included inflammatory ratio. Our patients were selected from a single center and therefore, our results should be validated in multi‐center prospective studies. Finally, a distinct genetic background affected patient survival, however, we did not detect microsatellite instability(MSI) status, RAS and BRAF mutations, or the status of CpG island methylator phenotype. Thus, we did not know the influence of these genetic alternations on FPR in predicting the survival of metastatic CMA patients.
In conclusion, FPR was better than the other three inflammatory ratios in predicting clinical outcome of patients with metastatic CMA. The combined CA19‐9‐FPR score was a practical, effective, and independent prognostic metric for the metastatic disease. Future multi‐center prospective studies are needed to validate our results.
ETHICAL APPROVAL
Written informed consent was obtained from each enrolled patient, and the study was approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University.
ACKNOWLEDGEMENT
We would like to thank Editage (www.editage.cn) for English language editing.
Yu‐Cui Liao, Ming Fu and Xue‐Feng Wang were contributed equally to the study.
DATA AVAILABILITY STATEMENT
Readers can access the data supporting the conclusions of the study by contacting with author through Email.
REFERENCES
- 1. Papadopoulos VN, Michalopoulos A, Netta S, et al. Prognostic significance of mucinous component in colorectal carcinoma. Tech Coloproctol. 2004;8(Suppl 1):s123‐s125. [DOI] [PubMed] [Google Scholar]
- 2. Pande R, Sunga A, LeVea C, et al. Significance of signet‐ring cells in patients with colorectal cancer. Dis Colon Rectum. 2008;51(1):50‐55. [DOI] [PubMed] [Google Scholar]
- 3. Reynolds IS, O'Connell E, Fichtner M, et al. Mucinous adenocarcinoma of the colon and rectum: a genomic analysis. J Surg Oncol. 2019;120(8):1427‐1435. [DOI] [PubMed] [Google Scholar]
- 4. Song IH, Hong S‐M, Yu E, et al. Signet ring cell component predicts aggressive behaviour in colorectal mucinous adenocarcinoma. Pathology. 2019;51(4):384‐391. [DOI] [PubMed] [Google Scholar]
- 5. Taniguchi K, Karin M. NF‐κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309‐324. [DOI] [PubMed] [Google Scholar]
- 6. Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002;16(2):217‐226. [PubMed] [Google Scholar]
- 7. Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196(4):395‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang L, Chen Q‐G, Li S‐Q, et al. Preoperative fibrinogen to prealbumin ratio as a novel predictor for clinical outcome of hepatocellular carcinoma. Future Oncol. 2019;15(1):13‐22. [DOI] [PubMed] [Google Scholar]
- 9. Li S‐Q, You X‐H, Sun F, et al. Albumin to fibrinogen ratio and fibrinogen to pre‐albumin ratio are economical, simple and promising prognostic factors for solid malignancy. J Thorac Dis. 2019;11(Suppl 15):S2036‐S2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao J, Xu J, Zhang R. Clinical and prognostic significance of pathological and inflammatory markers in mucinous rectal cancer patients receiving neoadjuvant chemoradiotherapy and curative surgery. Med Sci Monit. 2017;23:4826‐4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun Y, Huang Z, Chi P. An inflammation index‐based prediction of treatment response to neoadjuvant chemoradiotherapy for rectal mucinous adenocarcinoma. Int J Clin Oncol. 2020;25(7):1299‐1307. [DOI] [PubMed] [Google Scholar]
- 12. Chen Q‐G, Zhang L, Sun F, et al. Elevated FPR confers to radiochemoresistance and predicts clinical efficacy and outcome of metastatic colorectal cancer patients. Aging (Albany NY). 2019;11(6):1716‐1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li S‐Q, Jiang Y‐H, Lin J, et al. Albumin‐to‐fibrinogen ratio as a promising biomarker to predict clinical outcome of non‐small cell lung cancer individuals. Cancer Med. 2018;7(4):1221‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tawfik B, Mokdad AA, Patel PM, et al. The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anticancer Drugs. 2016;27(9):879‐883. [DOI] [PubMed] [Google Scholar]
- 15. Zhang X, Zhao W, Chen X, et al. Combining the Fibrinogen‐to‐Pre‐Albumin Ratio and Prognostic Nutritional Index (FPR‐PNI) predicts the survival in elderly gastric cancer patients after gastrectomy. Onco Targets Ther. 2020;13:8845‐8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Li S‐Q, Liao Z‐H, et al. Prognostic value of a novel FPR biomarker in patients with surgical stage II and III gastric cancer. Oncotarget. 2017;8(43):75195‐75205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao Q‐F, Qiu J‐C, Huang X‐H, et al. The predictive and prognostic role of a novel ADS score in esophageal squamous cell carcinoma patients undergoing esophagectomy. Cancer Cell Int. 2018;18:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luo C, Cen S, Ding G, et al. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young‐onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89(2):216‐224. [DOI] [PubMed] [Google Scholar]
- 20. Sun F, Peng HX, Gao QF, et al. Preoperative circulating FPR and CCF score are promising biomarkers for predicting clinical outcome of stage II‐III colorectal cancer patients. Cancer Manag Res. 2018;10:2151‐2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossi S, Basso M, Strippoli A, et al. Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer. 2017;16(4):264‐274. [DOI] [PubMed] [Google Scholar]
- 22. Ying H‐Q, Deng Q‐W, He B‐S, et al. The prognostic value of preoperative NLR, d‐NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;1(12):305. [DOI] [PubMed] [Google Scholar]
- 23. Ying HQ, Liao YC, Sun F, et al. The role of cancer‐elicited inflammatory biomarkers in predicting early recurrence within stage II–III colorectal cancer patients after curable resection. J Inflamm Res. 2021;2021(14):115‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Readers can access the data supporting the conclusions of the study by contacting with author through Email.


