Abstract
Background
Different disease severities of COVID‐19 patients could be reflected on clinical laboratory findings.
Methods
In this single‐centered retrospective study, demographic, clinical, and laboratory indicators on and during admission were compared among 74 participants with mild, moderate, critical severe, or severe classification. Risk factors associated with disease severity were analyzed by multivariate analyses. The AUC and 95% CI of the ROC curve were calculated.
Results
The most common manifestations of these patients were fever and cough. Critical severe or severe group owned the longest length of stay (23 (19,31), p < 0.001). After multivariate logistic regression, independent influence factors on admission for severity of disease were CK‐MB (OR 0.674; 95% CI 0.489–0.928; p = 0.016), LDH (OR 1.111 or 1.107; 95% CI 1.026–1.204 or 1.022–1.199; p = 0.009 or 0.013), normal T‐BIL (OR 4.58 × 10−8; 95% CI 3.05 × 10−9–6.88 × 10−7; p < 0.001), LYM% (OR 0.008; 95% CI 0–0.602; p = 0.029), and normal ESR (OR 0.016; 95% CI 0–0.498; p = 0.019). Factors during hospitalization were normal T‐BIL (OR 8.56 × 10−9; 95% CI 8.30 × 10−10–8.83 × 10−8; p < 0.001), LYM (OR 0.068; 95% CI 0.005–0.934; p = 0.044), albumin (OR 0.565; 95% CI 0.327–0.977; p = 0.041), and normal NEU% (OR 0.013; 95% CI 0.000–0.967; p = 0.048). Combined indicators of AUC were 0.860 (LYM, LDH, and normal ESR on admission, p < 0.001) and 0.750 (CK‐MB, LDH, and normal T‐BIL during hospitalization, p = 0.020) when predicting for severe or critical severe patients.
Conclusion
To pay close attention to the progression of COVID‐19 and take measures promptly, we should be cautious of the laboratory indicators when patients on admission especially CK‐MB, LDH, LYM%, T‐BIL as well as ESR; and T‐BIL, LYM, albumin, NEU% with the process of disease.
Keywords: COVID‐19, disease severity, laboratory indicators, multivariate regression, risk factor
Outline of analyses of the COVID‐19 patients laboratory indicators.
1. INTRODUCTION
Since December 2019, a number of patients with unexplained pneumonia had been reported in Wuhan, China. Later in January 2020, the pathogen was isolated to be a kind of novel coronavirus and named as 2019‐nCoV. 1 The epidemic was initially found in mainland China; then, it spread rapidly and became a worldwide health concern. 2 Globally, as of June 1, 2020, there have been 6040 609 confirmed cases due to coronavirus disease 2019 (COVID‐19), including 370 657 deaths, reported to WHO (https://covid19.who.int/). Confirmed patients mainly present with fever, dry cough, fatigue, dyspnea, and bilateral ground‐glass opacities on chest CT scans, which are likely to the features of SARS‐CoV and MERS‐CoV infections. Some exhibit nausea and vomiting. 3 , 4 , 5 , 6 According to the latest guidance issued by National Health Commission of the People's Republic of China (NHC China), patients with confirmed COVID‐19 could be classified due to their different severity of disease.
This disease may lead to potential damage to vital organs such as lung, liver, kidney, and heart with a mechanism analogous to the SARS coronavirus involving ACE2, which could be reflected on many aspects including laboratory findings, manifestations, and radiological image. 7 Though there are numbers of reports related to COVID‐19, most of the previous studies focused on epidemiology and clinical characteristics of patients. Reported studies have explored risk factors associated with clinical outcomes, such as laboratory findings, immunological features, cytokine storm, and liver biochemistries. 8 , 9 , 10 , 11 However, some of those researches commonly conducted on critically ill cases with 2019‐nCoV infection; others payed more attention to mortality or monitoring rather than predicting the severity of disease in current patients. 12 , 13 , 14 Few studies have conducted multivariable regression to help identify the dependent risk factors of disease severity during the different periods of admission. 15
In order to investigate the possible association between laboratory indicators and disease severity, we conducted a single‐centered, retrospective study in Yueyang First People's Hospital, Hunan province, China. Here, we present detailed laboratory results of all hospitalized COVID‐19 patients and analyze risk factors when patients on and during admission via multivariate logistic regression.
2. MATERIALS AND METHODS
2.1. Study population
This retrospective study was conducted in Yueyang First People's Hospital, Hunan province, China. Patients with confirmed COVID‐19 during the period from January 2020 to March 15, 2020, were enrolled in our study. During the study period, there were 83 confirmed COVID‐19 cases in total and 74 were enrolled finally.
Inclusion criteria were as follows: The patients were confirmed with COVID‐19. The diagnostic criteria of COVID‐19 refer to the latest guidance issued by National Health Commission of the People's Republic of China (NHC China), including fever/or respiratory symptoms; pulmonary CT image features: multiple lobular shadows or ground‐glass shadows; and the novel coronavirus nucleic acid of respiratory specimens is positive.
Exclusion criteria were as follows: Patients with incomplete laboratory indicators were excluded.
2.2. Study design and data collection
According to the NHC (National Health Commission) China guidance for clinical classification, we assessed the severity of all participants within 24 h after diagnosis, then divided them into mild group, moderate group, and critical severe or severe group. Mild patients had only mild clinical symptoms and no pneumonia radiological manifestations. Moderate patients showed fever and respiratory symptoms with imaging findings of pneumonia. Critical severe or sever patients should meet any of these following conditions: respiratory distress, respiratory rate ≥30/min; oxygen saturation ≤93%; PaO2/FiO2 ≤300 mmHg; pneumonia radiological image manifesting the lesions significantly progressed over 50% within 24–48 h; respiratory failure requiring mechanical ventilation; shock; combined with other organ dysfunction admitted to intensive care unit.
We collected the laboratory findings on admission and the most abnormal results during hospitalization of COVID‐19 patients, including blood routine, clinical chemistry indicators, routine coagulation test, and other inflammation indicators, as well as baseline characteristics such as age, sex, concomitant disease, symptoms, and length of stay. Data were extracted from the electronic medical record system.
2.3. Statistical analysis
Statistical analyses were performed using SPSS 20.0. Continuous variables were compared through one‐way ANOVA or non‐parametric tests and presented as mean ± standard deviation [] or medians with interquartile range (IQR). Categorical variables were evaluated with Pearson chi‐square test or Fisher exact test, expressed in frequencies and percentages [n(%)]. Multivariate analyses were performed and reported as odds ratios (OR) with 95% CI. Using collinearity diagnosis to assess the correlation between factors and testing parallel lines to confirm the disordered category of variables. After that only qualified variables were entered into a multivariate logistic regression to identify independent risk factors for disease severity. The AUC and the 95% confidence interval of the receiver operator characteristic (ROC) curve were calculated using the predicted probability of the severity of COVID‐19. p < 0.05 was considered statistical significance.
2.4. Ethics approval
The study was approved by the Ethics Committees from Xiangya Third Hospital, Central South University (2019‐S000).
3. RESULTS
3.1. Cases collection and baseline characteristics of participants
During the study period, 83 hospitalized cases with confirmed COVID‐19 were identified. Nine patients with incomplete laboratory results were excluded, leaving 74 for final analysis in our study (Figure 1).
FIGURE 1.
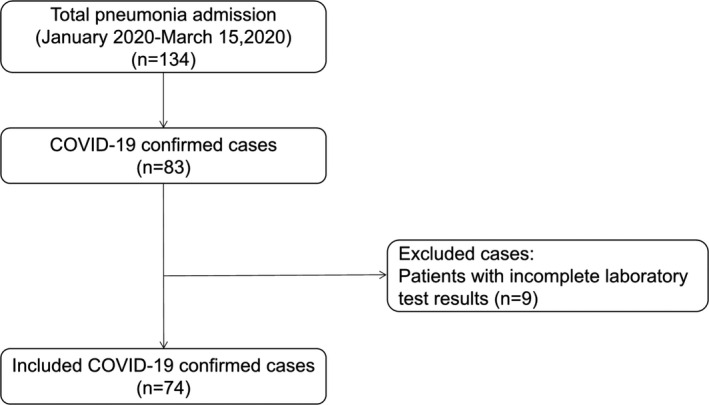
Process of case screening
The baseline characteristics of participants were shown in Table 1. COVID‐19 patients were categorized in three groups according to their severity of disease, including 18 mild patients (11 males and 7 females), 31 moderate patients (14 males and 17 females), and 25 critical severe or severe patients (13 males and 12 females). Mean age of these groups is 44.46 ± 18.43, 48.87 ± 15.25 and 53.88 ± 12.83 years, respectively. Proportion of patients with concomitant disease in each group account for 22.2%, 25.8%, and 20.0%. No difference in age, gender, and concomitant disease was found between three groups. As for clinical manifestations, 11 (44.0%) critical severe or severe patients manifested anhelation (p = 0.036). The length of stay is significantly different among groups (p < 0.001) and patients who were more seriously ill stayed longer.
TABLE 1.
Baseline characteristics of 2019 novel coronavirus pneumonia patients [n (%) /]
| Groups | Mild | Moderate | Critical severe or severe | p |
|---|---|---|---|---|
| Cases | 18 | 31 | 25 | |
| Age (years old) | 44.46 ± 18.43 | 48.87 ± 15.25 | 53.88 ± 12.83 | 0.141 |
| Sex | ||||
| Male | 11 (61.1) | 14 (45.2) | 13 (52.0) | 0.558 |
| Female | 7 (38.9) | 17 (54.8) | 12 (48.0) | |
| Concomitant disease | ||||
| With | 4 (22.2) | 8 (25.8) | 5 (20.0) | 0.873 |
| Without | 14 (77.8) | 23 (74.2) | 20 (80.0) | |
| Symptoms | ||||
| Fever | 16 (88.9) | 27 (87.1) | 24 (96.0) | 0.507 |
| Cough | 15 (83.3) | 23 (74.2) | 19 (76.0) | 0.756 |
| Anhelation | 3 (16.7) | 5 (16.1) | 11 (44.0) | 0.036 |
| Fatigue | 5 | 9 | 13 | 0.140 |
| Muscle ache | 3 | 6 | 6 | 0.829 |
| Headache | 7 | 9 | 9 | 0.749 |
| Sore throat | 5 | 9 | 7 | 0.994 |
| Nausea | 1 | 1 | 0 | 0.526 |
| Diarrhea | 2 | 2 | 0 | 0.267 |
| Length of stay (day) | 9 (8,10) | 14 (12,15) | 23 (19,31) | <0.001 |
3.2. Analysis of the laboratory indicators on admission among different groups
Blood routine, as well as clinical chemistry indicators, coagulation, and other inflammation indicators were obtained. We performed univariate analyses to determine indexes that could be further enrolled in multivariate logistic regression analysis. Indicators with statistically significance on admission are depicted in Table 2, and more details are shown in supplements (Tables S1–S5).
TABLE 2.
Indicators with statistically significant differences between three groups via univariate analysis on admission
| Variable | Mild group | Moderate group | Critical severe or severe group | F/H | p |
|---|---|---|---|---|---|
| LYM (×109) | 1.76(1.06,2.49) | 1.10 (0.75,1.61) | 1.17 (0.86,1.81) | 6.556 | 0.038 |
| NEU% (%) | 55.37 ± 13.90 | 64.87 ± 12.31 | 58.04 ± 11.66 | 3.879 | 0.025 |
| LYM% (%) | 33.34 ± 13.86 | 25.32 ± 9.09 | 30.23 ± 9.95 | 3.483 | 0.036 |
| Cys C (mg/L) | 0.67 (0.61,1.08) | 0.98 (0.76,1.11) | 1.00 (0.79,1.13) | 6.975 | 0.031 |
| UA (μmol/L) | 105.50 (93.15,251.93) | 194.7 (110.10,309.80) | 299.6 (231.95,387.58) | 12.888 | 0.002 |
| CK‐MB (U/L) | 8.10 (6.25,10.85) | 10.10 (7.80,11.70) | 13.00 (10.85,17.85) | 17.404 | <0.001 |
| LDH (U/L) | 131.00 (123.68,154.65) | 179.80 (156.30,221.30) | 200.70 (174.70,241.25) | 20.288 | <0.001 |
| Mb (ng/L) | 50.80 (29.00,68.95) | 61.9 0(51.30,80.40) | 84.80 (70.30,109.35) | 19.738 | <0.001 |
| Elevated T‐BIL | 0 (0.0) | 8 (25.8) | 1 (4.0) | 9.326 | 0.009 |
| Elevated ESR | 12 (66.7) | 17 (54.8) | 22 (88.0) | 7.064 | 0.029 |
The levels of LYM, NEU%, LYM%, CysC, UA, CK‐MB, LDH, and Mb have statistical significance among three groups. Proportion of T‐BIL elevation and ESR elevation cases also show differences. We find that LYM and LYM% levels are much lower in moderate and critical severe or severe patients than in mild [1.76 (1.06, 2.49), p = 0.038; 33.34 ± 13.86, p = 0.036]. On the contrary, NEU% is less in mild patients (55.37 ± 13.90). With aggravation of the disease, levels of CysC, UA, CK‐MB, LDH, and Mb increase and achieved maximum in critical severe or severe group (p < 0.05). The proportion of cases with elevated T‐BIL is higher in moderate patients (8, 25.8%). Critical severe or severe patients have the highest elevated ESR proportion (22, 88.0%). No difference in other indicators has been found.
We investigated the relationship between the first‐time laboratory examination indicators when patients on admission and disease severity, based on the univariate analyses above and consideration of professional evidence. Multivariate logistic regression analysis was performed to identify those variables that were independently associated with severity of patients. Results are shown in Table 3. Mild group served as reference. Most relevant risk factors on admission for more serious disease in the logistic regression model were CK‐MB (OR 0.674; 95%CI 0.489–0.928; p = 0.016), LDH (OR 1.111 or 1.107; 95%CI 1.026–1.204 or 1.022–1.199; p = 0.009 or 0.013), normal T‐BIL (OR 4.58 × 10−8; 95%CI 3.05 × 10−9–6.88 × 10−7; p < 0.001), LYM% (OR 0.008; 95%CI 0–0.602; p = 0.029), and normal ESR (OR 0.016; 95%CI 0–0.498; p = 0.019).
TABLE 3.
Multivariate logistic regression analysis of initial laboratory indicators
| Group a | Variable | B | SE | Wald | p | OR | 95%CI | |
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| Moderate | LYM (×109) | 0.065 | 0.097 | 0.446 | 0.504 | 1.067 | 0.882 | 1.29 |
| LYM% (%) | −2.984 | 2.12 | 1.982 | 0.159 | 0.051 | 0.001 | 3.222 | |
| Cys C (mg/L) | 1.251 | 2.181 | 0.329 | 0.566 | 3.496 | 0.049 | 251.223 | |
| UA (μmol/L) | −0.002 | 0.005 | 0.101 | 0.75 | 0.998 | 0.988 | 1.009 | |
| CK‐MB (U/L) | −0.395 | 0.163 | 5.838 | 0.016 | 0.674 | 0.489 | 0.928 | |
| LDH (U/L) | 0.106 | 0.041 | 6.746 | 0.009 | 1.111 | 1.026 | 1.204 | |
| Mb (ng/L) | 0.064 | 0.034 | 3.531 | 0.06 | 1.066 | 0.997 | 1.14 | |
| T‐BIL (μmol/L) | ||||||||
| Normal | −16.899 | 1.382 | 149.506 | <0.001 | 4.58 × 10−8 | 3.05 × 10−9 | 6.88 × 10−9 | |
| Elevated | 0 | |||||||
| Mb | ||||||||
| Normal | 4.952 | 2.555 | 3.757 | 0.053 | 141.465 | 0.946 | 21153.7 | |
| Elevated | 0 | |||||||
| ESR | ||||||||
| Normal | −1.997 | 1.606 | 1.546 | 0.214 | 0.136 | 0.006 | 3.162 | |
| Elevated | 0 | |||||||
| Critical severe or severe | LYM (×109) | 0.176 | 0.102 | 2.958 | 0.085 | 1.192 | 0.976 | 1.456 |
| LYM% (%) | −4.84 | 2.211 | 4.793 | 0.029 | 0.008 | 0 | 0.602 | |
| Cys C (mg/L) | −2.808 | 2.566 | 1.198 | 0.274 | 0.06 | 0 | 9.219 | |
| UA (μmol/L) | 0.005 | 0.005 | 0.816 | 0.366 | 1.005 | 0.994 | 1.015 | |
| CK‐MB (U/L) | −0.162 | 0.14 | 1.354 | 0.245 | 0.85 | 0.647 | 1.118 | |
| LDH (U/L) | 0.102 | 0.041 | 6.178 | 0.013 | 1.107 | 1.022 | 1.199 | |
| Mb (ng/L) | 0.038 | 0.034 | 1.231 | 0.267 | 1.039 | 0.971 | 1.111 | |
| T‐BIL (μmol/L) | ||||||||
| Normal | −15.283 | 0 | 2.31 × 10−9 | 2.31 × 10−9 | 2.31 × 10−9 | |||
| Elevated | 0 | |||||||
| Mb | ||||||||
| Normal | 1.098 | 2.592 | 0.18 | 0.672 | 3 | 0.019 | 482.365 | |
| Elevated | 0 | |||||||
| ESR | ||||||||
| Normal | −4.162 | 1.768 | 5.545 | 0.019 | 0.016 | 0 | 0.498 | |
| Elevated | 0 | |||||||
Reference:Mild group.
ROC curve was drawn, and we calculated the AUC of CK‐MB (0.286, p = 0.007), LDH (0.158, p < 0.001), and normal T‐BIL (0.420, p = 0.307), which were used to predict for moderate COVID‐19 on admission. And the AUC of combined indicators containing CK‐MB, LDH, and normal T‐BIL was 0.687 (p = 0.017), which could not predict the severity of COVID‐19 well. The AUC of combined indicators including LYM, LDH, and normal ESR was 0.860 (p < 0.001) when predicting for severe or critical severe patients, and it could better predict the severity of disease (Figure S1, Tables S6,S7.
3.3. Analysis of the most abnormal laboratory indicators during admission among different groups
The most abnormal indicators with statistical significance during admission are shown in Table 4, and more details are shown in supplements (Tables S8–S12). The levels of LYM, NEU%, LYM%, albumin, ALT, and LDH have statistical significance among three groups. Proportion of cases with decreased LYM and albumin, elevated NEU%, ALT, T‐BIL, and Mb also show statistical differences. We find that LYM and LYM% levels are much higher in mild patients than others [1.31(1.15, 1.75), p = 0.006; 33.00 ± 14.35, p = 0.007]. NEU% is statistically different among three groups with less in mild patients (54.94 ± 14.65, p = 0.009). This is the similar to our former analysis of indicators on admission. Albumin level has a significant difference among three groups with lower level in moderate and critical severe or severe patients. LDH level, proportion of cases with decreased LYM, decreased albumin, elevated NEU%, elevated ALT, and elevated Mb increase with aggravation of disease (p < 0.05) are significantly higher in critical severe or severe patients. Furthermore, ALT and elevated T‐BIL proportion are statistically different among groups (p = 0.003, p = 0.012 respectively). No difference in other indicators has been found.
TABLE 4.
Indicators with statistically significant differences between three groups via univariate analysis during admission
| Variable | Mild group | Moderate group | Critical severe or severe group | F/H | p |
|---|---|---|---|---|---|
| LYM (×109) | 1.31 (1.15, 1.75) | 0.95 (0.74, 1.33) | 1.01 (0.56, 1.39) | 10.258 | 0.006 |
| NEU% (%) | 54.94 ± 14.65 | 69.913 ± 12.8816 | 66.03 ± 20.12 | 5.021 | 0.009 |
| LYM% (%) | 33.00 ± 14.35 | 20.37 ± 10.00 | 23.43 ± 15.57 | 5.354 | 0.007 |
| Albumin (g/L) | 41.76 ± 5.85 | 39.08 ± 3.16 | 36.58 ± 3.87 | 8.106 | 0.001 |
| ALT (U/L) | 26.00 (25.40, 29.89) | 17.35 (13.64, 31.22) | 39.10 (23.93, 72.65) | 11.718 | 0.003 |
| LDH (U/L) | 158.60 (131.00, 213.33) | 179.80 (156.00, 213.33) | 195.20 (178.00, 239.80) | 6.100 | 0.047 |
| Elevated NEU% | 1 (5.6) | 10 (32.2) | 13 (52.0) | 10.163 | 0.006 |
| Decreased LYM | 3 (16.7) | 16 (51.6) | 15 (60.0) | 8.486 | 0.014 |
| Decreased Albumin | 6 (33.3) | 20 (64.5) | 20 (80.0) | 9.683 | 0.008 |
| Elevated ALT | 3 (16.7) | 6 (19.4) | 12 (48.0) | 7.095 | 0.029 |
| Elevated T‐BIL | 0 (0.0) | 9 (29.0) | 2 (8.0) | 8.869 | 0.012 |
| Elevated Mb | 1 (5.6) | 8 (25.8) | 15 (60.0) | 15.019 | 0.001 |
Based on the univariate analyses, collinearity diagnosis, and test of parallel lines, as well as consideration of professional evidence, we identified several variables that could be enrolled in further study (Table 5). After multivariate logistic regression, the most abnormal factors during admission for more serious disease were normal T‐BIL (OR 8.56 × 10−9; 95%CI 8.30 × 10−10–8.83 × 10−8; p < 0.001), LYM (OR 0.068; 95%CI 0.005–0.934; p = 0.044), albumin (OR 0.565; 95%CI 0.327–0.977; p = 0.041), and normal NEU% (OR 0.013; 95%CI 0.000–0.967; p = 0.048).
TABLE 5.
Multinomial logistic regression analysis of the most abnormal laboratory indicators
| Group a | Variable | B | SE | Wald | p | OR | OR 95%CI | |
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| Moderate | LYM (×109) | 0.023 | 1.062 | 9.825 | 0.983 | 1.024 | 0.128 | 8.202 |
| LYM% (%) | −0.086 | 0.054 | 0.084 | 0.114 | 0.918 | 0.825 | 1.021 | |
| Albumin (g/L) | −0.011 | 0.191 | 0 | 0.954 | 0.989 | 0.681 | 1.437 | |
| ALT (U/L) | 0.002 | 0.034 | 2.498 | 0.953 | 1.002 | 0.938 | 1.071 | |
| LDH (U/L) | −0.004 | 0.013 | 0.084 | 0.772 | 0.996 | 0.971 | 1.022 | |
| NEU% (%) | ||||||||
| Normal | −3.278 | 2.131 | 2.368 | 0.124 | 0.038 | 0.001 | 2.454 | |
| Elevated | 0 | – | – | – | – | – | – | |
| LYM (×109) | ||||||||
| Normal | −0.038 | 1.224 | 0.001 | 0.975 | 0.963 | 0.087 | 10.604 | |
| Decreased | 0 | – | – | – | – | – | – | |
| Albumin (g/L) | ||||||||
| Normal | −1.637 | 1.351 | 1.467 | 0.226 | 0.195 | 0.014 | 2.75 | |
| Decreased | 0 | – | – | – | – | – | – | |
| T‐BIL (μmol/L) | ||||||||
| Normal | −18.576 | 1.19 | 243.523 | 0.000 | 8.56 × 10−9 | 8.30 × 10−10 | 8.83 × 10−8 | |
| Elevated | 0 | – | – | – | – | – | – | |
| ALT (U/L) | ||||||||
| Normal | −1.046 | 1.644 | 0.405 | 0.524 | 0.351 | 0.014 | 8.803 | |
| Elevated | 0 | – | – | – | – | – | – | |
| Mb (U/L) | ||||||||
| Normal | −0.882 | 1.383 | 0.407 | 0.524 | 0.414 | 0.028 | 6.228 | |
| Elevated | 0 | – | – | – | – | – | – | |
| Critical severe or severe | LYM (×109) | −2.686 | 1.336 | 4.045 | 0.044 | 0.068 | 0.005 | 0.934 |
| LYM% (%) | 0.133 | 0.071 | 3.483 | 0.062 | 1.143 | 0.993 | 1.315 | |
| Albumin (g/L) | −0.571 | 0.279 | 4.176 | 0.041 | 0.565 | 0.327 | 0.977 | |
| ALT (U/L) | 0.011 | 0.034 | 0.112 | 0.738 | 1.011 | 0.947 | 1.08 | |
| LDH (U/L) | −0.005 | 0.014 | 0.143 | 0.706 | 0.995 | 0.969 | 1.022 | |
| NEU% (%) | ||||||||
| Normal | −4.311 | 2.183 | 3.901 | 0.048 | 0.013 | 0.000 | 0.967 | |
| Elevated | 0 | – | – | – | – | – | – | |
| LYM (×109) | ||||||||
| Normal | 0.19 | 1.356 | 0.02 | 0.889 | 1.209 | 0.085 | 17.237 | |
| Elevated | 0 | – | – | – | – | – | – | |
| Albumin (g/L) | ||||||||
| Normal | 0.189 | 1.606 | 0.014 | 0.906 | 1.208 | 0.052 | 28.148 | |
| Elevated | 0 | – | – | – | – | – | – | |
| T‐BIL (μmol/L) | ||||||||
| Normal | −18.904 | 0 | 6.168 × 10−9 | 6.168 × 10−9 | 6.168 × 10−9 | |||
| Elevated | 0 | – | – | – | – | – | – | |
| ALT (U/L) | ||||||||
| Normal | −2.311 | 1.788 | 1.67 | 0.196 | 0.099 | 0.003 | 3.299 | |
| Elevated | 0 | – | – | – | – | – | – | |
| Mb (U/L) | ||||||||
| Normal | −2.12 | 1.346 | 2.482 | 0.115 | 0.12 | 0.009 | 1.678 | |
| Elevated | 0 | – | – | – | – | – | – | |
Reference:Mild group.
According to the ROC curve and AUC results during hospitalization, the AUC of LYM, albumin, normal NEU% that were used to predict the severe or critical severe COVID‐19 was 0.739 (p = 0.007), 0.684 (p = 0.002), and 0.322(p = 0.002), respectively. And the AUC of combined indicators containing CK‐MB, LDH, and normal T‐BIL was 0.750 (p = 0.024), which could better predict the more severe COVID‐19 (Figure S2, Tables S13,S14.
4. DISCUSSION
In this single‐center retrospective study, we found that several laboratory indicators were associated with the severity of COVID‐19 disease. LYM% (OR 0.008; 95%CI 0–0.602; p = 0.029), CK‐MB (OR 0.674; 95%CI 0.489–0.928; p = 0.016), LDH (OR 1.111 or 1.107; 95%CI 1.026–1.204 or 1.022–1.199; p = 0.009 or 0.013), normal T‐BIL (OR 4.58 × 10−8; 95%CI 3.05 × 10−9–6.88 × 10−7; p < 0.001), and normal ESR (OR 0.016; 95%CI 0–0.498; p = 0.019) are independent risk factors for severity when confirmed patients on admission, while LYM (OR 0.068; 95%CI 0.005–0.934; p = 0.044), NEU% (OR 0.013; 95%CI 0.000–0.967; p = 0.048), normal T‐BIL (OR 8.56 × 10−9; 95%CI 8.30 × 10−10–8.83 × 10−8; p < 0.001), and albumin (OR 0.565; 95%CI 0.327–0.977; p = 0.041) are factors during hospitalization. Overall, blood routine, liver, cardiac, and coagulation function indexes could reflect the condition of patients in the present series.
Clinical and epidemiological features of COVID‐19 patients have already been reported, but existing studies comprehensively discussing the hematologic parameters were not sufficient enough. Thus, we detailed the laboratory findings to investigate indicators in different patients including 18 mild cases, 31 moderate cases, and 25 critical severe or severe cases. Among baseline characteristics, we found differences in anhelation (p = 0.036) and length of stay (p < 0.001) between groups. A study conducted in Tongji Hospital found that severe cases more frequently had dyspnea and tachypnea, which is consistent with our study. 11 Mild patients manifested lighter clinical symptoms, having the shortest hospitalization time of median 9 days. It is rational that more seriously ill cases need longer length of stay for treatment.
Routine blood tests for COVID‐19 patients have been described in the previous studies, mostly consistent with our study. Guang Chen et al. 11 analyzed patients with different severity and found decrease in lymphocyte was more common in severe cases than in moderate cases. An early research conducted in Jin Yin‐tan Hospital showed leukopenia and lymphopenia in confirmed COVID‐19 patients. 6 Other studies found that lymphocyte decreasing occurred while WBC counts remained normal or not. 16 , 17 In a logistic regression analysis, peripheral blood lymphocyte count <0.8 × 109/L was found to be a risk factor for mortality of pneumonia patients. 18 Similarly, in our univariate analyses whether on admission or during hospitalization, we found that LYM and LYM% level went lower with a more serious illness situation. But the WBC counts in our study were in a normal range (Table S1,S8). According to laboratory findings, LYM level in most patients was reduced, which is result from the possible role of ACE in hematopoiesis regulating. 19 Angiotensin‐converting enzyme 2 (ACE2) is able to bind with the receptor‐binding domain of SARS‐CoV‐2 spike protein. 20 , 21 Researchers proposed that COVID‐19 could lead to ACE2 reduction and increasing ACE expression in myeloid precursors, then worsen immune response and T‐cell consumption. 17
We found difference in the most abnormal NEU% level among groups (p = 0.009), and elevated NEU% proportion was higher in severe cases. Multivariate logistic regression showed that normal NEU% was an independent influence factor for disease severity (OR 0.013; 95%CI 0.000–0.967; p = 0.048). This is consistent with other studies. Huang et al. 6 reported that elevated NEU% was more frequent with the disease progression. Neutrophil counts were significantly higher in severe cases than moderate cases. 22 One systematic review described that neutrophil counts could be used as a predictor for severe COVID‐19. 23 Furthermore, leukocytosis and neutrophilia are hallmarks of acute infection, and lung infiltration of neutrophils was found in patient succumbed to COVID‐19. 24 These neutrophil changes may due to the infiltration mechanistically regulated by ACE2. 25 Researchers observed that ACE2 negatively regulates AngII, thus increasing vascular permeability, lung edema, and neutrophil infiltration. 26
Liver injury is frequent in COVID‐19 patients and manifest various degrees of function abnormalities. 27 In the present analysis, we found albumin and elevated T‐BIL during hospitalization to be independent influence factors for disease severity. Mild patients had higher albumin, and it went lower with the aggravation of infection. Moderate and severe cases showed more proportion of elevated T‐BIL than mild group. This is supported by the study by Qingxian Cai et al., 28 who found cases with abnormal liver tests were at higher risk of progressing to severe pneumonia. They mentioned that drug usage was the most important influence factor for liver damage after admission. Another study reported that using serum albumin might be helpful to identify patients at higher risk of death. Hypoalbuminemia is a feature of acute and chronic inflammation, and albumin may protect against the cytokine storm and anticoagulant, thus giving a possible explanation for this relationship. 29 Yang et al. 30 reported that SARS‐CoV could cause direct cytopathic liver injury which means direct viral effect on organ. One study showed that SARS‐CoV‐2 might directly bind to ACE2‐positive cholangiocytes and cause liver damage. 27 However, we did not find other obvious liver indicator changes like other studies, including GGT and zymogram. 31 We only found that ALT level was statistically significant in univariate analysis but generally remained in a normal range. Possible explanation is that the participants were slightly different among researches. Some of them analyzed deceased or ICU cases, while in our study we chose the current cases. Besides, various sample sizes, pre‐existing diseases and drugs could also be the possible reasons.
The LDH level when on and during admission elevated with the severity increasing, and critical severe or severe patients had the highest results. Logistic regression analysis indicated that LDH was a relevant risk factor for infection severity. Similarly, Guang Chen et al. 11 found LDH was significantly higher in more severe cases. Davide Ferrari et al. 32 analyzed 207 emergency participants and found a strong association for LDH with pandemic. Another study revealed that most COVID‐19 patients had abnormal myocardial zymogram, 13% cases with elevation of creatine kinase and 76% LDH. 5 In our univariate analyses, both CK‐MB and LDH were statistically different among groups and had the same increasing trend when disease worsen. But later multivariate analysis showed opposite trend between CK‐MB (OR = 0.674) and LDH (OR > 1). Taking that 13% vs. 76% elevation into consideration, LDH seems to be a more credible indicator. We speculated that this contradictory result emerged when the two factors discussed from respectively to simultaneously, possibly owing to statistics and it was only analyzed theoretically. Anyway, COVID‐19 patients have a high prevalence of cardiovascular disease. Over 7% of them experience myocardial injury from the infection. 33 ACE2 is highly expressed in the heart as well, making it possible to be directly affected by the coronavirus. Cardiac damage can also result from the associated cytokine storm manifested by elevated LDH. 34 However, when using LDH as a reminding factor for disease severity, its organ specificity should be taken into consideration and exclude other organ damage first.
We found that ESR elevation on admission is possibly related to the severity and normal ESR is an independent influence factor (OR 0.016; 95%CI 0–0.498; p = 0.019) for disease progression. It was predictor of death and associated with thrombosis in another study. Interestingly, we did not observe differences in other coagulation and inflammation indicators among groups including D‐dimer, which has been reported frequently. Al‐Samkari et al. 35 found that elevations in D‐dimer on admission predicted critical illness. Other studies showed significant D‐dimer elevation in more serious patients, and ICU patients had higher D‐dimer level on admission than non‐ICU. 6 , 11 Our analysis of D‐dimer may not be accurate enough since participants enrolled in the present study performed few times of coagulation tests, thus lacking more available data. Further data collection should be conducted. Lippi et al. 36 summarized main laboratory abnormalities in patients with aggravation of COVID‐19, including increased NEU, LDH, T‐BIL, and decreased LYM, albumin, which are consistent with the present study. We did not find any statistical significance in renal function indicators among different severity cases.
As for the AUC results for those two logistic models, when predicting for critical severe or severe patients, combined indicators worked better (AUC > 0.700). Respectively, the AUC was 0.860 (LYM, LDH, and normal ESR on admission) and 0.750 (CK‐MB, LDH, and normal T‐BIL during hospitalization). However, when analyzing in moderate patients comparing with mild group, the AUC was not very satisfactory. We speculated that the different key points of clinical classification for disease severity might be the causes. In the current study, according to the National Health Commission of China, classification for mild group was mild clinical symptoms and no sign of pneumonia on imaging. Moderate cases showed fever and respiratory symptoms with imaging findings. But critical severe or severe group had more serious manifestations such as respiratory distress even requiring mechanical ventilation, organ dysfunctions, and so on. Thus, the difference between moderate and mild groups may not be as great as that between more severe group and mild group. Improving the group design and maximizing the difference might be helpful to the further optimization of the prediction results.
Longitudinal evaluation of two analyses conducted on the first‐time examination when admitted and the most abnormal findings during hospitalization; indicators including CK‐MB, LDH, and normal ESR lost their status as influence factors with disease severity in the later analysis, while albumin and normal NEU% emerged predictive value. Considering many aspects of factors, possible explanations are listed.
First of all, participants were not treated when admitted and severity of disease was evaluated according to clinical manifestations after diagnosis. After that, all patients received appropriate treatment. The purpose of analyzing the most abnormal findings is to make a comprehensive prediction under a circumstance of disease development and treatment intervention. Therefore, this circumstance may lead to the predictive value change in those indicators.
Second, adjusted therapies for different clinical classified cases may result in different curative effects. According to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia from National Health Commission of China, there are general treatment, treatment of severe and critical cases, and traditional Chinese medicine treatment for different classified patients. 37 The effectiveness varies and then reflect on those indicators.
The characteristics of the indicators and statistical analysis are also potential causes. Detailed discussion is as follows:
After univariate analyses, CK‐MB and normal ESR were enrolled in multivariate logistic regression model on admission but were not enrolled when analyzed during hospitalization. CK‐MB [on admission: 8.10 (6.25, 10.85), 10.10 (7.80, 11.70), 13.00 (10.85, 17.85), p < 0.001 vs during hospitalization: 8.65 (4.58, 12.86), 10.70 (8.30, 12.64), 11.00 (9.75, 15.10), p = 0.295, mild group, moderate group, and critical severe or severe group respectively] and elevated ESR [on admission: 12 (66.7), 17 (54.8), 22 (88.0), p = 0.029 vs during hospitalization: 12 (66.7), 18 (58.1), 17 (68.0), p = 0.711] showed statistical significance among three groups in univariate analyses when on admission and finally were assessed as influence factors. Possible explanation for the changes may involve sample size or potential information bias, and clinical treatment, especially for severe patients. More data should be collected further to credibly discuss the clinical significance of indicators for COVID‐19 severity.
Clinical manifestations of COVID‐19 will surge at 7–14 days from the onset of disease symptoms. 38 Mild participants were diagnosed in time at the initial stage and then admitted to hospital. Their LDH level could elevate with the progression of disease during hospitalization and become higher than the first‐time laboratory findings. Besides, LDH level of more severe patients could remain higher since the coronavirus affects many essential organs. LDH presents in major organs and could be abnormal in many disorders. 39 , 40 Thus, comprehensively considering the periods of disease, treatment, and non‐specificity of the indicator, difference in LDH activity among three groups could be narrower during hospitalization [158.60 (131.00, 213.33), 179.80 (156.00, 213.33), 195.20 (178.00, 239.80), p = 0.047, mild group, moderate group, and critical severe or severe group, respectively] than on admission [131.00 (123.68, 154.65), 179.80 (156.30, 221.30), 200.70 (174.70, 241.25), p < 0.001], then could not serve as an influence factor for severity. LDH isozyme analyses may reflect specific organ effects better. The normalization of the predictive value of LDH may reflect the further organ damages or effective treatment.
Albumin and normal NEU% emerged as influence factors when analyzed during hospitalization. Possible explanation for the change in albumin may involve the usage of drugs. Antibiotics, interferon, herbal medications, and so on could induce liver injury during treatment. 28 Severe patients usually required more treatment, causing the albumin level to worsen than when untreated. Difference among groups became more obvious and significant. As for normal NEU%, peripheral blood lymphocytes progressively decrease in severe COVID‐19 cases, 41 making NEU% relatively elevate. NEU counts could also be hallmark of acute infection and higher during progression of disease. 22 , 24 Therefore, the emergence of the predictive value of albumin and normal NEU% may indicate the liver injury, medication, or progression of lymphocytopenia.
Our study has several limitations. Considering its retrospective design, possible information bias was inherently present in this single‐center study. Moreover, only laboratory tests included may not represent the patient condition properly, and clinical manifestations and radiologic images also could be involved to identify disease severity. And because of the small sample size, we need to further collect more available data to investigate the association between laboratory indexes and disease severity among different patients.
5. CONCLUSION
In conclusion, to predict the progression of disease severity and take measures promptly, when the patients on admission, we should be cautious of the blood routine and biochemical indicators especially CK‐MB, LDH, LYM%, T‐BIL, and ESR. Elevation of LDH and T‐BIL may be the independent risk factors for aggravation while less reduction of LYM% and more normal ESR could be protective. The exact role of CK‐MB in our study remained uncertain but it deserves attention. With the process of disease, we should continually be cautious of the blood routine and biochemical indicators especially T‐BIL, LYM, albumin, and NEU%. Clinicians should rise awareness of disease severity when LYM and albumin decreasing or T‐BIL and NEU% becoming abnormal.
CONFLICT OF INTEREST
The authors declare no conflict of interest or personal relationships that could have appeared to influence the work reported in this paper.
AUTHOR CONTRIBUTIONS
J. Zhang involved in conception, design, or planning of the study; J. Zhang, S. Chen, and T. Li involved in acquisition of the data; X. Shang, S. Chen, and H. Liu curated and analyzed the data; X. Shang and H. Liu drafted the manuscript; J. Zhang critically reviewed and edited the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We extend our gratitude to the colleagues in the department of laboratory medicine, Yueyang First People’s Hospital, Yueyang, Hunan, China, for data collection.
Haiting Liu and Xueling Shang contributed equally to this work.
Funding information
This work was supported by the Natural Science Foundation of Hunan Province under Grant number 2019JJ50909; the Fundamental Research Funds for the Innovation Project of Central South University of China under Grant number 2019zzts829 and the Fundamental Research Funds for the Universities of Hunan Province under Grant number 1021‐0001017106
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Yueyang First People's Hospital, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Yueyang First People's Hospital.
REFERENCES
- 1. Wu F, Zhao SU, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect Dis. 2020;20(6):669‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986‐1994. [DOI] [PubMed] [Google Scholar]
- 4. Assiri A, Al‐Tawfiq JA, Al‐Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20(6):689‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 COVID‐19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71(16):2089‐2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang Y, Shen C, Li J, et al. Plasma IP‐10 and MCP‐3 levels are highly associated with disease severity and predict the progression of COVID‐19. J Allergy Clin Immunol. 2020;146(1):119‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bloom PP, Meyerowitz EA, Reinus Z, et al. Liver biochemistries in hospitalized patients with COVID‐19. Hepatology. 2020.73(3):890–900. [DOI] [PubMed] [Google Scholar]
- 11. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu T, Zhang J, Yang Y, et al. The role of interleukin‐6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12(7):e12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liang W‐H, Guan W‐J, Li C‐C, et al. Clinical characteristics and outcomes of hospitalised patients with COVID‐19 treated in Hubei (epicenter) and outside Hubei (non‐epicenter): a Nationwide Analysis of China. Eur Respir J. 2020;55(6):2000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95(6):E131‐E134. [DOI] [PubMed] [Google Scholar]
- 17. Liu X, Zhang R, He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann Hematol. 2020;99(7):1421‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haznedaroglu IC, Beyazit Y. Local bone marrow renin‐angiotensin system in primitive, definitive and neoplastic haematopoiesis. Clin Sci. 2013;124(5):307‐323. [DOI] [PubMed] [Google Scholar]
- 20. Khan S, Siddique R, Shereen MA, et al. Emergence of a novel coronavirus, severe acute respiratory syndrome coronavirus 2: biology and therapeutic options. J Clin Microbiol. 2020;58(5):e00187‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu J, Zhao S, Teng T, et al. Systematic comparison of two animal‐to‐human transmitted human coronaviruses: SARS‐CoV‐2 and SARS‐CoV. Viruses. 2020;12(2):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J, Liu Y, Xiang P, et al. Neutrophil‐to‐lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid‐19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnes BJ, Adrover JM, Baxter‐Stoltzfus A, et al. Targeting potential drivers of COVID‐19: Neutrophil extracellular traps. J Exp Med. 2020;217(6):e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomar B, Anders H‐J, Desai J, Mulay SR. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID‐19. Cells. 2020;9(6):1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alqahtani SA, Schattenberg JM. Liver injury in COVID‐19: the current evidence. United European Gastroenterol J. 2020;8(5):509‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai Q, Huang D, Yu H, et al. COVID‐19: abnormal liver function tests. J Hepatol. 2020;73(3):566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Violi F, Cangemi R, Romiti GF, et al. Is albumin predictor of mortality in COVID‐19? Antioxid Redox Signal. 2020. 10.1089/ars.2020.8142. [DOI] [PubMed] [Google Scholar]
- 30. Yang Z, Xu M, Yi J‐Q, Jia W‐D. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. HBPD INT. 2005;4(1):60‐63. [PubMed] [Google Scholar]
- 31. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferrari D, Motta A, Strollo M, Banfi G, Locatelli M. Routine blood tests as a potential diagnostic tool for COVID‐19. Clin Chem Lab Med. 2020;58(7):1095‐1099. [DOI] [PubMed] [Google Scholar]
- 33. Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID‐19 and cardiovascular disease. Circulation. 2020;141(20):1648‐1655. [DOI] [PubMed] [Google Scholar]
- 34. Tikellis C, Thomas MC. Angiotensin‐Converting Enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012;2012:256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Al‐Samkari H, Karp Leaf RS, Dzik WH, et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood. 2020;136(4):489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lippi G, Plebani M. Laboratory abnormalities in patients with COVID‐2019 infection. Clin Chem Lab Med. 2020;58(7):1131‐1134. [DOI] [PubMed] [Google Scholar]
- 37. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7). Chin Med J. 2020;133(9):1087‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020;95(7):834‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khan AA, Allemailem KS, Alhumaydhi FA, Gowder SJT, Rahmani AH. The biochemical and clinical perspectives of lactate dehydrogenase: an enzyme of active metabolism. Endocr Metab Immune Disord Drug Targets. 2020;20(6):855‐868. [DOI] [PubMed] [Google Scholar]
- 40. Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen‐Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9(8):1736‐1742. [DOI] [PubMed] [Google Scholar]
- 41. Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID‐19 disease progression. Crit Rev Clin Lab Sci. 2020;57(6):389‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from Yueyang First People's Hospital, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Yueyang First People's Hospital.


