Abstract
Background
There is still a lack of tools to assess the prognosis of ischemic stroke patients induced by hypertension. In this study, we built a novel prognostic assessment model for ischemic stroke in the Chinese hypertensive population.
Methods
Mass spectrometry technique was used to analyze the changes in serum protein profiles of hypertensive patients with ischemic stroke. A total of 314 hypertensive patients were divided into the testing group (206 patients) and the validation group (108 patients).
Results
Compared with hypertensive patients without ischemic stroke, serum cytotoxic T lymphocyte‐associated antigen‐4 (CTLA‐4), ischemia‐modified albumin (IMA), lipoprotein‐associated phospholipase A2 (Lp‐PLA2), glial fibrillary acidic protein (GFAP), and homocysteine (HCY) levels were significantly increased among hypertensive patients with ischemic stroke (p < 0.05). Then, we built a novel prognostic assessment model for hypertensive patients with ischemic stroke [Logit(P) = 29.172–1.088*CTLA‐4–0.952*IMA‐0.537*Lp‐PLA2 −0.066*GFAP −0.149*HCY]. It showed higher efficiency (AUC = 0.981, sensitivity = 95.5%, specificity = 93.8%) than any single marker. The estimated probability was 0.739, which means if higher than 0.739, it was classified into poor prognosis. Compared with the estimated probability ≤0.739 group, the survival rate of hypertensive patients with ischemic stroke in the estimated probability >0.739 group was significantly decreased (χ 2 = 40.001, p < 0.001). In the validation group, our novel prognostic assessment model still showed good efficiency (AUC = 0.969, sensitivity = 89.4%, specificity = 92.5%; χ 2 = 47.551, p < 0.001).
Conclusion
Current novel prognostic assessment model we have built is of great value in the prognostic evaluation for ischemic stroke in the Chinese hypertensive population.
Keywords: hypertension, inflammation, ischemic stroke, mass spectrometry, prognosis
Mass spectrometry technique was used to analyze the changes in serum protein profiles of hypertensive patients with ischemic stroke. A total of 314 hypertensive patients were divided into the testing group (206 patients) and the validation group (108 patients). Compared with hypertensive patients without ischemic stroke, serum cytotoxic T lymphocyte‐associated antigen‐4 (CTLA‐4), ischemia‐modified albumin (IMA), lipoprotein‐associated phospholipase A2 (Lp‐PLA2), glial fibrillary acidic protein [GFAP], and homocysteine (HCY) levels were significantly increased among hypertensive patients with ischemic stroke (p < 0.05). Then, we built a novel prognostic assessment model for hypertensive patients with ischemic stroke [Logit(P) =29.172–1.088*CTLA‐4–0.952*IMA‐0.537*Lp‐PLA2 −0.066*GFAP −0.149*HCY]. It showed higher efficiency (AUC = 0.981, sensitivity = 95.5%, specificity = 93.8%) than any single marker. The estimated probability was 0.739, which means if higher than 0.739, it was classified into poor prognosis. Compared with estimated probability ≤0.739 group, the survival rate of hypertensive patients with ischemic stroke in the estimated probability >0.739 group was significantly decreased (χ 2 = 40.001, p < 0.001). In the validation group, our novel prognostic assessment model still showed good efficiency (AUC = 0.969, sensitivity = 89.4%, specificity = 92.5%; χ 2 = 47.551, p < 0.001).
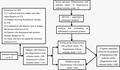
1. INTRODUCTION
With the aging of the population structure, the proportion of hypertension has increased substantially. According to relevant epidemiological data, the prevalence of hypertension in elderly Chinese is as high as 40%–60%. 1 If the blood pressure remains high for a long time, it can further cause irreversible damage to the heart, brain, kidney, and other organs, and will seriously threaten the health of the elderly population. 2 Hypertension is an independent risk factor for atherosclerosis, which can lead to stenosis of the vascular lumen and further cause complications such as coronary heart disease and thrombosis. 3 , 4 Therefore, monitoring the vascular status of hypertensive patients is conducive to the early detection and timely intervention of atherosclerosis, and is of great significance for reducing the complications of hypertension.
In recent years, the oxidative damage and abnormal lipid metabolism of hypertension have been a hot research topic. 5 , 6 It involves a variety of oxidative stress including lymphocyte DNA damage, myeloperoxidase, etc., which induces an inflammatory response, resulting in damage to various organs. 5 , 6 Among them, ischemic stroke is one of the more common complications of hypertension. 7 A series of pathological factors such as atherosclerosis causing arterial occlusion, body inflammatory response leading to cerebral vascular embolism, and brain damage leading to patients’ dysfunction have accelerated the occurrence and development of ischemic stroke. 8 , 9 , 10 At present, it is believed that the inflammation induced by hypertension can promote the damage of vascular endothelial cells, and then accelerate the deposition of atherosclerotic plaque on the surface of injured endothelial cells, and accelerate the formation of ischemic stroke. 11 , 12 Proteomics technology has explored ischemic stroke‐related proteins from a holistic level, which brings new opportunities for the treatment of ischemic stroke. 13 However, the current proteomics research on hypertension focuses on basic research, and there are still few studies on clinical interventions in the treatment of ischemic stroke in the hypertensive populations. 14 In particular, there has been no report on the clinical research related to proteomics technology of ischemic stroke in a hypertensive population. Moreover, there is still a lack of tools to assess the prognosis of ischemic stroke patients induced by hypertension. In the current study, we built a novel prognostic assessment model for ischemic stroke in the Chinese hypertensive population.
In this study, we included a total of 314 hypertensive patients through two groups (206 testing group and 108 validation group), including 200 hypertensive population with ischemic stroke (92 testing group and 108 validation group). First, 4D label‐free quantitative method‐diaPASEF (4DLFQ) was used to find differentially expressed protein molecules from the serum of 5 hypertensive patients with ischemic stroke and 5 hypertensive patients without ischemic stroke. Second, a logistic regression analysis was used to establish a prognostic model for ischemic stroke in the Chinese hypertensive population in the testing group. Finally, we further verify the established prediction model in the validation group.
Our results showed that eight differential proteins were screened through the 4DLFQ platform, including cytotoxic T lymphocyte‐associated antigen‐4 (CTLA‐4), lipoprotein‐associated phospholipase A2 (Lp‐PLA2), homocysteine (HCY), macrophage inflammatory protein (MIP), ischemia‐modified albumin (IMA), glial fibrillary acidic protein (GFAP), interleukin‐2 (IL‐2), and interleukin‐2 (IL‐2). Compared with hypertensive patients without ischemic stroke, serum CTLA‐4, IMA, Lp‐PLA2, (GFAP), and HCY levels were significantly increased among hypertensive patients with ischemic stroke. Then, we built a novel prognostic assessment model for hypertensive patients with ischemic stroke, and its estimated probability was 0.739, which means if higher than 0.739, it was classified into poor prognosis. Compared with the estimated probability ≤0.739 group, the survival rate of hypertensive patients with ischemic stroke in the estimated probability >0.739 group was significantly decreased. Besides, our findings were further confirmed in the validation group.
2. MATERIAL AND METHODS
2.1. Inclusion of subjects in the testing and validation groups
From September 2016 to February 2019, a total of 314 patients with hypertension were consecutively selected from the intensive care unit (ICU, 206 patients in the testing group) and the neurointensive care unit (NICU, 108 patients in the validation group) of Suzhou Kowloon Hospital Affiliated Shanghai Jiao Tong University. Moreover, we also included 5 hypertensive patients with ischemic stroke and 5 hypertensive patients without ischemic stroke for mass spectrometry analysis. Besides, we collected 100 healthy controls. There was no statistical difference between the healthy control group and the hypertension group in age and gender. The two groups are comparable. The exclusion criteria in this study were as follows: acute or chronic renal dialysis; malignant neoplasms; acute or chronic inflammatory diseases; angina or other cardiovascular diseases.
According to the Declaration of Helsinki, all subjects voluntarily signed an informed consent form. The project was approved by the Ethics Committee of Suzhou Kowloon Hospital Affiliated Shanghai Jiao Tong University (Ethics number: KS20160085).
2.2. Six‐month follow‐up of hypertensive patients with ischemic stroke
According to the follow‐up of hospitalized medical records, patients with hypertension who had an ischemic stroke during the treatment period were followed up for 6 months. The number of deaths and the time of death of patients with hypertension combined with ischemic stroke in 6 months were counted.
2.3. Collection of baseline data and serum samples
Collect basic patient information, including age, gender, course of the disease, smoking history, and body mass index (BMI). 5 mL of venous blood (over 8–10 h on an empty stomach) within 24 hours after admission of the hypertensive patients was collected using a gel tube containing separation. The gel tube was centrifuged at 3000× g for 5 minutes and serum was separated.
2.4. Quantitative proteomics analysis
The basic principle of quantitative proteomics is mainly to analyze the physical and chemical properties of the measured sample ions, and qualitative and quantitative results can be obtained based on the sample mass spectrum and related information. Mass spectrometry will identify and quantify peptide ions according to the four dimensions of retention time, the mass‐to‐charge ratio (m/z), ionic intensity, and ion mobility. The 4DLFQ quantitative proteomics analysis technology is provided by Hangzhou Jingjie Biotechnology Co., Ltd. The two‐tailed t test method was used to calculate the p‐value. When p‐value <0.05, the change of the differential expression amount exceeding 2 was used as the significant upregulation change threshold. 15
2.5. Detection methods of serum protein markers
Serum CTLA‐4 was detected by double‐antibody sandwich ABC‐ELISA method (Wuhan Boster Bioengineering Co., Ltd, Lot number: 200428), coated with anti‐human CTLA‐4 monoclonal antibody (Acris Antibodies GmbH Cat# PP1117P1, RRID: AB_975040) on the ELISA plate. CTLA‐4 in the standard and the sample was combined with the monoclonal antibody, and biotinylated anti‐human CTLA‐4 forms an immune complex and is connected to the plate. The secondary antibody labeled with horseradish peroxidase is combined with biotin and added with a substrate for color development. The optical density (OD) value was detected at 450 nm, and the CTLA‐4 concentration in the specimen was determined by the standard curve. Serum GFAP (Lot number: XY15264‐1), IL‐1α (Lot number: YY‐76253), and IL‐2 (Lot number: BS20044) were detected by Swiss Roche automatic biochemical analyzer E411 (Switzerland). MIP (Lot number: EWC101) was tested by Mindray Blood Cell Analyzer BC‐5000 (China). Serum IMA (Lot number: XY‐37111), Lp‐PLA2 (Lot number: XY‐00191), and HCY (Lot number: XY‐15491) were tested by Olympus AU5400 (Japan). The coefficient of variation of CTLA‐4, Lp‐PLA2, HCY, MIP, IMA, GFAP, IL‐2, and IL‐1α were 1.8%, 2.4%, 1.3%, 2.7%, 3.2%, 1.4%, 4.3%, and 1.2%, respectively. The coefficient of variation of each indicator is less than 5%, suggesting that the detection results of various indicators are reliable.
2.6. Statistical analysis
The statistical analysis was performed using SPSS version 22.0 (SPSSInc) and Stata version 13.0 (Stata Corporation). Two‐tailed significance values were applied, and statistical significance was defined as p < 0.05. The formula of the hypertensive patients with ischemic stroke regression model is: Logit (P) = ln[p/(1 − p)]. The “p” means the estimated probability. Normal distribution data are expressed as mean ± SD, and t test was used for comparison between groups. Count data is expressed as a percentage, and the chi‐square test is used for comparison between groups. Receiver operating characteristic (ROC) curves explore the predictive value of serum IMA, GFAP, HCY, Lp‐PLA2, and CTLA‐4 for the hypertensive populations with ischemic stroke. The predictive accuracy of each protein marker was assessed by calculation of the area under the curve (AUC). The survival curve explored the correlation between serum IMA, GFAP, HCY, Lp‐PLA2, and CTLA‐4 and the prognosis of the hypertensive population with ischemic stroke.
3. RESULTS
3.1. Technical route
From September 2016 to February 2019, we identified 417 subjects at the Suzhou Kowloon Hospital Affiliated Shanghai Jiao Tong University. After analyzing the subject's medical records, 103 subjects were excluded, including 35 that combined with liver, kidney, and other organ diseases, 3 that complicated with diseases such as tumors and serious infections, 19 that received thrombolytic therapy, 5 that combined with rheumatoid and systemic immune diseases, and 41 that did not sign the informed consent. Finally, 314 subjects including 200 hypertension combined with ischemic stroke and 114 subjects of hypertension without ischemic stroke were enrolled in our current study. Besides, 5 hypertensive patients with ischemic stroke and 5 hypertensive patients without ischemic stroke were also included for mass spectrometry analysis (Figure S1).
3.2. Analysis of the differentially expressed proteins by 4DLFQ mass spectrometry
The 4DLFQ platform was performed in this study. Five hypertensive patients with ischemic stroke and 5 hypertensive patients without ischemic stroke were subjected to a rustic analysis to quantify the proteome of the patient's serums. The two‐sample two‐tailed t test method was used to calculate p‐value. When p‐value <0.05, the change in differential expression was more than 2 as a significant upregulation change threshold, and <1/2 was a significantly downregulation change threshold (Figure 1A). We found that compared with hypertensive patients without ischemic stroke, the serum samples of hypertensive patients with ischemic stroke had 197 upregulated proteins and 132 downregulated proteins (Figure 1B). In this study, we included proteins whose differential expression levels changed by more than 4 or less than 1/4 for subsequent analysis. Finally, we included 8 proteins, including CTLA‐4, Lp‐PLA2, HCY, MIP, IMA, GFAP, IL‐2, and IL‐1α (Figure 1C).
FIGURE 1.
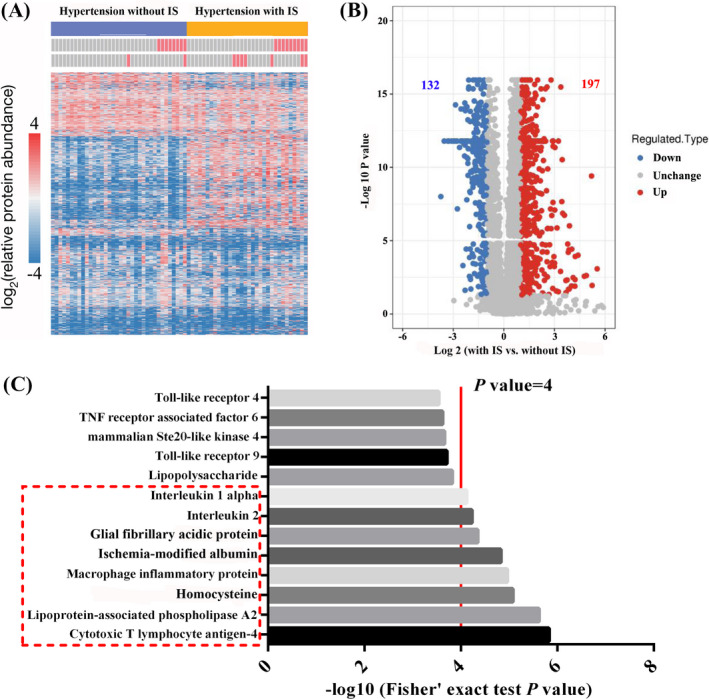
4DLFQ mass spectrometry and differential protein prediction. A and B, Results of 4DLFQ mass spectrometry between hypertensive patients with/without ischemic stroke. C, Proteins whose differential expression levels changed by more than 4 were included
3.3. Verification of differentially expressed proteins between hypertensive patients with or without ischemic stroke in the testing group
First, we compared the levels of CTLA‐4, Lp‐PLA2, HCY, MIP, IMA, GFAP, IL‐2, and IL‐1α between the testing group and the healthy control group (Figure S2). Compared with the healthy controls, serum IMA, GFAP, HCY, Lp‐PLA2, and CTLA‐4 levels were significantly increased among hypertensive patients (p < 0.05). However, there was no statistical difference between the two groups in MIP, IL‐2, and IL‐1α (p > 0.05).
To explore the relationship between the above eight protein levels and hypertension complicated with ischemic stroke, 206 hypertensive patients, including 92 patients with ischemic stroke, were included. Baseline data in the testing group was listed in Table 1. There was no significant difference in age, gender, body mass index, smoking, alcohol consumption, diabetes, serum MIP, IL‐2, and IL‐1α levels between hypertensive patients with ischemic stroke and those without ischemic stroke (p > 0.05, Table 1 and Figure 2D,E,H). Compared with hypertension without ischemic stroke, serum IMA, GFAP, HCY, Lp‐PLA2, and CTLA‐4 levels were significantly increased among hypertensive patients with ischemic stroke (p < 0.05, Figure 2A,B,C,F,G).
TABLE 1.
Baseline data in the testing group
| Variables | Hypertension with ischemic stroke | Hypertension without ischemic stroke | χ 2/t | p |
|---|---|---|---|---|
| Number of patients | 92 | 114 | ||
| Age, (mean ±SD) | 53.8 ± 6.2 | 54.0 ± 7.0 | −0.214 | 0.830 |
| Male (%) | 48 (52.2) | 54 (47.4) | 0.470 | 0.493 |
| Body mass index (mean ±SD, kg/m2) | 23.5 ± 1.7 | 23.7 ± 1.8 | −0.813 | 0.417 |
| Diabetes (%) | 13 (14.1) | 15 (13.2) | 0.041 | 0.840 |
| Smoking (%) | 6 (6.5) | 9 (7.9) | 0.142 | 0.706 |
| Drinking (%) | 25 (27.2) | 29 (25.4) | 0.079 | 0.778 |
| CTLA−4 concentrations (mean ±SD, ng/mL) | 5.3 ± 1.6 | 4.6 ± 1.5 | 3.232 | 0.001 |
| Lp‐PLA2 concentrations (mean ±SD, ng/mL) | 27.5 ± 7.3 | 17.8 ± 6.9 | 9.774 | <0.001 |
| HCY concentrations (mean ±SD, nmol/L) | 61.1 ± 8.8 | 55.7 ± 10.2 | 4.013 | <0.001 |
| MIP concentrations (mean ±SD, pg/mL) | 102.9 ± 21.5 | 97.5 ± 22.3 | 1.921 | 0.093 |
| IMA concentrations (mean ±SD, pg/mL) | 1.9 ± 0.6 | 1.2 ± 0.5 | 9.133 | <0.001 |
| GFAP concentrations (mean ±SD, mg/L) | 105.5 ± 24.1 | 93.7 ± 26.7 | 3.292 | 0.001 |
| IL−2 concentrations (mean ±SD, pg/mL) | 112.4 ± 28.5 | 108.7 ± 23.3 | 1.604 | 0.208 |
| IL−1α concentrations (mean ±SD, pg/mL) | 69.7 ± 14.7 | 66.1 ± 13.8 | 1.452 | 0.137 |
Abbreviation: CTLA‐4, cytotoxic T lymphocyte‐associated antigen‐4; IMA, ischemia‐modified albumin; IL‐1α, interleukin‐1 alpha; IL‐2, interleukin‐2; MIP, macrophage inflammatory protein; HCY, homocysteine; Lp‐PLA2, lipoprotein‐associated phospholipase A2; GFAP, glial fibrillary acidic protein.
FIGURE 2.
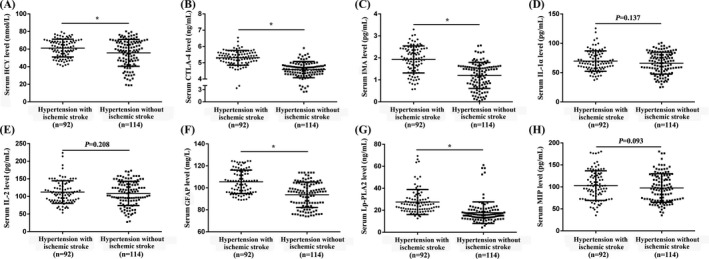
Serum CTLA‐4, Lp‐PLA2, HCY, MIP, IMA, GFAP, IL‐2, and IL‐1α levels between hypertensive patients with/without ischemic stroke. A, HCY. B, CTLA‐4. C, IMA. D, IL‐1α. E, IL‐2. F, GFAP. G, Lp‐PLA2. H, MIP. t test was used. *p < 0.05
3.4. Logistic regression analyses of serum IMA, GFAP, HCY, Lp‐PLA2, and CTLA‐4 in the prognosis assessment of hypertension with ischemic stroke in the testing group
Hypertension with/without ischemic stroke was treated as the dependent variable. Age, gender, body mass index, smoking, drinking, diabetes, serum IMA, GFAP, HCY, Lp‐PLA2, and CTLA‐4 levels were treated as independent variables for multiple linear regression analysis. The count data are assigned as follows: gender (male = 0, female = 1); smoking history (no = 0, yes = 1); drinking (no = 0, yes = 1); diabetes (no = 0, yes = 1). Results showed that IMA, GFAP, HCY, Lp‐PLA2, and CTLA‐4 were the main factors affecting hypertension complicated with ischemic stroke (p < 0.05), while age, gender, body mass index, smoking, drinking, diabetes, and other factors were not significantly correlated with the occurrence of hypertension combined with ischemic stroke (p > 0.05, Table 2).
TABLE 2.
Multiple linear regression analysis of risk factors for hypertension with ischemic stroke
| Independent variable | Hypertension with ischemic stroke | ||||
|---|---|---|---|---|---|
| β value | SE | Wald χ2 | p‐value | OR (95% CI) | |
| CTLA‐4 | 0.244 | 0.069 | 12.771 | 0.001 | 1.276 (1.117~1.459) |
| IMA | 0.195 | 0.078 | 6.250 | 0.009 | 1.213 (1.041~1.414) |
| Lp‐PLA2 | 0.142 | 0.051 | 7.863 | 0.004 | 1.152 (1.042~1.274) |
| GFAP | 0.134 | 0.062 | 4.591 | 0.032 | 1.145 (1.012~1.295) |
| HCY | 0.152 | 0.086 | 6.975 | <0.001 | 1.552 (1.156~2.937) |
| Age | 0.173 | 0.132 | 1.727 | 0.187 | 1.191 (0.916~1.556) |
| Gender | 0.142 | 0.127 | 1.282 | 0.256 | 1.158 (0.904~1.487) |
| Smoking | 0.013 | 0.126 | 0.011 | 0.918 | 1.013 (0.791~1.297) |
| Drinking | 0.069 | 0.083 | 0.691 | 0.406 | 1.071 (0.911~1.261) |
| Body mass index | 0.023 | 0.109 | 0.045 | 0.833 | 1.023 (0.826~1.267) |
| Diabetes | 0.018 | 0.127 | 0.029 | 0.862 | 1.026 (0.895~1.275) |
Abbreviation: OR, odd ratio; CI, confidence interval; CTLA‐4, cytotoxic T lymphocyte‐associated antigen‐4; IMA, ischemia‐modified albumin; IL‐1α, interleukin‐1 alpha; IL‐2: interleukin‐2; MIP: macrophage inflammatory protein; HCY: homocysteine; Lp‐PLA2: lipoprotein‐associated phospholipase A2; GFAP: glial fibrillary acidic protein.
The ROC results are shown in Figure 2. The serum IMA, GFAP, HCY, Lp‐PLA2, and CTLA‐4 at the time of admission were used for the identification of death from hypertensive patients with ischemic stroke, and the AUC were 0.911, 0.799, 0.842, 0.764, and 0.797, the sensitivity and specificity were 74.3%/92.6%, 63.9%/86.2%, 77.6%/79.9%, 48.1%/84.6%, and 83.0%/69.7%, respectively (Figure 3A–E).
FIGURE 3.
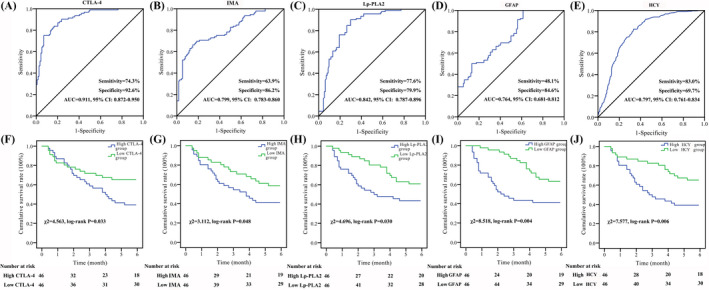
ROC and prognosis assessment of serum CTLA‐4, IMA, Lp‐PLA2, GFAP, and HCY among hypertensive patients with ischemic stroke in the testing group. A, ROC analysis of CTLA‐4. B, ROC analysis of IMA. C, ROC analysis of Lp‐PLA2. D, ROC analysis of GFAP. E, ROC analysis of HCY. F, Prognosis assessment of CTLA‐4. G, Prognosis assessment of IMA. H, Prognosis assessment of Lp‐PLA2. I, Prognosis assessment of GFAP. J, Prognosis assessment of HCY. The ROC curve and K‐M survival curve were used for differential diagnosis and prognostic evaluation
After 6 months of follow‐up, a total of 44 hypertensive patients with ischemic stroke died and there were no missing patients. Ninety‐two hypertensive patients with ischemic stroke were divided into high and low levels according to the median of serum IMA, GFAP, HCY, Lp‐PLA2, and CTLA‐4 levels. Compared with low CTLA‐4 group, low IMA group, low Lp‐PLA2 group, low GFAP group, and low HCY group, the survival rate of hypertensive patients with ischemic stroke in the high CTLA‐4 group, high IMA group, high Lp‐PLA2, high GFAP group, and high HCY group were significantly decreased (χ 2 = 4.563, p = 0.033; χ 2 = 3.112, p = 0.048; χ 2 = 4.696, p = 0.030; χ 2 = 8.518, p = 0.004; χ 2 = 7.577, p = 0.006, respectively, Figure 3F–J).
3.5. Establishment of the logistic regression model
A logistic regression model for the prognostic assessment of hypertensive patients with ischemic stroke was built. The predicting model was Logit(P) = 29.172–1.088*CTLA‐4–0.952*IMA ‐ 0.537*Lp‐PLA2 −0.066*GFAP −0.149*HCY. This predicting model has good value in identifying poor prognosis patients [sensitivity = 95.5% (42/44), and specificity = 93.8% (45/48), Figure 4A–C]. The estimated probability was 0.739, which means if higher than 0.739, it was classified into poor prognosis. Compared with the estimated probability ≤0.739 group, the survival rate of hypertensive patients with ischemic stroke in the estimated probability >0.739 group was significantly decreased (χ 2 = 40.001, p < 0.001, Figure 4D).
FIGURE 4.
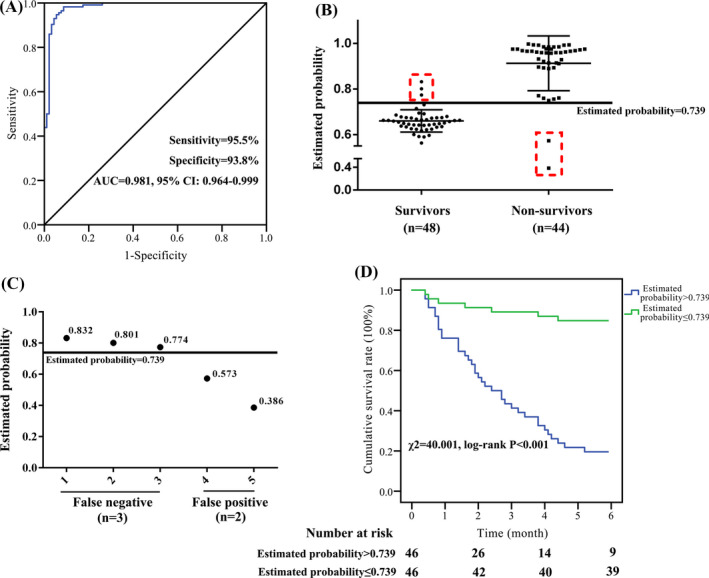
Logistic regression models for the prognostic assessment of hypertensive patients with ischemic stroke. A–C, ROC analysis of the prognostic assessment model [CTLA‐4, IMA, Lp‐PLA2, GFAP, and HCY] for hypertensive patients with ischemic stroke in the testing group. D, Compared with the estimated probability ≤0.739 group, the survival rate of hypertensive patients with ischemic stroke was significantly decreased in the estimated probability >0.739 group
3.6. Verification of the logistic regression model
To validate the clinical predictive value of the logistic regression model, we also tested it in a validation group, which including 108 hypertensive patients with ischemic stroke. The basic information of the two groups of patients in the test group and the validation group is shown in Table 3. There was no statistically significant difference between the two groups, indicating that the two groups are comparable. Results showed that this prognostic assessment model has a high value for the identification of death from hypertensive patients with ischemic stroke, and the AUC was 0.969, and the sensitivity/specificity was 89.4%/92.5% (Figure 5A). After 6 months of follow‐up, a total of 50 hypertensive patients with ischemic stroke died and there were no missing patients. Compared with the estimated probability ≤0.739 group, the survival rate of hypertensive patients with ischemic stroke in the estimated probability >0.739 group was significantly decreased (χ 2 = 47.551, p < 0.001, Figure 5B).
TABLE 3.
Comparison of the baseline data of hypertension patients with ischemic stroke between the testing group and validation group
| Variables | Hypertension with ischemic stroke (testing group) | Hypertension with ischemic stroke (validation group) | χ 2/t | P |
|---|---|---|---|---|
| Number of patients | 92 | 108 | ||
| Age, (mean ±SD) | 53.8 ± 6.2 | 53.7 ± 6.6 | 0.110 | 0.913 |
| Male (%) | 48 (52.2) | 55 (50.9) | 0.031 | 0.860 |
| Body mass index (mean ±SD, kg/m2) | 23.5 ± 1.7 | 23.4 ± 1.3 | 0.471 | 0.638 |
| Diabetes (%) | 13 (14.1) | 11 (10.2) | 0.732 | 0.392 |
| Smoking (%) | 6 (6.5) | 10 (9.3) | 0.506 | 0.477 |
| Drinking (%) | 25 (27.2) | 30 (27.8) | 0.009 | 0.924 |
| CTLA‐4 concentrations (mean ±SD, ng/mL) | 5.3 ± 1.6 | 5.5 ± 1.8 | −0.824 | 0.411 |
| Lp‐PLA2 concentrations (mean ±SD, ng/mL) | 27.5 ± 7.3 | 27.1 ± 6.5 | 0.410 | 0.682 |
| HCY concentrations (mean ±SD, nmol/L) | 61.1 ± 8.8 | 60.8 ± 9.6 | 0.229 | 0.819 |
| MIP concentrations (mean ±SD, pg/mL) | 102.9 ± 21.5 | 104.3 ± 20.1 | −0.475 | 0.635 |
| IMA concentrations (mean ±SD, pg/mL) | 1.9 ± 0.6 | 2.0 ± 0.4 | −1.404 | 0.162 |
| GFAP concentrations (mean ±SD, mg/L) | 105.5 ± 24.1 | 106.2 ± 21.9 | −0.215 | 0.830 |
| IL−2 concentrations (mean ±SD, pg/mL) | 112.4 ± 28.5 | 111.8 ± 27.0 | 0.153 | 0.879 |
| IL−1α concentrations (mean ±SD, pg/mL) | 69.7 ± 14.7 | 69.4 ± 13.7 | 0.149 | 0.882 |
Abbreviation: CTLA‐4, cytotoxic T lymphocyte‐associated antigen‐4; IMA, ischemia‐modified albumin; IL‐1α, interleukin‐1 alpha; IL‐2, interleukin‐2; MIP, macrophage inflammatory protein; HCY, homocysteine; Lp‐PLA2, lipoprotein‐associated phospholipase A2; GFAP, glial fibrillary acidic protein.
FIGURE 5.
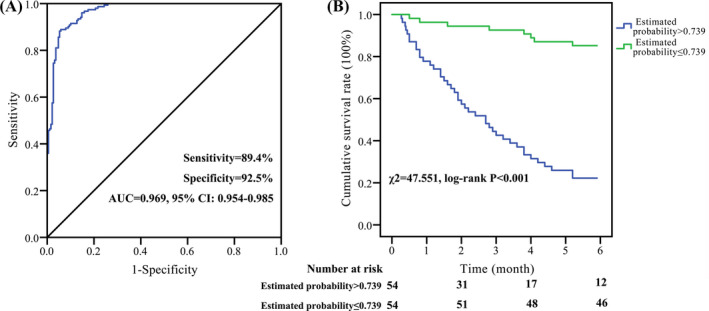
Verification of the prognostic assessment model. A, ROC analysis of the prognostic assessment model for hypertensive patients with ischemic stroke in the five protein markers [CTLA‐4, IL‐6, Lp‐PLA2, Lp(a), and TNF‐α] in the validation group. B, Compared with the estimated probability ≤0.739 group, the survival rate of hypertensive patients with ischemic stroke was significantly decreased in the estimated probability >0.739 group. The ROC curve and K‐M survival curve were used for differential diagnosis and prognostic evaluation
4. DISCUSSION
Ischemic stroke is a cerebrovascular disease with a high fatality rate and disability rate, which poses a great threat to human health. 16 , 17 The abnormal structure of blood lipids and inflammation in hypertensive patients is considered to be one of the promoting factors of cardiovascular events and has been widely confirmed. 18 , 19 High blood pressure can promote the damage of vascular endothelial cells and accelerate the deposition of lipid plaques. 19 Besides, atherosclerosis induced by hypertension can cause arterial occlusion; the body's inflammatory response can lead to cerebrovascular embolism; brain injury can lead to patient dysfunction, and these series of pathological factors accelerate the development of ischemic stroke. 18 , 19 Therefore, it is particularly important to evaluate the prognosis of hypertensive patients with ischemic stroke. At present, blood biomarkers related to ischemic stroke are auxiliary tools for disease prevention, screening, early diagnosis, and prognosis, and have a wide range of applications. 20 It is worth noting that six hours after the onset of ischemic stroke is called the "window period", the sooner the patient can receive thrombolytic therapy, the better the patient's prognosis. 21 However, there is still a lack of tools to assess the prognosis of hypertensive patients with ischemic stroke.
In this study, for the first time, we used the mass spectrometry technique to analyze the changes in serum protein profiles of hypertensive patients with ischemic stroke, and included IMA, GFAP, HCY, Lp‐PLA2, and CTLA‐4 proteins. CTLA‐4 has been shown to affect the development of hypertension. 22 CTLA‐4 participates in the negative regulation of the immune response by blocking CD28‐mediated co‐stimulatory molecules and is related to the activation and differentiation of Th2 cells. 23 Zheng et al. 24 pointed out that the CTLA‐4 gene + 49A/G polymorphism is an important risk factor for ischemic stroke susceptibility, especially in Asians, children, and atopic patients. IMA, GFAP, and HCY are important biological indicators to assess the degree of the inflammatory response in the body, and they have high sensitivity in early warning of the inflammatory response caused by oxidative stress. 25 , 26 , 27 Lp‐PLA2, also known as platelet‐activating factor acetylhydrolase, is mainly synthesized and secreted by mature macrophages and lymphocytes, and is a member of the phospholipase superfamily. 28 Lp‐PLA2 can effectively hydrolyze oxidized lecithin and promote and mediate the inflammatory response, leading to vascular endothelial cell damage. 29 We speculate that after the patient is complicated with ischemic stroke, Lp‐PLA2 in the cerebrospinal fluid enters the serum through the blood‐brain barrier, resulting in increased serum Lp‐PLA2 content. Besides, cerebral infarction can lead to acute cerebral edema, increased intracranial pressure, abnormal renal function through the mechanism of neuroendocrine disorder, increased secretion of angiotensin and antidiuretic hormone, decreased glomerular filtration rate, and reduced Lp‐PLA2 clearance rate in blood reduce. 30 , 31 This is another important cause of increased serum Lp‐PLA2 levels. Based on the above research basis, we explored the serum IMA, GFAP, HCY, Lp‐PLA2, and CTLA‐4 levels of hypertensive patients upon admission.
In our research, CTLA‐4 was the most effective marker (AUC = 0.911) than IMA (AUC = 0.799), Lp‐PLA2 (AUC = 0.842), GFAP (AUC = 0.764), and HCY (AUC = 0.797), but its sensitivity (74.3%) was unsatisfactory. Therefore, we built a novel prognostic assessment model [Logit(P) = 29.172–1.088*CTLA‐4–0.952*IMA‐0.537*Lp‐PLA2 −0.066*GFAP −0.149*HCY] which includes multiple protein markers. It showed higher efficiency (AUC = 0.981, sensitivity = 95.5%, specificity = 93.8%) than any single marker. Furthermore, we validated this prognostic assessment model in a validation group. This model still showed good efficiency (AUC = 0.969, sensitivity = 89.4%, specificity = 92.5%), indicating that this model can predict patients’ prognosis. Our study has the following potential application prospects: (1) Non‐invasive monitoring of the risk of stroke in severely hypertensive patients; (2) Prognostic assessment and clinical intervention for hypertensive patients who have had a stroke; and (3) It is expected to carry out health management for the general population.
In summary, the prognostic assessment model we have established is of great value in the prognosis evaluation of ischemic stroke patients induced by hypertension. However, our results require to be verified by multicenter studies with larger sample sizes.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
JM and QW conceived the study. JM, LB, HY, YW, HL, and LS were involved in gaining ethical approval, patient recruitment and data analysis. JM, LS, and QW wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ETHICAL APPROVAL
This study was approved by the Ethics Committee of Suzhou Kowloon Hospital Affiliated Shanghai Jiao Tong University (Ethics number: KS20160085).
Supporting information
Fig S1
Fig S2
ACKNOWLEDGMENTS
We thank our colleagues in the nursing department and the medical laboratory for their guidance.
Jin Ma and Likui Shen contributed equally to this work.
Funding information
This work was supported by the National Natural Science Foundation of China under Grant 81901343, and Kunshan Science Project under Grant KS18033
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Lu J, Lu Y, Wang X, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1·7 million adults in a population‐based screening study (China PEACE Million Persons Project). Lancet. 2017;390(10112):2549‐2558. [DOI] [PubMed] [Google Scholar]
- 2. Kameshima S, Okada M, Yamawaki H. Expression and localization of calmodulin‐related proteins in brain, heart and kidney from spontaneously hypertensive rats. Biochem Biophys Res Commun. 2016;469(3):654‐658. [DOI] [PubMed] [Google Scholar]
- 3. Ning BO, Chen Y, Waqar AB, et al. Hypertension enhances advanced atherosclerosis and induces cardiac death in watanabe heritable hyperlipidemic rabbits. Am J Pathol. 2018;188(12):2936‐2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al‐Sharea A, Lee MKS, Whillas A, et al. Chronic sympathetic driven hypertension promotes atherosclerosis by enhancing hematopoiesis. Haematologica. 2019;104(3):456‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reckelhoff JF, Romero DG, Yanes Cardozo LL. Sex, oxidative stress, and hypertension: insights from animal models. Physiology (Bethesda). 2019;34(3):178‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Massaro M, Scoditti E, Carluccio MA, et al. Oxidative stress and vascular stiffness in hypertension: a renewed interest for antioxidant therapies? Vascul Pharmacol. 2019;116:45‐50. [DOI] [PubMed] [Google Scholar]
- 7. Cipolla MJ, Liebeskind DS, Chan SL. The importance of comorbidities in ischemic stroke: impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab. 2018;38(12):2129‐2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alawieh A, Langley EF, Tomlinson S. Targeted complement inhibition salvages stressed neurons and inhibits neuroinflammation after stroke in mice. Sci Transl Med. 2018;10(441):6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yew WP, Djukic ND, Jayaseelan JSP, et al. Early treatment with minocycline following stroke in rats improves functional recovery and differentially modifies responses of peri‐infarct microglia and astrocytes. J Neuroinflammation. 2019;16(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris‐Blanco KC, Kim TaeHee, Lopez MS, et al. Induction of DNA hydroxymethylation protects the brain after stroke. Stroke. 2019;50(9):2513‐2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haeusler KG, Herm J, Konieczny M, et al. Impact of chronic inflammatory airway disease on stroke severity and long‐term survival after ischemic stroke–a retrospective analysis. BMC Neurol. 2015;15:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murphy TE, McAvay GJ, Allore HG, et al. Contributions of COPD, asthma, and ten comorbid conditions to health care utilization and patient‐centered outcomes among US adults with obstructive airway disease. Int J Chron Obstruct Pulmon Dis. 2017;12:2515‐2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou A. Proteomics in stroke research: potentials of the nascent proteomics. J Investig Med. 2016;64(8):1236‐1240. [DOI] [PubMed] [Google Scholar]
- 14. Montaner J, Ramiro L, Simats A, et al. Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke. Nat Rev Neurol. 2020;16(5):247‐264. [DOI] [PubMed] [Google Scholar]
- 15. Xu P, Wang L, Chen D, et al. The application of proteomics in the diagnosis and treatment of bronchial asthma. Ann Transl Med. 2020;8(4):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Can Demirdöğen B, Şahin E, Türkanoğlu Özçelik A, et al. Apolipoprotein A5 polymorphisms in Turkish population: association with serum lipid profile and risk of ischemic stroke. Mol Biol Rep. 2012;39(12):10459‐10468. [DOI] [PubMed] [Google Scholar]
- 17. Chen Y, Quddusi A, Harrison KathleenA, et al. Selection of preclinical models to evaluate intranasal brain cooling for acute ischemic stroke. Brain Circ. 2019;5(4):160‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang H‐Y, Shi W‐R, Yi X, et al. Assessing the performance of monocyte to high‐density lipoprotein ratio for predicting ischemic stroke: insights from a population‐based Chinese cohort. Lipids Health Dis. 2019;18(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng X, Zeng N, Wang A, et al. Elevated C‐reactive protein and depressed high‐density lipoprotein cholesterol are associated with poor function outcome after ischemic stroke. Curr Neurovasc Res. 2018;15(3):226‐233. [DOI] [PubMed] [Google Scholar]
- 20. Desai SM, Starr M, Molyneaux BJ, et al. Acute ischemic stroke with vessel occlusion‐prevalence and thrombectomy eligibility at a comprehensive stroke center. J Stroke Cerebrovasc Dis. 2019;28(11):104315. [DOI] [PubMed] [Google Scholar]
- 21. Branco JP, Oliveira S, Sargento‐Freitas J, et al. Assessing functional recovery in the first six months after acute ischemic stroke: a prospective, observational study. Eur J Phys Rehabil Med. 2019;55(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 22. Wanchoo R, Karam S, Uppal NN, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. 2017;45(2):160‐169. [DOI] [PubMed] [Google Scholar]
- 23. Chen YQ, Shi HZ. CD28/CTLA‐4–CD80/CD86 and ICOS–B7RP‐1 costimulatory pathway in bronchial asthma. Allergy. 2006;61(1):15‐26. [DOI] [PubMed] [Google Scholar]
- 24. Zheng Y, Wang H, Luo L, et al. A meta‐analysis of the association between CTLA‐4 genetic polymorphism and susceptibility of asthma. Medicine (Baltimore). 2018;97(28):e11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang M, Liang X, Cheng M, et al. Homocysteine enhances neural stem cell autophagy in in vivo and in vitro model of ischemic stroke. Cell Death Dis. 2019;10(8):561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Belayev L, Hong S‐H, Menghani H, et al. Docosanoids promote neurogenesis and angiogenesis, blood‐brain barrier integrity, penumbra protection, and neurobehavioral recovery after experimental ischemic stroke. Mol Neurobiol. 2018;55(8):7090‐7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Menon B, Ramalingam K, Krishna V. Study of ischemia modified albumin as a biomarker in acute ischaemic stroke. Ann Neurosci. 2018;25(4):187‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sairam SG, Sola S, Barooah A, et al. The role of Lp‐PLA2 and biochemistry parameters as potential biomarkers of coronary artery disease in Asian South‐Indians: a case‐control study. Cardiovasc Diagn Ther. 2017;7(6):589‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tibuakuu M, Kianoush S, DeFilippis AP, et al. Usefulness of lipoprotein‐associated phospholipase A2 activity and C‐reactive protein in identifying high‐risk smokers for atherosclerotic cardiovascular disease (from the atherosclerosis risk in communities study). Am J Cardiol. 2018;121(9):1056‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim JS, Caplan LR. Clinical stroke syndromes. Front Neurol Neurosci. 2016;40:72‐92. [DOI] [PubMed] [Google Scholar]
- 31. Tu W‐J, Dong X, Zhao S‐J, et al. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. J Neuroendocrinol. 2013;25(9):771‐778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


