Abstract
Objective
The current study aimed to investigate the prognostic value of T helper (Th) 1 and Th17 proportions in sepsis patients.
Methods
Th1 and Th17 cells in blood CD4+ T cells were detected by flow cytometry in 210 sepsis patients and 100 healthy controls (HCs). Besides, serum interferon‐γ (IFN‐γ), tumor necrosis factor‐α (TNF‐α), and interleukin‐17 (IL‐17) levels in the enrolled sepsis patients were determined with enzyme‐linked immunosorbent assay.
Results
Compared with HCs, Th1 and Th17 proportions were elevated in sepsis patients (both p < .001). Meanwhile, Th1 proportion was strongly correlated with IFN‐γ (p < .001, r = .484) but weakly correlated with TNF‐α (p = .024, r = .156) and IL‐17 (p = .002, r = .212), while Th17 proportion showed faint correlation with IFN‐γ (p = .015, r = .168), but strong correlations with TNF‐α (p < .001, r = .602) and IL‐17 (p < .001, r = .498) in sepsis patients. Besides, Th1 proportion was weakly associated with APACHE II score (p = .030, r = .150), but Th17 proportion was closely associated with APACHE II score (p < .001, r = .322) and SOFA score (p < .001, r = .337) in sepsis patients. Regarding their prognostic value, Th1 proportion (p = .042) was slightly, while Th17 proportion (p < .001) was dramatically, increased in septic deaths compared with survivors, and Th17 possessed good predictive value for 28‐day mortality risk (AUC: 0.748, 95% CI: 0.659–0.836).
Conclusion
Th1 and Th17 proportions are elevated in sepsis patients compared with HCs, and Th17 proportion is correlated with increased disease severity, higher inflammation level, and worse prognosis in sepsis patients.
Keywords: 28‐day mortality, Comprehensive disease severity scale, Sepsis, Th1, Th17
The current study aimed to investigate the prognostic value of T helper (Th) 1 and Th17 proportions in sepsis patients. Th1 and Th17 cells in blood CD4+ T cells were detected by flow cytometry in 210 sepsis patients and 100 healthy controls (HCs). Besides, serum interferon‐γ (IFN‐γ), tumor necrosis factor‐α (TNF‐α), and interleukin‐17 (IL‐17) levels in the enrolled sepsis patients were determined with enzyme‐linked immunosorbent assay. Compared with HCs, Th1 and Th17 proportions were elevated in sepsis patients (both p < .001). Meanwhile, Th1 proportion was strongly correlated with IFN‐γ (p < .001, r = .484) but weakly correlated with TNF‐α (p = .024, r = .156) and IL‐17 (p = .002, r = .212); while Th17 proportion showed faint correlation with IFN‐γ (p = .015, r = .168), but strong correlations with TNF‐α (p < .001, r = .602) and IL‐17 (p < .001, r = .498) in sepsis patients. Besides, Th1 proportion was weakly associated with APACHE II score (p = .030, r = .150), but Th17 proportion was closely associated with APACHE II score (p < .001, r = .322) and SOFA score (p < .001, r = .337) in sepsis patients. Regarding their prognostic value, Th1 proportion (p = .042) was slightly, while Th17 proportion (p < .001) was dramatically, increased in septic deaths compared with survivors, and Th17 possessed good predictive value for 28‐day mortality risk (AUC: 0.748, 95% CI: 0.659–0.836). Th1 and Th17 proportions are elevated in sepsis patients compared with HCs, and Th17 proportion is correlated with increased disease severity, higher inflammation level, and worse prognosis in sepsis patients.

1. INTRODUCTION
Sepsis is characterized by dysregulated immune system to the infection that attacks over 400 patients per 100,000 populations every year. 1 , 2 Meanwhile, sepsis leads to increased systematic inflammation, multiple organ injury, and even mortality; indeed, sepsis causes over 70 deaths per 100,000 populations annually and it is considered as a heavy burden both in humanity and economy. 3 , 4 , 5 Regarding the treatment for sepsis, antibiotics and other symptomatic managements (such as fluid therapy, mechanical ventilation, and continuous renal replacement treatment) should be administrated or conducted according to the specific organism that causes infection, patients’ disease severity, and symptoms. 5 , 6 Therefore, searching for novel biomarkers to reflect sepsis patients’ disease severity and prognosis might enhance the surveillance for sepsis patients, thus improving their overall prognosis.
The adaptive immune response is a critical defensive response that protects the host from infection, during which T cells are differentiated into several subsets, including T helper 1 (Th1) and Th17 cells. 7 However, the dysregulated immune response during sepsis might induce systematic inflammation and exacerbate organ injury, thus causes damage to the host in reverse. 8 It is supposed that Th1 cells release their related cytokine interferon‐γ (IFN‐γ) to exacerbate inflammation in the lipopolysaccharide‐induced sepsis cell model, 9 , 10 and Th17 cells release their related cytokines interleukin‐17 (IL‐17) and tumor necrosis factor‐α (TNF‐α) to directly participate in inflammation and multiple organ injury in sepsis model cells/mice. 11 , 12 , 13 , 14 Besides, previous studies also imply that Th1 and Th17 cells are dysregulated in sepsis patients, and they possess certain values in discriminating sepsis patients from healthy subjects. 15 , 16 However, the prognostic values of Th1 and Th17 cells in sepsis patients were largely unclear. Therefore, we aimed to detect Th1 and Th17 cells in sepsis patients, and evaluate their correlations with IFN‐γ, TNF‐α, IL‐17, disease severity, and prognosis in sepsis patients.
2. MATERIALS AND METHODS
2.1. Study subjects
Between January 2017 and August 2020, 210 sepsis patients were consecutively recruited in this prospective, observational study. The inclusion criteria of sepsis patients were as follows: (1) diagnosed as sepsis referring to the International Guidelines for Management of Sepsis and Septic Shock: 2016 17 ; (2) age above 18 years old; and (3) signed informed consent by patients or their legal guardians. Exclusion criteria included the following: (1) received immunosuppressive agents before being included in the study; (2) complicated with malignant tumor; and (3) history of hematological malignancies. Furthermore, this study also included 100 healthy subjects who underwent health examinations during the same period as health controls (HCs). The main inclusion criteria of HCs were as follows: (1) There were no obvious abnormalities in biochemical parameters; (2) no sepsis or malignancy history; (3) no active infection; and (4) signed informed consent. This study had been reviewed and approved by the institutional review board.
2.2. Data collection
Baseline demographics and clinical features of sepsis patients and HCs were collected, which included age, sex, body mass index (BMI), drink, smoke, history of diseases, primary infection site, and biochemical indexes. The primary organism was detected after the blood culture. Moreover, the acute physiology and chronic health evaluation II (APACHE II) score and sequential organ failure assessment (SOFA) score were also assessed for evaluation of the disease severity in sepsis patients. Patients were followed up until death or up to 28 days, and meanwhile, 28‐day mortality was calculated.
2.3. PBMC and serum collection
Totally, 5 ml of peripheral blood sample was acquired from every sepsis patients and 2.5 ml of peripheral blood sample was extracted from every HC. After extraction, the peripheral blood from sepsis patients was halved into two tubes, and ficoll solution was added to one half of the peripheral blood to isolate peripheral blood mononuclear cell (PBMC) by gradient density centrifugation immediately; meanwhile, the remaining half of the peripheral blood was separated by centrifugation at 3000 g for 15 min to separate serum. After extraction, PBMC was also isolated from the peripheral blood of HCs as previously described. Then, PBMC was used for detecting Th1 and Th17 cells. The serum was stored at −80°C for the detection of the levels of IFN‐γ (Th1‐secreted cytokine), IL‐17, and TNF‐α (Th17‐secreted cytokines).
2.4. Detection of Th1 and Th17 cell proportions
For sepsis patients and HCs, Th1 and Th17 were sorted from PBMC samples using Human Th1 or Th17 Phenotyping Kit (BD Pharmingen™) within 24 h after PBMC isolated from peripheral blood. Then, Th1 cell proportion in CD4+ T cell was counted by the ratio of IFN‐γ and CD4 co‐labeled cells to CD4 labeled cells using FACSARIA II flow cytometer (BD), and Th17 cell proportion in CD4+ T cell was counted by the ratio of IL‐17 and CD4 co‐labeled cells to CD4 labeled cells using FACSARIA II flow cytometer (BD).
2.5. IFN‐γ, IL‐17, and TNF‐α detection
For sepsis patients only, serum IFN‐γ, IL‐17, and TNF‐α levels were detected with commercial enzyme‐linked immunosorbent assay (ELISA) kits. The kits used in ELISA were as follows: human IFN‐γ ELISA kit (Thermo Fisher Scientific), human IL‐17A ELISA kit (Thermo Fisher Scientific), and human TNF‐α ELISA kit (Thermo Fisher Scientific). All procedures were performed based on the guidance of instruction. Finally, the optical density value was read at 450 nm by a microplate reader (BioTek).
2.6. Statistical analysis
SPSS 24.0 (IBM) was used for statistical analysis, and GraphPad Prism 8.01 (GraphPad Software Inc.) was applied for graph plotting. Normality test was performed for quantitative data by Kolmogorov–Smirnov (K‐S) test, and normally distributed quantitative data were described as mean ± standard deviation (M ± SD), while non‐normally distributed or unknown distributed quantitative data were shown as median with interquartile range (IQR). Qualitative data were described as counts with frequency. Comparison of Th1 and Th17 cell proportion between two groups (sepsis patients vs. HCs; survivors vs. death in sepsis patients) was determined by the Wilcoxon rank‐sum test. The receiver operating characteristic (ROC) analysis was performed to evaluate the effect of variables in distinguishing sepsis patients from HCs and for the prediction of 28‐day mortality risk in sepsis patients. Correlation analysis was determined by Spearman's rank correlation test. Accumulating mortality was displayed using Kaplan curve. Sepsis patients were termed as Th1 high group and Th1 low group by the cut off of the median value of Th1 cell proportion; meanwhile, patients were also termed as Th17 high group and Th17 low group by the cut off of the median value of Th17 cell proportion. Accumulating mortality between the two groups was analyzed by the log‐rank test. p value <.05 was considered as statistically significant.
3. RESULTS
3.1. Sepsis patients’ clinical characteristics
The enrolled sepsis patients had a mean age of 56.1 ± 12.3 years with 145 (69.0%) males and 65 (31.0%) females. Meanwhile, the enrolled HCs had a mean age of 53.9 ± 11.9 years with 64 (64.0%) males and 36 (36.0%) males. In sepsis patients, regarding the biochemical indexes, the median value of Scr was 1.5 (1.1–2.4) mg/dl, the median value of albumin was 26.9 (20.8–36.1) g/L, the median value of WBC was 20.0 (13.7–31.2) x 109/L, and the median value of CRP was 92.4 (51.2–132.7) mg/L in sepsis patients. Moreover, the mean value of APACHE II score was 13.1 ± 6.2 and the mean value of SOFA score was 6.0 ± 2.5. Further comparison analyses showed that Scr, WBC, and CRP were increased, while albumin was decreased in sepsis patients compared with HCs (all p < .001), while no difference was found in age, gender, BM1, smoke, or drink between the two groups (all p < .05). Table 1 presented sepsis patients’ and HCs’ basic characteristics in detail.
TABLE 1.
Sepsis patients’ and HCs’ characteristics
| Items | HCs (N = 100) | Sepsis patients (N = 210) | p value |
|---|---|---|---|
| Age (years), M ± SD | 53.9 ± 11.9 | 56.1 ± 12.3 | .140 |
| Gender, No. (%) | |||
| Male | 64 (64.0) | 145 (69.0) | .375 |
| Female | 36 (36Z`.0) | 65 (31.0) | |
| BMI (kg/m2), M ± SD | 22.7 ± 3.0 | 22.9 ± 3.6 | .708 |
| Smoke, No. (%) | 28 (28.0) | 71 (33.8) | .305 |
| Drink, No. (%) | 45 (45.0) | 86 (41.0) | .500 |
| History of hypertension, No. (%) | ‐ | 87 (41.4) | ‐ |
| History of hyperlipidemia, No. (%) | ‐ | 43 (20.5) | ‐ |
| History of diabetes, No. (%) | ‐ | 29 (13.8) | ‐ |
| History of CKD, No. (%) | ‐ | 23 (11.0) | ‐ |
| History of CCVDs, No. (%) | ‐ | 44 (21.0) | ‐ |
| Primary infection site, No. (%) | |||
| Abdominal infection | ‐ | 87 (41.4) | ‐ |
| Respiratory infection | ‐ | 44 (21.0) | ‐ |
| Skin and soft tissue infection | ‐ | 39 (18.6) | ‐ |
| Blood stream infection | ‐ | 22 (10.5) | ‐ |
| CNS infection | ‐ | 10 (4.8) | ‐ |
| Other infections | ‐ | 8 (3.8) | ‐ |
| Primary organism, No. (%) | |||
| Gram‐negative bacteria | ‐ | 116 (55.2) | ‐ |
| Gram‐positive bacteria | ‐ | 53 (25.2) | ‐ |
| Fungus | ‐ | 19 (9.0) | ‐ |
| Others | ‐ | 39 (18.6) | ‐ |
| Total culture negative | ‐ | 32 (15.2) | ‐ |
| Biochemical indexes, median (IQR) | |||
| Scr (mg/dl) | 0.8 (0.7–1.0) | 1.5 (1.1–2.4) | <.001 |
| Albumin (g/L) | 42.8 (39.1–47.4) | 26.9 (20.8–36.1) | <.001 |
| WBC (109/L) | 6.4 (5.4–7.5) | 20.0 (13.7–31.2) | <.001 |
| CRP (mg/L) | 3.7 (2.5–6.4) | 92.4 (51.2–132.7) | <.001 |
| Disease severity score, M ± SD | |||
| APACHE II score | ‐ | 13.1 ± 6.2 | ‐ |
| SOFA score | ‐ | 6.0 ± 2.5 | ‐ |
Abbreviations: APACHE II, acute physiology and chronic health evaluation II; BMI, body mass index; CCVDs, cardio‐cerebrovascular diseases;CKD, chronic kidney disease; CNS, central nervous system; CRP, C‐reactive protein; IQR, interquartile range; M ± SD, mean ± standard deviation; Scr, serum creatinine; SOFA, sequential organ failure assessment; WBC, white blood cell.
3.2. Th1 and Th17 cell proportions in sepsis patients and HCs
Th1 cell proportion showed an increase in sepsis patients (median value: 14.17% (11.94%–17.24%)) compared with HCs (median value: 10.06% [8.08%–12.34%]; p < .001; Figure 1A); meanwhile, it showed good potential for the discrimination of sepsis patients from HCs (AUC: 0.831, 95% CI: 0.783–0.878; Figure 1C). Besides, Th17 cell proportion was also elevated in sepsis patients (median value: 3.31% [2.63%–4.49%]) compared with HCs (median value: 1.72% [1.42%–1.98%]; p < .001; Figure 1B), and it presented great potential for the discrimination of sepsis patients from HCs (AUC: 0.927, 95% CI: 0.898–0.956; Figure 1D).
FIGURE 1.
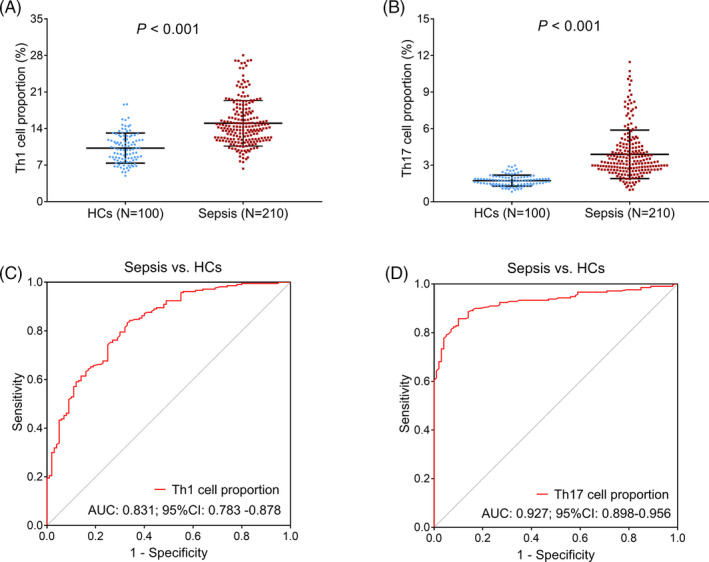
Detection of Th1 and Th17 cell proportions in sepsis patients and HCs. Comparison of Th1 (A) and Th17 (B) cell proportions between sepsis patients and HCs. The value of Th1 (C) and Th17 (D) cell proportions in discriminating sepsis patients from HCs by ROC curves. Th, T helper; HC, healthy control; AUC, area under the curve; CI, confidence interval; ROC, receiver's operating characteristic
3.3. Correlation of Th1 and Th17 cell proportions with their related cytokines in sepsis patients
A strong correlation was found in Th1 cell proportion with IFN‐γ (p < .001, r = .484; Figure 2A), while weak correlations were found in Th1 cell proportion with IL‐17 (p = .024, r = .156) and TNF‐α (p = .002, r = .212; Figure 2B,C) in sepsis patients. Meanwhile, Th17 cell proportion was faintly associated with IFN‐γ (p = .015, r = .168; Figure 2D), while it was closely associated with IL‐17 (p < .001, r = .602) and TNF‐α (p < .001, r = .498; Figure 2E,F) in sepsis patients.
FIGURE 2.
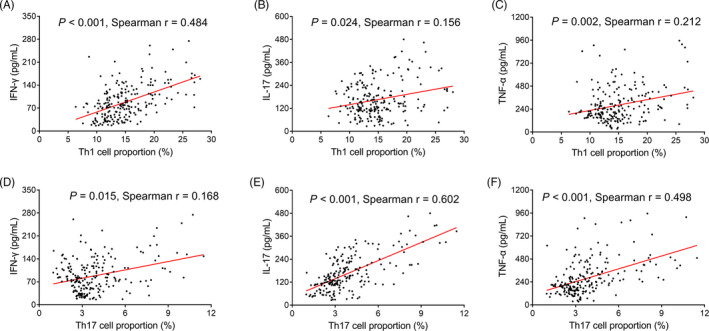
Correlation analysis in Th1 and Th17 cell proportions with their related cytokines in sepsis patients. Correlation in Th1 cell proportion with IFN‐γ (A), IL‐17 (B) and TNF‐α (C). Correlation in Th17 cell proportion with IFN‐γ (D), IL‐17 (E), and TNF‐α (F). Th, T helper; IFN‐γ, interferon‐γ; IL‐17, interleukin‐17; TNF‐α, tumor necrosis factor‐α
3.4. Correlation of Th1 and Th17 cell proportions with comprehensive disease severity scores in sepsis patients
A weak correlation was observed in Th1 cell proportion with APACHE II score (p = .030, r = .150; Figure 3A), while no correlation was found between Th1 cell proportion and SOFA score (p = .160, r = .097; Figure 3B) in sepsis patients. Besides, positive associations were found in Th17 cell proportion with both APACHE II score (p < .001, r = .322; Figure 3C) and SOFA score (p < .001, r = .337; Figure 3D) in sepsis patients.
FIGURE 3.
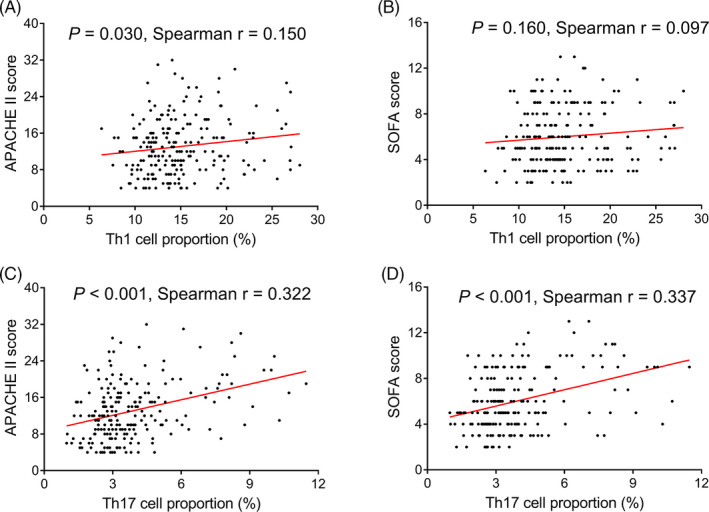
Correlation analysis in Th1 and Th17 cell proportions with disease severity in sepsis patients. Correlation in Th1 cell proportion with APACHE II score (A) and SOFA score (B). Correlation in Th17 cell proportion with APACHE II score (C) and SOFA score (D). Th, T helper; APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment
3.5. Th1 and Th17 cell proportions in septic survivors and deaths
Th1 cell proportion was slightly increased in septic deaths (median value: 15.48% [12.31%–18.75%]) compared with septic survivors (median value: 13.95% [11.78%–16.63%]; p = .042; Figure 4A). Meanwhile, Th17 cell proportion was dramatically elevated in septic deaths (median value: 4.81% [3.16%–7.70%]) compared with septic survivors (median value: 3.16% [2.60%–4.06%]; p < .001; Figure 4B).
FIGURE 4.
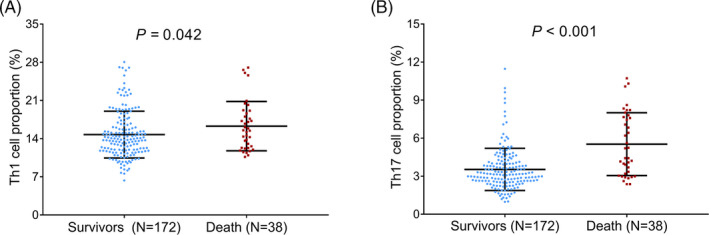
Detection of Th1 and Th17 cell proportions in septic deaths and survivors. Comparison of Th1 (A) and Th17 (B) cell proportions between septic deaths and survivors. Th, T helper
3.6. Ability of Th1 and Th17 cell proportions in predicting 28‐day mortality risk in sepsis patients
Th1 cell proportion showed only a weak ability in predicting 28‐day mortality risk in sepsis patients (AUC: 0.605, 95% CI: 0.509–0.701), while Th17 cell proportion presented a good ability in predicting that (AUC: 0.748, 95% CI: 0.659–0.836; Figure 5A). Meanwhile, IFN‐γ had no ability in predicting 28‐day mortality risk in sepsis patients (AUC: 0.568, 95% CI: 0.473–0.662), but IL‐17 and TNF‐α showed acceptable abilities in predicting that with AUC of 0.707 (95% CI: 0.608–0.807) and 0.702 (95% CI: 0.608–0.797), respectively (Figure 5B). Besides, both APACHE II score and SOFA score presented good value in predicting 28‐day mortality risk in sepsis patients with AUC of 0.839 (95% CI: 0.777–0.902) and 0.812 (95% CI: 0.733–0.891), respectively (Figure 5C).
FIGURE 5.
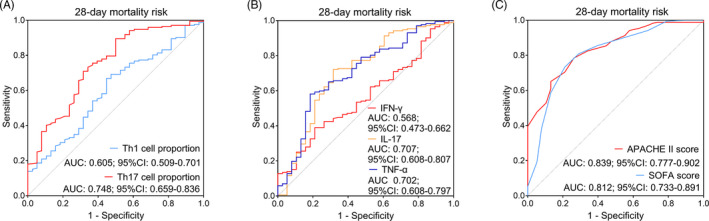
Value of Th1 and Th17 cell proportions in discriminating septic deaths from survivors. A, The value of Th1 and Th17 cell proportions in discriminating septic deaths from survivors; (B) The value of IFN‐γ, IL‐17, and TNF‐α in discriminating septic deaths from survivors; (C) The value of APACHE II score and SOFA score in discriminating septic deaths from survivors. Th, T helper; AUC, area under the curve; CI, confidence interval; IFN‐γ, interferon‐γ; IL‐17, interleukin‐17; TNF‐α, tumor necrosis factor‐α; APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment
3.7. Correlation of Th1 and Th17 cell proportions with accumulating mortality in sepsis patients
No statistical significance was observed in the correlation of Th1 cell proportion with accumulating mortality (p = .078; Figure 6A), while Th17 cell proportion was positively correlated with accumulating mortality in sepsis patients (p = .001; Figure 6B).
FIGURE 6.
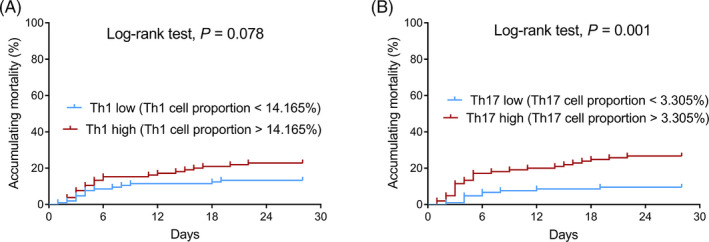
Accumulating mortality in Th1 high/low‐expression patients and Th17 high/low‐expression patients. A, Comparison of accumulating mortality between Th1 high‐expression patients and Th1 low‐expression patients; (B) Comparison of accumulating mortality between Th17 high‐expression patients and Th17 low‐expression patients. Th, T helper
4. DISCUSSION
Although it has been addressed that Th1 and Th17 cells play vital roles in the progression of sepsis, 9 , 10 , 11 , 12 , 13 , 14 the proportions of Th1 and Th17 cells in patients with sepsis remain largely unclear. One previous study suggested that RAR‐related orphan receptor‐γ protein and T‐bet are both reduced in elderly sepsis patients compared with elderly patients with infection alone, indicating decreased Th1 and Th17 cell proportions in elderly septic patients, 15 while another very recent study reported that in sepsis patients, Th1 and Th17 cell proportions are elevated compared with healthy subjects. 16 However, these studies are restricted by their small sample sizes, and Th1, Th17 cell proportions in sepsis patients should be further validated with larger sample sizes. Therefore, we enrolled 210 sepsis patients and 100 HCs to explore Th1 and Th17 cell proportions in them. Data suggested that elevations were found in both Th1 and Th17 cell proportions in sepsis patients compared with controls, which could be explained by that: (1) elevated Th1 and Th17 cell proportions increased IFN‐gamma, IL‐17 and TNF‐alpha levels (as demonstrated by the correlation analysis in Th1 or Th17 cell proportions with IFN‐gamma, IL‐17, and TNF‐alpha), which could lead to increased systematic inflammation and multiple organ injury, 9 , 10 , 11 , 12 , 13 , 14 thus led to sepsis; or (2) sepsis, an infectious disease characterized by the dysregulated immune response to infection, might cause elevated Th1 and Th17 cell proportions. To further clarify whether increased Th1 and Th17 cell proportions were caused by sepsis or they served as the trigger of sepsis, further prospective studies should be conducted. Meanwhile, we also found that increased Th1 and Th17 cell proportions discriminated sepsis patients from healthy subjects well, which implied that they possessed a certain value for supporting the diagnosis of sepsis patients, while further studies should validate that.
Regarding the correlation in Th1 and Th17 cell proportions with sepsis patients’ clinical characteristics, previous studies suggested that in sepsis patients, Th1 and Th17 cell proportions are positively correlated with APACHE II score. 16 In the present study, we found that Th1 cell proportion was strongly correlated with IFN‐γ but weakly correlated with TNF‐α and IL‐17, while Th17 cell proportion showed faint correlation with IFN‐γ, but strong correlations with TNF‐α and IL‐17. These data suggested that Th1 and Th17 cell proportions were positively correlated with inflammation in sepsis patients. Besides, partly in line with that previous study, we found that Th1 cell proportion was weakly correlated with APACHE II score, while Th17 cell proportion was positively associated with both APACHE II score and SOFA score with numerically higher correlation coefficients. These data indicated that Th17 cell proportion had a stronger correlation with disease severity in sepsis patients (which could also be revealed by that numerically higher correlation coefficients were observed in Th17 cell proportion with APACHE II score and SOFA score). Possible explanations for our data might be that: (1) increased Th1 and Th17 cell proportions might release related cytokines to induce systematic inflammation that exacerbated the severity of sepsis, 9 , 11 , 12 and Th17 cells might have stronger effect; (2) elevated Th1 and Th17 cell proportions might also release related cytokines to participate in multiple organ injury, 10 , 13 , 14 thus increased severity of sepsis patients.
A very recent study suggests that Th1 and Th17 cell proportions are associated with increased septic mortality risk. 16 In this study, it was observed that Th1 and Th17 cell proportions were both elevated in septic deaths compared with survivors. Meanwhile, Th1 cell proportion presented a weak predictive value, and its related cytokine IFN‐γ showed no predictive value for septic mortality risk; besides, Th17 cell proportion had good predictive value, and its related cytokines TNF‐α and IL‐17 presented acceptable predictive value for septic mortality. Although these predictive values for septic mortality were not as strong as those of APACHE II score and SOFA score, they might still serve as potential tools to improve the surveillance of septic death, thus enhanced the management for sepsis patients. Moreover, Th17 (but not Th1) cell proportion was positively associated with accumulating mortality in sepsis patients. One possible explanation for our data could be that: Th17 cell proportion showed strong correlations with disease severity, which might indirectly lead to increased mortality risk in sepsis patients. By contrast, Th1 cell proportion showed a relatively weak correlation with disease severity; thus, it might have little effect on mortality risk in sepsis patients. 18
The limitations of this study were as follows: Firstly, this study mainly focused on Th1 and Th17 cell proportions in sepsis patients; considering that other helper T cells and regulatory T cells also exert modulation on adaptive immune response, they may possess potential value in the clinical management of sepsis as well, and further studies should investigate that. Secondly, the specific mechanism of Th1 and Th17 cells in participating the occurrence and progression of sepsis was not investigated; Thirdly, the change of Th1 and Th17 cell proportions during 28 days of follow‐up duration was not investigated for it was not the main objective of this study.
To be collective, Th1 and Th17 cell proportions are increased in sepsis patients compared with HCs, and Th17 cell proportion correlates with worse disease severity, higher inflammation level, and unfavorable prognosis in sepsis patients, which could improve the management to sepsis patients by close surveillance of Th17 cell proportion.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
None.
Yu Liu and Xiaopin Wang contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Liao X, Du B, Lu M, et al. Current epidemiology of sepsis in mainland China. Ann Transl Med. 2016;4(17):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kempker JA, Martin GS. The changing epidemiology and definitions of sepsis. Clin Chest Med. 2016;37(2):165‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet. 2018;392(10141):75‐87. [DOI] [PubMed] [Google Scholar]
- 4. Tiru B, DiNino EK, Orenstein A, et al. The economic and humanistic burden of severe sepsis. Pharmacoeconomics. 2015;33(9):925‐937. [DOI] [PubMed] [Google Scholar]
- 5. Verdonk F, Blet A, Mebazaa A. The new sepsis definition: limitations and contribution to research and diagnosis of sepsis. Curr Opin Anaesthesiol. 2017;30(2):200‐204. [DOI] [PubMed] [Google Scholar]
- 6. Howell MD, Davis AM. Management of sepsis and septic shock. JAMA. 2017;317(8):847‐848. [DOI] [PubMed] [Google Scholar]
- 7. Rimmele T, Payen D, Cantaluppi V, et al. Immune cell phenotype and function in sepsis. Shock. 2016;45(3):282‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rendon JL, Choudhry MA. Th17 cells: critical mediators of host responses to burn injury and sepsis. J Leukoc Biol. 2012;92(3):529‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin CF, Tsai CC, Huang WC, et al. IFN‐gamma synergizes with LPS to induce nitric oxide biosynthesis through glycogen synthase kinase‐3‐inhibited IL‐10. J Cell Biochem. 2008;105(3):746‐755. [DOI] [PubMed] [Google Scholar]
- 10. Pan CH, Kim ES, Jung SH, et al. Tectorigenin inhibits IFN‐gamma/LPS‐induced inflammatory responses in murine macrophage RAW 264.7 cells. Arch Pharm Res. 2008;31(11):1447‐1456. [DOI] [PubMed] [Google Scholar]
- 11. Zhang M, Wang X, Bai B, Zhang R, Li Y, Wang Y. Oxymatrine protects against sepsis‐induced myocardial injury via inhibition of the TNF‐alpha/p38‐MAPK/caspase‐3 signaling pathway. Mol Med Rep. 2016;14(1):551‐559. [DOI] [PubMed] [Google Scholar]
- 12. Marton A, Kolozsi C, Kusz E, et al. Propylene‐glycol aggravates LPS‐induced sepsis through production of TNF‐alpha and IL‐6. Iran J Immunol. 2014;11(2):113‐122. [PubMed] [Google Scholar]
- 13. Cauvi DM, Williams MR, Bermudez JA, Armijo G, De Maio A. Elevated expression of IL‐23/IL‐17 pathway‐related mediators correlates with exacerbation of pulmonary inflammation during polymicrobial sepsis. Shock. 2014;42(3):246‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ding Q, Liu GQ, Zeng YY, et al. Role of IL‐17 in LPS‐induced acute lung injury: an in vivo study. Oncotarget. 2017;8(55):93704‐93711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coakley JD, Breen EP, Moreno‐Olivera A, et al. Dysregulated T helper type 1 (Th1) and Th17 responses in elderly hospitalised patients with infection and sepsis. PLoS ONE. 2019;14(10):e0224276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu D, Peng X, Li P. The correlation between Jun N‐terminal kinase pathway‐associated phosphatase and Th1 cell or Th17 cell in sepsis and their potential roles in clinical sepsis management. Ir J Med Sci. 2020. 10.1007/s11845-020-02382-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315(8):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin MD, Badovinac VP, Griffith TS. CD4 T cell responses and the sepsis‐induced immunoparalysis state. Front Immunol. 2020;11:1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


