Abstract
Background
Orthostatic hypotension is an excessive fall in blood pressure (BP) while standing and is the result of a decrease in cardiac output or defective or inadequate vasoconstrictor mechanisms. Fludrocortisone is a mineralocorticoid that increases blood volume and blood pressure. Fludrocortisone is considered the first‐ or second‐line pharmacological therapy for orthostatic hypotension alongside mechanical and positional measures such as increasing fluid and salt intake and venous compression methods. However, there has been no Cochrane Review of the benefits and harms of this drug for this condition.
Objectives
To identify and evaluate the benefits and harms of fludrocortisone for orthostatic hypotension.
Search methods
We searched the following databases on 11 November 2019: Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase and CINAHL. We also searched trials registries.
Selection criteria
We included all studies evaluating the benefits and harms of fludrocortisone compared to placebo, another drug for orthostatic hypotension, or studies without comparators, including randomized controlled trials (RCTs), quasi‐RCTs and observational studies. We included studies in people with orthostatic hypotension due to a chronic peripheral neuropathy, a central autonomic neuropathy, or autonomic failure from other causes, but not medication‐induced orthostatic hypotension or orthostatic hypotension from acute volume depletion or blood loss.
Data collection and analysis
We used Cochrane methodological procedures for most of the review. We developed and used a tool to prioritize observational studies that offered the best available evidence where there are gaps in the evidence from RCTs. We assessed the certainty of evidence for fludrocortisone versus placebo using GRADE.
Main results
We included 13 studies of 513 participants, including three cross‐over RCTs and 10 observational studies (three cohort studies, six case series and one case‐control study). The included RCTs were small (total of 28 participants in RCTs), short term (two to three weeks), only examined fludrocortisone for orthostatic hypotension in people with two conditions (diabetes and Parkinson disease), and had variable risk of bias (two had unclear risk of bias and one had low risk of bias). Heterogeneity in participant populations, comparators and outcome assessment methods prevented meta‐analyses of the RCTs.
We found very low‐certainty evidence about the effects of fludrocortisone versus placebo on drop in BP in people with diabetes (‐26 mmHg versus ‐39 mmHg systolic; ‐7 mmHg versus ‐11 mmHg diastolic; 1 cross‐over study, 6 participants). For people with Parkinson disease, we found very‐low certainty evidence about the effects of fludrocortisone on drop in BP compared to pyridostigmine (‐14 mmHg versus ‐22.1 mmHg diastolic; P = 0.036; 1 cross‐over study, 9 participants) and domperidone (no change after treatment in either group; 1 cross‐over study, 13 participants).
For orthostatic symptoms, we found very low‐certainty evidence for fludrocortisone versus placebo in people with diabetes (4 out of 5 analyzed participants had improvements in orthostatic symptoms, 1 cross‐over study, 6 participants), for fludrocortisone versus pyridostigmine in people with Parkinson disease (orthostatic symptoms unchanged; 1 cross‐over study, 9 participants) or fludrocortisone versus domperidone (improvement to 6 for both interventions on the Composite Autonomic Symptom Scale‐Orthostatic Domain (COMPASS‐OD); 1 cross‐over study, 13 participants). Evidence on adverse events was also very low‐certainty in both populations, but indicated side effects were minimal.
Observational studies filled some gaps in evidence by examining the effects in larger groups of participants, with more diverse conditions, over longer periods of time. One cohort study (341 people studied retrospectively) found fludrocortisone may not be harmful in the long term for familial dysautonomia. However, it is unclear if this translates to long‐term improvements in BP drop or a meaningful improvement in orthostatic symptoms.
Authors' conclusions
The evidence is very uncertain about the effects of fludrocortisone on blood pressure, orthostatic symptoms or adverse events in people with orthostatic hypotension and diabetes or Parkinson disease. There is a lack of information on long‐term treatment and treatment of orthostatic hypotension in other disease states. There is a need for standardized reporting of outcomes and for standardization of measurements of blood pressure in orthostatic hypotension.
Keywords: Humans; Bias; Diabetes Mellitus; Domperidone; Domperidone/therapeutic use; Dysautonomia, Familial; Dysautonomia, Familial/complications; Fludrocortisone; Fludrocortisone/therapeutic use; Hypotension, Orthostatic; Hypotension, Orthostatic/drug therapy; Observational Studies as Topic; Parkinson Disease; Parkinson Disease/complications; Pyridostigmine Bromide; Pyridostigmine Bromide/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Fludrocortisone for the treatment of orthostatic hypotension
Review question
In people with orthostatic hypotension, does fludrocortisone prevent or reduce a symptomatic drop in blood pressure that occurs with changes in position (for example, from sitting to standing) and does it have unwanted effects?
Background
Orthostatic, or postural, hypotension is a condition in which blood pressure falls when moving from a seated or lying flat position to a standing position. Regular, reproducible symptomatic postural hypotension is an abnormal bodily response.
Orthostatic hypotension can be due to the heart failing to supply enough blood to the brain after a change in position. It can also stem from failures in blood vessel response to a change in position, for which there are many causes. Medications, dehydration, poor physical health and age all contribute to the problem. It is more common among people older than 65 and increases with age.
Symptoms include dizziness, lightheadedness and feeling faint. Some people have more general complaints such as weakness, fatigue, headache, visual blurring, trouble thinking, leg buckling, neck pain, breathlessness or chest pain. Some episodes of postural hypotension result in a faint, fall or 'black‐out'. Symptoms decrease when blood pressure returns to normal, typically when the person sits, lies or, occasionally, falls down.
Fludrocortisone acetate is a man‐made steroid that increases blood volume and improves the ability of blood vessels to respond to changes in position. Fludrocortisone is taken by mouth. It has also been associated with high blood pressure, swelling, congestive heart failure, low potassium (a blood salt), headache, sleeplessness and increased sweating. Other common medications for postural hypotension include midodrine and droxidopa.
Because postural hypotension results from many underlying diseases and conditions, it is important to understand the effect of fludrocortisone depending upon the underlying physical cause (heart pumping action or blood vessel reaction), by other underlying disease (Parkinson disease, diabetes, etc.) and by age. In this way, we can better understand whether different groups of people will be helped or harmed by using this medication.
We reviewed all the evidence about the effects of use of fludrocortisone in people with orthostatic hypotension.
Search date
The evidence is up to date to 11 November 2019.
Study characteristics
After in‐depth screening of the scientific literature, we included 13 studies involving 513 participants. Only three studies used the most rigorous research design (randomized controlled trial [RCT]). Additionally, two non‐RCTs filled gaps in the evidence. The randomized studies were small (a total of 28) and short‐term (lasting three weeks or less). The randomized studies found that fludrocortisone reduced blood pressure drop compared to placebo (an inactive medication) in people with severe diabetic neuropathy, and compared to a medication called pyridostigmine when used in people with Parkinson disease. Among people with Parkinson disease, overall orthostatic symptoms were unchanged when fludrocortisone was compared to pyridostigmine but symptoms were reduced by both fludrocortisone and domperidone (an alternative medication) when compared. Side effects seen in these studies were minimal.
Study funding sources
Of the five studies that offered the best available evidence, two were at least partially funded by research foundations (such as the Dysautonomia Foundation), one had fludrocortisone tablets provided by the pharmaceutical company, and two did not report on funding.
Key results and certainty of the evidence
Based on considerable limitations in the available evidence, we cannot draw firm conclusions on the use of fludrocortisone for orthostatic hypotension in people with diabetes or Parkinson disease. The available evidence on blood pressure drop, orthostatic symptoms and adverse events in people with diabetes or Parkinson disease was very low‐certainty. We still need more and better information about long‐term use and about the effects of this medication among people with diseases besides Parkinson disease and diabetes.
Summary of findings
Summary of findings 1. Fludrocortisone compared to placebo for people with diabetes and orthostatic hypotension.
| Fludrocortisone compared to placebo for people with diabetes and orthostatic hypotension | |||||
|
Patient or population: people with diabetes and orthostatic hypotension
Setting: clinic for people with diabetes Intervention: fludrocortisone Comparison: placebo | |||||
| Outcomes | Placebo | Fludrocortisone | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Blood pressure outcomes Follow‐up: mean 3 weeks | Mean blood pressure drop was 39/11 mmHg | Mean blood pressure drop was 26/7 mmHg | 6 (1 RCT)a | Very low | The evidence is very uncertain about the effect of fludrocortisone on blood pressure outcomes in people with diabetes and orthostatic hypotension. |
| Orthostatic symptoms assessed with: self‐report Follow‐up: 3 weeks | Not reported | 4 out of 5 participants had improvements in orthostatic symptoms | 6 (1 RCT)a | Very low | The evidence is very uncertain about the effect of fludrocortisone on orthostatic symptoms in people with diabetes and orthostatic hypotension. |
| Frequency and severity of postural lightheadedness | Not measured | Not measured | 0 | No studies | |
| Frequency of syncope | Not measured | Not measured | 0 | No studies | |
| Quality of life | Not measured | Not measured | 0 | No studies | |
| Serious or severe adverse events Follow‐up: 3 weeks |
Not reported | No serious or severe adverse events reported | 6 (1 RCT)a | Very low | The evidence is very uncertain about serious or severe adverse events with fludrocortisone in people with diabetes and orthostatic hypotension. |
| RCT: randomized controlled trial | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | |||||
aWe downgraded the evidence three times: twice for imprecision (evidence based on 1 RCT of 6 people), and once for study limitations (process of randomization, allocation and masking not reported; 16% attrition in fludrocortisone group versus unclear attrition in placebo group).
Background
Description of the condition
Orthostatic, or postural, hypotension is a condition in which a prolonged fall in blood pressure occurs upon standing after sitting or lying down. The consensus definition is a sustained reduction of systolic blood pressure (BP) of at least 20 mmHg or diastolic BP of 10 mmHg within three minutes of standing or head‐up tilt to at least 60° on a tilt table (American Autonomic Society, American Academy of Neurology, Gibbons 2017). Orthostatic hypotension is detected by clinical examination, and may or may not cause symptoms (Freeman 2011). In people who have high blood pressure when lying down (supine hypertension), a reduction in systolic BP of 30 mmHg may be a more appropriate criterion for orthostatic hypotension because the size of the orthostatic BP fall is dependent on the baseline BP (Frith 2015).
The prevalence of orthostatic hypotension in people 65 years and older has been reported to be in the range of 5% to 30%, with higher prevalence linked to increasing age of the study population (Low 2008; Tilvis 1996). Orthostatic hypotension is more common in the institutionalized elderly (Freeman 2011). Orthostatic hypotension is frequently a comorbid factor for falls in the elderly (Gupta 2007). Recent evidence has suggested that traditional symptoms of orthostatic hypotension may be present in less than 50% of people with severe, chronic orthostatic hypotension (Arbogast 2009).
Orthostatic hypotension is the result of an excessive fall of cardiac output or defective or inadequate vasoconstrictor mechanisms, for which there are many causes and contributing mechanisms (Freeman 2011). Medications such as antihypertensive drugs and diuretics, antiparkinsonian medication (dopamine and dopamine agonists), antidepressants (particularly tricyclic agents), and sympatholytic agents and other vasodilators can cause or contribute to orthostatic hypotension (Gibbons 2017). Orthostatic hypotension can also occur because of inadequate fluid intake, particularly in elderly people. Other variables that affect orthostatic hypotension are gender, cardiac and vascular stiffness, prolonged lying, high ambient temperature and deconditioning (loss of strength and function that occurs when a person is very inactive) (Freeman 2011).
The autonomic nervous system contributes to the regulation of body states and processes, including blood pressure. Orthostatic hypotension can also therefore occur in people with neurodegenerative disorders affecting the autonomic nervous system. These conditions include multiple system atrophy (MSA), Parkinson disease and pure autonomic failure, and some neuropathies and ganglionopathies that affect autonomic nerves (Freeman 2007; Freeman 2011; Kaufmann 2003).
Characteristic symptoms of orthostatic hypotension include dizziness, lightheadedness, presyncope (faintness) and syncope (loss of consciousness) (Freeman 2008). Loss of consciousness is typically of gradual onset over seconds to minutes but can occur suddenly (Freeman 2011). Some people present with more general complaints such as weakness, fatigue, headache, visual blurring, cognitive slowing, leg buckling, neck pain, orthostatic dyspnea (breathlessness) or chest pain (Freeman 2008).
Symptoms of orthostatic hypotension subside as BP normalizes, typically when the person returns to a seated or a lying position (Wieling 1993).
Description of the intervention
Fludrocortisone acetate is a synthetic adrenocortical steroid with high mineralocorticoid activity that results in an increase in plasma volume and increased sensitivity of α‐adrenoreceptors. Fludrocortisone is dosed by mouth at between 50 µg and 300 µg per day. Adverse events associated with fludrocortisone include hypertension, edema (swelling), congestive heart failure, hypokalemia (low potassium), headache, insomnia and increased sweating.
Other than fludrocortisone, common therapies for orthostatic hypotension include midodrine and droxidopa. Midodrine is an α‐1 adrenergic agonist and is the most well established sympathomimetic agent used to treat orthostatic hypotension, approved by the US Food and Drug Administration (FDA) for this purpose (Low 1997). Its mechanism of action is direct stimulation of vascular α‐adrenergic receptors with a resultant increase in flow resistance and BP (McTavish 1989). Droxidopa (L‐threo‐dihydroxyphenylserine) or L‐DOPS, is a synthetic amino acid also FDA approved for treatment of orthostatic hypotension. Droxidopa is a precursor for norepinephrine requiring enzymatic conversion to the active amine by aromatic l‐amino acid decarboxylase. It raises norepinephrine levels in postganglionic sympathetic neurons and enhances central nervous system norepinephrine production. The increase in circulating norepinephrine may also act as a hormone with BP‐raising activity (Jordan 1998).
Less commonly‐used therapies include direct or indirect adrenoreceptor agonists and other sympathomimetic agents phenylephrine, ephedrine, pseudoephedrine, phenylpropanolamine, methylphenidate and dextroamphetamine (Biaggioni 1987; Davies 1978b; Freeman 1999; Ghrist 1928; Jordan 1998). Treatments with any of the sympathomimetic agents can result in severe supine hypertension, so should not be used while lying down (Sandroni 2001). Pyridostigmine may act through potentiation of sympathetic cholinergic ganglionic transmission, leading to increased vascular tone, but only in the upright position (Singer 2003). The benefit of this therapy is a lower risk of supine hypertension than is seen with agents that result in direct vasoconstriction (Schondorf 2003).
Additional agents that may have efficacy in the treatment of orthostatic hypotension through other mechanisms include erythropoietin, non‐steroidal anti‐inflammatory agents, octreotide, and vasopressin (Armstrong 1991; Freeman 2003).
How the intervention might work
Fludrocortisone acetate alters BP through a variety of mechanisms including mineralocorticoid‐induced sodium and water retention. Fludrocortisone binds to the aldosterone receptor, which increases activity of the distal tubule of the kidney, causing enhanced sodium ion and water transport into the plasma, and increasing urinary excretion of potassium and hydrogen ions (Campbell 1975b). Its effect on alleviating orthostatic hypotension is largely thought to be modulated through these actions. With chronic use, a BP‐raising effect may persist, even though sodium retention and overall plasma volume normalizes through increased peripheral vascular resistance (Armstrong 1991; Chobanian 1979b; Freeman 2003; Hoeldtke 1993). Other potential mechanisms may include sensitization of the vasculature to angiotensin II and norepinephrine (Hickler 1959; Van Lieshout 2000). Because orthostatic hypotension is a condition that results from multiple underlying diseases and conditions, it is important to analyze the effect of fludrocortisone by mechanism (peripheral versus central autonomic failure), by condition (Parkinson disease, diabetes, amyloid‐induced orthostatic hypotension, pure autonomic failure, Lewy body disease, or MSA), and by age, as both the benefits and potential for harm may vary by subgroups. As an example, the BP‐raising properties of fludrocortisone could potentially increase the risk of supine hypertension in certain subgroups, particularly the elderly.
Why it is important to do this review
There is inconsistent guidance on the use of fludrocortisone as first‐line pharmacological treatment for orthostatic hypotension (Lanier 2011; Moya 2009; NICE 2013; Zesiewicz 2010). The evidence for the efficacy of fludrocortisone has not been evaluated in a Cochrane Systematic Review. This review evaluates the current evidence available on the efficacy of fludrocortisone for the treatment of orthostatic hypotension to better inform treatment recommendations and to highlight gaps in knowledge that require further investigation.
Objectives
To identify and evaluate the benefits and harms of fludrocortisone for orthostatic hypotension.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) and quasi‐RCTs of fludrocortisone for orthostatic hypotension including cross‐over and cluster‐randomized trials. Quasi‐RCTs are studies that closely mirror the design of a randomized trial, but use systematic rather than truly random methods of allocation (e.g. allocation by clinic date, date of birth, case record number, etc.). When data from RCTs and quasi‐RCTs were lacking or at high risk of bias (that is, there are gaps in the evidence), we included certain types of non‐randomized studies (NRSs). Current guidance for treating orthostatic hypotension is based on a small number of short‐term RCTs (NICE 2013). Therefore, summarizing and evaluating NRSs could provide decision‐makers with information on long‐term benefits and harms, as well as dosing and other clinical approaches not adequately addressed in these trials. We utilized a hierarchical strategy to include NRSs in our analysis, to prioritize studies offering a higher certainty of evidence. To reduce the likelihood of NRSs' susceptibility to bias, we included prospective NRSs that compared distinct groups of participants who did and did not receive fludrocortisone (e.g. cohort or case‐control). If these sorts of studies were not available, we included other types of uncontrolled NRSs with more than one participant (e.g. case series). All our selected PICOs (population, intervention, comparators and outcomes) applied to RCTs, quasi‐RCTs and NRSs, with the exception of comparators, as we included uncontrolled NRSs if evidence from RCTs, quasi‐RCTs and controlled NRSs were lacking or at high risk of bias. We did not apply any limitations by language or publication status.
Types of participants
We included all participants with orthostatic hypotension due to a chronic (non‐acute, defined as present for at least eight weeks or more) peripheral neuropathy, a central autonomic neuropathy or autonomic failure from other causes (e.g. amyloid‐induced or due to diabetes). We excluded from evaluation participants with medication‐induced orthostatic hypotension and those with orthostatic hypotension from acute volume depletion or blood loss. We included both children and adults of any age.
We included studies that included only a subset of eligible participants if outcomes for eligible participants were reported separately, or at least 75% of the study population consisted of relevant participants.
Types of interventions
We included fludrocortisone compared to placebo, no treatment, another treatment or combination of treatments. If more than one treatment was included in the treatment arm, the same treatment (other than the fludrocortisone) had to be included in the comparator arm. In the event data from RCTs, quasi‐RCTs and controlled NRSs were lacking or at high risk of bias, we accepted studies without a comparator. Our preferred minimum treatment duration was three weeks; however, we accepted all durations of treatment. We accepted all treatment dosages.
Types of outcome measures
We included all studies that met the above criteria. For all outcomes, we gave preference to studies that measured the outcome at least three weeks after initiating treatment; however, we accepted all time points. We also gave preference to studies that use validated scales for measurement of outcomes; however, we accepted all measurements. We also included studies that fit our inclusion criteria but did not include our prespecified outcome criteria, although these were not included in any formal analysis.
We defined the frequency of an event (i.e. postural lightheadedness; dizziness or presyncope; syncope; and falling) as the number of times an event occurs during a given time period, which converted to a standard measurement (number of events per week).
The pre‐treatment to post‐treatment period refers to the time immediately before treatment initiation (pre‐treatment) to the last time a particular outcome measurement was collected for participants actively receiving treatment (post‐treatment), even if participants continued to receive treatment after the final measurement was collected.
We pre‐specified our definitions of minimum important differences. For systolic or diastolic BP, as no specific numerical value has been validated as the minimum important difference for change, we did not define a clinically important reduction in the change in BP but reported the change with 95% confidence intervals (CI). For severity of postural lightheadedness, dizziness or presyncope, quality of life or overall well‐being, functionality, and overall orthostatic symptoms, we accepted study definitions of minimum important difference when validated scales were used. When unvalidated measurements were used, we considered a 10% improvement as the minimum important difference. For change in frequency of postural lightheadedness, dizziness or presyncope, syncope, and falling, we considered a reduction of one event per week as the minimum important difference. For adverse events, we considered a statistically significant reduction in the likelihood of an event occurring as an important difference. We accepted any form of data aggregation (including but not limited to: median, mean or percentage of participants meeting a certain target).
Primary outcomes
BP outcomes: change between pre‐ and post‐treatment systolic BP and diastolic BP in the upright (standing or tilt) position, two to three minutes after standing or tilting was our preferred BP measurement after at least three weeks of treatment. However, we accepted any BP measurement method, including the pre‐ to post‐treatment change in the drop in systolic BP and diastolic BP after moving from supine to upright, or from sitting to upright, and measurements taken outside the two to three minute range (that is, any time between one and five minutes).
Secondary outcomes
Change in frequency and severity of postural lightheadedness, dizziness, or presyncope between pre‐ and post‐treatment, measured as number of events per week (frequency) and with a validated tool or other measurement (severity)
Change in frequency of syncope between pre‐and post‐treatment, measured as number of events per week
Change in frequency of falling between pre‐ and post‐treatment, measured as number of events per week
Change in quality of life or overall well‐being between pre‐ and post‐treatment, measured with a validated scale (e.g. 36‐item or 12‐item Short Form Health Survey (SF‐36 or SF‐12), EuroQol five dimensions questionnaire (EQ‐5D), Clinical Global Impression rating scale (CGI), Patient Global Impression of Change (PGIC)) or other measurement
Change in functionality between pre‐ and post‐treatment, measured with a validated scale or other measurement
Change in overall orthostatic symptoms between pre‐ and post‐treatment measured with a validated scale (e.g. Composite Autonomic Symptom Scale‐Orthostatic Domain (COMPASS‐OD)) or other similar measurement
Adverse events due to treatment, regardless of association to treatment. We analyzed categories of: all adverse events, severe or serious adverse events that lead to hospitalization or death, and adverse events leading to cessation of treatment.
Search methods for identification of studies
Electronic searches
We searched the following databases on 11 November 2019.
Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web; Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL) via CRS‐Web (Appendix 2).
MEDLINE Ovid (1946 to November 2019; Appendix 3).
Embase Ovid (1974 to November 2019; Appendix 4).
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to November 2019; Appendix 5).
We also searched MEDLINE and CENTRAL on 30 October 2017 (search strategies available in Appendix 6).
Searching other resources
We searched US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov); the World Health Organization International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch); and the ISRCTN registry (www.isrctn.com) on 23 September 2019. We also reviewed the bibliographies of the relevant RCTs and non‐randomized studies identified as well as relevant textbooks. We contacted known experts in the field.
Data collection and analysis
Selection of studies
Two review authors, including one clinician (YA or KAC) and one methodologist (KP or SV), independently reviewed titles and abstracts retrieved by the searches to identify relevant articles using the eligibility criteria described above. We retrieved full‐text articles of potentially relevant citations and both reviewers independently assessed them for inclusion. If there was disagreement, a third review author (MH, CHG, or SRR) adjudicated. There were no language restrictions and we translated non‐English articles into English as needed. We developed and used the Portland Observational Study Screening Tool for Interventions (POST‐I) (Figure 1) to identify observational studies that offered the best evidence (Veazie 2019).
1.
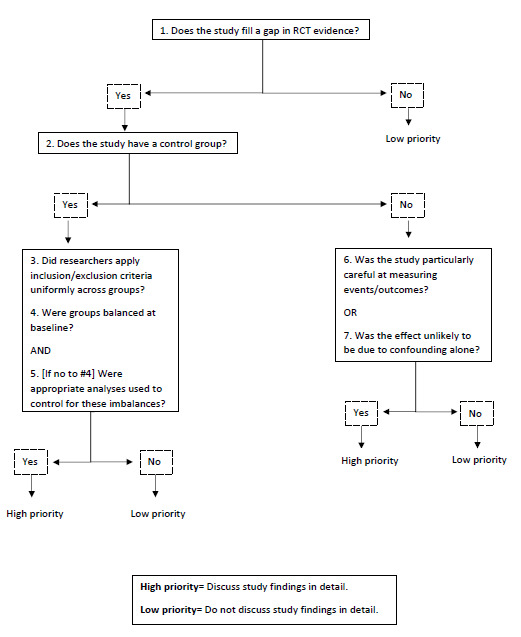
Portland Observational Study Screening Tool for Interventions (POST‐I)
Data extraction and management
Two review authors, including one clinician (YA or KAC) and one methodologist (KP or SV), independently extracted data on population characteristics using a data extraction form that had been piloted. We gathered information on interventions, comparators, participant characteristics, outcomes, follow‐up durations, settings, study design, eligibility criteria, conflicts of interest and funding. If there was disagreement, a third review author (MH, CHG, or SRR) adjudicated. We only extracted data from the studies selected as above. One author (SV) entered 'Characteristics of studies' data into Review Manager (RevMan 2020).
Assessment of risk of bias in included studies
Two review authors, including one clinician (YA or KAC) and one methodologist (KP or SV), independently assessed risk of bias of selected studies according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (hereafter referred to as the Cochrane Handbook;Higgins 2017). For RCTs, we addressed the following domains as described in the Cochrane Handbook: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias. For non‐randomized studies, we used the Risk of Bias in Non‐randomized Studies of Interventions (ROBINS‐I) assessment tool to assess bias due to confounding, participant selection, classification of interventions, deviation from intended interventions, missing data, outcome measurement and reporting of results (Sterne 2016). Key confounders included: disease state; severity of underlying illness; and presence of cardiac disease, smoking, diabetes or other co‐morbidities. Relevant co‐interventions included both non‐pharmacological interventions (such as wearing compression socks, maintaining hydration, sleeping with the head elevated, and behavioural changes such as rising slowly) and pharmacological interventions (such as direct or indirect sympathomimetic pressor agents). If there was disagreement about judgments of risk of bias, a third review author (MH, CHG or SRR) adjudicated.
Measures of treatment effect
We analyzed all the primary and secondary outcomes under consideration. Because we did not identify two sufficiently similar studies, we did not undertake any meta‐analysis in this version of the review. We used our clinical and methodological expertise when examining included studies’ populations, interventions, comparators and outcomes to determine if studies were adequately homogeneous. See Appendix 7 for details of our planned meta‐analyses from our protocol.
Unit of analysis issues
The unit of analysis was the participant. For RCTs we took into account both the level at which randomization occurred, and the trial design, such as cross‐over trials, cluster‐randomized trials and multiple observations for the same outcome. For cross‐over trials, we preferred results from the first randomization period when available. For cluster‐randomized trials, we only included data from those studies that used statistical methods that correctly account for the clustering in the data. For studies with multiple observations of the same outcome, we included all time points. We intended to conduct separate meta‐analyses for each intervention‐comparator pair of interest if at least two adequately homogeneous studies were identified.
Dealing with missing data
In the case of missing data that we could identify, we requested data from study authors. We analyzed the potential impact of missing data as part of the 'Risk of bias' assessment in included studies.
Assessment of heterogeneity
Because we did not identify sufficiently similar data to compare, we did not formally assess heterogeneity using the Chi² test and I² statistic (Higgins 2003).
Assessment of reporting biases
Because we did not identify sufficiently similar data to create a funnel plot, we did not formally assess reporting bias.
Data synthesis
Because we did not identify sufficiently similar studies to compare, we did not combine data from included RCTs in meta‐analyses. We thus used a narrative synthesis approach to review the literature. We grouped results by population groups, and organized findings so that evidence from the studies with the highest certainty of evidence appears first. In the review, for each population group, we first described findings from RCTs, then we described results from NRSs that offered the best available evidence. Then we briefly described the studies that do not provide the best evidence, including the reason why.
Subgroup analysis and investigation of heterogeneity
Because we had insufficient data on subgroups, we did not conduct formal subgroup analyses.
Sensitivity analysis
Because we identified such limited evidence, we did not perform formal sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
Two review authors, including one clinician (YA or KAC) and one methodologist (KP or SV), independently rated the certainty of the evidence for the important outcomes across studies according to the GRADE approach, which considers risk of bias (study limitations), consistency, precision, directness, and reporting bias as criteria for downgrading the evidence, and a large magnitude of effect, dose response, and the effect of all possible confounding would be to reduce the effect, as criteria for upgrading the evidence (GRADEPro GDT). We justified all decisions to downgrade or upgrade the certainty of evidence using footnotes and we made comments to aid the reader's understanding of the review where necessary. If there was disagreement, a third review author (MH, CHG, or SRR) adjudicated.
We summarized strength of evidence ratings in a 'Summary of findings' table using GRADEpro GDT software. The 'Summary of findings' table includes the following outcome measurements for our primary comparison of interest (fludrocortisone versus placebo).
BP outcomes: change in systolic BP and diastolic BP between pre‐ and post‐treatment, as measured in the upright (standing or tilt) position, two to three minutes after standing/tilting.
Change in frequency and severity of postural lightheadedness, dizziness or presyncope between pre‐ and post‐treatment, measured as number of events per week (frequency) and through a validated scale (severity).
Change in frequency of syncope between pre‐ and post‐treatment, measured as number of events per week.
Change in quality of life or overall well‐being between pre‐ and post‐treatment, measured through validated scales (e.g. SF‐36 or SF‐12, EQ‐5D, CGI, or PGIC).
Change in overall orthostatic symptoms between pre‐ and post‐treatment using a validated scale (e.g. COMPASS‐OD) or other similar measurement.
Serious or severe adverse events due to treatment.
We included a 'Summary of findings' table for the comparison of fludrocortisone versus placebo.
Results
Description of studies
Results of the search
Our searches in the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL, and clinical trials registries identified 3004 records. From the 2535 studies remaining after removal of duplicates, we excluded an additional 2411 during title and abstract screening. After screening the full text of the remaining 124 studies, we excluded 111 (see Excluded studies for reasons for exclusion). We included 13 studies of 513 participants (three cross‐over RCTs (Campbell 1975; Schoffer 2007; Schreglmann 2017), three cohort studies (Axelrod 2005; Pathak 2005a; Pathak 2005b), six case series (Campbell 1976; Chobanian 1979; Kochar 1978; Matsubara 1990; Schatz 1976; Ten Harkel 1992) and one case‐control study (Axelrod 1997)).
The three RCTs (Campbell 1975; Schoffer 2007; Schreglmann 2017), one cohort study (Axelrod 2005) and one case series (Ten Harkel 1992) offer the best evidence (see Appendix 8 for details on why these five studies were selected). Specifically, we included and discussed these two observational studies in detail as they met the POST‐I criteria and addressed gaps in RCT evidence by examining long‐term outcomes. A flow diagram summarizing the study selection process is shown in Figure 2.
2.
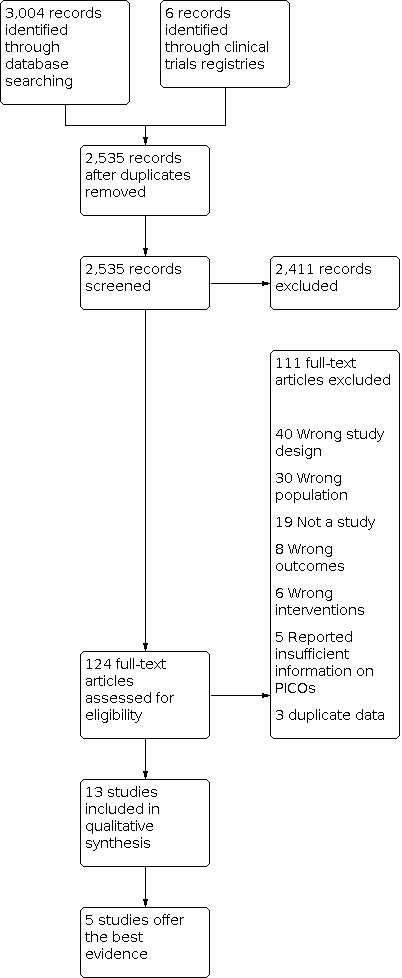
Included studies
We included 13 studies, of which only three were RCTs. We considered two more worthy of inclusion using the POST‐I tool. We 'de‐prioritized' eight studies, and we have not presented the data from these studies in the results apart from a brief description below.
Among our 13 studies, study size varied from five participants (in Kochar 1978) to 341 participants (in Axelrod 2005). Two studies were conducted in people with diabetes (Campbell 1975; Campbell 1976), two in those with Parkinson disease (Schoffer 2007; Schreglmann 2017), two in those with familial dysautonomia (Axelrod 1997; Axelrod 2005), one in those with Shy‐Drager syndrome (Matsubara 1990) and six included multiple conditions including idiopathic orthostatic hypotension (Chobanian 1979; Kochar 1978; Pathak 2005a; Pathak 2005b; Schatz 1976; Ten Harkel 1992). All studies had a mix of men and women (range: 14% to 86% male), except for one study that only included men (Campbell 1975).
Five studies were conducted in the USA (Axelrod 1997; Axelrod 2005; Chobanian 1979; Kochar 1978; Schatz 1976), two studies in the UK (Campbell 1975; Campbell 1976), one in the Netherlands (Ten Harkel 1992), one in Switzerland (Schreglmann 2017), two in France (Pathak 2005a; Pathak 2005b), one in Australia (Schoffer 2007) and one in Japan (Matsubara 1990). Only two studies reported on the participants' race or ethnicity: in one, 80% of participants were white (Kochar 1978), and in another, 100% of participants were Ashkenazi Jewish (Axelrod 1997). Four studies were conducted in specialized clinics, those being for dysautonomia (Axelrod 1997; Axelrod 2005), diabetes (Campbell 1975), or movement disorders (Schoffer 2007). Four were conducted in outpatient settings (Campbell 1976; Pathak 2005a; Pathak 2005b; Schreglmann 2017), one was conducted in both hospital and outpatient settings (Ten Harkel 1992), three were conducted in research hospitals (Chobanian 1979; Kochar 1978; Schatz 1976), and one did not report the setting (Matsubara 1990).
Studies generally did not comment on comorbidities such as smoking and cardiac disease, although two commented that they excluded people with cardiac disease or who were on antihypertensive medications (Campbell 1975; Schreglmann 2017). One study compared fludrocortisone to placebo (Campbell 1975). Eight compared fludrocortisone to another treatment (midodrine (Axelrod 1997), domperidone (Schoffer 2007), pyridostigmine (Schreglmann 2017), indometacin (Kochar 1978), L‐threo‐3,4‐dihydroxyphenylserine (Matsubara 1990 ), or compared fludrocortisone to multiple treatments (Axelrod 2005; Pathak 2005a; Pathak 2005b)). Four were observational studies of fludrocortisone alone (Campbell 1976; Chobanian 1979; Schatz 1976; Ten Harkel 1992). Six studies did not report or reported incomplete details on the regimen of fludrocortisone treatments (Axelrod 2005; Chobanian 1979; Kochar 1978; Pathak 2005a; Pathak 2005b; Schatz 1976). Among studies that did report regimen details on fludrocortisone treatments, the dosage ranged from 0.1 mg to 1.0 mg, administered once to three times daily. All but two studies measured BP (Pathak 2005b; Pathak 2005a), although a range of timings for measurements were used (for example one, three, five or 10 minutes after moving from supine to standing or tilting) and all but three studies measured or commented on orthostatic symptoms (Axelrod 2005; Campbell 1975; Campbell 1976; Chobanian 1979; Kochar 1978; Pathak 2005b; Schoffer 2007; Schreglmann 2017; Ten Harkel 1992). Four studies measured or commented on serious adverse events or survival (Axelrod 2005; Campbell 1976; Pathak 2005a; Pathak 2005b). Additional details of the five included studies that provide the best evidence are available in the Characteristics of included studies section. We have provided basic information on the eight studies that were de‐prioritized in Appendix 9.
Excluded studies
Details of excluded studies are available in the Characteristics of excluded studies tables. The reasons for exclusion were:
wrong design (40);
not a study (19);
wrong population (30);
wrong intervention (6);
no relevant data (8);
reported insufficient information on PICOs (5); and
duplicate data (3).
Risk of bias in included studies
Studies varied in terms of risk of bias. Among the three RCTs, one was at low risk of bias (Schreglmann 2017), and two were at unclear risk of bias (Campbell 1975; Schoffer 2007). Of the two observational studies that provided the best evidence, both were at high risk of bias (Axelrod 2005; Ten Harkel 1992). The primary methodological limitations of the RCTs were a lack of reporting of randomization, allocation and blinding procedures, as well as high overall attrition rates or differential attrition rates between groups.
The two observational studies were at high risk of bias because of their methodology in a number of areas. The primary methodological limitation of the observational studies was inadequate reporting on all important confounders. Detailed risk of bias ratings for prioritized studies are presented in the Characteristics of included studies section.
Allocation
All three RCTs were cross‐over design, meaning each participant received both treatments and, therefore, there were no baseline differences between groups that could have affected results. However, the RCTs were variable in terms of whether they adequately described how random sequences were generated and how allocation was concealed (Campbell 1975; Schoffer 2007; Schreglmann 2017). Bias could have been introduced if participants knew which drug they received during each phase of the study. As a result, the risk of bias due to randomization and allocation procedures ranged between low and unclear for these three studies.
Blinding
Among the RCTs, the risk of bias due to measurement of outcomes was low for objective outcomes such as BP measurements but unclear for subjective outcomes such as orthostatic symptoms (Campbell 1975). An exception to this was when the treatment arm was masked and dummy capsules were used for fludrocortisone and controls to maintain masking, as we felt this adequately controlled for this area of bias (Schoffer 2007; Schreglmann 2017).
Incomplete outcome data
The risk of bias due to incomplete outcome data ranged from low (Schoffer 2007; Schreglmann 2017) to unclear (Campbell 1975) among RCTs. Attrition was between 16% to 24% (Campbell 1975; Schoffer 2007; Schreglmann 2017).
Selective reporting
The risk of bias due to selection of reported results was unclear for two RCTs that did not have protocols (Campbell 1975; Schoffer 2007), and low for the last RCT, which did have a protocol (Schreglmann 2017).
Other potential sources of bias
First period data for Campbell 1975 were not available and there was no assessment for period effect. We identified no other potential sources of bias in Schoffer 2007 or Schreglmann 2017.
Risk of bias in non‐randomized controlled trials
Bias in selection of participants
In the two observational studies (Axelrod 2005; Ten Harkel 1992), clinicians prescribed treatments based on clinical judgment. These decisions were likely influenced by participant characteristics, such as disease severity, that could have affected the likelihood of treatment success. In addition, in one study (Axelrod 2005), the sample was prevalent users, not new users, of fludrocortisone. The risk of bias due to selection bias is therefore high for these two observational studies.
Bias in classification of interventions
The risk of bias due to classification of interventions was low among the observational studies as either the intervention was well described (Ten Harkel 1992), or was appropriately ascertained from an international database which drew from annual examinations of participants (Axelrod 2005).
Bias due to departure from intended interventions
The risk of bias due to departure from intended interventions was low in Axelrod 2005 and unclear in Ten Harkel 1992.
Bias due to measurement of outcomes
Among the observational studies, the risk of bias due to measurement of outcomes was low for survival outcomes (Axelrod 2005) but unclear for other types of outcomes (Ten Harkel 1992).
Bias due to confounding
The observational studies were at unclear risk of bias due to confounding, as there was either no information about or control of key confounders (Axelrod 2005; Ten Harkel 1992).
Bias due to missing data
In one observational study (Axelrod 2005), analyses likely excluded 37% of data (high risk of bias) and in another (Ten Harkel 1992), all outcome data were likely included.
Bias due to selection of reported results
For one observational study, the risk of bias due to selection of reported results was high as analyses were retrospective and could have been the results of multiple analyses (Ten Harkel 1992), while the other was low as it only looked at a prespecified survival outcome (Axelrod 2005).
Effects of interventions
See: Table 1
Diabetes
Fludrocortisone versus placebo in people with diabetes
Campbell 1975 investigated short‐term use of fludrocortisone 0.2 mg compared to placebo in a three‐week cross‐over RCT of six people with diabetic neuropathy and symptomatic orthostatic hypotension. Our certainty in the findings is very low as they are supported only by a single, very small RCT with important methodological weaknesses. See Table 1 for full details on outcomes.
Primary outcome: BP outcomes
We found very low‐certainty evidence about the effects of fludrocortisone on drop in BP in people with diabetes. Campbell 1975, three weeks after initiating treatment, reported mean systolic BP for fludrocortisone versus placebo while lying (180 mmHg versus 149 mmHg; P < 0.05, exact P value not reported) and tilted (154 mmHg versus 110 mmHg; P < 0.005, exact P value not reported), and mean diastolic BP while tilted (88 mmHg versus 76 mmHg; P < 0.05, exact P value not reported). The trial also reported data for fludrocortisone versus placebo on mean drop in BP when moving from lying to tilt (−26 mmHg versus −39 mmHg systolic; −7 mmHg versus −11 mmHg diastolic; 6 participants; very low‐certainty evidence).
Secondary outcomes: postural lightheadedness, syncope, falling, quality of life, functionality, orthostatic symptoms, adverse events
For orthostatic symptoms, we found very low‐certainty evidence for fludrocortisone versus placebo in people with diabetes. Four out of five participants who completed the study reported improvements in symptoms of orthostatic hypotension while on fludrocortisone, although these symptoms were not well described.
Fludrocortisone was associated with peripheral edema in two participants with hypoalbuminemia as well as a trend towards decreased potassium, but no side effects in the other three participants were reported. The study authors did not measure outcomes of quality of life or functionality.
Parkinson disease and related disorders
Clinically meaningful evidence is lacking about the comparative effectiveness of fludrocortisone to manage orthostatic hypotension in people with Parkinson disease. This is primarily because studies compared fludrocortisone to alternatives that are infrequently used in practice; namely, pyridostigmine and domperidone. Thus, the two available RCTs provide information that is not directly relevant to everyday practice (Schoffer 2007; Schreglmann 2017). Additionally, the accuracy and stability of the findings from these studies are questionable because the studies are so small (total N = 22) and short term (two to three weeks). Because no studies compared fludrocortisone to placebo in this population group, we did not include a 'Summary of findings' table for this population, but the evidence is of very low certainty because of multiple GRADE concerns, including very serious indirectness and imprecision.
One two‐week cross‐over RCT tested 0.1 mg of fludrocortisone for one day then 0.2 mg for 11 days versus 30 mg of pyridostigmine for three days then 60 mg for 11 days in nine people (Schreglmann 2017). Schreglmann 2017 reported the mean diastolic BP drop from supine to standing at two weeks for fludrocortisone versus pyridostigmine (‐14 mmHg versus ‐22.1 mmHg; P = 0.036), but neither treatment was associated with improvements in overall orthostatic symptoms as measured through the Orthostatic Hypotension Daily Activities Scale (OHDAS) or Orthostatic Hypotension Severity Assessment (OHSA).
The other cross‐over RCT tested 0.1 mg of fludrocortisone compared to 10 mg of domperidone in 13 people for three weeks (Schoffer 2007). It reported that neither domperidone nor fludrocortisone were associated with improvements in drop in systolic BP or diastolic BP from baseline, but both were associated with improved symptoms of orthostatic hypotension (Composite Global Impression of Change) (improvement to 1 for both); and the orthostatic domain of the Composite Autonomic Symptom Scale (COMPASS‐OD) (improvement to 6 for both). Side effects were minimal in both studies: neither of the two studies detected hypokalemia, and one detected supine hypertension (Schreglmann 2017).
Familial dysautonomia
We found no RCTs of fludrocortisone in people with familial dysautonomia, but did find one cohort study (Axelrod 2005), and one case‐control study (Axelrod 1997). We included Axelrod 2005 as it currently fills a gap in the evidence. We de‐prioritized Axelrod 1997 as it was lower in the hierarchy and will be included in the discussion.
When fludrocortisone use in familial dysautonomia was compared to those who had never taken it, fludrocortisone was associated with better survival time (median 32.8 years versus 22.2 years), improved mean supine systolic BP and standing systolic BP after initiating treatment (98 mmHg versus 117 mmHg supine BP; 77 mmHg versus 64 mmHg standing BP at five minutes) and reduced dizziness, in a large retrospective registry study of 341 people with familial dysautonomia (Axelrod 2005). Fludrocortisone was not associated with improvement in syncope (syncope recurred in 38% of people on fludrocortisone who had syncope prior to the study, and 19% of those who did not have syncope prior to the study developed it). However, important limitations of these findings include that information was lacking on baseline severity of disease and whether there were differences in severity between treatment groups that may have confounded the effects of fludrocortisone. Because no studies compared fludrocortisone to placebo in this population group, we did not include a 'Summary of findings' table for fludrocortisone compared to placebo for people with familial dysautonomia.
Axelrod 2005 included participants with a mean standing BP lower than 60 mmHg, or the presence of syncope or presyncope, or both. The intent of the study was to examine the effects of fludrocortisone on mortality and other long‐term outcomes associated with familial dysautonomia. The strengths of this study are its large sample size (N = 341), long follow‐up period (nearly 40 years), and careful analysis methods. Overall, we trust the estimates on survival but are less certain about the estimates on BP change in this study. As an outcome, survival is less likely to be affected by biases in study design than BP. The authors of Axelrod 2005 were also more careful about how they measured and calculated survival than BP. For example, authors did not report the mean time from initiation of treatment at which BP drop was measured, making it difficult to determine the applicability of the results. However, it should be noted that both outcomes were at risk of confounding bias, as authors acknowledge that assignment to the fludrocortisone group was likely based on clinical characteristics such as symptom severity.
Studies in people with various forms of orthostatic hypotension
Five studies included a variety of participants with different forms of orthostatic hypotension, including Parkinson disease, pure autonomic failure, MSA, Lewy body disease, secondary diabetic and nondiabetic peripheral neuropathies, autonomic failure, undifferentiated nervous system disease and amyloidosis (Chobanian 1979; Pathak 2005a; Pathak 2005b; Schatz 1976; Ten Harkel 1992). We included Ten Harkel 1992 as it currently fills a gap in evidence, but we de‐prioritized the remaining four studies as they were lower in the hierarchy (Chobanian 1979; Pathak 2005a; Pathak 2005b; Schatz 1976). We discuss these studies in the Discussion.
Ten Harkel 1992 is a case series that reported that fludrocortisone increased standing BP both acutely (increase from 58/42 mmHg to 95/57 mmHg) and in long‐term follow‐up (99/63 mmHg) (mean of 14 months, range 8 to 70 months). Participants in this study experienced some ankle edema and hypokalemia but otherwise no serious long‐term harms.
Discussion
Summary of main results
Our review identified little useful, and no high certainty, evidence for evaluating the benefits and harms of fludrocortisone for orthostatic hypotension. Randomized controlled trials (RCTs) were few (N = 3; Campbell 1975; Schoffer 2007; Schreglmann 2017), very small (28 participants in total) and short term (two to three weeks). Use of meta‐analysis to combine data across trials was not possible because of heterogeneity in participant populations, comparators and outcome assessment methods.
The evidence about the effects of fludrocortisone on BP drop and orthostatic symptoms compared to placebo in people with diabetes, and to pyridostigmine and domperidone in people with Parkinson disease was very uncertain. The evidence from these trials on adverse events of fludrocortisone is also very uncertain.
A hypothetical concern for the use of mineralocorticoids is risk of hypokalemia. In one RCT, fludrocortisone was associated with peripheral edema in two people with hypoalbuminemia as well as a trend towards decreased potassium, but no other unusual side effects (Campbell 1975). Neither of the two studies conducted in people with Parkinson disease detected hypokalemia. Supine hypertension is another potential unintended side effect of treatment. While supine hypertension is harder to define, a significant increase in supine systolic BP was noted in two of three studies (Campbell 1975; Schreglmann 2017). Overall, however, adverse events were tolerable.
Because of gaps in RCT evidence, we also included two observational studies in our results. Among those, the most useful information came from one large cohort study that partially filled the RCT gap in evidence by examining the long‐term effects (nearly 40 years) of fludrocortisone in people with familial dysautonomia (Axelrod 2005). Findings from this study indicate that fludrocortisone may not be harmful in the long term for some rare population groups. However, it is unclear if this translates to long‐term improvements in BP drop or a meaningful improvement in orthostatic symptoms. Because this study did not report baseline disease severity or confounders, we cannot conclude that fludrocortisone is associated with long‐term improvements in BP or orthostatic symptoms. However, we can conclude that fludrocortisone may not be harmful to people with familial dysautonomia, as survival was better in those who received fludrocortisone than those who did not. Study authors noted that those who received fludrocortisone had more long‐term problems, which could be due to confounding, as these people were living longer and may naturally have more health problems as they age. Another small case series that followed a mixed population of people with orthostatic hypotension over an average of 14 months found minor side effects of fludrocortisone, including ankle edema and hypokalemia, but no other serious events (Ten Harkel 1992), supporting our conclusion that fludrocortisone is may not be harmful in the long term.
Additional evidence from de‐prioritized studies
Diabetes
For the use of fludrocortisone in diabetic orthostatic hypotension, one very small RCT provided very low certainty evidence of a short‐term benefit but offered no long‐term data (Campbell 1975).
In one de‐prioritized case series of 14 people with diabetes and symptomatic hypotension, initial treatment of 0.1 mg of fludrocortisone for a mean of 12 months (range: 6 to 30 months) was associated with increased standing systolic BP and diastolic BP, as well as decreased BP drop, but no increase in supine BP (Campbell 1976). The study also reported that 13 out of 14 participants noted an improvement in symptoms. However, this study had major methodological limitations, including insufficient information about participants’ baseline characteristics and stability of other potential conditions, or treatments to rule out potential sources of important confounding, or both.
Movement disorders
The two RCTs exploring orthostatic hypotension in Parkinson disease were very limited, as above.
There are no RCTs or high quality evidence for the use of fludrocortisone in multiple system atrophy (MSA). The evidence is limited to two small, de‐prioritized studies (total N = 13) that lacked concurrent comparison groups and adequate control for important confounding due to participant‐level and temporal trend factors (Kochar 1978; Matsubara 1990). On the fourth day of treatment in four people with MSA, fludrocortisone was associated with increased systolic BP and diastolic BP and symptom improvement in two participants, while indomethacin was associated with improvements for all four participants (Kochar 1978). In nine people with MSA, two weeks of fludrocortisone was associated with improvements in BP drop and L‐DOPS (droxidopa) was associated with improvements in both BP drop and cerebral blood flow when moving from supine to sitting (Matsubara 1990).
Familial dysautonomia
While Axelrod 2005 provided some data on the possible effect of fludrocortisone on survival, Axelrod 1997 was de‐prioritized as it fell lower in the evidence hierarchy. This study compared 10 participants with familial dysautonomia to eight healthy control participants on measures of BP, heart rate, and corrected QT Interval (QTc) to determine if autonomic dysfunction involved peripheral vascular integrity in addition to cardiac integrity (Axelrod 1997). One hour after receiving fludrocortisone, participants with familial dysautonomia had decreased mean BP in both supine and tilt position, while one hour after receiving midodrine, participants had increased supine BP and decreased tilt BP. A strength of Axelrod 1997 is that it provides the only evidence about the most relevant comparison of fludrocortisone versus midodrine. However, it is very short term and reported baseline differences between intervention groups that were not adequately controlled for in analyses. Therefore, the findings of Axelrod 1997 should be considered very low certainty.
Various forms of orthostatic hypotension
One case series provided limited information on long‐term harms of fludrocortisone among those with orthostatic hypotension due to a variety of conditions (Ten Harkel 1992). Four additional, de‐prioritized studies provide additional data on populations with a range of different conditions and orthostatic hypotension (Chobanian 1979; Pathak 2005a; Pathak 2005b; Schatz 1976). Two are cohort studies (Pathak 2005a; Pathak 2005b), and two are case series (Chobanian 1979; Schatz 1976).
One case series found fludrocortisone increased standing BP in the short term and long term (Chobanian 1979), which aligns with findings from Ten Harkel 1992. A second case series found that fludrocortisone was tolerable for most (18/23) participants with a range of conditions causing orthostatic hypotension (Schatz 1976). Five participants, however, discontinued their treatment due to either recumbent hypertension (N = 4) or congestive heart failure (N = 1). We de‐prioritized these studies as researchers were not particularly careful at measuring outcomes and we could not rule out the possibility that effects were due to confounding.
Additionally, a prospective study of 121 people that had a primary objective of systematically evaluating adverse drug reactions found no significant differences between the frequency of adverse drug reactions in those taking midodrine alone, fludrocortisone alone, or combined treatment (Pathak 2005a). Findings from the other cohort study (N = 31) suggested that heat exposure is a risk factor for worsening orthostatic hypotension and that those taking fludrocortisone may have a higher risk of clinical events than those taking heptaminol, midodrine or combination therapy (Pathak 2005b). A strength of these cohort studies is that they had comparison groups. However, we de‐prioritized them because they had baseline differences between intervention groups that were not controlled for in analyses.
Overall completeness and applicability of evidence
The best evidence on fludrocortisone is limited to those with diabetes, Parkinson disease, familial dysautonomia and those with a variety of conditions underlying orthostatic hypotension, with each supported by one or two studies. We only identified observational evidence for people with MSA, Lewy body disease or amyloid‐induced orthostatic hypotension, which was too low certainty to warrant inclusion in the Results section of this review. The best evidence compared fludrocortisone to placebo, pyridostigmine, domperidone or no fludrocortisone treatment. None of these studies compared fludrocortisone to midodrine, which is typically considered either first‐ or second‐line treatment for orthostatic hypotension. Studies also examined a limited number of outcomes ‐ primarily some measurement of orthostatic BP (such as mean supine BP and standing BP, or mean drop when moving from supine to standing), some measurement of orthostatic or general symptoms (such as through the COMPASS‐OD, OHSA, or CGI) and some measurement of side effects. No studies examined frequency of syncope, falling or quality of life. Studies generally were not long enough to detect serious adverse events, although one long‐term observational study examined mortality (Axelrod 2005). Due to the heterogeneity in populations, comparators and outcomes, we did not have sufficient numbers of similar studies to conduct meta‐analyses.
Given the limited available evidence on the efficacy of fludrocortisone treatment, particularly on long‐term health outcomes, it may be surprising that fludrocortisone is considered first‐line pharmacological treatment for orthostatic hypotension. However, the most promising alternative treatment (midodrine) has historically been associated with side effects, including supine hypertension, which can sometimes be serious (NICE 2013). Our review further iterates that fludrocortisone is likely not harmful in the long term for some patient groups, but we could not determine if it is effective long term for any patients.
Quality of the evidence
The certainty of the evidence is mixed and, in general, low or very low. The main limitations of the RCTs were small sample sizes (less than 15 participants), short duration (three weeks or less), insufficient detail to assess adequacy of randomization, allocation and masking procedures, as well as high overall attrition rates or differential attrition rates between groups, and clinically irrelevant comparator treatments. The main methodological limitation of the observational studies was not reporting or accounting for important confounders that may have influenced results. Additional limitations of the observational evidence include a lack of detailed information on fludrocortisone dosage or mixed dosages across participants, as well as a lack of measurement of patient‐important outcomes.
Potential biases in the review process
We worked to reduce bias by applying a rigorous searching process (including searching multiple databases, trials registries, talking to experts and translating non‐English articles into English) as well as a rigorous article selection process (one clinician and one methodologist reviewed each article for eligibility and resolved disagreements through consensus). Nevertheless, it is still possible we missed eligible studies. It is possible but unlikely that evidence has emerged since we searched for studies, as new studies are infrequent. We also worked to reduce bias in our selection of the best observational evidence by creating the POST‐I screening tool that we could apply across studies. It is possible that other reviewers could have applied a different approach and selected different studies. However, it is likely that inclusion of the additional available observational studies would not meaningfully change our conclusions as the risk of bias was at best high due to use of inadequate methods for selecting participants and controlling for important confounding.
Agreements and disagreements with other studies or reviews
This review includes two RCTs (Campbell 1975; Schoffer 2007) also discussed in Ong 2013, the systematic review that formed the basis of the most recent NICE guidance (NICE 2013). The Ong 2013 review concluded that fludrocortisone and midodrine are associated with improvement in standing and head‐up tilt BP but there is limited evidence of an improvement in other clinical outcomes. Although we cannot safely draw any conclusions, our review adds to the evidence by adding a recently published RCT that reported improvement with fludrocortisone on BP but not orthostatic symptoms in people with Parkinson disease (Schreglmann 2017), as well as the best observational evidence from two observational studies that indicate fludrocortisone may not be associated with long‐term harms for people with familial dysautonomia or those with either diabetes or a variety of conditions underlying orthostatic hypotension (Axelrod 2005; Ten Harkel 1992).
Authors' conclusions
Implications for practice.
The evidence is very uncertain about the effects of fludrocortisone on blood pressure drop, orthostatic symptoms or adverse events in people with orthostatic hypotension and diabetes or Parkinson disease. We still need larger and longer‐term studies that compare fludrocortisone to midodrine, the most promising alternative treatment, on other important health outcomes.
Fludrocortisone is likely to continue to be considered one pharmacological treatment option for people with orthostatic hypotension in the short term. Clinicians should monitor patient progress to determine if an improvement in BP leads to an improvement in other outcomes, and look for potential side effects such as hypokalemia and supine hypertension. Long‐term use should be accompanied by careful monitoring, as there is a paucity of data on long‐term fludrocortisone use.
Implications for research.
This review identified several research gaps. First, there is a need for RCTs evaluating the effects of fludrocortisone on conditions such as Lewy body disease, multiple system atrophy, amyloid‐induced orthostatic hypotension and familial dysautonomia. None of these patient groups which have orthostatic hypotension were evaluated in RCTs, so we cannot say for certain if fludrocortisone is associated with an improvement in outcomes in these groups. Second, there is a need for a head‐to‐head RCT of fludrocortisone versus midodrine, as these represent pharmacological treatment options. Including a third placebo arm would help control for the expectancy effects associated with receiving treatment. Third, RCTs should assess the effect of fludrocortisone on other important patient outcomes such as syncope, falls and quality of life, as these outcomes are important to informing clinical decision‐making. Fourth, RCTs of a longer duration (at least six months) are needed to see if short‐term changes in BP drop correspond to longer‐term changes in other outcomes. Fifth, observational studies should evaluate whether fludrocortisone is associated with adverse events when used for long periods of time for conditions other than familial dysautonomia. Sixth, observational studies should evaluate different clinical approaches to fludrocortisone (e.g. fludrocortisone administered as the initial pharmacological approach versus as a second or third approach if other treatments are unsuccessful). Seventh, observational studies should report on baseline disease severity and measure potential confounders, and RCTs should report randomization, allocation and masking procedures.
Lastly, there is a great need for more rigorous research and use of a standardized, core set of outcomes. Specifically, researchers may want to consider using a higher threshold of orthostatic hypotension for inclusion in research studies than they would use to diagnose people with orthostatic hypotension in practice. There are people that meet clinical criteria for orthostatic hypotension, but who do not require treatment. A higher threshold for orthostatic hypotension (e.g. 30/15 mmHg) would decrease the possibility of overtreatment.
More generally, studies of orthostatic hypotension should use a core set of outcome measures, including both objective and subjective outcomes measures. In terms of objective measures, we propose all authors report mean supine BP and mean standing or tilted BP at three minutes so that data can be combined across studies. For people with such severe orthostatic symptoms they cannot stand for three minutes, BP should be measured as long as they are able to stand or tilt. In addition, the use of real‐world measurements such as ambulatory BP may provide useful information to clinical practice, although we acknowledge the technologies necessary to measure these outcomes can be expensive and are not always readily accessible. Lastly, observational studies should report on baseline disease severity and measure potential confounders, and RCTs should report randomization, allocation and masking procedures.
The use of a core set of subjective measures in orthostatic hypotension is also important. One of the biggest challenges in studying orthostatic hypotension is that orthostatic symptoms are difficult to measure, as people with subtle cognitive symptoms can be unaware of symptoms, unable to describe them or misinterpret one as another (i.e. imbalance as dizziness). This makes it difficult to determine whether differences in symptoms over time are due to treatment, or are just being reported in different ways. The use of standardized measurements (versus asking about overall symptoms) are one way to counteract this challenge. It may be feasible to create these types of standardized measures for more common diseases such as Parkinson disease and diabetes, but more challenging for rare diseases such as familial dysautonomia, as it is challenging to recruit the numbers of participants necessary to validate a measure. Initiatives such as the USA‐based Patient‐Centered Outcomes Research Institute (PCORI) are starting to bringing together researchers working on patient‐reported outcome (PRO) tools, including for rare diseases, for exactly this purpose, and may be a useful resource for researchers going forward. Another major challenge in studying orthostatic hypotension is that it is difficult to recruit sufficient numbers of participants into trials of orphan drug indications. Collaboration between centers may be one way to increase the number of participants in these trials (Schreglmann 2018), although we acknowledge this approach presents its own set of logistical challenges.
What's new
| Date | Event | Description |
|---|---|---|
| 24 May 2021 | Amended | Corrections to sources of support and author affiliation |
History
Protocol first published: Issue 12, 2017 Review first published: Issue 5, 2021
Acknowledgements
We thank Dr Michael Benatar and Dr Hans Lahrmann, co‐authors with SR and CG of the Cochrane protocol 'Pharmacological treatments for postural hypotension' (Gibbons 2010), which we revised to create the protocol for this review. We also thank Cochrane Neuromuscular Group staff including Angela Gunn, Information Specialist, for conducting searches, and Ruth Brassington, Managing Editor, and Anne‐Marie Stephani, acting Assistant Managing Editor, for their editorial guidance. Finally, we thank Ed Reid for his help with editing the review, and gratefully acknowledge Corneliu Antonescu, Katharina Greulich, Hebatullah Mohamed, Roland Buchter, Frank Sandmann, Tess Kristine Dalsbo and Barbara Fowler for their help with translation.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Diseases.
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register (CRS Web) search strategy
Search run on 11 November 2019
#1 orthostatic NEAR hypotens* AND INREGISTER
#2 orthostatic NEAR intolerance AND INREGISTER
#3 postural NEAR hypotens* AND INREGISTER
#4 neurally NEAR mediated NEAR hypotens* AND INREGISTER
#5 autonomic NEAR hypotens* AND INREGISTER
#6 #1 OR #2 OR #3 OR #4 OR #5 AND INREGISTER
#7 fludrocortisone* or florinef* or fluorocortisol* or fluorohydrocortisone* AND INREGISTER
#8 #6 AND #7 and INREGISTER
Appendix 2. Cochrane Register of Controlled Trials (CENTRAL in CRS Web Online) search strategy
Search run on 11 November 2019
#1 orthostatic NEAR hypotens* AND CENTRAL:TARGET
#2 orthostatic NEAR intolerance AND CENTRAL:TARGET
#3 postural NEAR hypotens* AND CENTRAL:TARGET
#4 neurally NEAR mediated NEAR hypotens* AND CENTRAL:TARGET
#5 autonomic NEAR hypotens* AND CENTRAL:TARGET
#6 #1 OR #2 OR #3 OR #4 OR #5 AND CENTRAL:TARGET
#7 fludrocortisone* or florinef* or fluorocortisol* or fluorohydrocortisone* AND CENTRAL:TARGET
#8 #6 AND #7 AND CENTRAL:TARGET
Appendix 3. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to November 08, 2019>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Hypotension, Orthostatic/ (5558)
2 orthostatic hypotens*.mp. (5119)
3 postural hypotens*.mp. (1471)
4 orthostatic intolerance.mp. (1325)
5 neurally mediated hypotens*.mp. (53)
6 autonomic hypotens*.mp. (1)
7 or/1‐6 (9928)
8 (fludrocortisone* or florinef*).mp. (2182)
9 (fluorocortisol* or fluorohydrocortisone*).mp. (262)
10 8 or 9 (2275)
11 7 and 10 (317)
12 exp animals/ not humans/ (4641527)
13 11 not 12 (317)
14 remove duplicates from 13 (317)
Appendix 4. Embase (OvidSP) search strategy
Database: Embase <1974 to 2019 November 08>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 orthostatic hypotension/ (19939)
2 orthostatic hypotens*.tw. (7694)
3 postural hypotens*.mp. (1925)
4 orthostatic intolerance.mp. (2199)
5 neurally mediated hypotens*.mp. (73)
6 autonomic hypotens*.mp. (4)
7 or/1‐6 (23568)
8 (fludrocortisone* or florinef*).mp. (6967)
9 (fluorocortisol* or fluorohydrocortisone*).mp. (170)
10 8 or 9 (7002)
11 7 and 10 (1325)
12 exp animal/ or exp invertebrate/ or animal.hw. or non human/ or nonhuman/ (26597824)
13 human/ or human cell/ or human tissue/ or normal human/ (20333794)
14 12 not 13 (6329961)
15 11 not 14 (1323)
16 limit 15 to (conference abstracts or embase) (1286)
Appendix 5. CINAHL (EBSCOhost) search strategy
Monday, November 11, 2019 11:20:59 AM
S9 S6 AND S7 Limiters ‐ Exclude MEDLINE records
Search modes ‐ Boolean/Phrase 13
S8 S6 AND S7 39
S7 fludrocortisone* or florinef* or fluorocortisol* or fluorohydrocortisone* 168
S6 S1 OR S2 OR S3 OR S4 OR S5 2,166
S5 "neurally mediated hypotens*" 11
S4 "orthostatic intolerance" 292
S3 "postural hypotens*" 233
S2 "orthostatic intolerance" 292
S1 orthostatic N3 hypotens* 1,825
Appendix 6. Additional search strategy
Searches
EMBASE.com
Date Searched: 9 August 2017
#1 'crossover procedure'/exp
#2 'double blind procedure'/exp
#3 'single blind procedure'/exp
#4 'randomized controlled trial'/exp
#5 random*:ti,ab OR crossover*:ti,ab OR ((cross NEXT/1 over*):ti,ab) OR placebo*:ti,ab OR ((doubl* NEAR/1 blind*):ti,ab) OR allocat*:ti,ab
#6 trial:ti
#7 'controlled clinical trial'/exp
#8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
#9 'animal'/exp OR 'invertebrate'/exp OR animal:de OR 'nonhuman'/exp
#10 'human'/exp OR 'human cell'/exp OR 'human tissue'/exp OR 'normal human'/exp
#11 #9 NOT #10
#12 #8 NOT #11
#13 #12 AND [embase]/lim
#14 'orthostatic hypotension'/exp
#15 (orthostatic NEXT/1 hypotension):ti,ab
#16 postural NEXT/1 hypotension
#17 orthostatic NEXT/1 intolerance
#18 neurally NEXT/1 mediated NEXT/1 hypotension
#19
MEDLINE (OvidSP) search strategy
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Ovid MEDLINE(R) Daily Update 29 October 2017
|
Searches 1 Hypotension, Orthostatic/ 2 orthostatic hypotens*.tw,kf. 3 postural hypotens*.tw,kf. 4 orthostatic intolerance.tw,kf. 5 neurally mediated hypotens*.tw,kf. 6 autonomic hypotens*.tw,kf. 7 or/1‐6 8 (fludrocortisone* or florinef*).tw,kf. 9 (fluorocortisol* or fluorohydrocortisone*).tw,kf. 10 8 or 9 11 (exp animals/ not humans/) or (bovine or canine or capra or cat or cats or cattle or cow or cows or dog or dogs or equine or ewe or ewes or feline or goat or goats or horse or horses or invertebrate or invertebrates or macaque or macaques or mare or mares or mice or monkey or monkeys or mouse or ovine or pig or pigs or porcine or primate or primates or rabbit or rabbits or rat or rats or rattus or rhesus or sheep or simian or sow or sows or vertebrate or vertebrates).ti. 12 10 not 11 13 remove duplicates from 12 |
Cochrane Central Register of Controlled Trials (OvidSP EBM Reviews)
September 2017
|
Searches 1 orthostatic hypotens*.ti,ab,kw. 2 orthostatic intolerance.ti,ab,kw. 3 postural hypotens*.ti,ab,kw. 4 "neurally mediated hypotens*".ti,ab,kw. 5 autonomic hypotens*.ti,ab,kw. 6 or/1‐5 7 (fludrocortisone* or florinef*).ti,ab,kw. 8 (fluorocortisol* or fluorohydrocortisone*).ti,ab,kw. 9 or/7‐8 10 and/6‐9 |
Appendix 7. Methods relating to meta‐analysis included in the protocol
Data collection and analysis
Data extraction and management
One author will enter outcome data into RevMan and a second author will check data entry.
Measures of treatment effect
For dichotomous outcome measures, we will calculate the risk ratios (RR) and risk differences (RD) with 95% confidence intervals (CIs). For continuous measures, we will report the mean difference (MD) and 95% CI, or standardized mean difference (SMD) and 95% CI when different measurement scales are used for the same outcome. We will use the Cochrane statistical package, Review Manager 5 (RevMan 5; RevMan 2020) for data analysis.
Dealing with missing data
If data are unavailable, we will assess whether data are likely to be missing at random. If yes, we will only analyze the available data. If data are not missing at random, we will perform sensitivity analyses to assess how sensitive results are to imputations of the missing data, with all good outcomes, poor outcomes and the mean. We will then analyze the potential impact of missing data as part of the 'Risk of bias' assessment in included studies.
Assessment of heterogeneity
We will assess heterogeneity using the Chi² test and I² statistic (Higgins 2003). Where significant heterogeneity is present, we will attempt to determine potential reasons by examining population and study characteristics and perform subgroup analyses to explore their influence when possible (Deeks 2020).
Assessment of reporting biases
For quantitative analyses, we will inspect forest plots and prepare funnel plots to look for evidence of reporting bias. We will present funnel plots if 10 or more studies are identified (Page 2020).
Data synthesis
When available and sufficiently clinically homogeneous, we will combine data from included RCTs in meta‐analyses. As we are anticipating some natural heterogeneity among the study populations, we have chosen a random‐effects model for our statistical analyses. We will conduct analyses using RevMan 5 software (RevMan 2020) for all statistical analyses. We will use a narrative synthesis approach to review the literature for all non‐randomized studies and for randomized studies that provide insufficient data to conduct a meta‐analysis. Results from RCTs and non‐randomized studies will be reported in the results section (Deeks 2020).
Subgroup analysis and investigation of heterogeneity
As data permits, we will carry out the following a priori subgroup analyses on all outcomes.
Peripheral autonomic failure versus central autonomic failure
Disease state (e.g. Parkinson Disease, diabetes, amyloid‐induced orthostatic hypotension, pure autonomic failure, Lewy body disease, or multiple system atrophy)
Age 65 years or over versus under 65 years
To detect differences between two subgroups, we will consider both the difference in the magnitude of effect as well as the overlap in confidence intervals. To detect differences between three or more subgroups, we will use the formal test for subgroup interactions in RevMan 5 (RevMan 2020).
Sensitivity analysis
As data permits, we will perform sensitivity analyses that are restricted to studies with unclear to low risk of bias, those conducted in the USA, UK or other high‐income country, those with non‐industry funding, or English‐language studies, those with attrition rates greater than 20%, those with missing data, those more than 3 weeks long, and those that use validated measurements, to explore their influence on effect size.
Summary of findings and assessment of the certainty of the evidence
If sensitivity analyses indicate outcomes measured at less than three weeks are unreliable (an important source of heterogeneity), in the 'Summary of findings' table we will only report measurements taken at three weeks or longer. We will use the assumed risks as reported in included studies.
Appendix 8. Supporting information for prioritization of five out of 13 studies as best evidence
| Study | Best evidence? | Rationale |
| Axelrod 2005 | Yes | Observational study and met all applicable POST‐I criteria. Addresses gap in evidence by examining the long‐term effects (nearly 40 years) of fludrocortisone in people with familial dysautonomia. |
| Axelrod 1997 | No | Observational study but did not report baseline differences between groups and didn't account for it in analysis (“No” to POST‐I item #5) |
| Campbell 1975 | Yes | RCT |
| Campbell 1976 | No | Observational study but did not use repeated measures at baseline and follow‐up (“No” to POST‐I item #6) and could not rule out that effect was due to confounding (“No” to POST‐I item #7) |
| Chobanian 1979 | No | Observational study but was not particularly careful at measuring outcomes (“No” to POST‐I item #6) and could not rule out that effect was due to confounding (“No” to POST‐I item #7) |
| Kochar 1978 | No | Observational study but was not particularly careful at measuring outcomes (“No” to POST‐I item #6) and could not rule out that effect was due to confounding (“No” to POST‐I item #7) |
| Matsubara 1990 | No | Observational study but was not particularly careful at measuring outcomes (“No” to POST‐I item #6) and could not rule out that effect was due to confounding (“No” to POST‐I item #7) |
| Pathak 2005a | No | Observational study but did not report baseline differences between groups and didn't account for it in analysis (“No” to POST‐I item #5) |
| Pathak 2005b | No | Observational study but did not report baseline differences between groups and didn't account for it in analysis (“No” to POST‐I item #5) |
| Schatz 1976 | No | Observational study but was not particularly careful at measuring outcomes (“No” to POST‐I item #6) and could not rule out that effect was due to confounding (“No” to POST‐I item #7) |
| Schoffer 2007 | Yes | RCT |
| Schreglmann 2017 | Yes | RCT |
| Ten Harkel 1992 | Yes | Observational study and met all applicable POST‐I criteria. Addresses gap in evidence by examining long‐term (14 months) effects and harms of fludrocortisone in people with orthostatic hypotension from a variety of underlying conditions. |
Appendix 9. Basic study characteristics of eight de‐prioritized studies
| Study | Study design | Sample size | Description of study |
| Axelrod 1997 | Case‐control | N = 18 | Evaluates response to non‐invasive tests among 10 people with family dysautonomia and 8 healthy controls while supine and at 90 degrees tilt. Goal was to determine if autonomic dysfunction involves cardiac as well as peripheral vascular integrity. The participants with family dysautonomia were tested before and 1 hour after receiving oral fludrocortisone or midodrine. |
| Campbell 1976 | Case series | N = 13 | Describes long‐term follow‐up (mean 12 months) of 13 people receiving fludrocortisone for diabetic postural hypotension. |
| Chobanian 1979 | Case series | N = 7 | Describes effects of fludrocortisone among 7 people with orthostatic hypotension in terms of hypotension while in recumbent and standing positions. |
| Kochar 1978 | Case series | N = 5 | Describes effects of indomethacin in 5 people compared to either propranolol or fludrocortisone. |
| Matsubara 1990 | Case series | N = 9 | Describes effects of fludrocortisone in 9 people with Shy‐Drager syndrome when moving from supine to sitting up. |
| Pathak 2005a | Cohort | N = 121 | Examines adverse drug reactions to drugs used in France for orthostatic hypotension, including fludrocortisone. |
| Pathak 2005a | Cohort | N = 31 | Examines effects of heat on people with neurogenic orthostatic hypotension of varying etiologies, a subgroup of which received fludrocortisone. |
| Schatz 1976 | Case series | N = 23 | Describes response (satisfactory, excellent, good, fair, unsatisfactory) to fludrocortisone treatment among 23 people with orthostatic hypotension of varying etiologies (i.e. idiopathic, diabetes, Shy‐Drager, undifferentiated CNS disease, previous gastric surgery, amyloidosis). |
Appendix 10. Clinical trials registries search strategies
We searched the US National Institutes for Health Clinical Trials Registry, ClinicalTrials.gov (www.clinicaltrials.gov/), the World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) and ISRCTN registry (www.isrctn.com) on 23 September 2019.
| Database or Resource | Strategy | Results |
| Clinicaltrials.gov | [Advanced Search] Condition: (orthostatic OR postural) AND hypotension Other terms: fludrocortisone OR florinef OR fluorocortisol |
6 studies |
| apps.who.int/trialsearch/ | [Advanced Search] Condition: orthostatic hypotension OR postural hypotension AND Intervention: fludrocortisone OR florinef OR fluorocortisol Recruitment status: ALL |
2 studies |
| www.isrctn.com | [Advanced Search] Intervention: fludrocortisone |
3 studies |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Axelrod 1997.
| Study characteristics | ||
| Methods | See Appendix 9: Basic study characteristics of 8 de‐prioritized studies | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Axelrod 2005.
| Study characteristics | ||
| Methods | Retrospective analysis of US data from Familial Dysautonomia database | |
| Participants |
Setting: Dysautonomia Treatment and Evaluation Center at New York University Medical Center Number of participants: 341 Group selection: clinical decision to administer fludrocortisone or not Age (mean): 14.7 Diagnostic criteria of orthostatic hypotension: indication for fludrocortisone therapy included 1. supine or erect mean BP < 60 mmHg, 2. the presence of presyncope or syncope, or both 1 and 2. Severity of orthostatic hypotension: for fludrocortisone, drop of systolic BP 28 mmHg at 1 min, 34 mmHg at 5 min Severity of underlying illness: not reported Inclusion/exclusion criteria: met diagnostic criteria for Familial Dysautonomia and had information from annual examinations |
|
| Interventions |
Intervention/exposure: fludrocortisone Comparator: no fludrocortisone |
|
| Outcomes |
Time points reported: baseline, last recorded observation Primary outcome: mean systolic BP in supine and erect position (1 min and 5 min) Secondary outcome: dizziness, survival |
|
| Notes | Conflicts of interest not reported. Funding from Dysautonomia Foundation, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection Bias | High risk | Assignment to fludrocortisone group was based on clinical characteristics such as symptom severity. Sample was prevalent users |
| Bias in measurement of interventions | Low risk | Worldwide Familial Dysautonomia database classification |
| Bias due to departure from intended interventions | Low risk | Worldwide Familial Dysautonomia database classification. Intent is to assess effect of assignment, not adherence. No reason to expect deviations due to departures from usual care |
| Bias due to measurement of outcomes | Low risk | No information about assessment methods, but differential misclassification less likely for survival outcome |
| Bias due to confounding | Unclear risk | No information about or control for key confounders |
| Bias due to missing data | Unclear risk | No information about completeness of data for survival. For systolic BP erect at 5 min, df = 109, which implies authors excluded 37% of data |
| Bias in the selection of reported results | Low risk | Survival analyzed both continuously and categorically. The 10‐year categorization was prespecified |
Campbell 1975.
| Study characteristics | ||
| Methods | Cross‐over RCT | |
| Participants |
Setting: Diabetic and Dietetic Department, University Department of Medicine, The Royal Infirmary, Edinburgh Number of participants: 6 Unit of randomization: individual Age (mean): 50 Diagnostic criteria of orthostatic hypotension: symptomatic postural hypotension with a fall of systolic BP of 30 mmHg or greater immediately on changing from the lying to standing position Severity of OH: see “diagnostic criteria of orthostatic hypotension” Severity of underlying illness: severe diabetic neuropathy Inclusion/exclusion criteria: see “diagnostic criteria of orthostatic hypotension” |
|
| Interventions |
Intervention: 0.1 mg of fludrocortisone twice a day for 3 weeks Comparator: placebo twice a day for 3 weeks |
|
| Outcomes |
Time points reported: baseline, 3 weeks Primary outcome: mean systolic and diastolic BP in lying and tilted position at 1‐min increments Secondary outcomes: orthostatic symptoms |
|
| Notes | Conflicts of interest not reported. Fludrocortisone and placebo tablets were supplied by ER Squibb and Son, Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Low for BP; unclear for symptoms |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Low for BP; unclear for symptoms |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low for BP; unclear for symptoms, 16% attrition while taking active drug for reason of "defaulted" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Unclear risk | First period data not available. No assessment for period effect |
Campbell 1976.
| Study characteristics | ||
| Methods | See Appendix 9: Basic study characteristics of 8 de‐prioritized studies | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Chobanian 1979.
| Study characteristics | ||
| Methods | See Appendix 9: Basic study characteristics of 8 de‐prioritized studies | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Kochar 1978.
| Study characteristics | ||
| Methods | See Appendix 9: Basic study characteristics of 8 de‐prioritized studies | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Matsubara 1990.
| Study characteristics | ||
| Methods | See Appendix 9: Basic study characteristics of 8 de‐prioritized studies | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Pathak 2005a.
| Study characteristics | ||
| Methods | See Appendix 9: Basic study characteristics of 8 de‐prioritized studies | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Pathak 2005b.
| Study characteristics | ||
| Methods | See Appendix 9: Basic study characteristics of 8 de‐prioritized studies | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Schatz 1976.
| Study characteristics | ||
| Methods | See Appendix 9: Basic study characteristics of 8 de‐prioritized studies | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Schoffer 2007.
| Study characteristics | ||
| Methods | Double‐blind, randomized, active‐control, cross‐over trial | |
| Participants |
Setting: 2 movement disorder clinics in Australia Number of participants: 17 Unit of randomization: individual Age (mean): 69.5 Diagnostic criteria of orthostatic hypotension: 20 mmHg drop in systolic BP, 10 mmHg drop in diastolic BP at baseline (but including people who met criteria at any prior time, as long as still symptomatic) Severity of orthostatic hypotension: at baseline, average maximum systolic drop over 5 min was 34 mmHg. Diastolic BP average maximum drop was 16 mmHg Severity of underlying illness: mean United Parkinson's Disease Rating Scale motor score of included participants was 25.5. Inclusion criteria: participants with diagnosis of idiopathic Parkinson disease; stable doses of medications for Parkinson disease. Exclusion criteria: participants with multiple system atrophy, acute coronary syndrome, inability to give informed consent, another cause of autonomic failure, systolic BP > 200 mmHg or diastolic BP > 100 mmHg |
|
| Interventions |
Intervention: 0.1 mg of fludrocortisone once a day for 3 weeks Comparator: 10 mg domperidone 3 times a day for 3 weeks |
|
| Outcomes |
Time points reported: baseline, 3 weeks Primary outcome: 1) Median maximum change in supine systolic BP and diastolic BP in the 5 min after a 5‐min 80° head‐up tilt. 2) Change in systolic BP and diastolic BP at 3 min. 3) Supine systolic BP Secondary outcomes: composite Global Impression of Change (CGI), composite Autonomic Symptom Scale‐Orthostatic Domain (Compass‐OD) |
|
| Notes | Conflicts of interest not reported. Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization with "Research Randomizer” |
| Allocation concealment (selection bias) | Low risk | Dummy capsules to maintain blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Dummy capsules to maintain blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Dummy capsules to maintain blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 13 participants reached drug treatment (4 withdrew from side effects) |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | No other bias identified |
Schreglmann 2017.
| Study characteristics | ||
| Methods | Double‐blind, randomized, active‐control, cross‐over, phase II non‐inferiority trial | |
| Participants |
Setting: outpatient clinics of participating centers Number of participants: 13 Unit of randomization: individual Age (mean): 71.3 Diagnostic criteria of orthostatic hypotension: ≥ 20 mmHg drop systolic or 10 mmHg diastolic peripheral BP within 3 min of standing on routine follow‐up. Severity of orthostatic hypotension: average drop of 22.9 ± 12.6 mmHg Severity of underlying illness: mean United Parkinson's Disease Rating Scale of included participants 24.2 ± 8.6, Montreal Cognitive Assessment 23.9 ± 3.3, Parkinson’s Disease Questionnaire 24.9 ± 18.6 Inclusion/exclusion criteria: Parkinson disease patients (using UK Brain Bank criteria) with symptomatic, established orthostatic hypotension were included. Patients on medication affecting BP regulation, systemic diseases such as diabetes, infection, renal or kidney failure, malignancy, with weight gain or loss or who were underweight or overweight, and those with multiple system atrophy were excluded |
|
| Interventions |
Intervention: 0.1 mg of fludrocortisone once a day for 3 days then 0.2 mg for 11 days Comparator: 30 mg pyridostigmine bromide 3 times a day for 3 days then 60 mg for 11 days |
|
| Outcomes |
Time points reported: 14 days duration with 21 days of wash out before crossover Primary outcome: mean systolic and diastolic BP when moving from supine to standing at 10 min Secondary outcomes: orthostatic symptoms (Orthostatic Hypotension Daily Activity Scale), subjective assessment of symptom severity |
|
| Notes | Authors report no conflicts of interest. Study supported by HSM‐II initiative, Canton of Zurich and Parkinson Schweiz | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Low risk | Randomization performed by Cantonal Pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Drugs prepared in identical capsules at separate site |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Drugs prepared in identical capsules at separate site |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20% attrition, but the investigators have appropriate data analysis strategy to account for missing data |
| Selective reporting (reporting bias) | Low risk | Outcomes from ClinicalTrials.gov are reported |
| Other bias | Low risk | No other bias identified |
Ten Harkel 1992.
| Study characteristics | ||
| Methods | Case series | |
| Participants |
Setting: hospital and outpatient at medical clinic Number of participants: 6 Group selection: 6 consecutive patients referred for evaluation of orthostatic hypotension were all given fludrocortisone Age (mean): 51.83 Diagnostic criteria of orthostatic hypotension: not reported Severity of orthostatic hypotension: severe (orthostatic hypotension was generally described as “incapacitating” or “progressive” over several years) Severity of underlying illness: not reported Inclusion/exclusion criteria: Hypoadrenergic orthostatic hypotension |
|
| Interventions |
Intervention/exposure: 0.1 mg or 0.2 mg daily fludrocortisone with diet containing 150 mmol Na+ to 200 mmol Na+ and minimal water and sleeping in 12% head‐up position Comparator: diet and head‐up sleeping position alone |
|
| Outcomes |
Time points reported: baseline, weekly for 3 weeks and again at 14 months Primary outcome: standing BP and drop in BP Secondary outcome: orthostatic disability score, standing time |
|
| Notes | Conflicts of interest not reported. Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection Bias | High risk | Selection based on referral to academic center for evaluation, so this was not an inception cohort at the same point of their disease process |
| Bias in measurement of interventions | Low risk | Intervention groups clearly defined and not affected by knowledge of outcome |
| Bias due to departure from intended interventions | Unclear risk | No information on co‐interventions or adherence |
| Bias due to measurement of outcomes | Unclear risk | No information on whether outcome assessors were aware of intervention, but measurement methods were well‐described and objective |
| Bias due to confounding | Unclear risk | No information on potential confounders |
| Bias due to missing data | Low risk | Outcome data available for all participants |
| Bias in the selection of reported results | High risk | Effect estimates not prespecified and possible to generate multiple types based on measures collected |
BP: blood pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbruzzese 1981 | Wrong study design |
| Agrawal 1999 | Wrong study design |
| Ahmad 1990 | Wrong study design |
| Andreelli 1999 | Wrong study design |
| Anlauf 1975a | No relevant data |
| Anlauf 1975b | Duplicate data |
| Anonymous 1958 | Wrong population |
| Bagatin 1995 | Wrong study design |
| Ballabio 1956 | Wrong population |
| Bannister 1969 | No relevant data |
| Bannister 1979 | Wrong study design |
| Barnett 1968 | Wrong study design |
| Barnett 1976 | Wrong study design |
| Bodemer 1986 | Wrong study design |
| Bodemer 1987 | No relevant data |
| Borck 1974 | Wrong population |
| Bowen 1988 | Wrong study design |
| Bradshaw 1986 | Wrong study design |
| Brown 1968 | Wrong study design |
| Castillo 1982 | Wrong study design |
| Charlier 1980 | Wrong study design |
| Chung 2016 | Only title/abstract available and insufficient reporting on PICOs to determine eligibility |
| Cohen 1991 | Wrong population |
| Colombat 1977 | Only title/abstract available and insufficient reporting on PICOs to determine eligibility |
| Comi 1988 | Wrong study design |
| Davidson 1976 | Wrong study design |
| Davies 1978 | Duplicate data |
| Davies 1979 | No relevant data |
| Davies 1982 | Wrong study design |
| Decaux 1979 | Wrong study design |
| Decourt 1958 | Wrong population |
| Degennes 1955 | Wrong study design |
| Devuyst 1992 | Wrong study design |
| Finke 1975 | Wrong population |
| Fouad 1986 | Wrong population |
| Freidenberg 2013 | Wrong intervention |
| Frewin 1973 | Wrong study design |
| Frick 1966 | Wrong population |
| Goovaerts 1984 | Wrong study design |
| Grijalva 2017 | Wrong population |
| Hakamaki 1998 | Wrong population |
| Hall 1961 | Wrong population |
| Hamwi 1955 | Wrong population |
| Hawkins 1982 | Wrong study design |
| Herpin 1979 | Not a study |
| Hickler 1959 | Wrong study design |
| Hohl 1965 | Wrong study design |
| Hohler 2012 | Wrong intervention |
| Hussain 1996 | Wrong population |
| Ibrahim 1975 | Not a study |
| Irvin 1970 | Wrong study design |
| Jay 2001 | Wrong study design |
| Jorg 1983 | Wrong study design |
| Khurana 1987 | Wrong study design |
| Kochar 1977 | Wrong intervention |
| Lange 1983 | Not a study |
| Legeler 1971 | Wrong population |
| Lennox 1980 | Wrong population |
| Lewis 1972 | Wrong intervention |
| Low 1978 | Not a study |
| MacLean 1959 | Wrong study design |
| Mathias 1998 | Not a study |
| Maximilian 1977 | Wrong population |
| Meijer 1977 | Wrong study design |
| Miller 1976 | Wrong population |
| Miskiewicz 1982 | Wrong study design |
| Mittal 2007 | Not a study |
| Molnar 1963 | Wrong study design |
| NCT00103597 | Duplicate data |
| Oldenburg 1999 | Wrong study design |
| Perkins 1978 | Wrong study design |
| Peterson 1998 | Wrong population |
| Poppe 1980 | Wrong intervention |
| Radian 1976 | Only title/abstract available and insufficient reporting on PICOs to determine eligibility |
| Robertson 1979 | No relevant data |
| Robertson 1985 | Not a study |
| Salim 2005 | Wrong population |
| Schafer 1957 | Wrong study design |
| Schatz 1984 | Not a study |
| Schatz 2001 | Not a study |
| Schirger 1964 | No relevant data |
| Schreglmann 2018 | Not a study |
| Scott 1995 | Wrong population |
| Seagar 1963 | Wrong study design |
| Seller 1969 | Wrong study design |
| Selye 1955 | Wrong population |
| Selye 1956 | Wrong population |
| Shear 1968 | No relevant data |
| Shibao 2011 | Only title/abstract available and insufficient reporting on PICOs to determine eligibility |
| Shibao 2013a | Only title/abstract available and insufficient reporting on PICOs to determine eligibility |
| Shibao 2013b | Wrong population |
| Silvestrini 1964 | Wrong study design |
| Simoes 2018 | Not a study |
| Sivertssen 1967 | Not a study |
| Stoll 1971 | Wrong population |
| Tantengco 1986 | Not a study |
| Tarazi 1978 | Not a study |
| Tifft 1985 | Not a study |
| Vassallo 2013 | Not a study |
| Villa 1955 | Wrong population |
| Volk 1976 | Wrong population |
| Von Mundy 1956 | Wrong population |
| Walter 1979 | Not a study |
| Warne 1987 | Not a study |
| Wasielewski 2001 | Wrong population |
| Watson 1987 | Not a study |
| Watt 1980 | Wrong intervention |
| Watt 1981 | No relevant data |
| Wenting 1981 | Wrong population |
| Whyte 2014 | Wrong study design |
| Williams 1985 | Wrong population |
Differences between protocol and review
We developed and used a screening tool (Portland Observational Study Screening Tool for Interventions [POST‐I]; Figure 1) to prioritize studies that offered the best available evidence to address our review question. We only conducted formal risk of bias and data extraction on prioritized studies. We described the process for data entry and checks.
Contributions of authors
SRR and CHG conceived the review and drafted the initial protocol and the search strategy with assistance from the Cochrane team.
All review authors contributed to and revised the draft. All review authors have approved publication of the review.
Dr Mark Helfand will be the guarantor of the review.
Sources of support
Internal sources
-
The authors completed this review within their own time, as there is no allocated time or funding for such activities within their jobs. This material is the result of work supported with resources and the use of facilities at VA Portland Medical Center, USA
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
External sources
No sources of support provided
Declarations of interest
MH: none known
YA: none known
KAC: none known
CHG: Dr Gibbons has served on an advisory board for Lundbeck on the treatment of orthostatic hypotension.
SRR: Dr Raj has served on the Board of Directors of multiple professional scientific organizations (American Autonomic Society and the Association of Clinical & Translational Sciences) and as a Medical Advisor for multiple Patient Advocacy Groups. He was not financially compensated for these activities. He has received grants from the National Institutes of Health (USA) and Canadian Institutes for Health Research for studies on Postural Tachycardia Syndrome and his colleague has received funding from the Canadian Institutes for Health Research for a study of fludrocortisone in vasovagal syncope. Dr Raj has also received study drug from Apotex Pharmaceuticals for a study of atomoxetine in vasovagal syncope. He has been compensated for and served as a consultant for Lundbeck Pharmaceuticals, GE Healthcare and Medtronic Inc. Dr Raj has been compensated for and served as an expert witness in a case involving whether a car accident caused Postural Tachycardia Syndrome in a patient.
KP: none known
SV: none known
Edited (no change to conclusions)
References
References to studies included in this review
Axelrod 1997 {published data only}
- Axelrod FB, Putman D, Berlin D, Rutkowski M. Electrocardiographic measures and heart rate variability in patients with familial dysautonomia. Cardiology 1997;88(2):133-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Axelrod 2005 {published data only}
- Axelrod B, Goldberg JD, Rolnitzky L, Mull J, Mann SP, Gold von Simson G, et al. Fludrocortisone in patients with familial dysautonomia--assessing effect on clinical parameters and gene expression. Clinical Autonomic Research 2005;15(4):284-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Campbell 1975 {published data only}
- Campbell IW, Ewing DJ, Clarke BF. 9-alpha-fluorohydrocortisone in the treatment of postural hypotension in diabetic autonomic neuropathy. Diabetes 1975;24(4):381-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Campbell 1976 {published data only}
- Campbell IW, Ewing DJ, Clarke BF. Therapeutic experience with fludrocortisone in diabetic postural hypotension. British Medical Journal 1976;1(6014):872-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chobanian 1979 {published data only}
- Chobanian AV, Volicer L, Tifft CP, Gavras H, Liang CS, Faxon D. Mineralocorticoid-induced hypertension in patients with orthostatic hypotension. New England Journal of Medicine 1979;301(2):68-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kochar 1978 {published data only}
- Kochar MS, Itskovitz HD. Treatment of idiopathic orthostatic hypotension (Shy-Drager syndrome) with indomethacin. Lancet 1978;1(8072):1011-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Matsubara 1990 {published data only}
- Matsubara S, Sawa Y, Yokoji H, Takamori M. Shy-Drager syndrome. Effect of fludrocortisone and L-threo-3,4-dihydroxyphenylserine on the blood pressure and regional cerebral blood flow. Journal of Neurology, Neurosurgery, and Psychiatry 1990;53(11):994-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pathak 2005a {published data only}
- Pathak A, Raoul V, Montastruc JL, Senard JM. Adverse drug reactions related to drugs used in orthostatic hypotension: a prospective and systematic pharmacovigilance study in France. European Journal of Clinical Pharmacology 2005;61(5-6):471-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pathak 2005b {published data only}
- Pathak A, Lapeyre-Mestre M, Montastruc JL, Senard JM. Heat-related morbidity in patients with orthostatic hypotension and primary autonomic failure. Movement Disorders 2005;20(9):1213-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schatz 1976 {published data only}
- Schatz IJ, Miller MJ, Frame B. Corticosteroids in the management of orthostatic hypotension. Cardiology 1976;61(Suppl 1):280-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schoffer 2007 {published data only}
- Schoffer KL, Henderson RD, O'Maley K, O'Sullivan JD. Nonpharmacological treatment, fludrocortisone, and domperidone for orthostatic hypotension in Parkinson's disease. Movement Disorders 2007;22(11):1543-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schreglmann 2017 {published data only}
- Schreglmann SR, Buchele F, Sommerauer M, Epprecht L, Kagi G, Hagele-Link S, et al. Pyridostigmine bromide versus fludrocortisone in the treatment of orthostatic hypotension in Parkinson's disease - a randomized controlled trial. European Journal of Neurology 2017;24(4):545-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ten Harkel 1992 {published data only}
- Ten Harkel AD, Van Lieshout JJ, Wieling W. Treatment of orthostatic hypotension with sleeping in the head-up tilt position, alone and in combination with fludrocortisone. Journal of Internal Medicine 1992;232(2):139-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbruzzese 1981 {published data only}
- Abbruzzese JL, Griffin JW. Postural hypotension. Johns Hopkins Medical Journal 1981;148(3):127-131. [PubMed] [Google Scholar]
Agrawal 1999 {published data only}
- Agrawal A, Saran R, Khanna R. Management of orthostatic hypotension from autonomic dysfunction in diabetics on peritoneal dialysis. Peritoneal Dialysis International 1999;19(5):415-417. [PubMed] [Google Scholar]
Ahmad 1990 {published data only}
- Ahmad RA, Watson RD. Treatment of postural hypotension. A review. Drugs 1990;39(1):74-85. [DOI] [PubMed] [Google Scholar]
Andreelli 1999 {published data only}
- Andreelli F, Plotton I, Thivolet C, Riou JP. A new hope in the nightmare of diabetic orthostatic hypotension: the midodrine-fludrocortisone association. Diabetes, Obesity and Metabolism 1999;1(5):297-8. [DOI] [PubMed] [Google Scholar]
Anlauf 1975a {published data only}
- Anlauf M, Werner U, Merguet P, Nitzs T, Graben N, Bock KD. Clinical experimental studies in patients with asympathicotonic hypotension. Deutsche Medizinische Wochenschrift (1946) 1975;100(17):924-33. [DOI: 10.1055/s-0028-1106316] [DOI] [PubMed] [Google Scholar]
Anlauf 1975b {published data only}
- Anlauf M, Werner U, Merguet P, Nitzs T, Graben N, Bock KD. Clinical investigations in asympathicotonic hypotension. Deutsche Medizinische Wochenschrift 1975;100(17):924-933. [DOI] [PubMed] [Google Scholar]
Anonymous 1958 {published data only}
- Anonymous. Fludrocortisone acetate. Pharmaceutisch Weekblad 1958;93(24):1075-6. [PubMed] [Google Scholar]
Bagatin 1995 {published data only}
- Bagatin J, Sardelic S, Rumboldt Z, Polic S, Pivac N, Ljutic D. An approach to a symptomatic hypotensive patient. Orthostatic test as a guide to diagnostic and therapeutic procedure. Pharmaca 1995;33(1-2):77-86. [Google Scholar]
Ballabio 1956 {published data only}
- Ballabio CB, Sala G, Villa L. Clinical and metabolic effects of delta 1-dehydro-9-alpha-fluoro hydrocortisone acetate. Annals of the Rheumatic Diseases 1956;15(3):237-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bannister 1969 {published data only}
- Bannister R, Ardill L, Fentem P. An assessment of various methods of treatment of idiopathic orthostatic hypotension. Quarterly Journal of Medicine 1969;38(152):377-95. [PubMed] [Google Scholar]
Bannister 1979 {published data only}
- Bannister R. Chronic autonomic failure with postural hypotension. Lancet (London, England) 1979;2(8139):404-6. [PubMed] [Google Scholar]
Barnett 1968 {published data only}
- Barnett AJ. Idiopathic orthostatic hypotension: a pharmacological study of the action of sympathomimetic drugs. Medical Journal of Australia 1968;1(6):213-6. [PubMed] [Google Scholar]
Barnett 1976 {published data only}
- Barnett AJ. Letter: Idiopathic orthostatic hypotension. Medical Journal of Australia 1976;1(6):172-3. [PubMed] [Google Scholar]
Bodemer 1986 {published data only}
- Bodemer C, Bletry O. Orthostatic hypotension. Concours Medical 1986;108(4):178-87. [Google Scholar]
Bodemer 1987 {published data only}
- Bodemer C, Bletry O, Guillevin L, Dumas P, Brunet P, Godeau P. Amyloidosis and orthostatic hypotension. Physiopathology, therapeutic attempts. Apropos of 5 cases. Annales de Medecine Interne 1987;138(8):625-30. [PubMed] [Google Scholar]
Borck 1974 {published data only}
- Borck C, Dienz K, Schneider E. Therapy of orthostatic circulatory dysregulation (9alpha fluorohydrocortisone in a cross over double blind trial) [Therapie der Orthostatischen Kreislauf Fehlregulation (9alpha Fluorhydrokortison im Gekreuzten Doppelblindversuch)]. Herz Kreislauf 1974;6(3):117-9. [Google Scholar]
Bowen 1988 {published data only}
- Bowen J. Orthostatic hypotension. Comprehensive Therapy 1988;14(10):6-10. [PubMed] [Google Scholar]
Bradshaw 1986 {published data only}
- Bradshaw MJ, Edwards RT. Postural hypotension--pathophysiology and management. Quarterly Journal of Medicine 1986;60(231):643-57. [PubMed] [Google Scholar]
Brown 1968 {published data only}
- Brown JW, Monroe LS, Palmer L. Intractable vomiting, diarrhea, and orthostatic hypotension in amyloidosis of the peripheral nervous system. American Journal of Digestive Diseases 1968;13(9):836-41. [DOI] [PubMed] [Google Scholar]
Castillo 1982 {published data only}
- Castillo J, Garcia-Martinez I, Lema M, Noya M. Chronic idiopathic orthostatic hypotension. Revista Clinica Espanola 1982;166(5):185-9. [PubMed] [Google Scholar]
Charlier 1980 {published data only}
- Charlier M, Franck G. Chronic, neurogenic orthostatic hypotension. Medecine et Hygiene 1980;38(1388):2655-61. [Google Scholar]
Chung 2016 {published data only}
- Chung D, Chen D, Semik P. Midodrine versus fludrocortisone for management of orthostatic hypotension after spinal cord injury: a retrospective review of efficacy. Journal of Spinal Cord Medicine 2016;39(5):601-2. [Google Scholar]
Cohen 1991 {published data only}
- Cohen JA, Miller L, Polish L. Orthostatic hypotension in human immunodeficiency virus infection may be the result of generalized autonomic nervous system dysfunction. Journal of Acquired Immune Deficiency Syndromes 1991;4(1):31-3. [PubMed] [Google Scholar]
Colombat 1977 {published data only}
- Colombat P, Bienvenu P, Morand P. Orthostatic hypotension. Revue de Medecine de Tours 1977;11(9):1297-1307. [Google Scholar]
Comi 1988 {published data only}
- Comi R, Mazzini I, Maniscalco A, Simonelli F. Mineral-active therapy for treatment of arterial hypotension. Policlinico - Sezione Medica 1988;95(4):236-56. [Google Scholar]
Davidson 1976 {published data only}
- Davidson C, Smith D, Morgan DB. Diurnal pattern of water and electrolyte excretion and body weight in idiopathic orthostatic hypotension. The effect of three treatments. American Journal of Medicine 1976;61(5):709-15. [DOI] [PubMed] [Google Scholar]
Davies 1978 {published data only}
- Davies IB, Bannister RG, Sever PS, Wilcox CS. Fludrocortisone in the treatment of postural hypotension: altered sensitivity to pressor agents. British Journal of Clinical Pharmacology 1978;6(5):444P-445P. [PubMed] [Google Scholar]
Davies 1979 {published data only}
- Davies B, Bannester R, Sever P, Wilcox C. The pressor actions of noradrenaline, angiotensin II and saralasin in chronic autonomic failure treated with fludrocortisone. British Journal of Clinical Pharmacology 1979;8(3):253-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 1982 {published data only}
- Davies IB. Chronic hypotension. Journal of the Royal Society of Medicine 1982;75(8):577-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Decaux 1979 {published data only}
- Decaux G. Fludrocortisone in orthostatic hypotension. New England Journal of Medicine 1979;301(20):1121-2. [PubMed] [Google Scholar]
Decourt 1958 {published data only}
- Decourt J, Jayle MF, Michard P, Mauvais P. Clinical applications of the adrenal cortex inhibition test using delta 1-9 alpha-fluorohydrocortisone; 8 cases. Annales d'Endocrinologie 1958;19(2):358-69. [PubMed] [Google Scholar]
Degennes 1955 {published data only}
- De Gennes L, Deltour G. 9-alpha-fluorohydrocortisone. La Presse Medicale 1955;63(60):1214-5. [PubMed] [Google Scholar]
Devuyst 1992 {published data only}
- Devuyst O, Lefebvre C, Coche E. The combination fludrocortisone-indomethacin in the treatment of idiopathic orthostatic hypotension. Revue de Medecine Interne 1992;13(7):S392. [Google Scholar]
Finke 1975 {published data only}
- Finke J, Sagemuller I. Fludrocortisone in the treatment of orthostatic hypotension: ophthalmodynamography during standing. Deutsche Medizinische Wochenschrift (1946) 1975;100(36):1790-2. [DOI: 10.1055/s-0028-1106461] [DOI] [PubMed] [Google Scholar]
Fouad 1986 {published data only}
- Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Annals of Internal Medicine 1986;104(3):298-303. [DOI] [PubMed] [Google Scholar]
Freidenberg 2013 {published data only}
- Freidenberg DL, Shaffer LET, MacAlester S, Fannin EA. Orthostatic hypotension in patients with dementia: clinical features and response to treatment. Cognitive and Behavioral Neurology 2013;26(3):105-20. [DOI: 10.1097/WNN.0000000000000003] [DOI] [PubMed] [Google Scholar]
Frewin 1973 {published data only}
- Frewin DB, Robinson SM, Willing RL. The use of a new mode of therapy in the management of orthostatic hypotension. Australian and New Zealand Journal of Medicine 1973;3(2):180-3. [DOI] [PubMed] [Google Scholar]
Frick 1966 {published data only}
- Frick MH. 9-alpha-fluorohydrocortisone in the treatment of postural hypotension. Acta Medica Scandinavica 1966;179(3):293-9. [DOI] [PubMed] [Google Scholar]
Goovaerts 1984 {published data only}
- Goovaerts J, Verfaillie C, Knockaert D, Fagard R, Fiocchi R, Amery A, et al. Prenalterol in the treatment of orthostatic hypotension in Shy-Drager syndrome. Acta Cardiologica 1984;39(2):147-55. [PubMed] [Google Scholar]
Grijalva 2017 {published data only}
- Grijalva CG, Biaggioni I, Griffin MR, Shibao CA. Fludrocortisone Is associated with a higher risk of all-cause hospitalizations compared with midodrine in patients with orthostatic hypotension. Journal of the American Heart Association 2017;6(10):e006848. [DOI: 10.1161/JAHA.117.006848] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hakamaki 1998 {published data only}
- Hakamaki T, Rajala T, Lehtonen A. Ambulatory 24-hour blood pressure recordings in patients with Parkinson's disease with or without fludrocortisone. International Journal of Clinical Pharmacology and Therapeutics 1998;36(7):367-9. [PubMed] [Google Scholar]
Hall 1961 {published data only}
- Hall CE, Hall O. Cardiac and renal lesions due to fludrocortisone and factors influencing their development. Texas Reports on Biology and Medicine 1961;19:774-84. [PubMed] [Google Scholar]
Hamwi 1955 {published data only}
- Hamwi GJ, Goldberg RF. Clinical use of fludrocortisone acetate; preliminary report. Journal of the American Medical Association 1955;159(17):1598-601. [DOI] [PubMed] [Google Scholar]
Hawkins 1982 {published data only}
- Hawkins S, Prout BJ. Postural hypotension and its management. The Practitioner 1982;226(1365):420-6. [PubMed] [Google Scholar]
Herpin 1979 {published data only}
- Herpin D, Pouget-Abadie JF, Becq-Giraudon B. Investigation and current treatment of orthostatic hypotension. Ouest Medical 1979;32(4):163-70. [Google Scholar]
Hickler 1959 {published data only}
- Hickler RB, Thompson GR, Fox LM, Hamlin JT 3rd. Successful treatment of orthostatic hypotension with 9-alpha-fluorohydrocortisone. New England Journal of Medicine 1959;261:788-91. [DOI: 10.1056/NEJM195910152611604] [DOI] [PubMed] [Google Scholar]
Hohl 1965 {published data only}
- Hohl RD, Frame B, Schatz IJ. The Shy-Drager variant of idiopathic orthostatic hypotension. American Journal of Medicine 1965;39:134-41. [DOI] [PubMed] [Google Scholar]
Hohler 2012 {published data only}
- Hohler AD, Amariei DE, Katz DI, DePiero TJ, Allen VB, Boyle S, et al. Treating orthostatic hypotension in patients with Parkinson's disease and atypical Parkinsonism improves function. Journal of Parkinson's Disease 2012;2(3):235-40. [DOI: 10.3233/JPD-2012-012101] [DOI] [PubMed] [Google Scholar]
Hussain 1996 {published data only}
- Hussain RM, McIntosh SJ, Lawson J, Kenny RA. Fludrocortisone in the treatment of hypotensive disorders in the elderly. Heart (British Cardiac Society) 1996;76(6):507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibrahim 1975 {published data only}
- Ibrahim MM, Tarazi RC, Dustan HP. Orthostatic hypotension: mechanisms and management. American Heart Journal 1975;90(4):513-20. [DOI] [PubMed] [Google Scholar]
Irvin 1970 {published data only}
- Irvin CW Jr. Orthostatic hypotension. Journal of the South Carolina Medical Association (1975) 1970;66(8):285-7. [PubMed] [Google Scholar]
Jay 2001 {published data only}
- Jay SJ. Orthostatic hypotension and chronic fatigue syndrome. JAMA 2001;285(11):1442-3. [PubMed] [Google Scholar]
Jorg 1983 {published data only}
- Jorg J. Therapeutic concept in Parkinson disease. Deutsche Medizinische Wochenschrift (1946) 1983;108(28-29):1116-22. [DOI: 10.1055/s-2008-1069705] [DOI] [PubMed] [Google Scholar]
Khurana 1987 {published data only}
- Khurana RK. Orthostatic hypotension-induced autonomic dysreflexia. Neurology 1987;37(7):1221-4. [DOI] [PubMed] [Google Scholar]
Kochar 1977 {published data only}
- Kochar MS, Itskovitz HD. Hemodynamic and hormonal alterations in idiopathic orthostatic hypotension (IOH) and response to various modalities of treatment. Clinical Research 1977;25(3):230A. [Google Scholar]
Lange 1983 {published data only}
- Lange L. Drug therapy for orthostatic dysregulation (primary essential form). Die Therapiewoche 1983;33(1):34-7. [Google Scholar]
Legeler 1971 {published data only}
- Legeler HJ, Stoll KD. Report on the clinical trial of 9alpha-fluorhydrocortisone in the treatment of hypotensive and orthostatic circulation regulation disorders. Arzneimittel-Forschung 1971;21(8):Suppl-9. [PubMed] [Google Scholar]
Lennox 1980 {published data only}
- Lennox IM, Williams BO. Postural hypotension in the elderly. Journal of Clinical and Experimental Gerontology 1980;2(4):313-28. [Google Scholar]
Lewis 1972 {published data only}
- Lewis RK, Hazelrig CG, Fricke FJ, Russell RO Jr. Therapy of idiopathic postural hypotension. Archives of Internal Medicine 1972;129(6):943-9. [PubMed] [Google Scholar]
Low 1978 {published data only}
- Low PA. Levodopa in combination therapy of idiopathic hypotension. Neurology 1978;28(2):203. [DOI] [PubMed] [Google Scholar]
MacLean 1959 {published data only}
- MacLean K, Schurr PH. Reversible amyotrophy complicating treatment with fludrocortisone. Lancet (London, England) 1959;1(7075):701-3. [DOI] [PubMed] [Google Scholar]
Mathias 1998 {published data only}
- Mathias CJ, Kimber JR. Treatment of postural hypotension. Journal of Neurology, Neurosurgery and Psychiatry 1998;65(3):285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maximilian 1977 {published data only}
- Maximilian VV, Oancea R, Lazar V. A mineralocorticoid effective by oral administration: 9-alpha-fluorohydrocortisone (Astonin H). Revista de Medicina Interna, Neurologe, Psihiatrie, Neurochirurgie, Dermato-Venerologie. Medicina Interna 1977;29(5):469-72. [PubMed] [Google Scholar]
Meijer 1977 {published data only}
- Meijer K, Siegenbeek Van Heukelom LH, Struyvenberg A. Idiopathic orthostatic hypotension. Nederlands Tijdschrift voor Geneeskunde 1977;121(38):1453-8. [PubMed] [Google Scholar]
Miller 1976 {published data only}
- Miller MJ, Schatz IJ, Frame B. 9 Alphaflourohydrocortisone (9 AE) in the management of orthostatic hypotension. Clinical Pharmacology and Therapeutics 1976;19(1):112. [Google Scholar]
Miskiewicz 1982 {published data only}
- Miskiewicz Z, Januszewicz W, Chlebus H. Current possibilities in the treatment of idiopathic orthostatic hypotension. Kardiologia Polska 1982;25(5-6):499-505. [PubMed] [Google Scholar]
Mittal 2007 {published data only}
- Mittal S. Managing orthostatic hypotension: is this inspiration the answer? Heart Rhythm 2007;4(2):136-7. [DOI] [PubMed] [Google Scholar]
Molnar 1963 {published data only}
- Molnar GD, Mattox VR, Maher FT. Adrenocortical dysfunction: reappraisal after 4 years' therapy. Metabolism: Clinical and Experimental 1963;12:399-403. [PubMed] [Google Scholar]
NCT00103597 {published data only}
- NCT00103597. Efficacy of therapeutic Interventions for orthostatic hypotension in Parkinson's disease and multiple system atrophy. www.clinicaltrials.gov (accessed 14 February 2005).
Oldenburg 1999 {published data only}
- Oldenburg O, Sack S, Erbel R, Philipp T, Kribben A. Therapy of orthostatic hypotension. Deutsche Medizinische Wochenschrift (1946) 1999;124(41):1209-12. [DOI: 10.1055/s-2007-1024515] [DOI] [PubMed] [Google Scholar]
Perkins 1978 {published data only}
- Perkins CM, Lee MR. Flurbiprofen and fludrocortisone in severe autonomic neuropathy. Lancet (London, England) 1978;2(8098):1058. [DOI] [PubMed] [Google Scholar]
Peterson 1998 {published data only}
- Peterson PK, Pheley A, Schroeppel J, Schenck C, Marshall P, Kind A, et al. A preliminary placebo-controlled crossover trial of fludrocortisone for chronic fatigue syndrome. Archives of Internal Medicine 1998;158(8):908-14. [DOI] [PubMed] [Google Scholar]
Poppe 1980 {published data only}
- Poppe G, Ludewig W, Ludewig H, Poppe W. The Shy-Drager syndrome, a disease of second half of life. Zeitschrift fur Alternsforschung 1980;35(3):193-203. [PubMed] [Google Scholar]
Radian 1976 {published data only}
- Radian N, Dinulescu E, Paunescu Vaida E. Treatment of the essential arterial hypotension syndrome and of arterial hypotension in Addison's disease by 9 alpha fluorocortisol. Revue Roumaine de Medecine - Serie Endocrinologie 1976;14(1):45-50. [Google Scholar]
Robertson 1979 {published data only}
- Robertson D. Enhancement by fludrocortisone of the pressor response to phenylephrine in idiopathic orthostatic hypotension. Federation Proceedings 1979;38 (3 I):129. [Google Scholar]
Robertson 1985 {published data only}
- Robertson D, Robertson RM. Orthostatic hypotension: diagnosis and therapy. Modern Concepts of Cardiovascular Disease 1985;54(2):7-12. [Google Scholar]
Salim 2005 {published data only}
- Salim MA, Di Sessa TG. Effectiveness of fludrocortisone and salt in preventing syncope recurrence in children: a double-blind, placebo-controlled, randomized trial. Journal of the American College of Cardiology 2005;45(4):484-8. [DOI: 10.1016/j.jacc.2004.11.033] [DOI] [PubMed] [Google Scholar]
Schafer 1957 {published data only}
- Schafer EL. 9-alpha-fluorhydrocortisone. Deutsche Medizinische Wochenschrift 1957;82(17):657-8. [DOI] [PubMed] [Google Scholar]
Schatz 1984 {published data only}
- Schatz IJ. Orthostatic hypotension: diagnosis and treatment. Hospital Practice (Office Edition) 1984;19(4):59-69. [DOI] [PubMed] [Google Scholar]
Schatz 2001 {published data only}
- Schatz IJ. Treatment of severe autonomic orthostatic hypotension. Lancet 2001;357(9262):1060-1. [DOI] [PubMed] [Google Scholar]
Schirger 1964 {published data only}
- Schirger A, Molnar GD. Idiopathic orthostatic hypotension. comparison of the effect of 9-alpha-fluorohydrocortisone and aldosterone in two patients. Geriatrics 1964;19:434-41. [PubMed] [Google Scholar]
Schreglmann 2018 {published data only}
- Schreglmann SR, Buchele F, Kagi G, Baumann CR. Study a. Pyridostigmine bromide versus fludrocortisone in the treatment of orthostatic hypotension in Parkinson's disease - reply. European Journal of Neurology 2018;25(2):e27-8. [DOI] [PubMed] [Google Scholar]
Scott 1995 {published data only}
- Scott WA, Pongiglione G, Bromberg BI, Schaffer MS, Deal BJ, Fish FA, et al. Randomized comparison of atenolol and fludrocortisone acetate in the treatment of pediatric neurally mediated syncope. American Journal of Cardiology 1995;76(5):400-2. [DOI] [PubMed] [Google Scholar]
Seagar 1963 {published data only}
- Seagar LH. Pressor action of fluorohydrocortisone in orthostatic hypotension. Tufts Folia Medica 1963;9:56-61. [PubMed] [Google Scholar]
Seller 1969 {published data only}
- Seller RH. Idiopathic orthostatic hypotension. Report of successful treatment with a new form of therapy. American Journal of Cardiology 1969;23(6):838-44. [DOI] [PubMed] [Google Scholar]
Selye 1955 {published data only}
- Selye H. On the toxicity of 9 alpha-fluorohydrocortisone and 9 alpha-chlorohydrocortisone. Journal of Clinical Endocrinology and Metabolism 1955;15(3):384-6. [DOI: 10.1210/jcem-15-3-384] [DOI] [PubMed] [Google Scholar]
Selye 1956 {published data only}
- Selye H, Bois P. Anticortisol action of 2-methyl-9-(alpha)-fluorocortisol. Proceedings of the Society for Experimental Biology and Medicine 1956;92(2):362-4. [DOI] [PubMed] [Google Scholar]
Shear 1968 {published data only}
- Shear L. Orthostatic hypotension. Treatment with sodium chloride and sodium retaining steroid hormones. Archives of Internal Medicine 1968;122(6):467-71. [DOI] [PubMed] [Google Scholar]
Shibao 2011 {published data only}
- Shibao C, Grijalva CG, Biaggioni I, Griffin MR. Short duration of treatment with fludrocortisone and midodrine in patients with orthostatic hypotension. Hypertension 2011;58(5):e131. [Google Scholar]
Shibao 2013a {published data only}
- Shibao C, Grijalva CG, Lipsitz LA, Biaggioni I, Griffin MR. Midodrine is associated with a lower risk of all-cause hospitalizations compared with fludrocortisone in patients with orthostatic hypotension and heart failure. Hypertension 2013;62(Suppl. 1):Abstract 366. [Google Scholar]
Shibao 2013b {published data only}
- Shibao C, Grijalva CG, Lipsitz LA, Biaggioni I, Griffin MR. Early discontinuation of treatment in patients with orthostatic hypotension. Autonomic Neuroscience: Basic and Clinical 2013;177(2):291-6. [DOI: 10.1016/j.autneu.2013.08.064] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silvestrini 1964 {published data only}
- Silvestrini F, Cecchetti G, Littamodignani R, Delprete S. Idiopathic orthostatic hypotension. Semiological data, hemodynamic investigations and therapeutic results. Archivio di Patologia e Clinica Medica 1964;41:61-88. [PubMed] [Google Scholar]
Simoes 2018 {published data only}
- Simoes RM, Castro Caldas A, Ferreira JJ. Pyridostigmine bromide versus fludrocortisone in the treatment of orthostatic hypotension in Parkinson's disease - a randomized controlled trial. European Journal of Neurology 2018;25(1):e4. [DOI] [PubMed] [Google Scholar]
Sivertssen 1967 {published data only}
- Sivertssen E. Postural hypotension. Tidsskrift for den Norske laegeforening: Tidsskrift for Praktisk Medicin, ny Raekke 1967;87(20):Suppl-9. [PubMed] [Google Scholar]
Stoll 1971 {published data only}
- Stoll KD, Legeler HJ. Clinical and statistical evaluation of Schellong's standing test for the objective assessment of the therapeutic results of 9 -fluorhydrocortisone in orthostatic circulation regulation disorders. Arzneimittel-Forschung 1971;21(8):Suppl-2. [PubMed] [Google Scholar]
Tantengco 1986 {published data only}
- Tantengco MV, Onrot J, Robertson D. Orthostatic hypotension: clinical spectrum and approach to treatment. Comprehensive Therapy 1986;12(6):41-7. [PubMed] [Google Scholar]
Tarazi 1978 {published data only}
- Tarazi RC. Familial dysautonomia and prolonged corticosteroid therapy. Journal of the American Medical Association 1978;240(3):285. [Google Scholar]
Tifft 1985 {published data only}
- Tifft CP, Chobanian AV. Evaluation and treatment of orthostatic hypotension. Practical Cardiology 1985;11(5):103-17. [Google Scholar]
Vassallo 2013 {published data only}
- Vassallo M, Sharma JC. Management of orthostatic hypotension. International Journal of Clinical Practice 2013;67(7):600-2. [DOI] [PubMed] [Google Scholar]
Villa 1955 {published data only}
- Villa L, Ballabio CB, Sala G. Further study of the therapeutic use of prednisone (metacortandracin) and 9-alpha-fluorohydrocortisone. Revue du Rhumatisme et des Maladies Osteo-Articulaires 1955;22(5):413-20. [PubMed] [Google Scholar]
Volk 1976 {published data only}
- Volk W, Stoll KD. Double blind study on the therapy of postural hypotension in psychotic patients under psychotropic medication. Arzneimittel-Forschung/Drug Research 1976;26(6):1188-9. [PubMed] [Google Scholar]
Von Mundy 1956 {published data only}
- Von Mundy VG. Results with fluorocortisone acetate. Medizinische Klinik 1956;51(50):2130-2. [PubMed] [Google Scholar]
Walter 1979 {published data only}
- Walter R. Fludrocortisone in orthostatic hypotension. New England Journal of Medicine 1979;301(20):1121. [DOI: 10.1056/NEJM197911153012014] [DOI] [PubMed] [Google Scholar]
Warne 1987 {published data only}
- Warne RW. Postural hypotension. Mechanisms and management. Current Therapeutics 1987;28(10):81-96. [Google Scholar]
Wasielewski 2001 {published data only}
- Wasielewski S. Chronic fatigue syndrome: fludrocortisone is not effective in neurally mediated hypotension. Deutsche Apotheker Zeitung 2001;141(6):44-5. [Google Scholar]
Watson 1987 {published data only}
- Watson RDS. Treating Postural Hypotension. British Medical Journal 1987;294(6569):390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watt 1980 {published data only}
- Watt SJ, Tooke JE, Perkins CM, Lee MR. Flurbiprofen and fludrocortisone in idiopathic postural hypotension. Clinical Science 1980;59(3):4P. [Google Scholar]
Watt 1981 {published data only}
- Watt SJ, Tooke JE, Perkins CM, Lee MR. The treatment of idiopathic orthostatic hypotension: a combined fludrocortisone and flurbiprofen regime. Quarterly Journal of Medicine 1981;50(198):205-12. [PubMed] [Google Scholar]
Wenting 1981 {published data only}
- Wenting GJ, Man in 't Veld AJ, Schalekamp MA. Time-course of vascular resistance changes in mineralocorticoid hypertension of man. Clinical Science 1981;61(Suppl. 7):97s-100s. [DOI] [PubMed] [Google Scholar]
Whyte 2014 {published data only}
- Whyte IM, Prior FH, Emslie IK, Davies PEJ, Cosgrove J. Posterior subcapsular cataract in association with fludrocortisone acetate therapy. Clinical and Experimental Ophthalmology 2014;42(8):799-800. [DOI: 10.1111/ceo.12326] [DOI] [PubMed] [Google Scholar]
Williams 1985 {published data only}
- Williams BO, Caird FI, Lennox IM. Haemodynamic response to postural stress in the elderly with and without postural hypotension. Age and Ageing 1985;14(4):193-201. [DOI] [PubMed] [Google Scholar]
Additional references
Arbogast 2009
- Arbogast SD, Alshekhlee A, Hussain Z, McNeeley K, Chelimsky TC. Hypotension unawareness in profound orthostatic hypotension. American Journal of Medicine 2009;122(6):574-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Armstrong 1991
- Armstrong E, Mathias CJ. The effects of the somatostatin analogue, octreotide, on postural hypotension, before and after food ingestion, in primary autonomic failure. Clinical Autonomic Research 1991;1(2):135-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Biaggioni 1987
- Biaggioni I, Onrot J, Stewart CK, Robertson D. The potent pressor effect of phenylpropanolamine in patients with autonomic impairment. Journal of the American Medical Association 1987;258(2):236-9. [PMID: ] [PubMed] [Google Scholar]
Campbell 1975b
- Campbell IW, Ewing DJ, Clarke BF. 9-Alpha-fluorohydrocortisone in the treatment of postural hypotension in diabetic autonomic neuropathy. Diabetes 1975;24(4):381-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chobanian 1979b
- Chobanian AV, Volicer L, Tifft CP, Gavras H, Liang CS, Faxon D. Mineralocorticoid-induced hypertension in patients with orthostatic hypotension. New England Journal of Medicine 1979;301(2):68-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Davies 1978b
- Davies B, Bannister R, Sever P. Pressor amines and monoamine-oxidase inhibitors for treatment of postural hypotension in autonomic failure. Limitations and hazards. Lancet 1978;1(8057):172-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Freeman 1999
- Freeman R, Landsberg L, Young J. The treatment of neurogenic orthostatic hypotension with 3,4-DL-threo-dihydroxyphenylserine: a randomized, placebo-controlled, crossover trial. Neurology 1999;53(9):2151-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Freeman 2003
- Freeman R. Treatment of orthostatic hypotension. Seminars in Neurology 2003;23(4):435-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Freeman 2007
- Freeman R. Autonomic peripheral neuropathy. Neurologic Clinics 2007;25(1):277-301. [PMID: ] [DOI] [PubMed] [Google Scholar]
Freeman 2008
- Freeman R. Clinical practice. Neurogenic orthostatic hypotension. New England Journal of Medicine 2008;358(6):615-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Freeman 2011
- Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clinical Autonomic Research 2011;21(2):69-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Frith 2015
- Frith J. Diagnosing orthostatic hypotension: a narrative review of the evidence. British Medical Bulletin 2015;115(1):123-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ghrist 1928
- Ghrist DG, Brown GE. Postural hypotension with syncope: its successful treatment with ephedrine. American Journal of Medical Science 1928;175:336-49. [Google Scholar]
Gibbons 2017
- Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. Journal of Neurology 2017;264(8):1567-82. [DOI: 10.1007/s00415-016-8375-x] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEPro GDT [Computer program]
- GRADEpro GDT. Version accessed 30 May 2017. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Gupta 2007
- Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. American Journal of Medicine 2007;120(10):841-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hoeldtke 1993
- Hoeldtke RD, Streeten DH. Treatment of orthostatic hypotension with erythropoietin. New England Journal of Medicine 1993;329(9):611-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jordan 1998
- Jordan J, Shannon JR, Biaggioni I, Norman R, Black BK, Robertson D. Contrasting actions of pressor agents in severe autonomic failure. American Journal of Medicine 1998;105(2):116-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaufmann 2003
- Kaufmann H, Biaggioni I. Autonomic failure in neurodegenerative disorders. Seminars in Neurology 2003;23(4):351-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lanier 2011
- Lanier JB, Mote MB, Clay EC. Evaluation and management of orthostatic hypotension. American Family Physician 2011;84(5):527-36. [PMID: ] [PubMed] [Google Scholar]
Low 1997
- Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. Journal of the American Medical Association 1997;277(13):1046-51. [PMID: ] [PubMed] [Google Scholar]
Low 2008
- Low PA. Prevalence of orthostatic hypotension. Clinical Autonomic Research 2008;18 Suppl 1:8-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
McTavish 1989
- McTavish D, Goa KL. Midodrine. A review of its pharmacological properties and therapeutic use. Drugs 1989;38(5):757-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moya 2009
- Moya A, Sutton R, Ammirati F, Blanc J-J, Brignole M, Dahm JB, et al. Guidelines for the diagnosis and management of syncope (version 2009). European Heart Journal 2009;30(21):2631-71. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- NICE. Postural hypotension in adults: fludrocortisone. Evidence summary [ESUOM20]. 01 October 2013. Available at www.nice.org.uk/advice/esuom20/resources/postural-hypotension-in-adults-fludrocortisone-pdf-54116458994857669.
Ong 2013
- Ong AC, Myint PK, Shepstone L, Potter JF. A systematic review of the pharmacological management of orthostatic hypotension. International Journal of Clinical Practice 2013;67(7):633-46. [DOI] [PubMed] [Google Scholar]
Page 2020
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
RevMan 2020 [Computer program]
- Review Manager (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Sandroni 2001
- Sandroni P, Benarroch EE, Wijdicks EF. Caudate hemorrhage as a possible complication of midodrine-induced supine hypertension. Mayo Clinic Proceedings 2001;76(12):1275. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schondorf 2003
- Schondorf R. Acetylcholinesterase inhibition in the treatment of hypotension. Journal of Neurology, Neurosurgery, and Psychiatry 2003;74(9):1187. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singer 2003
- Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. Journal of Neurology, Neurosurgery, and Psychiatry 2003;74(9):1294-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tilvis 1996
- Tilvis RS, Hakala SM, Valvanne J, Erkinjuntti T. Postural hypotension and dizziness in a general aged population: a four-year follow-up of the Helsinki Aging Study. Journal of the American Geriatrics Society 1996;44(7):809-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Lieshout 2000
- Van Lieshout JJ, Ten Harkel AD, Wieling W. Fludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failure. Clinical Autonomic Research 2000;10(1):35-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Veazie 2019
- Veazie S, Peterson K, Helfand M. Development of the Portland Observational Study Screening Tool of Interventions (POST-I) to prioritize studies for a Cochrane Systematic Review. In: Cochrane Colloquium Santiago. Embracing diversity; 2019 Dec 2-6; Santiago, Chile. www.colloquium2019.cochrane.org/abstracts/development-portland-observational-study-screening-tool-interventions-post-i-prioritize: Cochrane, 2019. [ID: P2-080]
Wieling 1993
- Wieling W, Van Lieshout JJ, Van Leeuwen AM. Physical manoeuvres that reduce postural hypotension in autonomic failure. Clinical Autonomic Research 1993;3(1):57-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zesiewicz 2010
- Zesiewicz TA, Sullivan KL, Arnulf I, Chaudhuri KR, Morgan JC, Gronseth GS, et al. Practice Parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010;74(11):924-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gibbons 2010
- Gibbons CH, Raj SR, Lahrmann H, Benatar M. Pharmacological treatments of postural hypotension. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD008269. [DOI: 10.1002/14651858.CD008269] [DOI] [Google Scholar]


