Abstract
Background
Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of ulcerative colitis (UC). Here, we performed an integrated analysis of malignancy events from the tofacitinib phase 3 UC clinical development program (excluding nonmelanoma skin cancer [NMSC]).
Methods
Data (up to May 2019) were pooled from two phase 3 induction studies, a phase 3 maintenance study, and an ongoing, open-label, long-term extension (OLE) study, and analyzed as 3 cohorts: induction (N = 1139), maintenance (N = 592), and overall (induction, maintenance, and ongoing OLE study; N = 1124). Proportions and incidence rates (IRs; unique patients with events per 100 patient-years [PY] of exposure) for malignancies confirmed by adjudication were calculated.
Results
The overall cohort consisted of patients who received at least 1 dose of tofacitinib at 5 or 10 mg twice daily, for up to 6.8 years, with an exposure of 2576.4 PY. Of the 1124 overall cohort tofacitinib-treated patients, 20 developed a malignancy (excluding NMSC; IR, 0.75; 95% confidence interval, 0.46–1.16), of which 17 occurred in patients treated with tofacitinib 10 mg twice daily; importantly, more than 80% of patients predominantly received this dose. Furthermore, there was no apparent clustering of malignancy types, and IRs were stable over time.
Conclusions
In the tofacitinib UC clinical development program, malignancy events were infrequent, and rates were comparable with those in the tofacitinib rheumatoid arthritis and psoriatic arthritis clinical development programs, and for biologic UC treatments. ClinicalTrials.gov: NCT01465763, NCT01458951, NCT01458574, and NCT01470612.
Keywords: ulcerative colitis, inflammatory bowel disease, cancer
INTRODUCTION
Ulcerative colitis (UC) is a chronic, idiopathic inflammatory disorder of the colon, with an unknown etiology.1 Compared with the general population, patients with UC are at increased risk of developing a malignancy; this may be due to the immunosuppressive treatments used, the underlying immune dysfunction of UC, or a combination of both factors.2, 3 A serious complication of UC is the development of colorectal cancer, the pathogenesis of which is driven by inflammation-dependent mechanisms.4 Most patients with UC require life-long treatment, one of the goals of which is to reduce inflammation and thereby reduce the risk of developing colorectal cancer.5 Cervical dysplasia has also been reported at an increased rate in females with inflammatory bowel disease (IBD) compared with females without IBD.6
Treatments for UC, such as thiopurines and tumor necrosis factor inhibitors (TNFi), have been associated with increased risk of malignancy.7–9 This increased risk attributed to TNFi therapy is thought to be driven by concomitant immunomodulatory therapy, with no evidence to suggest an increased risk in those treated with TNFi alone.10, 11 Compared with no exposure to azathioprine, when adjusted for age, treatment of patients with IBD with azathioprine was shown to increase the risk of developing cancer (rate ratio 1.43).7 Whether or not stopping thiopurine treatment reduces a patient’s cancer risk is unknown, with evidence suggesting a risk reduction after cessation7 and conflicting data proposing an ongoing increased risk after cessation.12 A nationwide cohort study based on French National Health Insurance databases concluded that the use of thiopurine or TNFi monotherapy was associated with an increased risk of lymphoma compared with no exposure to either agent, and combination therapy with these agents increased the risk further.9 A recent meta-analysis emphasized the age-adjusted nature of lymphoma in patients using thiopurines, with the highest risk in patients older than 50 years and in males.13 In patients with IBD, an increased risk of nonmelanoma skin cancer (NMSC) has been associated with past or concurrent thiopurine therapy.12, 14
Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of UC. The safety of tofacitinib in adults with moderate to severe active UC was evaluated in clinical studies, including a phase 2 dose-ranging study,15 two phase 3 induction studies,16 a maintenance study,16 and an ongoing, open-label, long-term extension study.17
Sandborn et al, as part of a report on the integrated safety analyses of tofacitinib-treated patients with moderate to severe UC, reported malignancy (excluding NMSC) and NMSC rates for the overall cohort (all patients who received tofacitinib) of the UC tofacitinib clinical development program up to December 2016.18 Here, we further characterize the safety of tofacitinib in patients with UC. We present updated (up to May 2019) integrated interim analyses of malignancy (excluding NMSC) events along with supplemental analyses to evaluate the relationship between malignancy (excluding NMSC) events and average daily tofacitinib dose levels, changes over time, and to identify potential risk factors that may predispose patients to malignancies.
MATERIALS AND METHODS
Tofacitinib Ulcerative Colitis Clinical Development Program
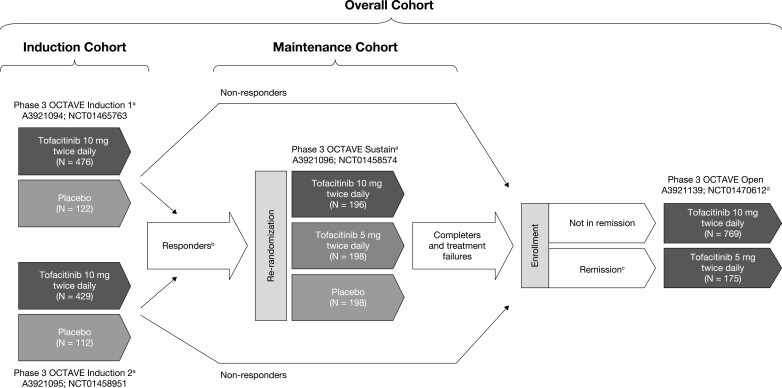
Data from patients treated with tofacitinib 5 mg twice daily and tofacitinib 10 mg twice daily in the tofacitinib UC clinical development program (including three phase 3 studies16 and an ongoing, phase 3, open-label, long-term extension study17) were pooled (Fig. 1, Table 1). Data generated from the dose-ranging phase 2 study (ClinicalTrials.gov: NCT00787202) informed the dose selection for evaluation in the phase 3 studies.15 The phase 3 program evaluated the efficacy and safety of tofacitinib in 2 identical 8-week induction studies (ClinicalTrials.gov: NCT01465763, NCT01458951), a 52-week maintenance study (NCT01458574), and an ongoing, open-label, long-term extension study (NCT01470612; Fig. 1).16, 17
FIGURE 1.
Overview of the tofacitinib UC clinical development program. aFinal complete efficacy assessment at week 8 of 52. Treatment continued up to week 9 of 53. bClinical response in OCTAVE Induction 1 and 2 was defined as a decrease from induction study baseline total Mayo score of ≥3 points and ≥30%, plus a decrease in rectal bleeding subscore of >1 point or an absolute rectal bleeding subscore of 0 or 1. cRemission was defined as a total Mayo score of ≤2 with no individual subscore >1, and a rectal bleeding subscore of 0. dStudy A3921139 (OCTAVE Open) is ongoing. Abbreviations: N, number of patients included in the cohort analysis; UC, ulcerative colitis.
TABLE 1.
Summary of the Cohorts Used for Analysis of Malignancies
| Cohort | Description | |
|---|---|---|
| Induction | Patients | Patients who received either placebo or tofacitinib 10 mg twice daily in OCTAVE Induction 1 or 2 for 8 weeks |
| Treatment groups | Placebo, tofacitinib 10 mg twice daily | |
| Exposure time | 0–9 weeks | |
| Maintenance | Patients | Patients who completed induction treatment with either placebo or tofacitinib 10 mg twice daily demonstrated a clinical response and entered the phase 3 maintenance study |
| Treatment groups | Placebo, tofacitinib 5 mg twice daily, tofacitinib 10 mg twice daily | |
| Exposure time | 0–53 weeks | |
| Overall | Patients | All patients who received at least one dose of tofacitinib 5 mg or 10 mg twice daily in induction studies, the maintenance study, and ongoing OLE studies |
| Treatment groups | Tofacitinib All, predominant-dose tofacitinib 5 mg twice daily, predominant-dose tofacitinib 10 mg twice daily | |
| Exposure time | Up to 6.8 years |
Abbreviations: OLE, open-label, long-term extension
Safety Analysis Cohorts for Malignancy Events
For evaluation of safety, data from the tofacitinib UC clinical development program were pooled and analyzed as 3 cohorts (Table 1).
The overall cohort allows for the evaluation of malignancies up to 6.8 years. To evaluate the potential relationship between malignancies and tofacitinib dose level, patients were analyzed as 2 groups based on their average daily tofacitinib dose: predominant-dose tofacitinib 5 mg twice daily (average daily dose: <15 mg; patients who received tofacitinib 5 mg twice daily for most of their treatment duration) and predominant-dose tofacitinib 10 mg twice daily (average daily dose: ≥15 mg; patients who received tofacitinib 10 mg twice daily for most of their treatment duration). For overall cohort analyses, the start of data capture for tofacitinib exposure was set as the first day of tofacitinib treatment, regardless of whether the patient was initially randomized to receive tofacitinib or placebo in OCTAVE Induction 1 or 2 or OCTAVE Sustain. Occurrence of malignancies in patients who only received placebo during OCTAVE Induction 1 or 2 or OCTAVE Sustain was not included in the overall cohort analyses.
In each cohort, a patient only contributed once to the denominator of each treatment.
Malignancy Adjudication Committee
Biopsies of all potentially malignant tumors were submitted for blinded, central over-read of histopathology. To increase objectivity and control variability, an independent, external, blinded malignancy adjudication committee (consisting of US board-certified oncologists) reviewed all potential malignancy events in the phase 3 studies and the ongoing, open-label, long-term extension study to assess if the possible event met the criteria for malignancy classification. Potential malignancy events were identified for adjudication by review of all adverse events (AEs) and serious AEs coded to the malignant tumor standard Medical Dictionary for Regulatory Activities queries, events submitted for histopathology review as potential malignancies, and any additional cases nominated by investigators or the study sponsor for review. Identification of potential malignancy cases were based on all available study data, including output from the histopathology review, where available.
Proportions and Incidence Rates
For the induction cohort, due to the short treatment duration (up to 9 weeks) and similar mean durations of treatment between the treatment groups, evaluation of malignancies was based on the proportions of patients with events. In contrast, for the maintenance and overall cohorts, incidence rates (IRs) were calculated based on the number of events per 100 patient-years [PY] of exposure. All IR calculations were based on patient censoring at the time of first event or study withdrawal/death.
The changes in IR over time were calculated using 6-month exposure intervals.
Risk Factor Analysis
Summary subgroup analysis was used to identify potential risk factors that could be associated with malignancy events. Potential risk factors included oral corticosteroid use at baseline (yes, no), prior TNFi use (yes, no), TNFi failure (yes, no), prior thiopurines use (yes, no), and thiopurines failure (yes, no).
Cox proportional regression models were used to assess the association of demographic and clinical factors with the risk of malignancies (excluding NMSC). Models were applied to the overall cohort, which included all patients exposed to tofacitinib in the UC program and excluded any time periods and events experienced while receiving placebo. Evaluated baseline factors included (but were not limited to) age, sex, race, body weight, prior TNFi use, prior TNFi failure, prior immunosuppressant use, oral corticosteroid use at baseline, and predominant-dose tofacitinib (a full list of the factors considered is provided in Supplementary Table 1).
The modeling approach for baseline characteristics first applied univariate models to identify individual risk factors with a statistically significant relationship to malignancy; factors with P < 0.10 were then included in a stepwise multivariable model. The final model included all factors from the stepwise model with P < 0.05.
Ethical Considerations
All studies were conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines and were approved by the Institutional Review Boards and/or Independent Ethics Committees at each of the investigational centers participating in the studies, or a central Institutional Review Board. All patients provided written informed consent.
Inclusion and exclusion criteria for OCTAVE Induction 1 and 2 and OCTAVE Sustain have been described previously (see the Supplementary Data).16 OCTAVE Open enrolled patients who were nonresponders after completing OCTAVE Induction 1 or 2, and patients who completed, or were treatment failures in, OCTAVE Sustain.
RESULTS
Patients
Baseline characteristics and demographics are shown in Table 2 for the 3 cohorts. The overall cohort consisted of 1124 patients who received at least 1 dose of tofacitinib at 5 mg or 10 mg twice daily in the UC clinical development program for up to 6.8 years, as of May 27, 2019, with an exposure of 2576.4 PY (mean, 837.2 days; median, 685.5 days; range, 1–2494 days). Due to the design of the OCTAVE studies, the majority (926 of 1124; 82%) of patients received a predominant dose of tofacitinib 10 mg twice daily
TABLE 2.
Demographics and Baseline Characteristics of the Induction, Maintenance, and Overall Cohorts
| Induction Cohort (phase 3) | Maintenance Cohort (phase 3) | Overall Cohort (phase 3/open-label, long-term extension) | ||||
|---|---|---|---|---|---|---|
| Placebo(N = 234) | Tofacitinib10 mg twice daily (N = 905) | Placebo(N = 198) | Tofacitinib5 mg twice daily (N = 198) | Tofacitinib10 mg twice daily (N = 196) | Tofacitinib All (N = 1124)a | |
| Total PY of exposure | 38.2 | 151.2 | 100.4 | 146.2 | 154.3 | 2576.4 |
| Age (years), mean (SD) | 41.1 (14.4) | 41.2 (13.8) | 43.4 (14.0) | 41.9 (13.7) | 43.0 (14.4) | 41.2 (13.9) |
| Male, n (%) | 132 (56.4) | 536 (59.2) | 116 (58.6) | 103 (52.0) | 110 (56.1) | 658 (58.5) |
| Race, n (%) | ||||||
| White | 186 (79.5) | 726 (80.2) | 155 (78.3) | 164 (82.8) | 153 (78.1) | 897 (79.8) |
| Asian | 28 (12.0) | 114 (12.6) | 26 (13.1) | 23 (11.6) | 25 (12.8) | 144 (12.8) |
| Geographical region, n (%) | ||||||
| Asia | 26 (11.1) | 95 (10.5) | 20 (10.1) | 22 (11.1) | 21 (10.7) | 123 (10.9) |
| Eastern Europe | 67 (28.6) | 260 (28.7) | 57 (28.8) | 66 (33.3) | 63 (32.1) | 319 (28.4) |
| North America | 53 (22.6) | 187 (20.7) | 45 (22.7) | 39 (19.7) | 44 (22.4) | 241 (21.4) |
| Western Europe | 68 (29.1) | 274 (30.3) | 55 (27.8) | 47 (23.7) | 57 (29.1) | 337 (30.0) |
| Rest of the world | 20 (8.5) | 89 (9.8) | 21 (10.6) | 24 (12.1) | 11 (5.6) | 104 (9.3) |
| Extent of disease, n (%)b,c | ||||||
| Proctosigmoiditis | 35 (15.0)d | 132 (14.6)d | 21 (10.6)d | 28 (14.3)d | 33 (16.9)d | 163 (14.5)d |
| Left-sided colitis | 76 (32.6)d | 307 (34.0)d | 68 (34.3)d | 66 (33.7)d | 60 (30.8)d | 380 (33.9)d |
| Pancolitis | 122 (52.4)d | 463 (51.3)d | 108 (54.5)d | 102 (52.0)d | 102 (52.3)d | 577 (51.5)d |
| Duration of UC (years), mean (SD) | 8.1 (7.0) | 8.1 (7.0) | 8.8 (7.5) | 8.3 (7.2) | 8.7 (7.0) | 8.2 (7.0) |
| Mean total Mayo score (SD) | 9.0 (1.5)e | 9.0 (1.4)e | 3.3 (1.8) | 3.3 (1.8) | 3.4 (1.8) | 8.6 (2.0)e |
| Median C-reactive protein, mg/L | 4.7f | 4.6f | 1.0 | 0.7 | 0.9 | 4.5f |
| Prior TNFi treatment, n (%)c | 130 (55.6) | 488 (53.9) | 92 (46.5) | 90 (45.5) | 100 (51.0) | 612 (54.4) |
| Prior TNFi failure, n (%)c | 124 (53.0) | 465 (51.4) | 89 (44.9) | 83 (41.9) | 92 (46.9) | 583 (51.9) |
| Prior thiopurine treatment, n (%)c | 160 (68.4) | 683 (75.5) | 134 (67.7) | 149 (75.3) | 144 (73.5) | 838 (74.6) |
| Oral corticosteroid use at baseline, n (%)g | 113 (48.3) | 412 (45.5) | 100 (50.5) | 101 (51.0) | 86 (43.9) | 505 (44.9) |
| Mean oral corticosteroid daily dose at baseline –prednisone equivalent, mg/day (SD)g | 16.5 (6.0) | 16.1 (6.4) | 15.9 (6.2) | 14.9 (6.2) | 14.5 (5.9) | 16.0 (6.3) |
| Smoker status, n (%) | ||||||
| Current smoker | 9 (3.8) | 47 (5.2) | 12 (6.1) | 7 (3.5) | 6 (3.1) | 58 (5.2) |
| Never smoked | 161 (68.8) | 569 (62.9) | 113 (57.1) | 142 (71.7) | 127 (64.8) | 716 (63.7) |
| Ex-smoker | 64 (27.4) | 289 (31.9) | 73 (36.9) | 49 (24.7) | 63 (32.1) | 350 (31.1) |
Abbreviations: N, number of patients in the treatment group; n, number of unique patients with characteristic; PY, patient-years; SD, standard deviation; TNFi, tumor necrosis factor inhibitor; UC, ulcerative colitis.
aAll patients who received tofacitinib 5 or 10 mg twice daily in phase 3 trials (OCTAVE Induction 1 and 2, OCTAVE Sustain, and OCTAVE Open).
bOne patient with proctitis who received tofacitinib in OCTAVE Induction and placebo in OCTAVE Sustain, and was enrolled as a protocol deviation.
cData were collected at the start of the phase 3 induction studies.
dInduction cohort: placebo, N = 233, tofacitinib 10 mg twice daily, N = 903. Maintenance cohort: placebo, N = 198, tofacitinib 5 mg twice daily, N = 196, tofacitinib 10 mg twice daily, N = 195. Overall cohort: N = 1121.
eInduction cohort: placebo, N = 233, tofacitinib 10 mg twice daily, N = 903. Overall cohort: N = 1122.
fInduction cohort: placebo, N = 233, tofacitinib 10 mg twice daily, N = 891. Overall cohort: N = 1110.
gBased on prednisone-equivalent total daily dose and excludes medications such as budesonide and beclomethasone.
Malignancy Events
The malignancy adjudication process was not used in the phase 2 study, although no AEs with a neoplasm or malignancy preferred term were reported in the phase 2 study. In the induction cohort, no malignancy (excluding NMSC) events arose in either treatment group, and in the maintenance cohort, there were no malignancy events in patients treated with either tofacitinib at 5 mg or 10 mg twice daily. However, a single event of breast cancer occurred in a placebo-treated patient with no prior tofacitinib exposure.
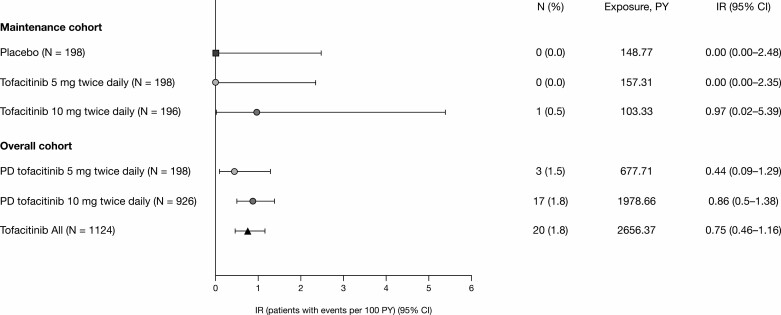
In total, 20 patients had malignancies (excluding NMSC) in the overall cohort as of May 2019; the overall IR for all tofacitinib-treated patients in the UC clinical development program was 0.75/100 PY (95% confidence interval [CI], 0.46–1.16; Fig. 2). All malignancy events occurred during OCTAVE Open; 17 patients had received predominant-dose tofacitinib 10 mg twice daily, resulting in an IR of 0.86 (95% CI, 0.50–1.38), and 3 patients had received predominant-dose tofacitinib 5 mg twice daily (IR 0.44; 95% CI, 0.09–1.29; Fig. 2). These included 1 event each of cholangiocarcinoma (also with peritoneal metastases), leukemia, lung cancer (ex-smoker), essential thrombocythemia, renal cancer, hepatic angiosarcoma, cancer of the penis, esophageal adenocarcinoma, and cutaneous leiomyosarcoma; 2 reports each of colorectal cancer, cervical cancer, lymphoma, and melanoma; and 2 reports of breast cancer (patient listings for malignancies are shown in Supplementary Table 2).
FIGURE 2.
Proportions and IRs of adjudicated malignancies (excluding nonmelanoma skin cancer) in the maintenance and overall cohorts. Abbreviations: CI, confidence interval; IR, incidence rate (unique patients with events per 100 PY of exposure); N, number of patients; PD, predominant dose; PY, patient-years.
Colorectal Cancer
Two patients in the predominant-dose tofacitinib 10 mg twice daily group reported colorectal cancer, resulting in a colorectal cancer IR for the overall cohort of 0.08 (95% CI, 0.01–0.27; Table 3). One of the colorectal cancer events occurred in a patient (male, late 40s) with pancolitis who was diagnosed with UC 16 years before enrollment and received placebo during OCTAVE Induction and due to nonresponse progressed to OCTAVE Open and received tofacitinib 10 mg twice daily. Colon biopsy at week 8 of OCTAVE Induction identified this patient to have high-grade dysplasia of the cecum; the patient subsequently underwent a colectomy. The second colorectal cancer event occurred in a patient (male, mid-30s) with pancolitis who was diagnosed with UC 25 years before enrollment and received placebo during OCTAVE Induction and due to nonresponse progressed to OCTAVE Open and received tofacitinib 10 mg twice daily. After a surveillance biopsy, the patient was identified to have adenocarcinoma with metastases to the liver; the patient subsequently had a subtotal colectomy without primary anastomosis and liver metastasectomy.
TABLE 3.
Proportions and Incidence Rates of Malignancies (Excluding Nonmelanoma Skin Cancer) of Interest in the Overall Cohorta
| Tofacitinib All | Predominant-dose Tofacitinib 5 mg Twice Daily | Predominant-dose Tofacitinib 10 mg Twice Daily | |
|---|---|---|---|
| Total number of patients | 1124 | 198 | 926 |
| Primary analysis | |||
| Total PY of exposure | 2576.4 | 664.1 | 1912.2 |
| Patients with events, n (%) | 20 (1.8) | 3 (1.5) | 17 (1.8) |
| IR (95% CI) | 0.75 (0.46–1.16) | 0.44 (0.09–1.29) | 0.86 (0.50–1.38) |
| Colorectal cancer | |||
| Total PY of exposure | 2661.70 | 677.87 | 1983.83 |
| Patients with events, n (%) | 2 (0.2) | 0 (0.0) | 2 (0.2) |
| IR (95% CI) | 0.08 (0.01–0.27) | 0.00 (0.00–0.54) | 0.10 (0.01–0.36) |
| Lymphoma and lymphoproliferative disease | |||
| Total PY of exposure | 2661.76 | 677.76 | 1984.00 |
| Patients with events, n (%) | 2 (0.2) | 1 (0.5) | 1 (0.1) |
| IR (95% CI) | 0.08 (0.01–0.27) | 0.15 (0.00–0.82) | 0.05 (0.00–0.28) |
Abbreviations: CI, confidence interval; IR, incidence rate (unique patients with event per 100 PY of exposure); n, number of patients with the event; PY, patient-years.
aAll events, including those that are outside the 28-day risk period, are included.
Lymphoma
As of the May 2019 data cut, 2 events of lymphoma were reported in tofacitinib-treated patients, and the overall IR was 0.08 (95% CI, 0.01–0.27).
Non-Hodgkin lymphoma (Epstein-Barr virus-positive) was reported in a patient with refractory disease who received tofacitinib 10 mg twice daily during OCTAVE Induction, progressed directly to OCTAVE Open as a nonresponder, and continued to receive tofacitinib 10 mg twice daily. However, this patient failed to respond. After colectomy due to a perforated sigmoid colon and subsequent colonic tissue examination, a diagnosis of non-Hodgkin lymphoma was made, suggesting that colonic lesions might be a manifestation of the lymphoproliferative disease. The patient had a history of prior UC treatment with TNFi and thiopurines; this patient had been receiving prednisone long term (doses between 10–40 mg/day) before the event.
A patient who had received predominant-dose tofacitinib 5 mg twice daily developed a diffuse large B-cell lymphoma that originated in the testis (not associated with Epstein-Barr virus). This patient also had a history of prior UC treatment with TNFi but no history of treatment with thiopurines.
Malignancy Cause of Deaths
Four patients who received predominant-dose tofacitinib 10 mg twice daily died, 3 of whom had a cause of death reported to be due to malignancy (excluding NMSC) that occurred >28 days after the last dose of tofacitinib. Malignancy events reported included 1 event each of hepatic angiosarcoma (this patient had fatal complications after a liver biopsy 232 days after first tofacitinib dose), acute myeloid leukemia (398 days after first tofacitinib dose), and melanoma (1518 days after first tofacitinib dose). The fourth patient died from pulmonary embolism after developing cholangiocarcinoma and metastases to the peritoneum and undergoing an endoscopic retrograde cholangiopancreatography (384 days after first tofacitinib dose).
Malignancy Events Over Time and Risk Factors
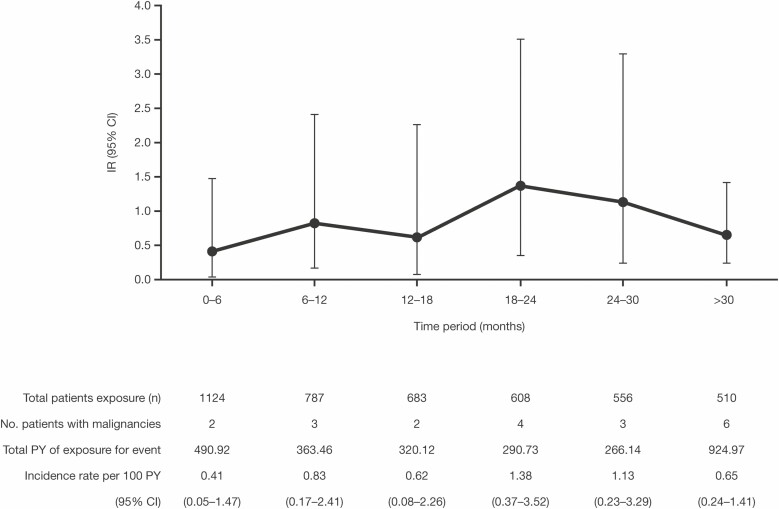
The interval overall IR of malignancies (excluding NMSC) by 6-month intervals was stable over the duration of exposure, with an IR of 0.41 (95% CI, 0.05–1.47) within the first 6 months and an IR of 0.65 (95% CI, 0.24–1.41) at >30 months (Fig. 3).
FIGURE 3.
Incidence rates for malignancies (excluding nonmelanoma skin cancer) in the overall cohort, by 6-monthly intervals. Abbreviations: CI, confidence interval; IR, incidence rate (unique patients with events per 100 PY of exposure); PY patient-years.
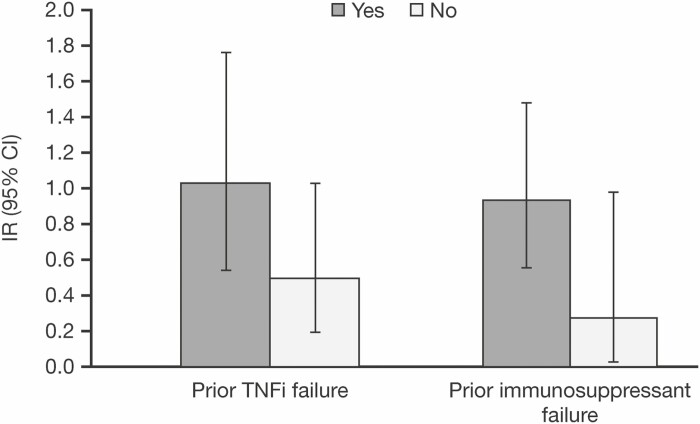
In the overall cohort, there appeared to be a trend towards an increased risk of malignancy (excluding NMSC) in those patients with prior treatment and/or treatment failure with thiopurines or TNFi, compared with those without (Fig. 4). Of the 20 patients with malignancy (excluding NMSC) events, 13 had prior TNFi treatment and failure, 19 had received prior thiopurines treatment, and 18 had thiopurines treatment failure (see Supplementary Table 2). Ten patients with malignancy (excluding NMSC) events had prior treatment with both TNFi and thiopurines (see Supplementary Table 2).
FIGURE 4.
Incidence rates for malignancies (excluding nonmelanoma skin cancer) in the overall cohort, by subgroup. Abbreviations: CI, confidence interval; IR, incidence rate (unique patients with event per 100 PY of exposure); PY, patient-years; TNFi, tumor necrosis factor inhibitor.
When the multivariable Cox proportional hazard model was applied for the overall cohort (tofacitinib all doses), increasing age (every 10 years) and disease duration were identified as significant risk factors for malignancies (excluding NMSC; hazard ratio 1.45; 95% CI, 1.03–2.04 and 1.05; 95% CI, 1.00–1.09, respectively). No other variables analyzed (see Supplementary Table 1), including prior treatment and/or treatment failure with thiopurines or TNFi, were identified as significant risk factors. Results of the univariate analyses are shown in Supplementary Table 1.
DISCUSSION
Malignancies (excluding NMSC) occurred infrequently in the tofacitinib UC clinical development program, with no clustering of specific types and no increase in risk over time. As of May 2019, all malignancy (excluding NMSC) events in tofacitinib-treated patients occurred during OCTAVE Open; 17 patients who had experienced a malignancy had received a predominant dose of tofacitinib 10 mg twice daily (due to the study design, 82% of patients fell into this category), and 3 had received a predominant dose of tofacitinib 5 mg twice daily. In OCTAVE Sustain, 1 placebo-treated patient developed breast cancer. The use of a malignancy adjudication committee across the phase 3 studies ensured systematic application of definitions and validity of data. Investigators reported the relationship between a malignant event and tofacitinib, and relatedness was established by the event-onset date, with a long period to onset required to associate the event with treatment.
The malignancy (excluding NMSC) IR reported here (0.75 overall) is similar to those reported with other UC treatments; a malignancy (excluding NMSC) IR of 0.6 was determined from a pooled analysis of 10 infliximab UC studies,19 and a malignancy IR of 0.5 was reported in patients with UC treated with vedolizumab.20 The IR of malignancies can be contextualized further by comparison to the US Truven MarketScan claims database trial criteria cohort of patients receiving TNFi, for whom a comparable malignancy (excluding NMSC) IR of 0.63 was determined.21 Malignancy (excluding NMSC) events observed in the tofacitinib UC clinical development program are consistent with data from the tofacitinib rheumatoid arthritis and psoriatic arthritis clinical programs, with IRs of 0.8 and 0.55, respectively.21, 22 Data from the rheumatoid arthritis long-term extension study represents over 9 years of tofacitinib clinical experience and ~16,000 PY of exposure, and did not report an IR increase over time.22
Over a 6.8-year period, 20 patients had a confirmed malignancy (excluding NMSC) in the tofacitinib UC clinical development program, 2 of which were colorectal cancer with an IR of 0.08. In one case, the diagnosis of colorectal cancer was made upon colectomy for high-grade dysplasia identified during protocol-mandated endoscopy at the end of the 8-week induction period, during which this patient received placebo. Therefore, it can be assumed malignancy was present before tofacitinib exposure.
Data from 6 vedolizumab clinical trials in patients with UC and Crohn’s disease (2830 patients in total) reported 13 malignancy (excluding NMSC) events; 3 of the 13 events were colorectal cancer with an IR of 0.1.23
NMSC events occurred across the UC clinical development program and will be reported separately. A greater proportion of NMSC events occurred in patients who received predominant-dose tofacitinib 10 mg twice daily compared with patients who received predominant-dose tofacitinib 5 mg twice daily. NMSC events are being reported separately in consideration of the risk factors associated with NMSC, including impact of prior therapies.
Female patients with UC have been identified to be at increased risk of cervical dysplasia, compared with those without UC.6 Two events of cervical cancer occurred over 6.8 years in the tofacitinib UC clinical development program. Treatment of UC with immunosuppressive agents has been associated with an increased risk of lymphoma.24 Two patients developed lymphoma in the tofacitinib UC clinical development program; both patients had prior exposure to TNFi, and one had previously received thiopurines. Thiopurine or TNFi exposure is associated with an increased lymphoma risk compared with no exposure and a further increase in risk with combination therapy compared with monotherapy.9 The IR of lymphoma after thiopurine discontinuation has been shown to gradually decline over time.25 Kotlyar et al showed the lymphoma risk after thiopurine exposure did not persist after thiopurine cessation, with a lower IR in former users (1.42; 95% CI, 0.86–2.34) compared with current users (5.71; 95% CI, 3.72–10.1).13
In the overall cohort, half of the patients with malignancy (excluding NMSC) events had prior treatment with both thiopurines and TNFi. A pooled safety analysis of infliximab treatment in patients with UC showed the malignancy IRs of those treated with infliximab and placebo to be similar between patients with and without immunosuppressant use.19 However, there was a trend for a higher malignancy IR (although not significant) in patients with prior immunosuppressant treatment, compared with immunosuppressant-naïve patients,19 supporting data suggesting that thiopurines are associated with a moderately increased risk of malignancy.
In the UC clinical development program, there was no dose-dependent association with numerically similar rates of malignancy (excluding NMSC) in the 2 predominant-dose groups. However, caution should be exercised when evaluating these data, with most patients categorized to predominant-dose tofacitinib 10 mg twice daily due to the OCTAVE UC clinical development program design. Data from the tofacitinib clinical development program (including rheumatoid arthritis, psoriatic arthritis, psoriasis, and UC) does not show a dose-dependent association with the risk of malignancies (excluding NMSC).21 Within the rheumatoid arthritis clinical program, the IR of malignancies (excluding NMSC) was similar between doses, with an IR of 0.8 and 0.9 for patients who received constant tofacitinib 5 mg or 10 mg twice daily, respectively.26 Further data on malignancy events in patients with rheumatoid arthritis will be gained from the ongoing, postmarketing, open-label study designed to compare the safety of tofacitinib vs TNFi with respect to major adverse cardiovascular events and malignancies (excluding NMSC; NCT02092467).
In the UC clinical development program, there was no evidence of an increase in risk of malignancy (excluding NMSC) with increased duration of tofacitinib treatment as shown by IR analysis by 6-month intervals. Furthermore, the 20 (1.8%) patients with malignancies (excluding NMSC) and an IR of 0.75 (95% CI, 0.46–1.16) reported here is comparable with previous data reported up to December 2016 of 11 (1.0%) patients with malignancies with an IR of 0.7 (95% CI, 0.3–1.2).18 This was also true in the larger tofacitinib rheumatoid arthritis clinical development program, during which the IR remained relatively stable across time intervals at approximately 1.0/100 PY through to 9.4 years.26, 27
An important limitation of these analyses was the small size of the patient population in the UC clinical development program; IRs were based on relatively few events observed in 1124 patients, therefore determining risk factors for malignancy was challenging. Furthermore, approximately three-quarters of patients in the overall cohort had prior immunosuppressant exposure, which is known to carry a long-term risk of malignancy7–9 and may have affected the analysis of these data. Moreover, data concerning the duration of exposure to thiopurines were not available for the patients analyzed; therefore, it was not possible to further stratify the effect of prior immunosuppressant exposure on malignancy risk.
The data presented both here and previously from patients with UC demonstrate the difficulties in evaluating risk factors in a population with prior exposure to multiple treatments that are themselves potential risk factors. The safety profile of tofacitinib in UC integrated from 5 clinical studies with treatment durations of up to 6.8 years, showed that malignancies occurred infrequently, with no apparent clustering of type, and the risk did not increase with time. The IR of malignancies (excluding NMSC) with tofacitinib was comparable with that seen in the rheumatoid arthritis and psoriatic arthritis programs21, 26 and with that reported for biologic therapies for UC.19, 23 Continued monitoring of risk for malignancies over time is needed to fully assess the risk of malignancies including NMSC in patients treated with tofacitinib; larger studies with longer exposure, including analysis of registry data, will enable further understanding of the long-term safety profile of tofacitinib for patients with UC. Before initiation of tofacitinib treatment, the risks and benefits of tofacitinib should be considered in patients with known malignancies other than successfully treated NMSC, or when considering continuation of tofacitinib treatment in those patients who develop a malignancy.28
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the patients, investigators, and study teams involved in the tofacitinib ulcerative colitis clinical development program: phase 2 Induction, OCTAVE Induction 1, OCTAVE Induction 2, OCTAVE Sustain, and OCTAVE Open. These studies were sponsored by Pfizer Inc. Medical writing support, under the guidance of the authors, was provided by Helen Findlow, PhD, CMC Connect, McCann Health Medical Communications and was funded by Pfizer Inc, New York, NY, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015;163:461–464).
Author Contribution: JP and GL planned the trial(s). GL, JP, GR, MC, NL, and CS conducted the trial(s). GL, JP, GR, MC, CS, GC, RP, NL, DQ, CN, and AT collected or interpreted the data. GL, JP, GR, MC, CS, GC, RP, NL, DQ, CN, and AT drafted and edited the manuscript. All authors approved the final version of the manuscript.
Supported by: The clinical studies described in this article were sponsored by Pfizer Inc. Funding for editorial support was provided by Pfizer Inc.
Presented at: Data in this manuscript were originally presented at UEG Week (October 2019) and American College of Gastroenterology (October 2019).
Conflicts of Interest: GL has received research support and/or funding from Celgene, Janssen Orthobiotech, Pfizer Inc, Shire, Takeda, and UCB; has received consultancy fees from AbbVie, American Regent, Cellceutix, Celgene, Eli Lilly, Endo Pharmaceuticals, Ferring, Gilead, Janssen Orthobiotech, Merck, Morphic Therapeutics, Pfizer Inc, Prometheus Laboratories Inc, Romark, Salix/Valeant, Shire, Takeda, and UCB; has received honoraria from American College of Gastroenterology, American Regent, Gastroenterology and Hepatology, Merck, Romark, Springer Science and Business Media and Up-To-Date; and has received royalties from Professional Communications Inc and SLACK Inc. GR has received consultancy fees from AbbVie, Augurix, Bristol-Myers Squibb, Boehringer Ingelheim, Calypso, Celgene, FALK, Ferring, Fisher, Genentech, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Phadia, Roche, Takeda, Tillots, UCB, Vifor, Vital Solutions, and Zeller; has received speaker’s honoraria from AbbVie, AstraZeneca, FALK, Janssen, MSD, Pfizer Inc, Phadia, Takeda, Tillots, UCB, Vifor, and Zeller; and has received educational grants and research grants from AbbVie, Ardeypharm, Augurix, Calypso, FALK, Flamentera, MSD, Novartis, Pfizer Inc, Roche, Takeda, Tillots, UCB, and Zeller. MC has received research support from AbbVie, Gilead, Incyte, and UCB; and has received speaker or consultancy fees from AbbVie, Incyte, Pfizer Inc, Takeda, Theravance, and UCB. JP has received unrestricted institutional grants, consultancy fees, or nonfinancial support (ie, travel support) from AbbVie, Boehringer Ingelheim, Celgene, Genentech, GoodGut, GSK, Janssen, MSD, Nestlé, Novartis, Oppilan, Pfizer Inc, Progenity, Shire, Robarts, Roche, Takeda, Theravance, and TiGenix. CS, GC, RP, NL, DQ, CN, and AT are employees and stockholders of Pfizer Inc.
DATA-SHARING STATEMENT
Upon request and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (a) for indications that have been approved in the US and/or EU or (b) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
REFERENCES
- 1. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017; 389:1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Long MD, Kappelman MD, Pipkin CA. Nonmelanoma skin cancer in inflammatory bowel disease: a review. Inflamm Bowel Dis. 2011;17:1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kappelman MD, Farkas DK, Long MD, et al. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol. 2014;12:265–273.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology . Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523. [DOI] [PubMed] [Google Scholar]
- 6. Rungoe C, Simonsen J, Riis L, et al. Inflammatory bowel disease and cervical neoplasia: a population-based nationwide cohort study. Clin Gastroenterol Hepatol. 2015;13:693–700.e1. [DOI] [PubMed] [Google Scholar]
- 7. Pasternak B, Svanström H, Schmiegelow K, et al. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013;177:1296–1305. [DOI] [PubMed] [Google Scholar]
- 8. Herrinton LJ, Liu L, Weng X, et al. Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2011;106:2146–2153. [DOI] [PubMed] [Google Scholar]
- 9. Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318:1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Annese V, Beaugerie L, Egan L, et al. ; ECCO. European evidence-based consensus: inflammatory bowel disease and malignancies. J Crohns Colitis. 2015;9:945–965. [DOI] [PubMed] [Google Scholar]
- 11. Dulai PS, Siegel CA. The risk of malignancy associated with the use of biological agents in patients with inflammatory bowel disease. Gastroenterol Clin North Am. 2014;43:525–541. [DOI] [PubMed] [Google Scholar]
- 12. Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. ; Cesame Study Group. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621–28.e1–5. [DOI] [PubMed] [Google Scholar]
- 13. Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol. 2015;13:847–858.e4. [DOI] [PubMed] [Google Scholar]
- 14. Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390–399.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandborn WJ, Ghosh S, Panés J, et al. ; Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–624. [DOI] [PubMed] [Google Scholar]
- 16. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 17. Lichtenstein GR, Loftus EV Jr, Bloom S, et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of ulcerative colitis: an interim analysis of an open-label, long-term extension study with up to 4.9 years of treatment (abstract). Am J Gastroenterol. 2018;113(Suppl 1):571. [Google Scholar]
- 18. Sandborn WJ, Panés J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019;17:1541–1550. [DOI] [PubMed] [Google Scholar]
- 19. Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107:1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yajnik V, Khan N, Dubinsky M, et al. Efficacy and safety of vedolizumab in ulcerative colitis and Crohn’s disease patients stratified by age. Adv Ther. 2017;34:542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. U.S. Food and Drug Administration. FDA Advisory Committee Meeting sNDA 203214 Supplement 018 Briefing Document, February 2018. 2018. Accessed April 8, 2020. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/GastrointestinalDrugsAdvisoryCommittee/UCM599514.pdf.
- 22. Wollenhaupt J, Lee EB, Curtis JR, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Afif W, Sandborn WJ, Faubion WA, et al. Risk factors for lymphoma in patients with inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 2013;19:1384–1389. [DOI] [PubMed] [Google Scholar]
- 25. Khan N, Abbas AM, Lichtenstein GR, et al. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology. 2013;145:1007–1015.e3. [DOI] [PubMed] [Google Scholar]
- 26. Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76:1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wollenhaupt J, Silverfield J, Lee EB, et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and efficacy in open-label, long-term extension studies over 9 years (abstract). Arthritis Rheumatol. 2017;69(Suppl 10):522. [Google Scholar]
- 28. Pfizer Inc. XELJANZ Prescribing Information. 2019. Accessed February 11, 2020. https://labeling.pfizer.com/ShowLabeling.aspx?id=959#S5.1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.