Abstract
Background
Fetal exposure risk factors are associated with increased autism spectrum disorder (ASD) risk. New hypotheses regarding multigenerational risk for ASD have been proposed, but epidemiological evidence is largely lacking. We evaluated whether parental birth characteristics, including preterm birth and low birthweight, were associated with ASD risk in offspring.
Methods
We conducted a nationwide register-based cohort study that included 230 174 mother-child and 157 926 father-child pairs in Denmark. Logistic regression models were used to estimate odds ratios (OR) and 95% confidence intervals (CI) for offspring ASD according to parental preterm (<37 weeks) and low birthweight (<2500 g) status, with or without adjustment for certain grandmaternal sociodemographic factors. Mediation analyses were performed for selected parental and offspring health-related factors.
Results
Offspring of mothers or fathers with adverse birth characteristics had about 31–43% higher risk for ASD (maternal preterm birth, OR = 1.31, 95% CI= 1.12, 1.55; maternal low birthweight, OR = 1.35, 95% CI: 1.17,1.57; paternal preterm birth, OR = 1.43, 95% CI = 1.18, 1.73; paternal low birthweight, OR = 1.38, 95% CI= 1.13, 1.70). Parents born very preterm (<32 weeks) marked a nearly 2-fold increase in ASD risk in their children. These associations were slightly attenuated upon adjustment for grandmaternal sociodemographic factors. Mediation analyses suggested that parental social-mental and offspring perinatal factors might explain a small magnitude of the total effect observed, especially for maternal birth characteristic associations.
Conclusions
Offspring of parents born with adverse characteristics had an elevated risk for ASD. Transmission of ASD risk through maternal and paternal factors should be considered in future research on ASD aetiology.
Keywords: Autism spectrum disorder, birth cohort, Denmark
Key Messages
Maternal and paternal birth characteristics, including preterm birth and low birthweight, were associated with an increased risk of autism spectrum disorder (ASD) in offspring.
Parents born very preterm (<32 weeks) marked a nearly 2-fold increase in ASD risk in their children.
Our mediation analysis suggested that parental social-mental risk and perinatal outcomes of the offspring might explain a small magnitude of the total effect between parental birth characteristics and offspring ASD risk.
Our findings provide new evidence for possible multigenerational factors influencing ASD risk that should be sought, in both men and women, before conception.
Introduction
Autism spectrum disorder (ASD) comprises a group of neurodevelopmental conditions characterized by atypical socialization, language impairments and restricted, repetitive behaviours and interests. The recent prevalence estimate of ASD is around 1.5% in developed countries and the prevalence is still on the rise.1,2 The aetiology of ASD remains largely unclear while both genetic3–5 and environmental6–8 risk factors are proposed to contribute.
Multigenerational risks for neurodevelopmental disorders have recently been suggested in epidemiological studies. For instance, in-utero exposure to diethylstilbestrol in female nurses is associated with elevated risk of attention-deficit/hyperactivity disorder (ADHD) in offspring.9 A Swedish population-based study has indicated that advancing grandparental age at delivery may influence ASD risk in successive generations.10 Maternal grandmother smoking during pregnancy has also been associated with social communication deficits and repetitive behaviours in grandchildren from a UK birth cohort.11
Preterm birth12,13 and low birthweight8 are well-established predictors for ASD. However, the relation between parental adverse birth characteristics, indicators of unfavourable intrauterine growth and development of parents,14 and ASD risk in their offspring has remained unexplored. We hypothesized several mechanisms through which these birth characteristics may influence ASD risk in the following generation. First, intrauterine exposures to harmful environmental stimuli that lead to prematurity and impaired fetal growth might induce epigenetic reprogramming in the germline and influence offspring ASD risk.15–17 Furthermore, unfavourable outcomes at birth have been associated with impaired overall physical health, social difficulties and poorer socioeconomic achievement persisting into adulthood.18–20 These characteristics of parents, in turn, could potentially affect the risk of ASD in their children. Finally, shared genetic and environmental influences on adverse birth characteristics of the parents and ASD risk in the family are also possible.21
Here, we conducted a population-based multigenerational cohort study in Denmark and evaluated whether parental preterm birth or low birthweight was associated with increased risk of ASD in offspring. The study was approved by the Danish Data Protection Agency (J.nr. 2013–41-2569) and the Institutional Review Board at Yale University (2000025260). By Danish law, no informed consent is required for a register-based study of anonymized data.
Methods
Study population
The source population consisted of Danish individuals born since 1978 who had a live-born offspring registered in Denmark during 1994–2013. We retrieved data on parents and their offspring from the Danish Medical Birth Register (DMBR) that has collected extensive information on pregnancy and birth outcomes across Denmark since 1973.22 Parent-child pairs were linked using the unique 10-digit civil registration identifier assigned to all Danish residents, and only singleton births were studied.23 Gestational age was recorded since 1978; thus parents born between 1973 and 1977 were excluded. We assessed offspring born 1994–2013 for ASD diagnosis using the International Classification of Diseases 10th edition (ICD-10) codes recorded in the Danish Psychiatric Central Registry (DPCR), which has registered electronic information on all psychiatric hospital admissions in Denmark since 1969 and all outpatient visits since 1995.24 All children were at least 3 years of age by the end of study follow-up in 2016. We identified 230 174 mother-child and 157 926 father-child pairs with complete information on parental gestational age (20–45 weeks) and birthweight (500–6800 g) for analysis.
Exposure
Low birthweight was defined according to the World Health Organization's standards as weight at birth less than 2500 g,25 and preterm birth was referred to as less than 37 completed weeks of gestation.26 We further classified being born before 32 completed weeks of gestation as very preterm birth, and defined weight at birth less than 2500 g but delivered at full term as term low birthweight. Gestational age recorded in the DMBR in most cases was estimated based on ultrasound examinations done before 24 weeks of gestation, conducted by midwives, and the first day of the last menstrual period (LMP).27
Outcome
ICD-10 codes, used for diagnostic reporting in Denmark since 1994, were used to define ASD in this study (F84.0, F84.1, F84.5, F84.8, and F84.9).
Covariates
Covariate data on offspring sex and grandmaternal age at delivery (<25, 25–29, 30–34, >35 years) were obtained from the DMBR. Year of birth of parents (1978–82, 1983–87, 1988–98), grandmaternal place of residence at parents’ birth in Denmark [Capital city (Copenhagen), other cities with more than 100 000 inhabitants (Aarhus, Odense, Aalborg) and other places], grandmaternal parity(1, 2 and ≥3) and grandmaternal education level (primary and lower secondary, upper secondary education and academy profession degree, bachelor and above) were retrieved from the Integreret Database for Arbejdsmarkedsforskning (IDA).28
Mediators
We obtained information on several considered established risk factors related to ASD from multiple Danish nationwide registers, and evaluated these factors as potential meditators. First, we assessed maternal chronic and pregnancy conditions using data from the Danish National Patient Registry,29,30 which has collected nationwide data on all somatic hospital admissions since 1977 and outpatient and emergency records since 1994. Maternal chronic and pregnancy-related conditions included hypertension (I10), preeclampsia (O14), diabetes (E08-E11, E13), gestational diabetes (O244) or thyroid dysfunction (E05.0-E05.9) diagnosed 1 year before and during pregnancy. Second, maternal and paternal history of mental illness, which was defined as either parent had a diagnosis of mental disorder before childbirth (F00-F99, from the DPCR). Third, adverse perinatal outcomes of the offspring were defined as the child being born preterm, low birthweight or receiving a diagnosis of congenital malformation (Q00-Q07, Q10-18, Q20-28, Q30-45, Q50-56, Q60-99). Fourth, maternal or paternal educational attainment, defined as whether the mother or the father has completed upper secondary schooling, was ascertained from the IDA.28
Statistical analysis
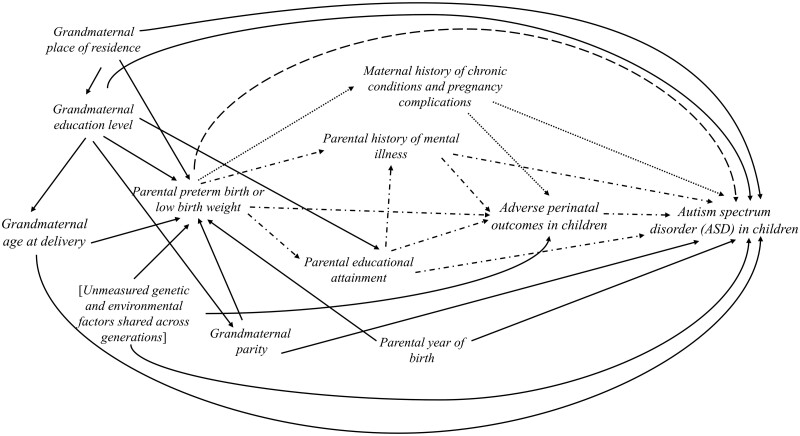
Logistic regression models were used to estimated odds ratios (OR) and 95% confidence intervals (CI) for evaluating offspring ASD risk according to predefined categories of parental birth characteristics. Specifically, we compared ASD risk in children of mothers or fathers born preterm (<37 weeks) or very preterm (<32 weeks) with those with gestational age ≥37 weeks as the reference. We then compared ASD risk in children of mothers or fathers with low birthweight (<2500 g) or term low birthweight (<2500 g and ≥37 weeks) compared with individuals whose birthweight was ≥2500 g as the reference. We first estimated the crude associations. Next, we constructed two adjusted models: the first adjusted for parent’s birth year and residential place at birth, and the second additionally included grandmaternal characteristics (parity, education and grandmaternal age) at delivery. Missing covariates (less than 1.5 %) were accounted for by multiple imputation using the SAS MI procedure. Ten replications without missing values were imputed, and results from these complete datasets were combined. The statistical models were guided by directed acyclic graphs (DAG) considering possible causal relationships among variables included in our analysis (Figure 1).
Figure 1.
The directed acyclic graph (DAG) for the main variables included in the analyses for parental preterm birth or low birthweight and autism spectrum disorder (ASD) risk in children.
The directed acyclic graph represents proposed causal relationships between the variables of interest. A crude (unadjusted) model includes all possible pathways between maternal or paternal birth outcomes and autism spectrum disorder (ASD) in children. First, the direct causal effect (indicated by the long dash line) summarizes possible epigenetic reprogramming of the germ cells that leads to multigenerational transmission of ASD risk. Second, several indirect or mediating paths from parental birth characteristics and ASD in children were evaluated (indicated by dash-dot lines). Maternal chronic diseases and pregnancy complications are not expected to mediate paternal exposures (indicated with dotted line). Finally, factors that could influence the intrauterine growth of the parents constitute possible backdoor paths (solid lines). We blocked these backdoor paths by controlling for several measured factors in the adjusted models. Possible influences of other unmeasured genetic and environmental factors shared across generations (denoted with a bracket) could not be directly evaluated in this project and warrant future investigation.
We carried out mediation analysis to investigate whether and the extent to which selected binary mediating variables mentioned above mediated the relationship between parental birth characteristics and offspring ASD after adjusting for the aforementioned covariates. Each mediating factor was evaluated separately. A regression-based approach under the counterfactual framework was used to perform the analysis, and the total effect of the exposure was decomposed to a controlled direct effect and an indirect effect.31,32 Here, the controlled direct effect indicated the change in offspring ASD risk when the mediator was controlled at the reference level (e.g. without the presence of the mediator) and the exposure was changed from the reference to the index level.33 The indirect effect indicated the effect of the exposure that acted through the pre-specified mediator when parental adverse birth characteristics were fixed to the reference.33 Proportion mediated estimate (PE), the measure of the proportion of the total effect of the exposure mediated by the intermediate variable on the log odds scale, was also calculated.34
Additionally, we conducted analyses stratified by offspring sex to evaluate potential sex-specific associations. Test of heterogeneity was performed by assessing the P-value and corresponding CI for the interaction term. We performed analyses restricted to firstborn children to address concerns that parents born in earlier periods were likely to have more than one child by the end of study follow-up. We also restricted our analyses to children with autistic disorder (F84.0), the most typical subtype, to address clinical and genetic heterogeneity of ASD.35 We then evaluated the results when adjusting for maternal and paternal birth characteristics simultaneously in the same model. Furthermore, we excluded parents born post-term (≥42 weeks) or high birthweight (≥4000 g) in the reference, and re-examined our results. To remove potential influences of recurrent risk genes associated with adverse birth characteristics on ASD risk, we conducted analyses excluding preterm or low birthweight parents whose offspring were also born preterm or low birthweight. Finally, we used different cut-offs to define preterm birth (<35 or <36 completed gestational weeks) and low birthweight (<2400 g or <2600 g) and evaluated the influences of potential misclassification of parental birth characteristics. All analyses were performed using SAS version 9.4 (SAS Institute Inc.).
Results
Study population
Sociodemographic characteristics of the study participants are presented in Table 1. Of all parents, 4.6% mothers (10 688) and 3.7 % fathers (5869) were born with low birthweight, and 3.7% mothers (8419) and 4.1% fathers (6453) were born preterm. Overall, ASD cases were more likely to be descendants of grandmothers with lower levels of education or grandmothers with relatively younger (<25) or older (>35) age at delivery (Supplementary Table S1).
Table 1.
Characteristics of parental birth outcomes in the mothers and fathers
| Parental birth outcomes |
|||
|---|---|---|---|
| Total | Low birthweight | Preterm birth | |
| Mother (n = 230 174) | |||
| Maternal year of birth | |||
| 1978–82 | 134 940 | 6418 (4.8%) | 4745 (3.5%) |
| 1983–87 | 73 718 | 3281 (4.5%) | 2807 (3.8%) |
| 1988–98 | 21 516 | 989 (4.6%) | 867 (4.0%) |
| Missing | 0 | 0 (0.0%) | 0 (0.0%) |
| Grandmaternal education level | |||
| Primary and low secondary | 116 829 | 6430 (5.5%) | 4937 (4.2%) |
| Upper secondary and academy profession | 78 381 | 3116 (4.0%) | 2451 (3.1%) |
| Bachelor+ | 32 076 | 920 (2.9%) | 873 (2.7%) |
| Missing | 2888 | 222 (7.7%) | 158 (5.5%) |
| Grandmaternal parity | |||
| 1 | 104 499 | 5497 (5.3%) | 4138 (4.0%) |
| 2 | 83 327 | 3221 (3.9%) | 2627 (3.2%) |
| ≥3 | 42 348 | 1970 (4.7%) | 1654 (3.9%) |
| Missing | 0 | 0 (0.0%) | 0 (0.0%) |
| Grandmaternal residence at birth | |||
| Copenhagen | 18 803 | 1094 (5.8%) | 846 (4.5%) |
| Aarhus, Odense, or Aalborg | 26 508 | 1177 (4.4%) | 932 (3.5%) |
| Other | 184 863 | 8417 (4.6%) | 6641 (3.6%) |
| Missing | 0 | 0 (0.0%) | 0 (0.0%) |
| Grandmaternal age at birth | |||
| <25 | 97 782 | 5036 (5.2%) | 3881 (4.0%) |
| 25–29 | 83 797 | 3461 (4.1%) | 2704 (3.2%) |
| 30–34 | 36 893 | 1515 (4.1%) | 1264 (3.4%) |
| ≥35 | 11 702 | 676 (5.8%) | 570 (4.9%) |
| Missing | 0 | 0 (0.0%) | 0 (0.0%) |
| Father (n = 157 926) | |||
| Paternal year of birth | |||
| 1978–82 | 102 442 | 3676 (3.6%) | 3928 (3.8%) |
| 1983–87 | 45 665 | 1785 (3.9%) | 2039 (4.5%) |
| 1988–98 | 9819 | 408 (4.2%) | 486 (4.9%) |
| Missing | 0 | 0 (0.0%) | 0 (0.0%) |
| Grandmaternal education level | |||
| Primary and low secondary | 76 656 | 3366 (4.4%) | 3503 (4.6%) |
| Upper secondary and academy profession | 54 922 | 1843 (3.4%) | 2108 (3.8%) |
| Bachelor+ | 24 448 | 545 (2.2%) | 745 (3.0%) |
| Missing | 1900 | 115 (6.1%) | 97 (5.1%) |
| Grandmaternal parity | |||
| 1 | 70 750 | 3144 (4.4%) | 3173 (4.5%) |
| 2 | 58 861 | 1732 (2.9%) | 2079 (3.5%) |
| ≥3 | 28 315 | 993 (3.5%) | 1201 (4.2%) |
| Missing | 0 | 0 (0.0%) | 0 (0.0%) |
| Grandmaternal residence at birth | |||
| Copenhagen | 12 894 | 588 (4.6%) | 585 (4.5%) |
| Aarhus, Odense, or Aalborg | 18 158 | 627 (3.5%) | 731 (4.0%) |
| Other | 126 874 | 4654 (3.7%) | 5137 (4.0%) |
| Missing | 0 | 0 (0.0%) | 0 (0.0%) |
| Grandmaternal age at birth | |||
| <25 | 64 851 | 2732 (4.2%) | 2810 (4.3%) |
| 25–29 | 58 465 | 1920 (3.3%) | 2186 (3.7%) |
| 30–34 | 26 494 | 880 (3.3%) | 1027 (3.9%) |
| ≥35 | 8116 | 337 (4.2%) | 430 (5.3%) |
| Missing | 0 | 0 (0.0%) | 0 (0.0%) |
Adverse parental birth characteristics and offspring ASD
Offspring of mothers or fathers born preterm or low birthweight were associated with 30–40% elevated ASD risk, compared with the reference. The associations did not change when adjusting for parental year of birth and grandmaternal place of residence. The effect estimates were slightly smaller upon adjustment for other grandmaternal factors (maternal preterm birth, OR = 1.26, 95% CI = 1.07, 1.49; maternal low birthweight, OR = 1.27, 95% CI = 1.10, 1.49; paternal preterm birth, OR = 1.41, 95% CI = 1.16, 1.71; paternal low birthweight, OR = 1.33, 95% CI= 1.09, 1.64). Notably, parents born very preterm marked a nearly 2-fold increase in ASD risk in their children (mother: OR = 1.97, 95% CI= 1.28, 3.05; father: OR = 2.13, 95% CI = 1.23, 3.71). The estimates for maternal term low birthweight were similar to those for maternal low birthweight, and the estimates for paternal term low birthweight were closer to the null (Table 2).
Table 2.
Odds ratios and 95% confidence intervals (CI) for autism spectrum disorder (ASD) in children according to parental preterm birth or low birthweight status
| Parental birth outcomes | No. |
Odds Ratios |
|||
|---|---|---|---|---|---|
| ASD cases | Non-cases | Crude | Adjusted | Adjusted | |
| (95% CI) | (95% CI) a | (95% CI) b | |||
| Mother (n = 230 174) | |||||
| Gestational age | |||||
| Non-preterm (≥37 weeks) | 3082 | 218 673 | Reference | Reference | Reference |
| Preterm (<37 weeks) | 153 | 8266 | 1.31 | 1.31 | 1.26 |
| (1.12, 1.55) | (1.11, 1.54) | (1.07, 1.49) | |||
| Very preterm (<32 weeks) | 21 | 732 | 2.04 | 2.04 | 1.97 |
| (1.32, 3.15) | (1.32, 3.16) | (1.28, 3.05) | |||
| Birthweight | |||||
| Non-low birthweight | 3036 | 216 450 | Reference | Reference | Reference |
| (≥2500 g) | |||||
| Low birthweight | 199 | 10 489 | 1.35 | 1.33 | 1.27 |
| (<2500 g) | (1.17, 1.57) | (1.15, 1.54) | (1.10, 1.49) | ||
| Term low birthweight | 98 | 5361 | 1.31 | 1.28 | 1.26 |
| (<2500 g and ≥37 weeks) | (1.07, 1.60) | (1.05, 1.57) | (0.95, 1.67) | ||
| Father (n = 157 926) | |||||
| Gestational age | |||||
| Non-preterm (≥37 weeks) | 1834 | 149 639 | Reference | Reference | Reference |
| Preterm (<37 weeks) | 111 | 6342 | 1.43 | 1.44 | 1.41 |
| (1.18, 1.73) | (1.18, 1.74) | (1.16, 1.71) | |||
| Very preterm (<32 weeks) | 13 | 492 | 2.16 | 2.18 | 2.13 |
| (1.24, 3.75) | (1.26, 3.79) | (1.23, 3.70) | |||
| Birthweight | |||||
| Non-low birthweight | 1847 | 150 210 | Reference | Reference | Reference |
| (≥2500 g) | |||||
| Low birthweight | 98 | 5771 | 1.38 | 1.37 | 1.33 |
| (<2500 g) | (1.13, 1.70) | (1.12, 1.69) | (1.09, 1.64) | ||
| Term low birthweight | 34 | 2335 | 1.19 | 1.18 | 1.12 |
| (<2500 g and ≥37 weeks) | (0.85, 1.68) | (0.83, 1.66) | (0.80, 1.59) | ||
Adjusted for parental year of birth and grandmaternal place of residence at birth.
Adjusted for parental year of birth, grandmaternal place of residence at birth, grandmaternal parity, grandmaternal education level and grandmaternal age at delivery.
Mediation analyses
Table 3 shows estimated direct and indirect effect ORs and proportion mediated estimates (PE). The total effects were approximately the same as the effect estimates reported in Table 2 and thus are not shown here. For maternal associations, educational attainment was the strongest mediator of the total effects (PE: 16.4% for preterm and 30.1% for low birthweight) on offspring ASD risk. A smaller magnitude of indirect effects was found for maternal mental illnesses and adverse perinatal outcomes in offspring (PE: 6.9% to 11.2%), and only a small portion (PE <3.4%) of the total effects was mediated by maternal pregnancy complications. For paternal associations, education level (PE = 11.1%) and mental illness (PE = 6.4%) also had some noticeable mediating effects for low birthweight whereas other estimated indirect effects were small (PE <4.3%). As expected, the estimated indirect effects of maternal pregnancy complications for paternal birth characteristics and offspring ASD risk were null.
Table 3.
The estimated direct and indirect effects of selected mediators on the association between parental adverse birth outcomes and risk of autism spectrum disorder (ASD) in offspring
| Parental birth outcomes and mediators | Controlled direct effecta | Indirect effectb | Proportion mediated estimate (PE) |
|---|---|---|---|
| Odds ratiosd (95% CI) | Odds ratiosd (95% CI) | ||
| Mother | |||
| Preterm birth | |||
| History of mental illness | 1.26 (1.07, 1.48) | 1.01 (1.01, 1.02) | 6.9% |
| Maternal complications | 1.27 (1.08, 1.50) | 1.01 (1.00, 1.01) | 2.5% |
| Adverse perinatal outcomes in offspringc | 1.26 (1.07, 1.48) | 1.02 (1.01, 1.02) | 7.1% |
| Maternal educational attainment | 1.22 (1.03, 1.44) | 1.03 (1.02, 1.05) | 16.4% |
| Low birthweight | |||
| History of mental illness | 1.26 (1.09, 1.46) | 1.02 (1.02, 1.03) | 10.1% |
| Maternal complications | 1.28 (1.11, 1.48) | 1.01 (1.00, 1.01) | 3.4% |
| Adverse perinatal outcomes in offspringc | 1.26 (1.09, 1.45) | 1.03 (1.02, 1.03) | 11.2% |
| Maternal education attainment | 1.20 (1.03, 1.38) | 1.07 (1.06, 1.08) | 30.1% |
| Father | |||
| Preterm birth | |||
| History of mental illness | 1.40 (1.15, 1.70) | 1.01 (1.00, 1.01) | 2.8% |
| Maternal complications | 1.41 (1.16, 1.71) | 1.00 (1.00, 1.00) | 0.7% |
| Adverse perinatal outcomes in offspringc | 1.40 (1.15, 1.69) | 1.01 (1.00, 1.01) | 3.1% |
| Paternal educational attainment | 1.39 (1.14, 1.70) | 1.03 (1.02, 1.04) | 4.3% |
| Low birthweight | |||
| History of mental illness | 1.32 (1.07, 1.62) | 1.02 (1.01, 1.02) | 6.4% |
| Maternal complications | 1.34 (1.09, 1.64) | 1.00 (1.00, 1.00) | 0.9% |
| Adverse perinatal outcomes in offspringc | 1.30 (1.06, 1.61) | 1.00 (1.00, 1.01) | 3.6% |
| Paternal educational attainment | 1.31 (1.07, 1.61) | 1.03 (1.02, 1.04) | 11.1% |
The controlled direct effect indicates the change in offspring ASD risk when the mediator was controlled at the reference level (e.g. without the presence of the mediating factor) and the exposure was changed from the reference to the index level.
The natural indirect effect indicates the effect of the exposure that acts through the pre-specified mediator when parental adverse birth outcomes were fixed to the reference.
These perinatal birth outcomes include congenital malformation, preterm birth (<37 weeks of gestational age) or low birthweight (<2500 g).
Adjusted for parental year of birth, grandmaternal place of residence at birth, grandmaternal parity, grandmaternal education level and grandmaternal age at delivery.
Sensitivity analyses
There were no apparent differences comparing these associations in male and female offspring (Supplementary Table S2, available as Supplementary data at IJE online). The effect estimates were less precise in girls due to a smaller number of ASD cases. The results were unchanged when only including firstborn offspring (Supplementary Table S3, available as Supplementary data at IJE online). Similar effect sizes were found in analyses restricted to children with autistic disorder (Supplementary Table S4, available as Supplementary data at IJE online). Despite a decrease in statistical precision when adjusted for the spouse’s birth characteristic in the same model, the maternal associations remained unchanged, whereas a slight attenuation was found for the paternal associations (except for paternal very preterm birth where the effect size was unchanged) (Supplementary Table S5, available as Supplementary data at IJE online). The results only changed slightly when excluding parents born post-term or high birthweight in the reference (Supplementary Table S6, available as Supplementary data at IJE online) or excluding preterm or low birthweight parents whose offspring were also born preterm or low birthweight (Supplementary Table S7, available as Supplementary data at IJE online). When varying the cut-offs to define birth characteristics, the estimates for the maternal associations were slightly strengthened when preterm was defined as <35 or 36 weeks and low birthweight defined as < 2400 g, and the results remained largely unchanged for the paternal associations (Supplementary Table S8, available as Supplementary data at IJE online).
Discussion
We found that adverse maternal or paternal birth characteristics were independently associated with elevated ASD risk in offspring. The strongest associations were observed in parents born very preterm. Parental social-mental risk, maternal pregnancy complications and perinatal outcomes of the child might act as potential mediators. However, the estimated indirect effects were generally small in magnitude. Future research is needed to replicate our findings and elucidate the mechanisms of these multigenerational ASD risk associations.
Possible explanation of findings
Our findings are consistent with studies demonstrating transmissions of environment-induced risk for developmental disease outcomes across generations. Both animal15–17 and human studies have indicated that female’s prenatal exposures to dichlorodiphenyltrichloroethane (DDT),16 endocrine disrupting chemicals,36 nicotine37,38 and stress17,39 might lead to epigenetic modifications and neurodevelopmental impairments in following generations. Less research has been conducted regarding the fathers, but paternal gestational exposures to environmental agents have been suggested to influence offspring well-being.40,41
The observed associations suggest several plausible pathways. First, epigenetic alterations marked by unfavourable parental birth characteristics might affect ASD risk in offspring through the germline by direct causal mechanisms.42 Ancestral environmental exposures could induce epigenetic reprogramming in the germ cell and regulate gene expression without changes in the underlying DNA sequence.43 Additionally, epigenetic inheritance might be mediated via both the egg and sperm cells,43 supporting our findings showing associations of offspring ASD with paternal adverse birth characteristics, independent of maternal factors. Specific underlying mechanisms should be evaluated in epigenetic studies.
Second, there might be indirect paths mediated via parent’s overall social and health status in adulthood and/or adverse perinatal birth outcomes in offspring. Individuals with unfavourable birth characteristics may experience persistent disadvantages in the physical, mental and social domains of health.18–20 Individuals born preterm were at higher risks of chronic diseases such as diabetes44 and hypertension,45 psychiatric disorders46 and poorer educational attainment47 compared with their term-born counterparts. Further along the trajectory, these characteristics of parents could potentially lead to impaired preconception and reproductive health that might affect ASD risk in the next generation.48,49 Our mediation analysis, conducted to disentangle the direct and indirect pathways, suggested that parental education and health-related factors, as well as perinatal outcomes of the index offspring, might explain a small proportion of the observed associations. However, limited by data availability, this study was not able to capture all indirect paths as only several established risk factors for ASD were included, warranting future research.
Third, grandparental factors that might influence the intrauterine growth of the parent generation have been the focuses of recent multigenerational autism risk research.10,11,50 The associations were slightly attenuated in some models controlled for grandmaternal demographic and pregnancy variables, requiring further understandings of the contributions from grandparental characteristics. Other unmeasured genetic and environmental factors tracked across generations can also play a role.51 Genetic factors might be involved in both the causes of adverse birth characteristics in family52 and risk for ASD.3,4 Our results remained robust after excluding mother-child or father-child pairs that were both born preterm or low birthweight, indicating that genetic risk might not be a primarily explanation for the observed associations. However, possibilities for shared genetic variations could not be ruled out based on current results. Influences from environmental, social and behavioural factors that persist through generations51 also need to be investigated in the future.
Strengths and limitations
The present study was based on a nationwide cohort comprising families of three generations in Denmark starting from 1978. All information on grandmaternal and parental birth characteristics was prospectively and independently registered at the time of birth of the parents, in the DMBR. The DMBR provides high quality obstetric data for research, and any measurement errors for parental birth characteristics were expected to be non-differential with respect to offspring ASD classification. This study included assessment of several potential mediators as physical, social and psychological measures related to parental and child health, using information from national registers in Denmark. The ASD diagnosis was ascertained by trained psychiatrists,53 based on standardized ICD-10 diagnostic criteria. A previous validation study was able to confirm 94% childhood autism diagnoses (F84.0) in 499 cases ascertained in the DPCR,54 but the validity of the diagnoses of other ASD subtypes has not been evaluated.
Several limitations should be noted. First, other factors associated with intrauterine experiences, such as maternal body mass index and smoking, were not collected until more recent years in Denmark, and thus we could not evaluate the effects of these exposures on parental birth characteristics.22 Moreover, individuals with unfavourable birth characteristics, who might have not had children at the time of study,55 were inevitably left out, leaving room for possible selection bias. In addition to the assumption of no uncontrolled confounding between the main exposure and the main outcome, the mediation analysis assumed no uncontrolled confounding between the mediator and the outcome, which might not be satisfied.33 These assumptions should be considered when interpreting direct and indirect effects in the mediation analysis. Moreover, we did not perform multiple mediator analysis because that would additionally require correct specifications of the relations among these mediators.56 Finally, we did not have information on genetic and epigenetic markers, which should be considered in future research.
Conclusions and implications
Parental adverse birth characteristics, including preterm birth and low birthweight, were associated with increased risk of ASD in offspring. Our findings present some arguments for multiple pathways regarding multigenerational influences on ASD risk. Future investigations with extended follow-up, and which examine both genomic and non-genomic mechanisms underlying possible transmission of ASD risk across generations, are recommended.
Supplementary Data
Supplementary data are available at IJE online.
Funding
Z.L. and J.X. were partly supported by the NIH/NIEHS Pathway to Independence Award (K99ES026729/R00ES026729). Y.G. was supported by the National Natural Science Foundation of China (81773387, 81872629) and the Science and Technology Commission of Shanghai Municipality (17ZR1415800). Y.Y. and J.L. were supported by unrestricted grants from the Lundbeck Foundation (R232-2016–2462 and R265-2017–4069), the Danish Council for Independent Research and Independent Research Fund Denmark (DFF-6110–00019, 9039–00010), the Karen Elise Jensens Fond (2016) and Novo Nordisk Fonden (NNF18OC0052029).
Supplementary Material
Acknowledgement
The data for this research were provided by the Danish Medical Birth Registry and the Danish Psychiatric Central Registry.
Author Contributions
Z.L. and JL had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Z.L., J.X. and J.L. conceived of and designed the study. J.X., Y.X. and Z.L. undertook the statistical analysis. J.X. and Z.L. drafted the manuscript. All authors provided critical input to the analyses, interpreted the data and revised the manuscript critically.
Conflict of interest
All authors have no potential or actual conflict of interest to disclose.
References
- 1. Lyall K, Croen L, Daniels J. et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health 2017;38:81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schendel DE, Thorsteinsson E.. Cumulative incidence of autism into adulthood for birth cohorts in Denmark, 1980-2012. JAMA 2018;320:1811–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim YS, Leventhal BL.. Genetic epidemiology and insights into interactive genetic and environmental effects in autism spectrum disorders. Biol Psychiatry 2015;77:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tick B, Bolton P, Happé F, Rutter M, Rijsdijk F.. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatr 2016;57:585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loke YJ, Hannan AJ, Craig JM.. The role of epigenetic change in autism spectrum disorders. Front Neurol 2015;6:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Modabbernia A, Velthorst E, Reichenberg A.. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism 2017;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Early life exposure to particulate matter air pollution (PM1, PM2.5 and PM10) and autism in Shanghai, China: A case-control study. Environ Int 2018;121:1121–27. [DOI] [PubMed] [Google Scholar]
- 8. Wang C, Geng H, Liu W, Zhang G.. Prenatal, perinatal, and postnatal factors associated with autism. Medicine (Baltimore) 2017;96:e6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kioumourtzoglou M-A, Coull BA, O’Reilly ÉJ, Ascherio A, Weisskopf MG.. Association of exposure to diethylstilbestrol during pregnancy with multigenerational neurodevelopmental deficits. JAMA Pediatr 2018;172:670–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frans EM, Sandin S, Reichenberg A. et al. Autism risk across generations: a population based study of advancing grandpaternal and paternal age. JAMA Psychiatry 2013;70:516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Golding J, Ellis G, Gregory S. et al. Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism. Sci Rep 2017;7:46179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirschberger RG, Kuban KCK, O'Shea TM. et al. Co-occurrence and severity of neurodevelopmental burden (cognitive impairment, cerebral palsy, autism spectrum disorder, and epilepsy) at age ten years in children born extremely preterm. Pediatric Neurol 2018;79:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agrawal S, Rao SC, Bulsara MK, Patole SK.. Prevalence of autism spectrum disorder in preterm infants: a meta-analysis. Pediatrics 2018;142:e20180134. [DOI] [PubMed] [Google Scholar]
- 14. Valero de Bernabé J, Soriano T, Albaladejo R. et al. Risk factors for low birthweight: a review. Eur J Obstetr Gynecol Reprod Biol 2004;116:3–15. [DOI] [PubMed] [Google Scholar]
- 15. Martinez ME, Duarte CW, Stohn P. et al. Thyroid hormone influences brain gene expression programs and behaviours in later generations by altering germ line epigenetic information. | Mol Psychiatry 2018;25:939–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Skinner MK, Mohan M, Tracey R. et al. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med 2013;11:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franklin TB, Russig H, Weiss IC. et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 2010;68:408–15. [DOI] [PubMed] [Google Scholar]
- 18. Johnson S, Marlow N.. Early and long-term outcome of infants born extremely preterm. Arch Dis Child 2017;102:97–102. [DOI] [PubMed] [Google Scholar]
- 19. Luu TM, Mian MOR, Nuyt AM.. Long-term impact of preterm birth: neurodevelopmental and physical health outcomes. Clin Perinatol 2017;44:305–14. [DOI] [PubMed] [Google Scholar]
- 20. Bilsteen JF, Taylor-Robinson D, Børch K, Strandberg-Larsen K, Andersen A-MN.. Gestational age and socioeconomic achievements in young adulthood: a Danish population-based study. JAMA Netw Open 2018;1:e186085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schendel D, Bhasin TK.. Birthweight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics 2008;121:1155–64. [DOI] [PubMed] [Google Scholar]
- 22. Bliddal M, Broe A, Pottegård A, Olsen J, Langhoff-Roos J.. The Danish medical birth register. Eur J Epidemiol 2018;33:27–36. [DOI] [PubMed] [Google Scholar]
- 23. Loos RJF, Derom C, Derom R, Vlietinck R.. Determinants of birthweight and intrauterine growth in liveborn twins. Paediatr Perinat Epidemiol 2005;19: 15–22. [DOI] [PubMed] [Google Scholar]
- 24. Mors O, Perto GP, Mortensen PB.. The Danish psychiatric central research register. Scand J Public Health 2011;39:54–57. [DOI] [PubMed] [Google Scholar]
- 25. Wardlaw TM. Low Birthweight: Country, Regional and Global Estimates. Geneva: WHO, UNICEF: 2004. [Google Scholar]
- 26. Blencowe H, Cousens S, Oestergaard MZ. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–72. [DOI] [PubMed] [Google Scholar]
- 27. Kristensen J, Langhoff-Roos J, Skovgaard LT, Kristensen FB.. Validation of the Danish birth registration. J Clin Epidemiol 1996;49:893–97. [DOI] [PubMed] [Google Scholar]
- 28. Li J, Vestergaard M, Obel C, Cnattingus S, Gissler M, Olsen J.. Cohort Profile: The Nordic Perinatal Bereavement Cohort. Int J Epidemiol 2011;40:1161–67. [DOI] [PubMed] [Google Scholar]
- 29. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT.. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH, The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull 1999;46:263–68. [PubMed] [Google Scholar]
- 31. VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 32. Richiardi L, Bellocco R, Zugna D.. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol 2013;42:1511–19. [DOI] [PubMed] [Google Scholar]
- 33. Valeri L, VanderWeele TJ.. Mediation analysis allowing for exposure–mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013;18:137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. VanderWeele TJ, Vansteelandt S.. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol 2010;172:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bruining H, Sonneville L. D, Swaab H. et al. Dissecting the clinical heterogeneity of autism spectrum disorders through defined genotypes. PLoS One 2010;5:e10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iqbal K, Tran DA, Li AX. et al. Deleterious effects of endocrine disruptors are corrected in the mammalian germline by epigenome reprogramming. Genome Biol 2015;16:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buck JM, Sanders KN, Wageman CR, Knopik VS, Stitzel JA, O'Neill HC.. Developmental nicotine exposure precipitates multigenerational maternal transmission of nicotine preference and ADHD-like behavioural, rhythmometric, neuropharmacological, and epigenetic anomalies in adolescent mice. Neuropharmacology 2019;149:66–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCarthy DM, Morgan TJ, Lowe SE. et al. Nicotine exposure of male mice produces behavioural impairment in multiple generations of descendants. PLoS Biol 2018;16:e2006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Serpeloni F, Radtke K, de Assis SG, Henning F, Nätt D, Elbert T.. Grandmaternal stress during pregnancy and DNA methylation of the third generation: an epigenome-wide association study. Transl Psychiatry 2017;7:e1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pembrey ME, Bygren LO, Kaati G, et al. ; The ALSPAC Study Team. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet 2006;14:159–66. [DOI] [PubMed] [Google Scholar]
- 41. Bygren LO, Tinghög P, Carstensen J. et al. Change in paternal grandmothers’ early food supply influenced cardiovascular mortality of the female grandchildren. BMC Genet 2014;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maddalena P. Long term outcomes of preterm birth: the role of epigenetics. Newborn Infant Nurs Rev 2013;13:137–39. [Google Scholar]
- 43. Nilsson EE, Sadler-Riggleman I, Skinner MK.. Environmentally induced epigenetic transgenerational inheritance of disease. Environ Epigenet 2018;4. doi : 10.1093/eep/dvy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crump C, Winkleby MA, Sundquist K, Sundquist J.. Risk of diabetes among young adults born preterm in Sweden. Diabetes Care 2011;34:1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lindström L, Skjaerven R, Bergman E. et al. Chronic hypertension in women after perinatal exposure to preeclampsia, being born small for gestational age or preterm. Paediatr Perinat Epidemiol 2017;31:89–98. [DOI] [PubMed] [Google Scholar]
- 46. Nosarti C, Reichenberg A, Murray RM. et al. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry 2012;69:610–17. [DOI] [PubMed] [Google Scholar]
- 47. Swamy GK, Østbye T, Skjærven R.. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. JAMA 2008;299:1429–36. [DOI] [PubMed] [Google Scholar]
- 48. Alhowikan AM, Al-Ayadhi LY, Halepoto DM.. Review Impact of environmental pollution, dietary factors and diabetes mellitus on Autism Spectrum Disorder (ASD). Pak J Med Sci 2019;35:1179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ornoy A, Weinstein-Fudim L, Ergaz Z.. Prenatal factors associated with autism spectrum disorder (ASD). Reprod Toxicol 2015;56:155–69. [DOI] [PubMed] [Google Scholar]
- 50. Gao Y, Yu Y, Xiao J. et al. Association of grandparental and parental age at childbirth with autism spectrum disorder in children. JAMA Netw Open 2020;3:e202868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chapman D, Scott KG.. The impact of maternal intergenerational risk factors on adverse developmental outcomes. Dev Rev 2001;21:305–25. [Google Scholar]
- 52. Crider KS, Whitehead N, Buus RM.. Genetic variation associated with preterm birth: a HuGE review. Genet Med 2005;7:593–604. [DOI] [PubMed] [Google Scholar]
- 53. Parner ET, Baron-Cohen S, Lauritsen MB. et al. Parental age and autism spectrum disorders. Ann Epidemiol 2012;22:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lauritsen MB, Jørgensen M, Madsen KM. et al. Validity of childhood autism in the Danish psychiatric central register: findings from a cohort sample born 1990–1999. J Autism Dev Disord 2010;40:139–48. [DOI] [PubMed] [Google Scholar]
- 55. Thorsted A, Lauridsen J, Høyer B. et al. Birthweight for gestational age and the risk of infertility: a Danish cohort study. Hum Reprod 2020;35:195–202. [DOI] [PubMed] [Google Scholar]
- 56. Daniel RM, Stavola BLD, Cousens SN, Vansteelandt S.. Causal mediation analysis with multiple mediators. Biometrics 2015;71:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.