Abstract
Background
Maternal diabetes has been associated with a risk of neurodevelopmental disorders (NDDs) in offspring, though the common co-occurrence of autism spectrum disorders (ASD), attention-deficit/hyperactivity disorder (ADHD) and intellectual disability (ID) is rarely considered, nor is the potential for confounding by shared familial factors (e.g. genetics).
Methods
This population-based cohort study used data from Psychiatry Sweden, a linkage of Swedish national registers, to follow 2 369 680 individuals born from 1987 to 2010. We used population-averaged logit models to examine the association between exposure to maternal type 1 diabetes mellitus (T1DM), pre-gestational type 2 diabetes mellitus (T2DM) or gestational diabetes mellitus (GDM), and odds of NDDs in offspring. Subgroup analysis was then performed to investigate the timings of GDM diagnosis during pregnancy and its effect on the odds of NDDs in offspring. We compared these results to models considering paternal lifetime T1DM and T2DM as exposures.
Results
Overall, 45 678 individuals (1.93%) were diagnosed with ASD, 20 823 (0.88%) with ID and 102 018 (4.31%) with ADHD. All types of maternal diabetes were associated with odds of NDDs, with T2DM most strongly associated with any diagnosis of ASD (odds ratioadjusted 1.37, 95% confidence interval 1.03–1.84), ID (2.09, 1.53–2.87) and ADHD (1.43, 1.16–1.77). Considering common co-morbid groups, the associations were strongest between maternal diabetes and diagnostic combinations that included ID. Paternal T1DM and T2DM diagnoses were also associated with offspring NDDs, but these associations were weaker than those with maternal diabetes. Diagnosis of GDM between 27 and 30 weeks of gestation was generally associated with the greatest risk of NDDs in offspring, with the strongest associations for outcomes that included ID.
Conclusion
The association of maternal diabetes with NDDs in offspring varies depending on the co-morbid presentation of the NDDs, with the greatest odds associated with outcomes that included ID. Results of paternal-comparison studies suggest that the above associations are likely to be partly confounded by shared familial factors, such as genetic liability.
Keywords: Autism spectrum disorders, intellectual disability, attention-deficit hyperactivity disorder, type 1 diabetes mellitus, type 2 diabetes mellitus, gestational diabetes mellitus
Key Messages
Maternal diabetes [i.e. type 1 diabetes mellitus, type 2 diabetes mellitus and gestational diabetes mellitus (GDM)] has been associated with an increased risk of neurodevelopmental disorders (NDDs) in offspring [i.e. autism spectrum disorders, intellectual disability (ID) and attention-deficit/hyperactivity disorder]. Though the co-occurrence of NDDs is common, previous studies have rarely taken this into account when exploring associations with maternal diabetes.
This study presents evidence that associations of different types of maternal diabetes vary in relation to the co-occurrence of NDDs in offspring, with the strongest associations generally found for diagnostic groups that included ID.
GDM diagnosed between 27 and 30 weeks of gestation was generally associated with the greatest risk of NDDs in offspring compared with GDM diagnosed earlier or later, especially for cases with co-occurring ID.
Introduction
Autism spectrum disorders (ASD), intellectual disability (ID) and attention-deficit/hyperactivity disorder (ADHD) are common neurodevelopmental disorders (NDDs) with lifelong impacts on affected children and their families.1–3 The co-occurrence of ASD, ADHD and ID is more common than would be expected by chance.1,2,4–9 Clinically, the co-occurrence of NDDs indicates more severe impairment, different needs and poorer prognoses of the affected children compared with children with a single NDD diagnosis.4,7,8,10
Globally, ≤15% of pregnancies are complicated by diabetes, as gestational diabetes mellitus (GDM) or as pre-existing type 1 mellitus (T1DM) or type 2 diabetes mellitus (T2DM).11 Multiple observational studies have reported that maternal diabetes is associated with an increased risk of NDDs in offspring.11–24 Most studies have considered the NDDs individually without regard for their common co-occurrence, with few exceptions.15,25 An adverse effect of a hyperglycaemic intrauterine environment on the developing nervous system has been proposed as one mechanism to explain these associations. Studies by Xiang et al. indicating that GDM diagnosed earlier than 26 weeks of gestation (wkGA) was associated with an increased risk of ASD in offspring,14,26 whereas GDM diagnosed after 26 wkGA was not, support the notion that hyperglycaemia during critical windows of development may impair the developing nervous system, though a similar pattern was not observed for ADHD.12 However, ASD, ADHD and ID are disorders with high heritability estimates27,28 and previous studies have reported that the relationship between maternal body mass index (BMI), a strong predictor for both GDM and T2DM, and offspring risk of ASD29,30 and ADHD31,32 is likely confounded by shared familial factors, such as genetic background. This is supported by studies indicating a genetic overlap between ADHD and body composition, particularly increased body-fat content.33 It has also been suggested that psychiatric disorders may share common genetic loci with autoimmune disorders like T1DM.34
We aimed to evaluate the relationships between maternal diabetes (i.e. T1DM, pre-gestational T2DM and GDM) and risk of NDDs (i.e. ASD, ID and ADHD) in offspring, taking into account the co-occurrence of NDDs. In addition, we evaluated the timing of GDM diagnosis and used a family-based study design employing paternal-offspring comparisons to evaluate evidence for alternative mechanisms to explain the relationship between maternal diabetes and offspring risk of NDDs.
Methods
Register linkage and study population
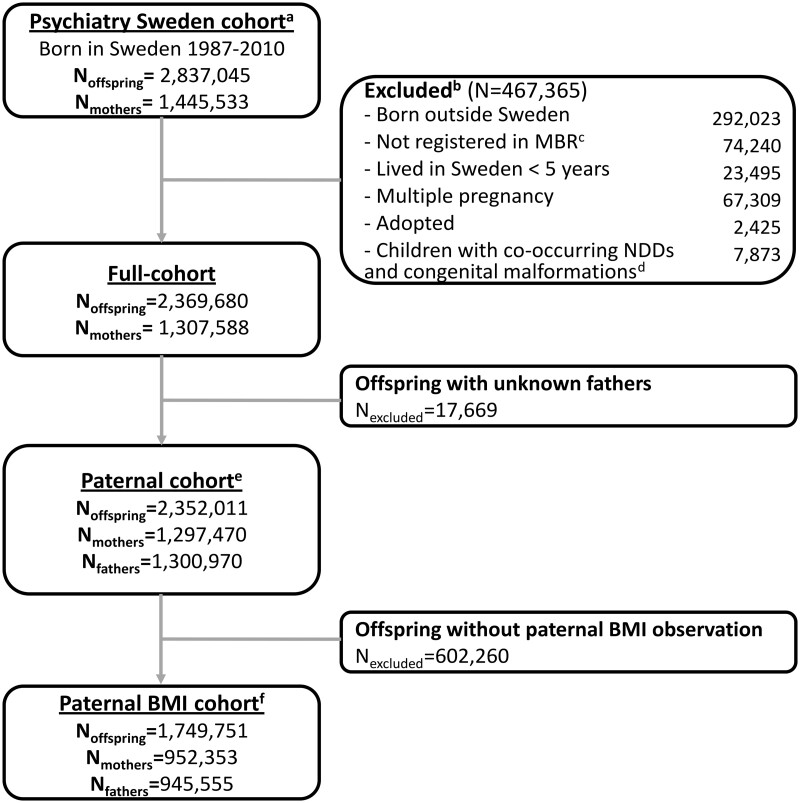
This national, population-based cohort study was based on ‘Psychiatry Sweden’, a comprehensive register linkage designed for studying the occurrence, determinants, and outcomes of psychiatric disorders (Supplementary Figure 1, available as Supplementary data at IJE online). The unique personal identification number assigned to each individual at birth (or upon arrival in Sweden for immigrant parents)35 was replaced by a study identity number by Statistics Sweden. Individual-level data from national registers containing routinely collected health and socio-demographic data were linked using the study identity number by staff statisticians (Supplementary Figure 1, available as Supplementary data at IJE online). We considered as eligible index persons all singleton children born in Sweden from 1 January 1987 to 31 December 2010 (n = 2 837 045), with follow-up to 31 December 2016 (Figure 1). Children who were born outside Sweden, not recorded in the Medical Birth Register (MBR), resident in Sweden for <5 years or adopted were excluded, as were children with both congenital malformations and diagnoses of ASD, ADHD or ID (Figure 1).36 More than one index person could be born to the same mother in this study. Our final study population included 2 369 680 offspring born to 1 307 588 mothers. Ethics approval was obtained from the Stockholm regional ethical review committee (DNR 2010/1185-31/5). Informed consent was not required for the analysis of anonymized register data.
Figure 1.
Study-sample derivation. a Children born before 1987 were excluded from the study population since the date of gestational diabetes mellitus (GDM) diagnosis was only available from 1987. b Individuals were excluded stepwise. c Individuals without information from the Medical Birth Register (MBR) were excluded. d Children with co-morbidities of congenital malformations (or inborn error of metabolism) and neurodevelopmental disorders (NDDs), as NDDs may be attributable to the congenital condition. e Those whose biological fathers were unknown were excluded. f Those whose biological fathers were not registered in the conscription register, were unknown or who lacked body mass index (BMI) observations were excluded.
Case ascertainment
ASD, ADHD and ID were identified using ICD-9 and ICD-10 codes in the National Patient Register (Supplementary Table 1, available as Supplementary data at IJE online),37–39 supplemented with information from the Prescription Drug Register for ADHD case ascertainment (Supplementary Table 1, available as Supplementary data at IJE online).40,41 We considered three potentially overlapping outcomes for comparability to previous studies (Supplementary Figure 2, available as Supplementary data at IJE online): any ASD diagnosis, any ADHD diagnosis and any ID diagnosis. In addition, five mutually exclusive outcomes were considered25,42 (Supplementary Figure 2, available as Supplementary data at IJE online): ‘ASD only’ (without co-occurring ADHD and/or ID); ‘ADHD only’ (without co-occurring ASD and/or ID); ‘ASD + ADHD’ (ASD co-occurring with ADHD, without co-occurring ID); ‘ID + ASD’ (ID co-occurring with ASD, including those with co-occurring ADHD); and ‘ID without ASD’ (ID only or ID co-occurring with ADHD, excluding those with co-occurring ASD). The majority [9587 (70.7%)] of individuals included in the ‘ID without ASD’ group had only an ID diagnosis, with the remainder [3976 (29.3%)] diagnosed with ID with co-occurring ADHD.
Parental diabetes
T1DM, pre-gestational T2DM or GDM were identified from the National Patient Register (Supplementary Table 1, available as Supplementary data at IJE online). If a mother’s diabetes diagnosis could not be clearly established as T1DM or T2DM, given the lack of distinction of these two diagnoses prior to ICD-10, the diagnosis was considered non-specified pre-gestational diabetes mellitus (PGDM-NOS). We classified offspring exposure to maternal diabetes into five mutually exclusive groups: ‘Unexposed’, ‘T1DM’, ‘Pre-gestational T2DM’, ‘GDM’ and ‘PGDM-NOS’ (Supplementary Table 1, available as Supplementary data at IJE online).
We calculated gestational weeks at GDM diagnosis using gestational week at delivery, GDM diagnostic date and birth date (0.11% individuals in the study population had missing values for gestational week at delivery and 13.94% of individuals with a diagnosis of GDM had missing values for date of GDM diagnosis). For the 0.11% of individuals with missing values for gestational week at delivery, we replaced these missing values with 40 wkGA. This resulted in 18 352 (86.06%) of GDM diagnoses with an estimated gestational week at diagnosis. We categorized GDM exposure according to gestational week at GDM diagnosis: ‘≤26 wkGA’, ‘27–30 wkGA’ and ‘>30 wkGA’.12,14
For paternal-comparison analyses, paternal lifetime diagnoses of T1DM and T2DM were used. We classified offspring exposure to paternal diabetes into four mutually exclusive groups: ‘Unexposed’, ‘T1DM’, ‘T2DM’ and ‘DM-NOS’.
Covariates
Parental covariates included parental age at delivery,43 income quintile at birth,35 immigration status,44 education level (highest level achieved by either parent before birth of the index child)35 and history of inpatient psychiatric care prior to the birth of the child (defined as neither parent, one parent or both parents with a psychiatric history); mothers’ diagnosis of Polycystic Ovarian Syndrome (PCOS)40; and paternal BMI measured at the time of conscription to the Swedish military (at age 18).29 Obstetric covariates included parity, smoking during pregnancy, maternal hypertensive diseases, maternal early gestation BMI29,45 and gestational weight gain (defined as ‘ideal’, ‘insufficient’ or ‘excessive’ according to the Institute of Medicine recommendations for each BMI category).46 Offspring covariates include sex, birth year, gestational age at birth, Apgar score at 5 minutes, size for gestational age and population density of birthplace in Sweden (according to the European population-density index,47 with additional categories for the three largest cities in Sweden: ‘Gothenburg and Malmö’, ‘Stockholm’ and ‘Stockholm Suburbs’).
Among these covariates, maternal hypertensive diseases, gestational age, size for gestational age and Apgar score were considered as being potentially on the causal pathways for the association between maternal diabetes and NDDs26 and thus were not included in statistical models.
Statistical analysis
Analyses were conducted using Stata/MP 14.0 (College Station, TX). We considered the neurodevelopmental outcomes to be dichotomous outcomes (i.e. present or absent). To account for clustering of multiple children born to the same mother and provide robust standard errors given this clustering, we used population average logit models, clustered on maternal identification number, to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the relationships between different forms of maternal diabetes (T1DM, T2DM, GDM and PGDM-NOS) and dates of GDM diagnosis (≤26, 27–30 and >30 wkGA) and each outcome.48 In Model 1, we adjusted for children’s sex and birth year. In Model 2, we additionally adjusted for parental educational level, income, immigration status and history of inpatient psychiatric care; children’s birthplace in Sweden; and maternal age, BMI, parity, smoking during pregnancy and PCOS.
The proportion of missing data for most of the covariates and the gestational week at delivery was low (<1%), except for smoking during pregnancy (5.15%), maternal pre-gestational BMI (24.63%) and gestational weight gain (65.62%). To address missing values, we used the missing-indicator method, replacing missing values (except in gestational-weight-gain observations) with a dummy category.49
We conducted several sensitivity analyses. To test for potential confounding by shared familial factors (e.g. genetic factors), we used paternal-comparison models, evaluating the associations of offspring NDDs in relation to paternal-diabetes diagnoses in a restricted population including individuals with an identified biological father (which was most of the population, as only 1.35% did not have an identified biological father). Models evaluating paternal DM as an exposure were adjusted analogously to models evaluating maternal diabetes (Models 1 and 2) and were further adjusted for maternal-diabetes exposure (Model 3). To test for potential biases introduced by the missing-indicator method, we repeated our analyses excluding subjects with missing values. We then adjusted estimates for gestational weight gain within the sub-cohort that had gestational-weight-gain observations. Finally, to investigate whether changes in GDM diagnostic criteria over time affected the odds of NDDs, we stratified GDM-exposed offspring into those born before (<1993) or after 1993 (≥1993).
Results
Study sample
After being followed for 6–29 years, 45 678 (1.93%) individuals were diagnosed with ASD, 20 823 (0.88%) with ID and 102 018 (4.31%) with ADHD, with considerable overlap between the diagnoses (Supplementary Figure 2, available as Supplementary data at IJE online). Individuals exposed to different types of maternal diabetes differed with regard to all covariates (P < 0.001) compared with the unexposed group, except offspring sex (P = 0.11, Table 1). This was also true for individuals exposed to different gestational weeks of GDM diagnosis (Supplementary Table 2, available as Supplementary data at IJE online; P < 0.001 except for sex, where P = 0.049) and paternal diabetes (Supplementary Table 3, available as Supplementary data at IJE online; P < 0.001 except for sex, where P = 0.069).
Table 1.
Characteristics of the cohort, born in 1987–2010, according to maternal-diabetes exposure during the index pregnancy
| Unexposedb | T1DMb | T2DMb | GDMb | PGDM-NOSb | |
|---|---|---|---|---|---|
| Totala | 2 326 033 | 17 444 | 1679 | 21 325 | 3199 |
|
Children’s characteristics | |||||
| NDDs diagnosis (n, %) | |||||
| Unaffected | 2 193 432 (94.3%) | 16 024 (91.9%) | 1523 (90.7%) | 19 808 (92.9%) | 2961 (92.6%) |
| Any ASD | 44 493(2.0%) | 482 (2.9%) | 51 (3.2%) | 577 (2.8%) | 75 (2.5%) |
| Any ID | 20 151 (0.9%) | 277 (1.7%) | 43 (2.7%) | 306 (1.5%) | 46 (1.5%) |
| Any ADHD | 99 665 (4.3%) | 1038 (6.1%) | 102 (6.3%) | 1035 (5.0%) | 178 (5.7%) |
| ASD only | 19 029 (0.9%) | 191 (1.2%) | 23 (1.5%) | 256 (1.3%) | 27 (0.9%) |
| ADHD only | 74 982 (3.3%) | 753 (4.5%) | 74 (4.6%) | 750 (3.6%) | 132 (4.3%) |
| ASD + ADHD | 18 439 (0.8%) | 199 (1.2%) | 16 (1.0%) | 205 (1.0%) | 33 (1.1%) |
| ID without ASDc | 13 126 (0.6%) | 185 (1.1%) | 31 (2.0%) | 190 (1.0%) | 31 (1.0%) |
| ID + ASDd | 7025 (0.3%) | 92 (0.6%) | 12 (0.8%) | 116 (0.6%) | 15 (0.5%) |
| Male sex (n, %) | 1 194 408 (51.3%) | 8924 (51.2%) | 826 (49.2%) | 11 096 (52.0%) | 1658 (51.8%) |
| Birth year (n, %) | |||||
| 1987–1991 | 542 753 (23.3%) | 3813 (21.9%) | 238 (14.2%) | 4292 (20.1%) | 978 (30.6%) |
| 1992–1996 | 508 881 (21.9%) | 3701 (21.2%) | 124 (7.4%) | 4880 (22.9%) | 1065 (33.3%) |
| 1997–2001 | 408 397 (17.6%) | 3205 (18.4%) | 227 (13.5%) | 3505 (16.4%) | 294 (9.2%) |
| 2002–2006 | 461 388 (19.8%) | 3714 (21.3%) | 474 (28.2%) | 4155 (19.5%) | 485 (15.2%) |
| 2007–2010 | 404 614 (17.4%) | 3011 (17.3%) | 616 (36.7%) | 4493 (21.1%) | 377 (11.8%) |
| Birthplace in Sweden (n, %) | |||||
| Stockholm | 213 925 (9.2%) | 1584 (9.1%) | 186 (11.1%) | 1432 (6.7%) | 359 (11.2%) |
| Stockholm Suburbs | 264 836 (11.4%) | 1981 (11.4%) | 217 (12.9%) | 1766 (8.3%) | 362 (11.3%) |
| Gothenburg and Malmö | 201 673 (8.7%) | 1413 (8.1%) | 192 (11.4%) | 2686 (12.6%) | 305 (9.5%) |
| Other cities | 329 077 (14.1%) | 2395 (13.7%) | 199 (11.9%) | 3656 (17.1%) | 414 (12.9%) |
| Other towns and suburbs | 630 402 (27.1%) | 4839 (27.7%) | 422 (25.1%) | 5648 (26.5%) | 719 (22.5%) |
| Rural areas | 675 646 (29.0%) | 5098 (29.2%) | 448 (26.7%) | 6058 (28.4%) | 1016 (31.8%) |
| Missing data | 10 474 (0.5%) | 134 (0.8%) | 15 (0.9%) | 79 (0.4%) | 24 (0.8%) |
|
| |||||
| Parental characteristics | |||||
|
| |||||
| Maternal age at birth (n, %) | |||||
| <25 | 442 481 (19.0%) | 3370 (19.3%) | 99 (5.9%) | 2124 (10.0%) | 398 (12.4%) |
| 25–29 | 792 177 (34.1%) | 5756 (33.0%) | 315 (18.8%) | 5401 (25.3%) | 916 (28.6%) |
| 30–34 | 719 640 (30.9%) | 5223 (29.9%) | 546 (32.5%) | 7083 (33.2%) | 1087 (34.0%) |
| 35–39 | 311 475 (13.4%) | 2557 (14.7%) | 505 (30.1%) | 5083 (23.8%) | 620 (19.4%) |
| ≥40 | 60 122 (2.6%) | 538 (3.1%) | 214 (12.7%) | 1633 (7.7%) | 178 (5.6%) |
| Missing data | 138 (0.01%) | 0 (0.0%) | 0 (0.0%) | 1 (<0.01%) | 0 (0.0%) |
| The highest educational level (either parent) (n, %) | |||||
| <9 years | 75 775 (3.3%) | 772 (4.4%) | 141 (8.4%) | 1146 (5.4%) | 187 (5.8%) |
| 9–12 years | 964 083 (41.4%) | 8123 (46.6%) | 825 (49.1%) | 9838 (46.1%) | 1568 (49.0%) |
| >12 years | 1 284 664 (55.2%) | 8546 (49.0%) | 708 (42.2%) | 10 311 (48.4%) | 1442 (45.1%) |
| Missing data | 1511 (0.1%) | 3 (0.02%) | 5 (0.3%) | 30 (0.1%) | 2 (0.1%) |
| Parental income quintile at birth (n, %) | |||||
| 1 (lowest) | 322 599 (13.9%) | 2411 (13.8%) | 389 (23.2%) | 4199 (19.7%) | 674 (21.1%) |
| 2 | 473 313 (20.3%) | 3797 (21.8%) | 488 (29.1%) | 5249 (24.6%) | 851 (26.6%) |
| 3 | 496 273 (21.3%) | 3876 (22.2%) | 362 (21.6%) | 4381 (20.5%) | 648 (20.3%) |
| 4 | 507 830 (21.8%) | 3896 (22.3%) | 263 (15.7%) | 3988 (18.7%) | 539 (16.8%) |
| 5 (highest) | 509 785 (21.9%) | 3328 (19.1%) | 162 (9.6%) | 3362 (15.8%) | 443 (13.8%) |
| Missing data | 16 233 (0.7%) | 136 (0.8%) | 15 (0.9%) | 146 (0.7%) | 44 (1.4%) |
| Parents born in Sweden (n, %) | |||||
| Only mother | 152 039 (6.5%) | 1141 (6.5%) | 134 (8.0%) | 1163 (5.5%) | 230 (7.2%) |
| Only father | 124 902 (5.4%) | 636 (3.6%) | 121 (7.2%) | 1754 (8.2%) | 184 (5.8%) |
| Both born in Sweden | 1 790 854 (77.0%) | 13 692 (78.5%) | 908 (54.1%) | 13 340 (62.6%) | 2264 (70.8%) |
| Both born outside Sweden | 258 238 (11.1%) | 1975 (11.3%) | 516 (30.7%) | 5068 (23.8%) | 521 (16.3%) |
| Parental history of inpatient psychiatric care (n, %) | |||||
| Neither | 1 959 617 (84.2%) | 13 457 (77.1%) | 1209 (72.0%) | 17 296 (81.1%) | 2544 (79.5%) |
| One parent | 318 642 (13.7%) | 3394 (19.5%) | 394 (23.5%) | 3513 (16.5%) | 566 (17.7%) |
| Both parents | 33 198 (1.4%) | 481 (2.8%) | 48 (2.9%) | 361 (1.7%) | 65 (2.0%) |
| Missing data | 14 576 (0.6%) | 112 (0.6%) | 28 (1.7%) | 155 (0.7%) | 24 (0.8%) |
| Mothers with PCOS (n, %) | 14 618 (0.6%) | 296 (1.7%) | 103 (6.1%) | 409 (1.9%) | 42 (1.3%) |
| Paternal diabetes (n, %) | |||||
| Unexposed | 224 9630 (96.7%) | 16 631 (95.3) | 1560 (92.9) | 20 258 (95) | 3028 (94.7) |
| T1DM | 19 167 (0.8%) | 201 (1.2%) | 7 (0.4%) | 171 (0.8%) | 34 (1.1%) |
| T2DM | 32 543 (1.4%) | 386 (2.2%) | 70 (4.2%) | 596 (2.8%) | 84 (2.6%) |
| DM-NOS | 7418 (0.3%) | 78 (0.5%) | 9 (0.5%) | 116 (0.5%) | 24 (0.8%) |
| With unknown fathers | 17 275 (0.7%) | 148 (0.9%) | 33 (2.0%) | 184 (0.9%) | 29 (0.9%) |
|
| |||||
| Maternal obstetric characteristics | |||||
|
| |||||
| Pre-gestational body mass index (n, %) | |||||
| Normal weight | 1 153 118 (49.6%) | 6483 (37.2%) | 276 (16.4%) | 5944 (27.9%) | 1146 (35.8%) |
| Underweight | 58 613 (2.5%) | 195 (1.1%) | 10 (0.6%) | 271 (1.3%) | 45 (1.4%) |
| Overweight | 391 062 (16.8%) | 3740 (21.4%) | 368 (21.9%) | 4839 (22.7%) | 623 (19.5%) |
| Obese | 150 831 (6.5%) | 2293 (13.1%) | 672 (40.0%) | 5073 (23.8%) | 424 (13.3%) |
| Missing during 1990–1991 | 230 970 (9.9%) | 1646 (9.4%) | 82 (4.9%) | 1799 (8.4%) | 389 (12.2%) |
| Otherwise missing | 341 439 (14.7%) | 3087 (17.7%) | 271 (16.1%) | 3399 (15.9%) | 572 (17.9%) |
| Gestational weight gain (n, %) | |||||
| Ideal | 309 229 (13.3%) | 1827 (10.5%) | 173 (10.3%) | 2478 (11.6%) | 401 (12.5%) |
| Inadequate | 197 679 (8.5%) | 1279 (7.3%) | 152 (9.1%) | 2540 (11.9%) | 271 (8.5%) |
| Excessive | 293 373 (12.6%) | 2348 (13.5%) | 210 (12.5%) | 2369 (11.1%) | 398 (12.4%) |
| Missing during 1990-1993 | 352 596 (15.2%) | 2616 (15.0%) | 126 (7.5%) | 3107 (14.6%) | 709 (22.2%) |
| Otherwise missing | 1 173 156 (50.4%) | 9374 (53.7%) | 1018 (60.6%) | 10 831 (50.8%) | 1420 (44.4%) |
| Smoking during pregnancy (n, %) | |||||
| No | 1 857 941 (79.9%) | 13 039 (74.7%) | 1253 (74.6%) | 17 236 (80.8%) | 2420 (75.6%) |
| Yes | 348 790 (15.0%) | 3050 (17.5%) | 323 (19.2%) | 3022 (14.2%) | 637 (19.9%) |
| Missing data | 119 302 (5.1%) | 1355 (7.8%) | 103 (6.1%) | 1067 (5.0%) | 142 (4.4%) |
| Maternal hypertensive diseases (n, %) | |||||
| Neither | 2 218 576 (95.4%) | 14 426 (82.7%) | 1370 (81.6%) | 19 302 (90.5%) | 2854 (89.2%) |
| Pre-gestational hypertension | 15 684 (0.7%) | 639 (3.7%) | 111 (6.6%) | 423 (2.0%) | 173 (5.4%) |
| Pre-eclampsia | 67 759 (2.9%) | 1908 (10.9%) | 137 (8.2%) | 1268 (5.9%) | 99 (3.1%) |
| Both | 5234 (0.2%) | 316 (1.8%) | 45 (2.7%) | 170 (0.8%) | 25 (0.8%) |
| Missing data | 18 780 (0.8%) | 155 (0.9%) | 16 (1.0%) | 162 (0.8%) | 48 (1.5%) |
| Parity (n, %) | |||||
| 1 | 1 000 242 (43.0%) | 7546 (43.3%) | 408 (24.3%) | 7233 (33.9%) | 658 (20.6%) |
| 2 | 842 920 (36.2%) | 6105 (35.0%) | 523 (31.1%) | 7130 (33.4%) | 1218 (38.1%) |
| ≥3 | 482 871 (20.8%) | 3793 (21.7%) | 748 (44.6%) | 6962 (32.6%) | 1323 (41.4%) |
|
| |||||
| Neonatal characteristics | |||||
|
| |||||
| Maturity at birth (n, %) | |||||
| Preterm | 111 937 (4.8%) | 2879 (16.5%) | 227 (13.5%) | 1761 (8.3%) | 212 (6.6%) |
| Term | 2 043 315 (87.8%) | 14 135 (81.0%) | 1405 (83.7%) | 18 671 (87.6%) | 2772 (86.7%) |
| Post-term | 168 224 (7.2%) | 389 (2.2%) | 47 (2.8%) | 880 (4.1%) | 210 (6.6%) |
| Missing data | 2557 (0.1%) | 41 (0.2%) | 0 (0.0%) | 13 (0.1%) | 5 (0.2%) |
| Size for gestational age (n, %) | |||||
| AGA | 1 121 277 (48.2%) | 6564 (37.6%) | 652 (38.8%) | 9429 (44.2%) | 1462 (45.7%) |
| SGA | 1 158 209 (49.8%) | 8703 (49.9%) | 879 (52.4%) | 10 428 (48.9%) | 1565 (48.9%) |
| LGA | 38 724 (1.7%) | 1873 (10.7%) | 126 (7.5%) | 1345 (6.3%) | 151 (4.7%) |
| Missing data | 7823(0.3%) | 304 (1.7%) | 22 (1.3%) | 123 (0.6%) | 21 (0.7%) |
| Apgar score at 5 minutes (n, %) | |||||
| <7 | 23 618 (1.0%) | 426 (2.4%) | 42 (2.5%) | 295 (1.4%) | 34 (1.1%) |
| ≥7 | 2 280 141 (98.0%) | 16 770 (96.1%) | 1630 (97.1%) | 20 797 (97.5%) | 3116 (97.4%) |
| Missing data | 22 274 (1.0%) | 248 (1.4%) | 7 (0.4%) | 233 (1.1%) | 49 (1.5%) |
T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; GDM, gestational diabetes mellitus; PGDM-NOS, pre-gestational diabetes mellitus-not specified; ASD, autism spectrum disorder; ID, intellectual disability; ADHD, attention-deficit hyperactivity disorder; NDD, neurodevelopmental disorders; PCOS, polycystic ovary syndrome; AGA, appropriate for gestational age; SGA, small for gestational age; LGA, large for gestational age.
Table 1 includes 2 369 680 children born to 1 307 588 mothers. Results are presented as either numbers and percentages (n, %) or median.
We applied the χ2 test for proportions to compare NDD diagnoses and the parental, obstetric and neonatal and children’s characteristics among the unexposed, T1DM, pre-gestational T2DM, GDM and PGDM-NOS. P-values were <0.001 for all covariates except P = 0.11 for children’s sex.
Includes individuals diagnosed with ID and ADHD.
Includes individuals diagnosed with ASD, ID and ADHD.
The number of pregnancies complicated by T2DM and GDM grew between 1987 and 2010, whereas the trend for T1DM was relatively stable (Supplementary Figure 4A, available as Supplementary data at IJE online). Additionally, the prevalence of different types of maternal diabetes varied across regions in Sweden, especially for GDM (Supplementary Figure 4B and C, available as Supplementary data at IJE online). The incidence of ASD, ADHD and ID also increased from 1987 to 2016 (Supplementary Figure 4D, available as Supplementary data at IJE online). Women were diagnosed with GDM at every week from ≤5 to 43 wkGA (Supplementary Figure 3, available as Supplementary data at IJE online). Furthermore, whereas there were several peaks in gestational week at GDM diagnosis, GDM cases were generally more likely to be diagnosed near the end of pregnancy.
Primary analysis
Maternal diabetes was associated with increased odds of ASD (‘Any ASD’), ADHD (‘Any ADHD’) and ID (‘Any ID’), with T2DM being associated with greater odds compared with GDM and T1DM (Table 2). Odds associated with all types of maternal diabetes were stronger in the ‘Any ID’ group compared with ‘Any ASD’ and ‘Any ADHD’. Estimates across exposure categories and outcomes were generally attenuated in adjusted models compared with unadjusted models (Table 2).
Table 2.
Odds ratios and 95% confidence intervals for the association between maternal diabetes and neurodevelopmental disorders for children born in Sweden in 1987–2010 (N = 2 369 680)
| Unexposed | T1DM |
T2DM |
GDM |
PGDM-NOS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. cases (%) | No. cases (%) | Model 1a | Model 2b | No. cases (%) | Model 1a | Model 2b | No. cases (%) | Model 1a | Model 2b | No. cases (%) | Model 1a | Model 2b | |
| Unaffected | 2 193 432 | 16 024 | Ref. | Ref. | 1523 | Ref. | Ref. | 19 808 | Ref. | Ref. | 2961 | Ref. | Ref. |
| Any ASD | 44 493 (1.99) | 482 (2.92) | 1.48 (1.35, 1.63) | 1.29 (1.17, 1.42) | 51 (3.24) | 1.85 (1.39, 2.48) | 1.37 (1.03, 1.84) | 577 (2.83) | 1.43 (1.32, 1.56) | 1.30 (1.20, 1.42) | 75 (2.47) | 1.21 (0.96, 1.53) | 1.13 (0.90, 1.43) |
| Any ID | 20 151 (0.91) | 277 (1.70) | 1.91 (1.69, 2.16) | 1.58 (1.40, 1.79) | 43 (2.75) | 3.57 (2.59, 4.92) | 2.09 (1.53, 2.87) | 306 (1.52) | 1.68 (1.50, 1.89) | 1.30 (1.15, 1.46) | 46 (1.53) | 1.49 (1.10, 2.02) | 1.24 (0.92, 1.67) |
| Any ADHD | 99 665 (4.35) | 1038 (6.08) | 1.42 (1.33, 1.53) | 1.21 (1.13, 1.29) | 102 (6.28) | 1.78 (1.44, 2.19) | 1.43 (1.16, 1.77) | 1035 (4.97) | 1.17 (1.10, 1.25) | 1.16 (1.08, 1.23) | 178 (5.67) | 1.22 (1.04, 1.43) | 1.14 (0.97, 1.34) |
| ASD only | 19 029 (0.86) | 191 (1.18) | 1.38 (1.20, 1.60) | 1.25 (1.08, 1.45) | 23 (1.49) | 2.00 (1.31, 3.03) | 1.54 (1.01, 2.34) | 256 (1.28) | 1.49 (1.32, 1.69) | 1.37 (1.21, 1.56) | 27 (0.90) | 1.02 (0.70, 1.49) | 0.97 (0.66, 1.42) |
| ADHD only | 74 982 (3.31) | 753 (4.49) | 1.37 (1.27, 1.49) | 1.17 (1.08, 1.26) | 74 (4.63) | 1.74 (1.37, 2.22) | 1.43 (1.12, 1.82) | 750 (3.65) | 1.13 (1.05, 1.22) | 1.14 (1.06, 1.23) | 132 (4.27) | 1.22 (1.02, 1.47) | 1.15 (0.96, 1.38) |
| ASD + ADHD | 18 439 (0.83) | 199 (1.23) | 1.46 (1.26, 1.69) | 1.23 (1.06, 1.42) | 16 (1.04) | 1.46 (0.88, 2.42) | 1.12 (0.68, 1.86) | 205 (1.02) | 1.26 (1.09, 1.44) | 1.20 (1.04, 1.38) | 33 (1.10) | 1.33 (0.94, 1.88) | 1.27 (0.89, 1.80) |
| ID without ASDc | 13 126 (0.59) | 185 (1.14) | 1.96 (1.68, 2.28) | 1.59 (1.37, 1.85) | 31 (1.99) | 4.22 (2.91, 6.12) | 2.42 (1.67, 3.49) | 190 (0.95) | 1.62 (1.40, 1.88) | 1.25 (1.08, 1.45) | 31 (1.04) | 1.50 (1.04, 2.17) | 1.22 (0.85, 1.76) |
| ID + ASDd | 7025 (0.32) | 92 (0.57) | 1.81 (1.46, 2.23) | 1.54 (1.25, 1.90) | 12 (0.78) | 2.74 (1.53, 4.90) | 1.61 (0.90, 2.88) | 116 (0.58) | 1.82 (1.51, 2.19) | 1.38 (1.14, 1.66) | 15 (0.50) | 1.54 (0.92, 2.57) | 1.28 (0.77, 2.14) |
T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; GDM, gestational diabetes mellitus; PGDM-NOS, pre-gestational diabetes mellitus-not specified; ASD, autism spectrum disorder; ID, intellectual disability; ADHD, attention-deficit hyperactivity disorder.
Model 1: adjusted for birth year and children’s sex.
Model 2: adjusted for birth year, children’s sex, maternal age, parity, the highest parental educational level, parental income quintile at birth, children’s birthplace in Sweden, parental immigration status, parental history of inpatient psychiatric care, smoking during pregnancy, PCOS and pre-gestational BMI. Missing values were replaced with dummy categories and a complete data set was used for this analysis.
Includes individuals diagnosed with ID and ADHD.
Includes individuals diagnosed with ASD, ID and ADHD.
Considering the mutually exclusive diagnostic categories containing individuals diagnosed with ASD (i.e. ‘ASD only’, ‘ASD + ADHD’ and ‘ASD + ID’) (Supplementary Figure 2, available as Supplementary data at IJE online), the odds of ‘ASD only’ (ORadjusted 1.25, 95% CI 1.08–1.45) and ‘ASD + ADHD’ (ORadjusted 1.23, 95% CI 1.06–1.42) among individuals exposed to T1DM were similar to each other (Table 2, Model 2) and compared with the odds of ‘Any ASD’ (ORadjusted 1.29, 95% CI 1.17–1.42. Table 2, Model 2). However, T1DM exposure was associated with somewhat higher odds of ASD co-occurring with ID (‘ID + ASD’) (ORadjusted 1.54, 95% CI 1.25–1.90). T2DM was associated with greater odds of ‘ID + ASD’ (ORadjusted 1.61, 95% CI 0.90–2.88) and ‘ASD only’ (ORadjusted 1.54, 95% CI 1.01–2.34) compared with ‘ASD + ADHD’ (ORadjusted 1.12, 95% CI 0.68–1.86). Odds associated with GDM were similar across the mutually exclusive outcome groups (Table 2) and comparable to estimates for ‘Any ASD’ (ORadjusted 1.30, 95% CI 1.20–1.42, Table 2).
Among the mutually exclusive categories containing individuals diagnosed with ID (i.e. ‘ID without ASD’ and ‘ID + ASD’) (Supplementary Figure 2, available as Supplementary data at IJE online), exposures to T1DM and GDM were associated with odds of ‘ID without ASD’ and ‘ID + ASD’ that were similar to each other and to ‘Any ID’ (TIDM: ORadjusted 1.58, 95% CI 1.40–1.79; GDM: ORadjusted 1.30, 95% CI 1.15–1.46). However, exposure to T2DM was associated with somewhat higher odds of ‘ID without ASD’ (ORadjusted 2.42, 95% CI 1.67–3.49) compared with ‘ID + ASD’ (ORadjusted 1.61, 95% CI 0.90–2.88) and ‘Any ID’ (ORadjusted 2.09, 95% CI 1.53–2.87) (Table 2).
Among the mutually exclusive categories containing individuals diagnosed with ADHD (‘ADHD only’, ‘ASD + ADHD’, ‘ID without ASD’ and ‘ID + ASD’) (Supplementary Figure 2, available as Supplementary data at IJE online), exposure to T1DM was associated with similar odds between the ‘ADHD only’ (ORadjusted 1.17, 95% CI 1.08–1.26) and ‘ASD + ADHD’ groups (which contain no cases of co-occurring ID) and to ‘Any ADHD’ (ORadjusted 1.21, 95% CI 1.13–1.29). However, exposure to T1DM was associated with greater odds of ‘ID without ASD’ and ‘ID + ASD’ (groups that contain individuals with co-occurring ADHD and ID). Similar patterns were observed for groups exposed to both T2DM and GDM (Table 2).
Timing of GDM
After stratifying exposure to GDM by gestational week of diagnosis, we found that GDM diagnosed at 27–30 wkGA was associated with the greatest odds of ‘Any ASD’, ‘Any ID’ and ‘Any ADHD’ compared with diagnosis at ≤26 and >30 wkGA (Table 3, Supplementary Figure 5, available as Supplementary data at IJE online), with the highest odds associated with the outcome of ‘Any ID’. Considering mutually exclusive groups, we found that, for all groups except ‘ASD + ADHD’, the highest odds were also associated with GDM between 27 and 30 wkGA (Table 3). Of these, the greatest odds were associated with outcomes including ID compared with outcomes without ID.
Table 3.
Odds ratios and 95% confidence intervals for the association between gestational diabetes mellitus and neurodevelopmental disorders, according to gestational week at diagnosis, in children born in Sweden in 1987–2010 (N = 2 347 358)
| Unexposed | ≤26 wkGA |
27-30 wkGA |
>30 wkGA |
Unknown date |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. cases (%) | No. cases (%) | Model 1a | Model 2b | No. cases (%) | Model 1a | Model 2b | No. cases (%) | Model 1a | Model 2b | No. cases (%) | Model 1a | Model 2b | |||
| Unaffected | 2 193 432 | 2371 | Ref. | Ref. | 2117 | Ref. | Ref. | 12 547 | Ref. | Ref. | 2773 | Ref. | Ref. | ||
| Any ASD | 44 493 (1.99) | 70 (2.87) | 1.55 (1.22, 1.97) | 1.35 (1.06, 1.72) | 70 (3.20) | 1.78 (1.41, 2.26) | 1.59 (1.25, 2.02) | 355 (2.75) | 1.37 (1.23, 1.53) | 1.25 (1.12, 1.39) | 82 (2.87) | 1.38 (1.11, 1.72) | 1.30 (1.04, 1.63) | ||
| Any ID | 20 151 (0.91) | 31 (1.29) | 1.50 (1.04, 2.16) | 0.98 (0.68, 1.40) | 51 (2.35) | 2.90 (2.19, 3.84) | 2.12 (1.60, 2.81) | 192 (1.51) | 1.65 (1.43, 1.91) | 1.32 (1.14, 1.52) | 32 (1.14) | 1.16 (0.82, 1.65) | 0.92 (0.65, 1.31) | ||
| Any ADHD | 99 665 (4.35) | 127 (5.08) | 1.33 (1.11, 1.59) | 1.25 (1.04, 1.51) | 110 (4.94) | 1.24 (1.03, 1.51) | 1.23 (1.01, 1.50) | 668 (5.05) | 1.18 (1.09, 1.27) | 1.16 (1.07, 1.26) | 130 (4.48) | 0.99 (0.83, 1.18) | 1.00 (0.84, 1.20) | ||
| ASD only | 19 029 (0.86) | 31 (1.29) | 1.62 (1.13, 2.31) | 1.47 (1.03, 2.10) | 29 (1.35) | 1.74 (1.21, 2.51) | 1.58 (1.10, 2.29) | 153 (1.20) | 1.39 (1.18, 1.63) | 1.27 (1.08, 1.50) | 43 (1.53) | 1.68 (1.24, 2.28) | 1.58 (1.17, 2.14) | ||
| ADHD only | 74 982 (3.31) | 89 (3.62) | 1.26 (1.02, 1.56) | 1.22 (0.98, 1.51) | 83 (3.77) | 1.25 (1.00, 1.56) | 1.29 (1.03, 1.61) | 483 (3.71) | 1.13 (1.03, 1.24) | 1.14 (1.04, 1.26) | 95 (3.31) | 0.96 (0.78, 1.17) | 0.99 (0.80, 1.21) | ||
| ASD + ADHD | 18 439 (0.83) | 26 (1.08) | 1.45 (0.98, 2.13) | 1.34 (0.91, 1.98) | 21 (0.98) | 1.27 (0.82, 1.96) | 1.19 (0.77, 1.84) | 128 (1.01) | 1.23 (1.03, 1.46) | 1.17 (0.98, 1.39) | 30 (1.07) | 1.23 (0.86, 1.77) | 1.24 (0.86, 1.78) | ||
| ID without ASDc | 13 126 (0.59) | 18 (0.75) | 1.40 (0.87, 2.25) | 0.88 (0.55, 1.41) | 31 (1.44) | 2.76 (1.92, 3.96) | 2.01 (1.40, 2.89) | 118 (0.93) | 1.58 (1.31, 1.90) | 1.26 (1.05, 1.51) | 23 (0.82) | 1.26 (0.84, 1.91) | 1.01 (0.67, 1.52) | ||
| ID + ASDd | 7025 (0.32) | 13 (0.55) | 1.77 (1.01, 3.08) | 1.16 (0.67, 2.02) | 20 (0.94) | 3.18 (2.05, 4.93) | 2.29 (1.47, 3.56) | 74 (0.59) | 1.82 (1.45, 2.29) | 1.41 (1.12, 1.78) | 9 (0.32) | 0.97 (0.50, 1.86) | 0.76 (0.39, 1.46) | ||
ASD, autism spectrum disorder; ID, intellectual disability; ADHD, attention-deficit hyperactivity disorder; GDM, gestational diabetes mellitus; wkGA, weeks of gestation.
Model 1: adjusted for birth year and children’s sex.
Model 2: adjusted for birth year, children’s sex, maternal age, parity, the highest parental educational level, parental income quintile at birth, children’s birthplace in Sweden, parental immigration status, parental history of inpatient psychiatric care, smoking during pregnancy, PCOS and pre-gestational BMI. Missing values were replaced with dummy categories and a complete data set was used for this analysis. Missing values in gestational week at delivery (Percentage missing = 0.11%) were replaced by 40 wkGA (280 days) for calculating the gestational week of exposure to GDM.
Includes individuals diagnosed with ID and ADHD.
Includes individuals diagnosed with ASD, ID and ADHD.
Sensitivity analysis
After excluding those with missing values in confounders (Supplementary Tables 4 and 5, available as Supplementary data at IJE online), the magnitude and direction of point estimates were largely similar in crude and adjusted models comparing the results of the sensitivity analysis with the main analysis, though CIs tended to be wider. The greatest variability in the point estimates in the sensitivity analysis compared with the main analysis occurred for the exposure of PGDM-NOS. Among individuals with gestational-weight-gain information, associations with maternal diabetes were similar after additionally adjusting for gestational weight gain (Supplementary Tables 6 and 7, Model 3, available as Supplementary data at IJE online) compared with adjusting for maternal BMI and other confounders only (Supplementary Tables 6 and 7, Model 2, available as Supplementary data at IJE online).
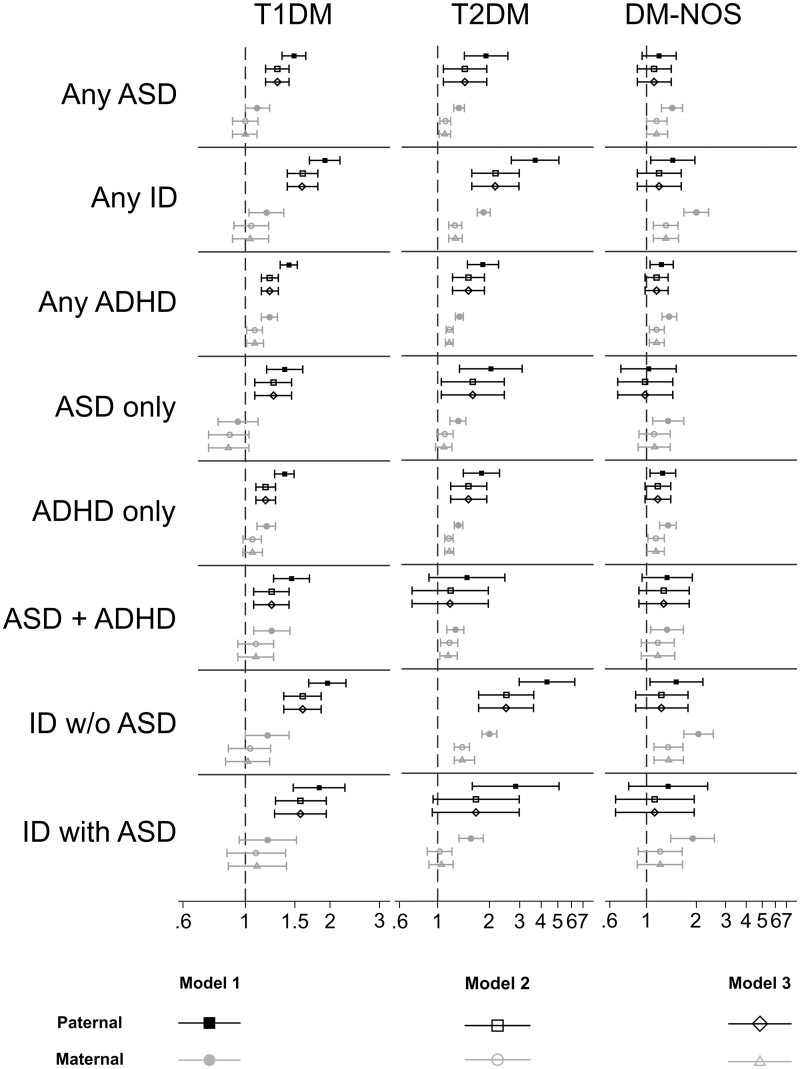
Paternal T2DM was associated with most NDD outcomes (Supplementary Table 8, available as Supplementary data at IJE online;Figure 2). However, the odds associated with paternal T1DM and T2DM were generally lower than the odds associated with maternal T1DM and T2DM. Adjusting for paternal T1DM or T2DM had no material effect on the association between maternal T1DM or T2DM and NDDs. Results were similar after excluding individuals whose biological fathers were not in the conscription register or were otherwise missing BMI information (Supplementary Table 9, available as Supplementary data at IJE online).
Figure 2.
Odds ratios (log-scale) and 95% confidence intervals for the associations between maternal/paternal diabetes and neurodevelopmental disorders in the offspring (see also Supplementary Table 8, available as Supplementary data at IJE online)
T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; GDM, gestational diabetes mellitus; PGDM-NOS, pre-gestational diabetes mellitus-not specified; ASD, autism spectrum disorder; ID, intellectual disability; ADHD, attention-deficit hyperactivity disorder.
After stratifying GDM-exposed offspring into those born <1993 and ≥1993, we found that, although the odds of NDDs in the <1993 group were generally slightly higher compared with those in the ≥1993 group, no drastic differences in the patterns of associations were observed (Supplementary Figure 6, available as Supplementary data at IJE online).
Discussion
In this large population-based cohort study, we found that exposure to maternal diabetes was associated with an increased risk of ASD, ID and ADHD in offspring. Exposure to T2DM was associated with a greater risk of all three NDDs in offspring compared with T1DM and GDM. After categorizing the NDD outcomes into mutually exclusive diagnostic groups, we found that T2DM was still associated with the greatest risks for most outcomes. The associations with T1DM and T2DM were strongest for outcomes that included a diagnosis of ID. Paternal lifetime history of T1DM and T2DM was also associated with offspring risk of NDDs, though the associations were weaker compared with associations with maternal T1DM and T2DM. Finally, considering the timing of GDM onset, we found that diagnosis of GDM between 27 and 30 wkGA was generally associated with the greatest risk of NDDs in offspring, with the strongest associations for outcomes including diagnoses of ID.
Strengths and limitations
Our study has several strengths. By considering the co-occurrence of ASD, ID and ADHD, we were able to compare and contrast the magnitude of associations between diagnostic groups—something few other studies have done despite the common co-occurrence of these disorders. Use of clinically diagnosed maternal diabetes to define exposure and clinically diagnosed outcomes recorded in population-based registers reduced biases in the ascertainment of both exposures and outcomes. We used a family-based study design to evaluate the potential for confounding of the relationship between maternal diabetes and offspring risk for NDDs by shared familial factors. We considered any diagnosis of each NDD as well as mutually exclusive groups reflecting commonly co-occurring diagnostic groups. This approach allowed us to question whether the relationships between maternal diabetes and neurodevelopmental disorders were unique to the individual diagnostic group. We observe that some relationships that were more apparent when we considered the mutually exclusive groups (e.g. the association between T2DM and ID without ASD) were somewhat attenuated compared with the analyses that considered overlapping diagnostic groups (e.g. T2DM and Any ID).
Our results ought to be interpreted in light of several limitations. The register data we used are generally considered to be of high quality.38 For example, in the Swedish National Patient Register, the positive predictive value is over 99% for diabetes and 94.3% for ASD.38,39 However, the ability of the registers to distinguish between the different types of maternal diabetes has not been validated.
Whereas records for most individuals were largely complete, we did have a large number of missing observations for two variables that are important in this context: BMI and gestational weight gain. Pre-gestational BMI is likely to be a strong modulating factor for the risks associated with T2DM50 and GDM.51 Although we generally found the same pattern of associations in the sensitivity analysis after excluding those with missing values in BMI and other confounders, variability in the point estimates and CIs suggests that there may be some residual confounding from individuals whose BMI could not be correctly classified in our main analysis. In addition, there is still the possibility for residual confounding by other factors not adequately captured by our analysis, particularly with regard to socio-demographic factors.
Sample sizes for different exposure groups varied, leading to a difference in statistical power across these groups. Additionally, when comparing results for different types of maternal diabetes, we have emphasized point estimates, though it is important to note that the 95% CIs between these groups sometimes overlap, so caution is necessary when interpreting differences between exposure groups relative to each other.
Given that individuals in Sweden have universal access to comprehensive healthcare, we assume that most of the GDM cases were detected and recorded promptly. Using the registered dates of diagnoses as a proxy for the onset of GDM may lead to misclassification if the true time of onset did not correspond to the registered date of diagnosis. Moreover, there was no information available regarding the methods and criteria used for diagnosing GDM, which have changed over time.52 We did not have information on the efficacy of treatment of hyperglycaemia, nor do we have any information regarding biomarkers of glycaemic control, which is a key limitation as the risks associated with maternal diabetes may well differ with the degree of control of diabetes. Similarly, we lacked any information regarding subclinical disease or pre-diabetes, as we relied on clinical diagnosis of overt diabetes in this study.
Another limitation is the generalizability of the study. The prevalence of different types of maternal diabetes varies across ethnicities and countries.53 Sweden has the second highest annual incidence of T1DM in the world next to Finland.54 The prevalence of overweight and obesity, however, and the corresponding prevalence of T2DM and GDM are considerably lower in Sweden compared with those in other parts of the developed world.13 Despite these differences, multiple other studies have reported similar patterns of associations,13,14,24,26 indicating that our results may well be applicable to other populations.
Comparison with previous studies
In accordance with previous studies, we showed that PGDM is associated with an increased risk of ASD in offspring.13,14,24,26 Xiang et al. observed an association between GDM and risk of ASD after stratification by gestational week at diagnosis and observed that exposure at ≤26 wkGA was associated with the greatest risk of ASD.14,26 Conversely, we found that exposure to cases diagnosed between 27 and 30 wkGA was associated with the greatest risk for ASD, though this difference may relate to the strategies for GDM screening in the two different healthcare setting. Few previous studies have investigated the association between maternal diabetes and ID in offspring.16,17 Mann et al. found a stronger association between PGDM and ID in offspring compared with GDM,16 consistently with our results for ID. A handful of studies have previously investigated the association between maternal diabetes and ADHD,12,25,55,56 with results largely consistent with our own, though Xiang et al. reported no associations between GDM and ADHD, even after the timing of exposure was considered.12
While investigating the association between maternal diabetes and ASD, ID and/or ADHD in offspring, few studies have accounted for the frequent co-occurrence of these three conditions.15,25 Li et al. reported that the risk of ASD associated with PGDM and GDM could mainly be explained by the co-occurrence of ASD and ID, suggesting that ASD with ID formed an etiologically distinct group.25 Our results support the idea that co-occurring ASD and ID may be etiologically distinct from ASD, though we observed the strongest associations with outcomes including ID regardless of other co-occurring NDDs. In another Swedish registered-based study, Ji et al. excluded ADHD cases with a secondary diagnosis of ID, ASD or Tourette syndrome and report an increased risk of ADHD in offspring whose mothers had T1DM,15 consistently with our findings when considering ADHD without ASD and/or ID.
Potential mechanisms and interpretation
ASD, ID and ADHD are complex NDDs with high estimated heritabilities.28 Both T1DM and T2DM are polygenic disorders57,58 and genes related to increased risks of both forms of diabetes may also be involved in the aetiology of NDDs.34,59–61 Shared genetic liability may therefore contribute to the associations between maternal T1DM and T2DM and offspring risk of NDDs reported here, given the elevated risk for NDDs in offspring in relation to paternal lifetime T1DM and T2DM diagnoses. However, maternal diabetes was more strongly associated with NDDs compared with paternal diabetes, indicating that genetic factors or other shared familial factors do not completely explain the associations between maternal diabetes and offspring risk of NDDs. Genetic studies have provided mixed evidence as to whether genetic correlations exist between neurodevelopmental disorders and diabetes. A study using polygenic risk scores found no evidence that shared genetic liability contributed significantly towards the association between maternal diabetes and NDDs in offspring, though the authors noted that the study may not have been adequately powered to detect such associations.62 Another study using data from genome-wide association studies showed a negative genetic association between T1DM and ADHD in the same individuals, and no association between T1DM and ASD.34 However, genetic correlations have been noted between ADHD and indicators of obesity (high body-fat percentage, fat mass and fat-free mass, which are risk factors for T2DM and GDM) and Mendelian randomization subsequently indicated that the relationship between fat mass and ADHD may be causal.33 The genetic overlap between body-fat composition and ADHD may support the idea for some cross-generational genetic liability33 in that mothers at greater risk of obesity (and thus T2DM and GDM) also have greater genetic liability for ADHD, and may therefore transmit a higher risk of ADHD to their children.
Besides genetic liability, multiple plausible mechanisms to link maternal diabetes and NDDs have been proposed. These mechanisms include oxidative stress, hypoxia, apoptosis, epigenetic changes and increased risk of pregnancy complications induced by the immunologic and metabolic disturbances associated with maternal diabetes.12–15,25,63–70 While plausible, our study provides no direct evidence for the involvement of any of these mechanisms. Our study design also does not allow conclusions to be drawn regarding direct causality in the associations we observed, which would need to be explored further in future studies.
We found that PGDM was associated with a greater risk of NDDs in offspring compared with GDM. The timing of environmental exposures may lead to different phenotypic outcomes with regard to neurodevelopment.28 Hyperglycemia due to PGDM may have a greater influence over gross development of the brain in the first trimester,63,71 whereas GDM usually occurs during the second half of pregnancy when the major developmental events for the cerebral cortex occur72 and may thus have a greater influence over the development of higher cognitive functions.63,64,73
We also observed that T1DM and T2DM were most strongly associated with outcomes including ID. Though we do not have direct evidence to suggest underlying mechanism for this observation, we speculate that it may be related to late-pregnancy fetal exposure to hyperglycaemia being associated with complications such as preterm birth, macrosomia and neonatal hyperinsulinaemia; which can occur with all forms of diabetes. These can subsequently lead to neonatal complications such as hypoxia/asphyxia.74
Whereas differences in risk for NDDs with regard to the timing of exposure lend plausibility to the notion that glycaemic control during distinct periods of pregnancy may influence the neurodevelopment of the offspring,75 it is important to note that the observations regarding timing of GDM are not entirely consistent between our study and the only other setting in which the timing of GDM diagnosis has been considered.12,14 The duration of diabetes exposure also did not have a large influence on the risk of NDDs in offspring, in the sense that risks associated with GDM diagnosed at earlier dates were not necessarily associated with higher risks of NDDs compared with GDM diagnosed at later dates.
Conclusion
Our results suggest that differences in the association between maternal diabetes and risk of NDDs in offspring depend on the type and timing of maternal diabetes and the co-occurring presentation of NDDs, with the strongest associations observed for outcomes including ID. These associations remained when parental genetic factors were accounted for. The underlying mechanisms of our findings remain unclear, thus future studies are needed to further explore whether neurodevelopmental disorders in children exposed to diabetic pregnancies may to some extent be related to exposure to hyperglycaemia during pregnancy.
Supplementary data
Supplementary data are available at IJE online.
Author Contributions
R.G. and C.D. proposed the research idea. S.C. conducted the statistical analysis and all authors participated in the interpretation of the statistical reports. S.C. and S.Z. wrote the first and subsequent drafts of the paper with important intellectual input and revision from all co-authors. Full access to the data, statistical reports and tables arising from the data analysis was granted to all authors, and all authors take responsibility for the integrity of the data and accuracy of the data analysis. All authors have approved the final version of the manuscript submitted for publication. R.G. and C.D. act as guarantors and assure that this manuscript is an honest, accurate and transparent account of the analysis undertaken.
Funding
This work was supported by grants from the Swedish Research Council [grant numbers 2017–02900 (to R.G.) and 523–2010-1052 (to C.D.)] and from StratNeuro [Strategic Research Area Neuroscience at the Karolinska Institutet) (to R.G.)]. The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication.
Supplementary Material
Acknowledgements
The data underlying this article cannot be shared publicly. The Swedish health and population register data used in this study are available from Statistics Sweden and the Swedish National Board of Health and Welfare. The authors are not allowed to distribute the data according to the ethical approval for this study and the agreements with Statistics Sweden and the Swedish National Board of Health and Welfare. Ethical approval was obtained from the Stockholm regional ethical review committee (DNR 2010/1185–31/5). Informed consent was not required for the analysis of anonymized register data.
Conflict of interest
None declared.
References
- 1. Thapar A, Cooper M.. Attention deficit hyperactivity disorder. Lancet 2016;387:1240–50. [DOI] [PubMed] [Google Scholar]
- 2. Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J.. Autism spectrum disorder. Lancet 2018;392:508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Batshaw ML. Mental retardation. Pediatr Clin North Am 1993;40:507–21. [DOI] [PubMed] [Google Scholar]
- 4. Murray MJ. Attention-deficit/hyperactivity disorder in the context of autism spectrum disorders. Curr Psychiatry Rep 2010;12:382–88. [DOI] [PubMed] [Google Scholar]
- 5. Rommelse NN, Franke B, Geurts HM, Hartman CA, Buitelaar JK.. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry 2010;19:281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. La Malfa G, Lassi S, Bertelli M, Salvini R, Placidi GF.. Autism and intellectual disability: a study of prevalence on a sample of the Italian population. J Intellect Disabil Res JIDR 2004;48:262–67. [DOI] [PubMed] [Google Scholar]
- 7. Matson JL, Shoemaker M.. Intellectual disability and its relationship to autism spectrum disorders. Res Dev Disabil 2009;30:1107–14. [DOI] [PubMed] [Google Scholar]
- 8. Lai M-C, Kassee C, Besney R. et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry 2019;6:819–29. [DOI] [PubMed] [Google Scholar]
- 9. Idring S, Rai D, Dal H. et al. Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PloS One 2012;7:e41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thapar A, Cooper M, Rutter M.. Neurodevelopmental disorders. Lancet Psychiatry 2017;4:339–46. [DOI] [PubMed] [Google Scholar]
- 11. Xu G, Jing J, Bowers K, Liu B, Bao W.. Maternal diabetes and the risk of autism spectrum disorders in the offspring: a systematic review and meta-analysis. J Autism Dev Disord 2014;44:766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiang AH, Wang X, Martinez MP. et al. Maternal gestational diabetes mellitus, type 1 diabetes, and type 2 diabetes during pregnancy and risk of ADHD in offspring. Diabetes Care 2018;41:2502–08. [DOI] [PubMed] [Google Scholar]
- 13. Xiang AH, Wang X, Martinez MP, Page K, Buchanan TA, Feldman RK.. Maternal type 1 diabetes and risk of autism in offspring. JAMA 2018;320:89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiang AH, Wang X, Martinez MP. et al. Association of maternal diabetes with autism in offspring. JAMA 2015;313:1425–34. [DOI] [PubMed] [Google Scholar]
- 15. Ji J, Chen T, Sundquist J, Sundquist K.. Type 1 diabetes in parents and risk of attention deficit/hyperactivity disorder in offspring: a population-based study in Sweden. Diabetes Care 2018;41:770–74. [DOI] [PubMed] [Google Scholar]
- 16. Mann JR, Pan C, Rao GA, McDermott S, Hardin JW.. Children born to diabetic mothers may be more likely to have intellectual disability. Matern Child Health J 2013;17:928–32. [DOI] [PubMed] [Google Scholar]
- 17. Leonard H, N de K, Bourke J, Bower C.. Maternal health in pregnancy and intellectual disability in the offspring: a population-based study. Ann Epidemiol 2006;16:448–54. [DOI] [PubMed] [Google Scholar]
- 18. Wan H, Zhang C, Li H, Luan S, Liu C.. Association of maternal diabetes with autism spectrum disorders in offspring: a systemic review and meta-analysis. Medicine (Baltimore) 2018;97:e9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burstyn I, Sithole F, Zwaigenbaum L.. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Inj Can 2010;30:125–34. [PubMed] [Google Scholar]
- 20. Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S.. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord 2011;41:891–902. [DOI] [PubMed] [Google Scholar]
- 21. Bytoft B, Knorr S, Vlachova Z. et al. Assessment of attention deficits in adolescent offspring exposed to maternal type 1 diabetes. PloS One 2017;12:e0169308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piven J, Simon J, Chase GA. et al. The etiology of autism: pre-, peri- and neonatal factors. J Am Acad Child Adolesc Psychiatry 1993;32:1256–63. [DOI] [PubMed] [Google Scholar]
- 23. Ornoy A, Ratzon N, Greenbaum C, Wolf A, Dulitzky M.. School-age children born to diabetic mothers and to mothers with gestational diabetes exhibit a high rate of inattention and fine and gross motor impairment. J Pediatr Endocrinol Metab 2001;14 (Suppl):681–89. [DOI] [PubMed] [Google Scholar]
- 24. Atladottir HO, Pedersen MG, Thorsen P. et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics 2009;124:687–94. [DOI] [PubMed] [Google Scholar]
- 25. Li M, Fallin MD, Riley A. et al. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 2016;137:e20152206–e20152206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiang AH. Association of maternal diabetes with autism in offspring. JAMA 2017;317:537–38. [DOI] [PubMed] [Google Scholar]
- 27. Yip BHK, Bai D, Mahjani B. et al. Heritable variation, with little or no maternal effect, accounts for recurrence risk to autism spectrum disorder in Sweden. Biol Psychiatry 2018;83:589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loo K. V, Martens GJM.. Genetic and environmental factors in complex neurodevelopmental disorders. Curr Genomics 2007;8:429–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gardner RM, Lee BK, Magnusson C. et al. Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: results from a Swedish total population and discordant sibling study. Int J Epidemiol 2015;44:870–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Surén P, Gunnes N, Roth C. et al. Parental obesity and risk of autism spectrum disorder. Pediatrics 2014;133:e1128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Q, Sjölander A, Långström N. et al. Maternal pre-pregnancy body mass index and offspring attention deficit hyperactivity disorder: a population-based cohort study using a sibling-comparison design. Int J Epidemiol 2014;43:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Musser ED, Willoughby MT, Wright S. et al. Maternal prepregnancy body mass index and offspring attention-deficit/hyperactivity disorder: a quasi-experimental sibling-comparison, population-based design. J Child Psychol Psychiatr 2017;58:240–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. HüBel C, Gaspar HA, Coleman JRI. et al. Genetic correlations of psychiatric traits with body composition and glycemic traits are sex- and age-dependent. Nat Commun 2019;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tylee DS, Sun J, Hess JL. et al. Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data. Am J Med Genet B Genet 2018;177:641–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rai D, Lewis G, Lundberg M. et al. Parental socioeconomic status and risk of offspring autism spectrum disorders in a Swedish population-based study. J Am Acad Child Adolesc Psychiatry 2012;51:467–76.e6. [DOI] [PubMed] [Google Scholar]
- 36. Homsy J, Zaidi S, Shen Y. et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science 2015;350:1262–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Socialstyrelsen. The National Patient Register [Internet]. 21 September 2020. https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/the-national-patient-register/ (16 October 2020, date last accessed).
- 38. Ludvigsson JF, Andersson E, Ekbom A. et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ludvigsson JF, Reichenberg A, Hultman CM, Murray JA.. A nationwide study of the association between celiac disease and the risk of autistic spectrum disorders. JAMA Psychiatry 2013;70:1224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kosidou K, Dalman C, Widman L. et al. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Mol Psychiatry 2016;21:1441–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Skoglund C, Chen Q, Franck J, Lichtenstein P, Larsson H.. Attention-deficit/hyperactivity disorder and risk for substance use disorders in relatives. Biol Psychiatry 2015;77:880–86. [DOI] [PubMed] [Google Scholar]
- 42. Wiegersma AM, Dalman C, Lee BK, Karlsson H, Gardner RM.. Association of prenatal maternal anemia with neurodevelopmental disorders. JAMA Psychiatry 2019;76:1294–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Idring S, Magnusson C, Lundberg M. et al. Parental age and the risk of autism spectrum disorders: findings from a Swedish population-based cohort. Int J Epidemiol 2014;43:107–15. [DOI] [PubMed] [Google Scholar]
- 44. Magnusson C, Rai D, Goodman A. et al. Migration and autism spectrum disorder: population-based study. Br J Psychiatry 2012;201(2):109–15. [DOI] [PubMed] [Google Scholar]
- 45. Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynaecol Obstet 2006;93:269–74. [DOI] [PubMed] [Google Scholar]
- 46.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Determining Optimal Weight Gain, In: Rasmussen KM, Yaktine AL (eds). Weight Gain during Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press (US; ), 2009, pp, 241–62 . http://www.ncbi.nlm.nih.gov/books/NBK32813/ (16 October 2020, date last accessed). [PubMed] [Google Scholar]
- 47.Eurostat [Internet]. https://ec.europa.eu/eurostat/web/degree-of-urbanisation/background (16 October 2020, date last accessed).
- 48. Zeger SL, Liang KY.. An overview of methods for the analysis of longitudinal data. Stat Med 1992;11:1825–39. [DOI] [PubMed] [Google Scholar]
- 49. Groenwold RHH, White IR, Donders ART, Carpenter JR, Altman DG, Moons KGM.. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CMAJ Can Med Assoc J 2012;184:1265–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Al-Goblan AS, Al-Alfi MA, Khan MZ.. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes Targets Ther 2014;7:587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chu SY, Callaghan WM, Kim SY. et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007;30:2070–76. [DOI] [PubMed] [Google Scholar]
- 52. Karagiannis T, Bekiari E, Manolopoulos K, Paletas K, Tsapas A.. Gestational diabetes mellitus: why screen and how to diagnose. Hippokratia 2010;14:151–54. [PMC free article] [PubMed] [Google Scholar]
- 53. Spanakis EK, Golden SH.. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep 2013;13:814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med J Br Diabet Assoc 2006;23:857–66. [DOI] [PubMed] [Google Scholar]
- 55. Nielsen PR, Benros ME, Dalsgaard S.. Associations between autoimmune diseases and attention-deficit/hyperactivity disorder: a nationwide study. J Am Acad Child Adolesc Psychiatry 2017;56:234–40.e1. [DOI] [PubMed] [Google Scholar]
- 56. Instanes JT, Halmøy A, Engeland A, Haavik J, Furu K, Klungsøyr K.. Attention-deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biol Psychiatry 2017;81:452–59. [DOI] [PubMed] [Google Scholar]
- 57. Todd JA. Etiology of type 1 diabetes. Immunity 2010;32:457–67. [DOI] [PubMed] [Google Scholar]
- 58. Zheng Y, Ley SH, Hu FB.. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14:88–98. [DOI] [PubMed] [Google Scholar]
- 59. Kumar S, Reynolds K, Ji Y, Gu R, Rai S, Zhou CJ.. Impaired neurodevelopmental pathways in autism spectrum disorder: a review of signaling mechanisms and crosstalk. J Neurodev Disord 2019;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Plummer JT, Gordon AJ, Levitt P.. The genetic intersection of neurodevelopmental disorders and shared medical comorbidities—relations that translate from bench to bedside. Front Psychiatry 2016;7:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Demontis D, Walters RK, Martin J. et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 2019;51:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leppert B, Havdahl A, Riglin L. et al. Association of maternal neurodevelopmental risk alleles with early-life exposures. JAMA Psychiatry 2019;76:834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ornoy A, Reece EA, Pavlinkova G, Kappen C, Miller RK.. Effect of maternal diabetes on the embryo, fetus, and children: congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defect Res C Embryo Today 2015;105:53–72. [DOI] [PubMed] [Google Scholar]
- 64. Hoirisch-Clapauch S, Nardi AE.. Autism spectrum disorders: let’s talk about glucose? Transl Psychiatry 2019;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hall DA, Wadwa RP, Goldenberg NA, Norris JM.. Maternal risk factors for term neonatal seizures: population-based study in Colorado, 1989—2003. J Child Neurol 2006;21:795–98. [DOI] [PubMed] [Google Scholar]
- 66. Glass HC, Pham TN, Danielsen B, Towner D, Glidden D, Wu YW.. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998-2002. J Pediatr 2009;154:24–28.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Negrato CA, Mattar R, Gomes MB.. Adverse pregnancy outcomes in women with diabetes. Diabetol Metab Syndr 2012;4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pappas A, Shankaran S, McDonald SA. et al. Cognitive outcomes after neonatal encephalopathy. Pediatrics 2015;135:e624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gardener H, Spiegelman D, Buka SL.. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 2011;128:344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sucksdorff M, Lehtonen L, Chudal R. et al. Preterm birth and poor fetal growth as risk factors of attention-deficit/hyperactivity disorder. Pediatrics 2015;136:e599–608. [DOI] [PubMed] [Google Scholar]
- 71. Ornoy A, Tsadok MA, Yaffe P, Zangen SW.. The Cohen diabetic rat as a model for fetal growth restriction: vitamins C and E reduce fetal oxidative stress but do not restore normal growth. Reprod Toxicol 2009;28:521–29. [DOI] [PubMed] [Google Scholar]
- 72. Tau GZ, Peterson BS.. Normal development of brain circuits. Neuropsychopharmacology 2010;35:147–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ornoy A. The impact of intrauterine exposure versus postnatal environment in neurodevelopmental toxicity: long-term neurobehavioral studies in children at risk for developmental disorders. Toxicol Lett 2003;140–141:171–81. [DOI] [PubMed] [Google Scholar]
- 74. Mitanchez D. Foetal and neonatal complications in gestational diabetes: perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab 2010;36:617–27. [DOI] [PubMed] [Google Scholar]
- 75. Griffith RJ, Harding JE, McKinlay CJD, Wouldes TA, Harris DL, Alsweiler JM.. Maternal glycemic control in diabetic pregnancies and neurodevelopmental outcomes in preschool aged children: a prospective cohort study. Early Hum Dev 2019;130:101–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.