Why was the consortium set up?
Considerable evidence exists that the risk determinants of many non-communicable diseases have their origins in early life, including cardiovascular disease, cancer, chronic obstructive lung disease, musculoskeletal diseases, diabetes and mental illnesses. The Developmental Origins of Health and Disease (DOHaD) research explores how the interplay between maternal and environmental factors programme fetal and child growth and influence developmental trajectories and susceptibility to disease later in life.1 Canada, as well as many other countries worldwide, have invested heavily in pregnancy and birth cohort studies supporting such research interests. However, the impact of single existing cohort databases could be expanded to provide more value to the research community, healthcare providers and policy-makers than each contributes individually. Cohort investigators recognize that individual studies often do not have the statistical power, specific data items or sufficient duration of follow-up needed to fully support the current and upcoming needs of research. Even the largest and best-designed cohorts often only generate enough participants to detect the most important relative risks, or to investigate relatively common health issues, and are limited in the potential to explore how the social and physical environment interacts with genetic factors to influence health. To address these issues and expedite discovery, we need a paradigm shift in the way we conduct our research. Enabling timely access to available data and samples, increasing the potential to share data across cohorts and promoting a multidisciplinary and collaborative approach to research are major assets of this new approach. They form the motivation behind the instigation of the Research Advancement through Cohort Cataloguing and Harmonization (ReACH) initiative.
Several national and international initiatives have been created to support DOHaD data access, integration and co-analysis. Birthcohorts.net,2 LifeCycle Project,3 Maternal, Infant, Child and Youth Research Network (MICYRN),4 Environmental Health Risks in European Birth Cohorts (ENRIECO)5 and the Global Pregnancy CoLab COLLECT database6 are very good examples of such initiatives. However, all these platforms face similar challenges. First, national, regional or organizational ethical and legal policies often limit access to individual participant data to external investigators. Second, even with many relatively well-known cohorts, information on the samples and data items collected is often not publicly available or is only available in a format that does not allow investigators to easily find the specific information they need to understand data content. The lack of accessible and structured documentation represents a major barrier for external investigators interested in using cohort-specific data. Third, because of the complexity and inevitable heterogeneity of the information collected across pre-existing studies and databases, valid comparison and/or integration of information presents major methodological challenges. Finally, the achievement of scientifically founded data harmonization and co-analysis requires access to funding, secure data environments, and specialized expertise and resources, fundamentals that are not always accessible.
Bringing together the expertise and tools developed by MICYRN4 and Maelstrom Research,7 ReACH was launched in 2016 to tackle these challenges. The initiative aims to leverage DOHaD research by providing the Canadian and international research community with a platform optimizing data discoverability and facilitating co-analysis across studies. Making use of the approach and tools developed by Maelstrom Research,8,9 ReACH implemented a centralized web-based catalogue documenting 26 Canadian pregnancy and birth studies relevant to DOHaD research. The initiative also offers support to investigators interested in harmonizing and co-analyzing data across studies. Building on data collected by the Canadian DOHaD studies, ReACH brings together cohort leaders, investigators requesting cohort data to achieve their research goals, and experts from different fields developing data discovery and integration resources.
Who is in the ReACH cohorts?
The ReACH project assembled studies with potential for collaborative DOHaD research. Inclusion criteria for the studies were: (i) recruit Canadian mothers and/or children; (ii) have a longitudinal design (i.e. at least one follow-up of participants after the initial collection event); (iii) have collected data (baseline or follow-up) after the year 2000; (iv) collect information on pregnancy and birth outcomes. Currently, 26 Canadian studies have been selected, but new studies are welcome to join the initiative. Table 1 provides an overview of the study characteristics.
Table 1.
Characteristics of the 26 member studiesa
| Study name | Main research focus | Province | Sub-populations | Number of participants | Number of participants with biospecimens |
|---|---|---|---|---|---|
| 3D Study - Design, Develop, Discover (3D) | Prenatal exposure, birth outcome, infant development | Quebec | Mothers | 2456 | 2357 |
| Partners | 2333 | 2333 | |||
| Children | 2456 | 1010 | |||
| Aboriginal Birth Cohort (ABC) | Adiposity, early life origins | Ontario | Mothers | 150 | 150 |
| Children | 150 | 150 | |||
| Grandmothers | 30 | ||||
| Alberta Pregnancy Outcomes and Nutrition (APrON) | Mental health, birth outcomes, neurodevelopment, nutrition | Alberta | Mothers | 2189 | 2041 |
| Partners | 1483 | 1343 | |||
| Children | 2169 | 1791 | |||
| All Our Babies and All Our Families (AOB/F) | Pregnancy and birth outcomes, child development | Alberta | Mothers | 3387 | 1862 |
| Children | 3387 | 1399 | |||
| CHILD Cohort Study (CHILD) | Allergy, asthma and chronic diseases, child and infant health, early life origins | British Columbia, Alberta, Manitoba, Ontario | Mothers | 3579 | 3487 |
| Fathers | 2841 | 2560 | |||
| Children | 3455 | 3417 | |||
| Early child development and the effects of prenatal antidepressant exposure (SRI_Exposure) | Prenatal exposure to antidepressants, fetal and childhood development | British Columbia | Mothers (6 sub-groups) | 274 | 271 |
| Children (6 sub-groups) | 276 | 264 | |||
| Early Pregnancy Markers for Pre-Eclampsia (PreMark) | Pre-eclampsia, homocysteine, pregnancy and birth outcomes | Nova Scotia | Mothers | 2200 | 2200 |
| Family Atherosclerosis Monitoring in Early Life (FAMILY) | Adiposity, cardiovascular risk factors, fetal and early childhood family-based determinants | Ontario | Mothers | 857 | 857 |
| Fathers | 530 | 530 | |||
| Children | 901 | 683 | |||
| Siblings | 264 | 264 | |||
| Feelings in Pregnancy and Motherhood (FPM) | Depression, prenatal and postpartum exposure to antidepressants | Saskatchewan | Mothers | 648 | |
| Children | 594 | ||||
| Fort McMurray Mommy Baby Study (Mommybabyfmm)b | Maternal psychopathologies, pregnancy outcomes, infant development, mother–child interactions | Alberta | Mothers (3 sub-groups) | 206 | |
| Children (3 sub-groups) | 206 | ||||
| GESTation and Environment (GESTE) | Thyroid function, exposure to polybrominated diphenyl ethers and polychlorinated biphenyls | Quebec | Mothers | 800 | 800 |
| Children | 761 | 761 | |||
| Kids, Families and Places (KFP) | Family life, biological exposures, neighbourhoods, child development | Ontario | Mothers | 668 | 405 |
| Children | 1579 | 935 | |||
| Life experiences and psychosocial development of the child: the role and quality of child care services (EMIGARDE) | Child care, child development | Quebec | Mothers | 515 | |
| Children | 515 | 157 | |||
| Maternal Adversity, Vulnerability and Neurodevelopment (MAVAN) | Mental health, gene–environment interactions, child development, child behaviour | Ontario, Quebec | Mothers (2 sub-groups) | 627 | |
| Children (2 sub-groups) | 622 | ||||
| Maternal–Infant Research on Environmental Chemicals (MIREC) | Infant and child health, prenatal exposure to environmental chemicals | British Columbia, Alberta, Manitoba, Ontario, Quebec, Nova Scotia | Mothers | 1983 | 1983 |
| Fathers | 1648 | ||||
| Children | 1959 | 1959 | |||
| Ontario Birth Study (OBS) | Maternal and infant health, developmental origins of health and diseases, pregnancy outcomes | Ontario | Mothers | 1374 | 1325 |
| Children | 1374 | 510 | |||
| Ottawa and Kingston Birth Cohort (OaK Birth Cohort) | Thrombophilias, folate supplementation, placenta-mediated pregnancy outcomes | Ontario | Mothers | 8085 | 7241 |
| Children | 2175 | 2175 | |||
| Oxidative stress, fetal growth and programming of the metabolic syndrome and cardiovascular disorders (MIROS C) | Type 2 diabetes, cardiovascular disorders, oxidative stress, prenatal exposure to antioxidant, pregnancy and infant outcomes | Quebec | Mothers | 307 | 307 |
| Children | 307 | 255 | |||
| Pre-Eclampsia New Emerging Team (PE-NET) | Cardiovascular risk, pre-eclampsia, neurodevelopment | Ontario | Mothers (2 sub-groups) | 235 | 235 |
| Children (2 sub-groups) | 235 | 235 | |||
| Prenatal Determinants of Inflammation- mediated Conditions Transdisciplinary Research (PreDICTR) | Determinants of inflammatory-mediated conditions | Saskatchewan | Mothers (4 sub-groups) | 35 | 35 |
| Children | 35 | 35 | |||
| Quebec Longitudinal Study of Child Development (QLSCD) | Factors occurring during early childhood, child development, lifestyle and housing | Quebec | Children | 2120 | 1000 |
| Quebec Newborn Twin Study (QNTS) | Developmental health, school and health outcomes, bio-social determinants | Quebec | Children (twins) | 1410 | 1043 |
| South Asian Birth Cohort (START) | Adiposity, diabetes, cardiovascular disease, environmental and genetic determinants, birth outcomes | Ontario (International) | Mothers (3 sub-groups) | 1512 | 1512 |
| Children (3 sub-groups) | 1512 | 1512 | |||
| Study of Asthma, Genes and the Environment (SAGE)c | Asthma, environmental exposures, early-life exposures, genetic determinants | Manitoba | Children (2 sub-groups) | 723 | 723 |
| TARGet Kids! | Obesity, early-life exposures, developmental trajectories | Ontario, Quebec | Children | 11 379 | 5248 |
| Twin Birth Study (TBS)b | Delivery and birth outcomes, method of delivery | All provinces (International) | Mothers (2 sub-groups) | 2804 | 2786 |
| Children | 5607 | 5607 |
Information as provided by the studies.
Clinical trial design.
Case–control design.
One of the participating studies started recruitment of participants preconception, 19 during pregnancy, 4 when the child was an infant (<1 year old) and 2 when the child was older (>1 year old). The majority (n = 18) of the studies recruited women through a care provider during their routine pregnancy visits at the clinic. The number of participants recruited ranged from 70 to 11 379, with 2 studies including specifically >5000 children (Table 1). Together, studies recruited 34 891 mothers, 45 907 children, 8835 fathers, 30 grandmothers and 264 siblings. Of the 26 participating studies, 21 followed up both mothers and children, 4 children only and one followed only mothers. Fathers were included in 5 studies, whereas one also included grandmothers and another included siblings. The scientific focus varied broadly across studies. The most frequent outcomes pertain to child development (12 studies), child health (10 studies) and pregnancy and birth outcomes (10 studies). The main risk factors of interest include environmental exposures, mothers’ lifestyle habits and behaviours, and familial and socio-economic environments.
How often have participants been followed up?
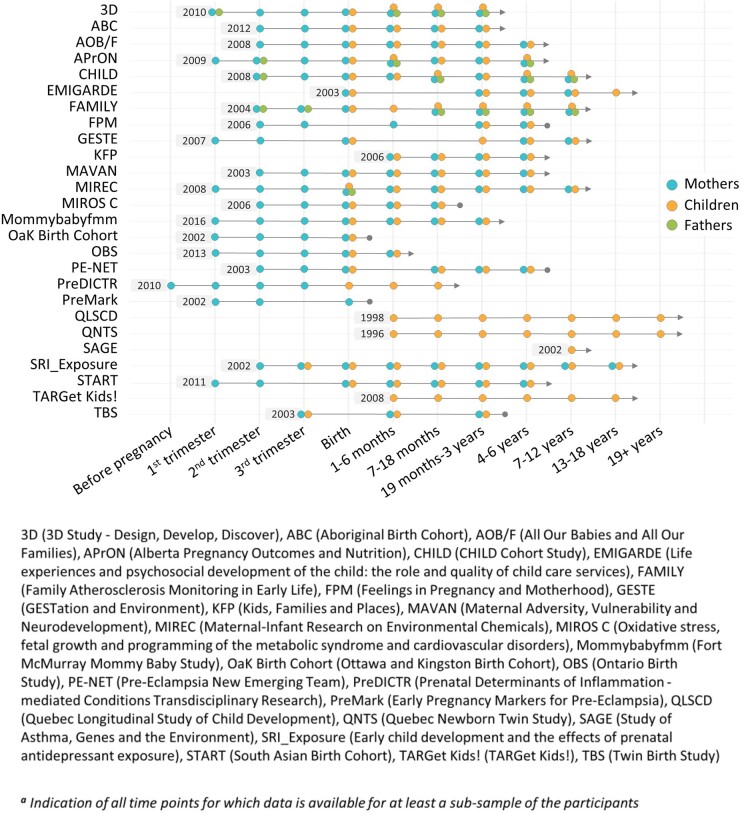
Significant variation is present across studies in years of recruitment, duration and frequency of the follow-up of participants (data collection time points). Two studies started recruitment before 2000, 18 from 2000 to 2009 and 6 in 2010 or later. Currently, 20 of the 26 studies continue to follow participants (Figure 1). The follow-up duration ranges between 16 months and 24 years. The number of data collection time points also varies across studies and ranges from 1 to 13 for mothers, 1 to 22 for children and 1 to 6 for fathers. Fifteen of the studies collected information from infancy to at least 5 years of age, but the time points of data collection differ across studies.
Figure 1.
Duration of participant follow-up by study.
What has been measured?
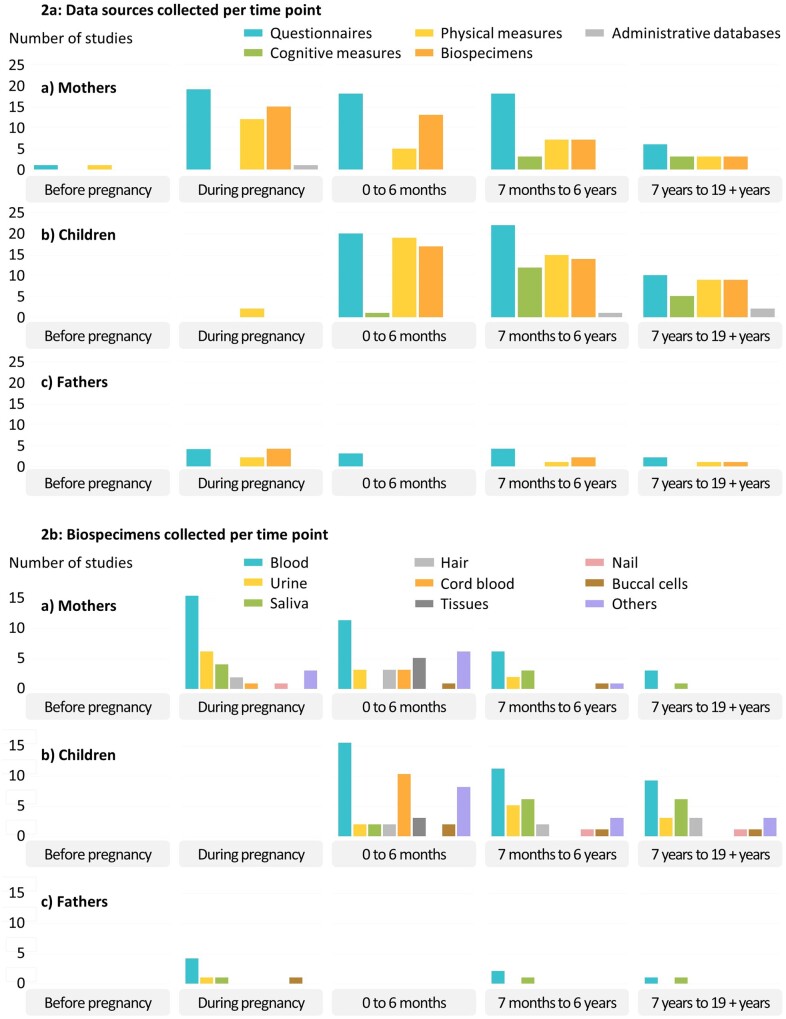
Figure 2a outlines the sources of data collected by sub-populations and time points. All 26 studies collected information from questionnaires, 23 also collected biospecimens, 21 performed physical measurements, 12 conducted cognitive assessments and 3 retrieved information from administrative databases at least once during the follow-up of participants. Blood was the most common biospecimen collected (Figure 2b), with 21 studies having collected blood at least once. Among the 23 studies that collected biospecimens for a given sub-population, the number of biospecimen collection time points varies from 1 to 6 for mothers, 1 to 20 for children and 1 to 3 for fathers.
Figure 2.
Data sources collected per time point (2a) and biospecimens collected per time point (2b) for (a) mothers, (b) children and (c) fathers
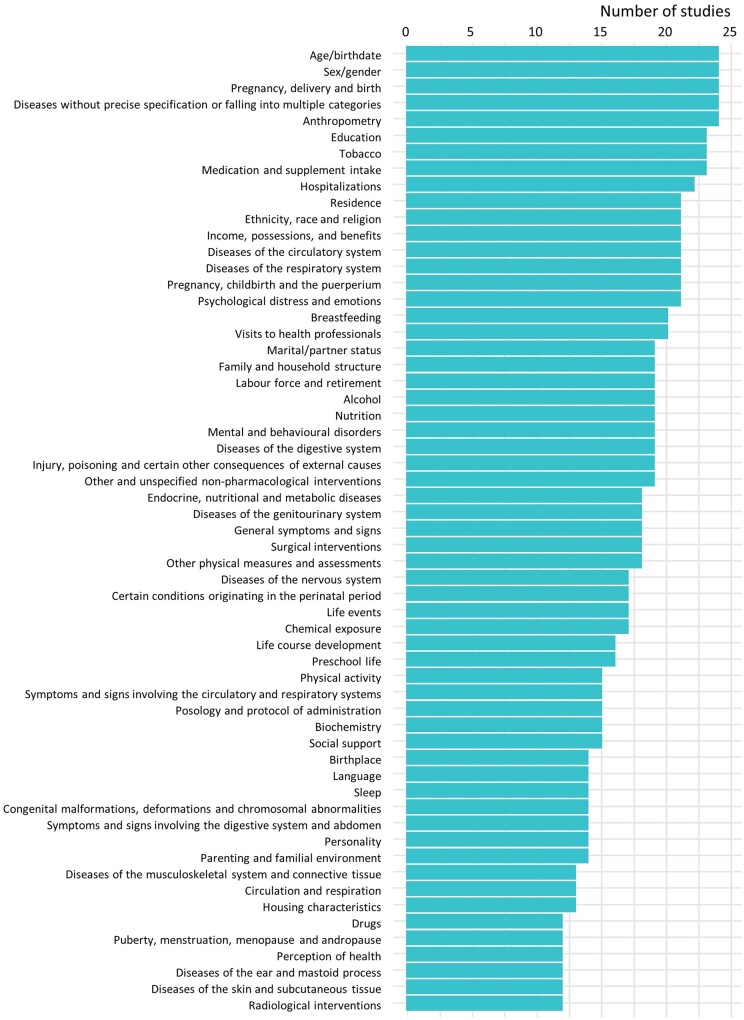
A broad range of data was collected by the studies. Details of specific information collected by at least 50% of the 24 studies with a documented list of variables is displayed in Figure 3. Based on the data dictionaries provided by the cohorts, all studies collected information about age, sex, anthropometric measures, and pregnancy and delivery outcomes. The majority of cohorts (>80%) also collected information about tobacco use, breastfeeding, diseases of the circulatory or respiratory systems, visits to health professionals, hospitalizations, medication intake, education, income, residence, ethnicity, and psychological distress and emotions. The information collected varies according to the sub-populations and the time of collection. As expected, life-course development was collected for the children sub-populations and pregnancy and delivery information was collected during the pregnancy and delivery visits or shortly after delivery. For more information about the domains of information covered in each sub-population for each of the follow-ups see Supplementary Material 1, Distribution of information collected for mothers, children and fathers, available as Supplementary data at IJE online, or visit the Maelstrom Research website.7
Figure 3.
Frequency of information collected by at least half of the studies
What has ReACH found? Resources and supported projects
Participating studies have collected an invaluable amount of data and samples particularly useful to support collaborative DOHaD research, and the tools (catalogue, methods and software) offered by ReACH can leverage the use of these scientific resources. The ReACH catalogue (https://www.maelstrom-research.org/mica/network/reach) documents the design of the 26 studies and the potential to access data and samples. It also offers a detailed description of each sub-population, data/biospecimen collection time points and variables collected. A user-friendly search engine (including comparison tables and a variable cart), as well as support from the ReACH team, facilitates data discovery. The catalogue resources allow identification of studies of interest, selection of variables and evaluation of the harmonization potential across studies. Furthermore, the methodological tools8,10 and open source software9 developed are freely accessible. The software has been successfully used by a number of Canadian and international projects to catalogue and disseminate metadata (Mica), store, curate and harmonize data (Opal) and, where relevant, perform federated data analysis (DataSHIELD).11 A case study illustrating usage of the ReACH resources to support co-analysis of data is included in Supplementary Material 2, Using the resources, a case example, available as Supplementary data at IJE online. Investigators interested in the available resources are also welcome to explore our website or contact the ReACH team.
The first project developed under the ReACH umbrella was launched in 2017 and the number of Canadian and international initiatives established or supported by the team is rapidly increasing. Some of these projects aim to harmonize and co-analyze existing data to answer various research questions, each initiative assembling data from 10 000 to >250 000 mothers. For example, the Prenatal Alcohol Exposure initiative (Table 2) brought together data from 5 Canadian cohorts (10 263 mothers) and allowed successful harmonization of information about alcohol intake, tobacco and drugs use, age, sex, marital status, education, ethnicity, working status, income, anthropometric measures, and pregnancy and birth outcomes. Analyses are currently underway to investigate the impact of alcohol consumption during pregnancy on birthweight and preterm birth, and identify the correlates of drinking before, during and after pregnancy. Other initiatives focus on supporting the prospective implementation of common measures to be collected across studies to facilitate future data sharing and co-analysis among specialized DOHaD cohort networks. One of the projects supported by ReACH, the Healthy Life Trajectories Initiative 12 (Table 2), is addressing the burden of non-communicable diseases by developing evidence-based interventions. It brings together cohorts from Canada, China, South Africa and India. ReACH helped the network to define >2000 variables to be collected across countries for mothers, children and fathers sub-populations at ten time points (one preconception, two during pregnancy, one at delivery and six from birth to 5 years old). Finally, some projects foster the development of specialized methods and accessible resources to improve cost efficiency of DOHaD harmonization initiatives and optimize their scientific impact. The resources developed include ethico-legal and methodological tools and software applications. ReACH investigators are also working towards joining efforts with the Birthcohorts.net,2 Lifecycle Project 3 and EUCAN-Connect13 projects to foster implementation of common standards and international research activities. Most of the projects developed or supported by ReACH are still ongoing and have not generated publications yet. An overview of some of these initiatives is provided in Table 2.
Table 2.
Examples of projects developed or supported
| Project | Objectives | Country | Start–end years | Number of studies and participants |
|---|---|---|---|---|
| Retrospective harmonization efforts | ||||
| Prenatal Alcohol Exposure initiative (A Bocking, Mount Sinai Hospital) |
|
Canada | 2019–21 |
Studies: 5 Mothers: 10 263 Children: 10 287 |
| Prenatal exposure to stress, preterm birth and development of toddlers (A-M Nybo Andersen, University of Copenhagen) |
|
Canada France Norway |
2020–22 |
Studies: 5 Mothers: 102 169 Children: 119 785 |
| Gestational age at birth and offspring body size from infancy to adolescence (A-M Nybo Andersen, University of Copenhagen) | Assess the impact of gestational age at birth on attained body size. |
Canada Europe |
2020–22 |
Studies: 16 Children: 293 619 |
| Prospective harmonization efforts | ||||
| The Healthy Life Trajectories Initiative (HeLTI) (C-L Dennis, S Matthews and C Birken, University of Toronto; H Huang, Shanghai Jiao Tong University; W Fraser, Université de Sherbrooke, K Kumaran, CSI Holdsworth Memorial Hospital; S Norris, University of Witwatersrand; S Lye, Lunenfeld-Tanenbaum Research Institute)12 | Define the core set of variables to be collected by the studies member of the HeLTI initiative, which addresses the burden of non-communicable diseases by developing evidence-based interventions from pre-conception to the postnatal period. |
Canada China India South Africa |
2017–26 |
Studies: 4 Mothers: 22 730a |
| Qualitative evaluation of different approaches to asking women about alcohol use during pregnancy (N Poole, Centre of Excellence for Women's health)14 | Identify the most commonly asked questions regarding alcohol consumption during pregnancy and explore standardized alcohol screening tools used to collect information in perinatal surveillance efforts, with the aim to develop recommendations for collection of information on alcohol intake during pregnancy. | Canada | 2018–19 |
Studies: 12 Mothers: 23 856 |
| Prospective harmonization of pregnancy cohorts to understand and mitigate the impact of the coronavirus disease 2019 (COVID-19) pandemic on the next generation: The RAINBOW Project (S Côté, Centre Hospitalier Universitaire Sainte-Justine) | Define the core set of variables to be collected by the studies member of the Rainbow initiative, which aims to investigate the impact of the COVID-19 pandemic on the mental and physical health of the mother, father and unborn child, identify factors that moderate the impact of the pandemic, and test the efficacy of psychological prevention programmes. | Canada | 2020–22 |
Studies: 7 Mothers: 25 000a |
| Tools or resources to support and leverage harmonization initiatives | ||||
| Development of generic consent for mother and child cohorts (BM Knoppers, McGill University)15 | Develop a series of generic consent forms for adults, adolescents, young children (parental consent for minors) to be used by future mother and child studies. | Canada | 2017 | – |
| Accessible R packages facilitating data harmonization [G Fabre, Research Institute of the McGill University Health Center (RI-MUHC)] | Develop a pipeline facilitating data harmonization, including structured rules to process data integrated in a system involving the Opal software and RStudio environment. | Canada | 2019–22 | – |
| Guidelines for Developmental Origins of Health and Disease (DOHaD) harmonization initiatives (I Fortier, RI-MUHC) | Develop guidelines to support international DOHaD harmonization initiatives from initiation of the project to publication of results. |
Canada Europe |
2020–21 | – |
| Outline of the harmonization potential across Canadian DOHaD mother and child cohorts (I Fortier, RI-MUHC) | Document the potential to generate a core set of variables across the ReACH member studies to facilitate implementation of collaborative research initiatives. | Canada | 2020–21 | Studies: 24 |
| Outline of the harmonization potential across mother and child cohorts’ part of the ReACH and LifeCycle Project initiatives (I Fortier, RI-MUHC) | Document the harmonization potential across ReACH and LifeCycle Project member studies to leverage the implementation of new European–Canadian research collaborations. |
Canada Europe |
2020–21 | Studies: 40 |
Number of mothers aimed to be recruited.
What are the main strengths and weaknesses?
Strengths
ReACH brings under a common umbrella (i) Canadian pregnancy and birth cohort studies having carefully conducted longitudinal follow-ups and collected well phenotyped participant data and high-quality biospecimens; (ii) DOHaD investigators with various research interests; and (iii) expert data scientists, ethicists and software developers. In addition to the comprehensive study and variable catalogue developed, the ReACH team provides open access to expertise and specialized software to support data harmonization, integration and co-analysis. Although primarily developed to leverage usage of existing data, the ReACH resources also provide useful tools supporting conceptualization and implementation of new cohorts.
A major strength of ReACH is the level of detail and standardization offered by the catalogue, combined with the capacity to search and easily extract information to (i) identify existing variables and studies of interest and (ii) explore harmonization potential across studies. The catalogue allows one to easily estimate whether data of interest is accessible, is suitable to answer the specific research questions addressed (e.g. level of physical activity measured with a specific scale), and is similar enough to enable co-analysis across multiple studies. In addition, the catalogue supports documentation of the harmonized data generated, helping investigators to learn from the rules and algorithms used by others and to improve the quality and efficiency of forthcoming data harmonization initiatives.
Weaknesses
ReACH does not offer access to a central data repository including study-specific and/or harmonized data from all participating studies. The platform was implemented as an adaptable resource aimed at leveraging and supporting discretionary harmonization initiatives. Researchers interested in using original data from specific studies need to contact the principal investigators and/or data access committees to seek permission for access to data, complete data transfer agreements and generate project-specific harmonized datasets. Although the process requires substantial effort and time for each project, the approach empowers achievement of projects requiring harmonization of comprehensive sets of data across a limited number of studies.
Although the ReACH catalogue is among the catalogues worldwide providing the most comprehensive information, its true value clearly depends on its regular improvement and long-term maintenance. Cohorts are, by definition, longitudinal studies, and most of the ReACH partners are still following participants. Information included in the catalogue will thus need to be regularly updated to be kept current. The quality of the catalogue also rests on the information provided by study investigators. Comprehensiveness of the data dictionaries provided (full list of variables collected and detailed variables description) is sometimes limited. For example, selected types of information such as genotypes or variables extracted from health administrative databases are not always provided. This can occasionally lead to bias in the catalogue content and limitations in the variables search. Continuous extension, in conjunction with the development of new search features, will thus be required to respond to the upcoming needs of the DOHaD research community.
Can I access the data? Where can I find out more?
The ReACH web-based metadata catalogue is freely accessible: https://www.maelstrom-research.org/mica/network/reach#/. The individual participant data cannot be downloaded from the website, users interested in accessing the original data must contact the principal investigator or the study access committee. Contact information and study website can be found on each study description page included in the catalogue. Researchers interested in obtaining more information about the ReACH initiative or in need of support to use the ReACH resources can contact the team (info@maelstrom-research.org).
Ethics Approval
All participating studies received ethical approvals from their respective institutions and informed consent from participants. Investigators requiring access to study-specific data need to obtain ethical approval from their home institution and follow study-specific data access rules and procedures.
Profile in a nutshell
The Research Advancement through Cohort Cataloguing and Harmonization (ReACH) initiative aims to optimize and expand the use of data and biospecimens from Canadian pregnancy and birth cohort studies by providing the Developmental Origins of Health and Disease (DOHaD) research community with the means to leverage and carry-out leading-edge collaborative research.
The initiative brings together 26 Canadian pregnancy and birth cohort studies of interest to support DOHaD research. Together, studies recruited 34 891 mothers, 45 907 children, 8835 fathers, and 294 grandmothers and siblings.
All studies collected longitudinal data from questionnaires, 23 also collected biospecimens, 21 performed physical measurements and 12 conducted cognitive assessments.
The ReACH catalogue provides access to the information collected by the participating studies at a level of detail allowing researchers to understand study designs, select variables of interest to answer specific research questions and evaluate the harmonization potential across studies.
Access to the catalogue and software resources is open and free.
Supplementary data
Supplementary data are available at IJE online.
Funding
This work is supported by the Canadian Institutes of Health Research (CIHR): operating grant for the Canadian DOHaD cohort registry (2016–2021) (OCR-144561) and the Research Institute of the McGill University Health Center.
Supplementary Material
Acknowledgements
The authors acknowledge the contribution and support of all participating studies as well as of the Maelstrom Research staff, particularly Guillaume Fabre, Karla Ordonez, Sara Samoely Lala, and Audrey Bégin Poissant.
Data Availability
Data from the ReACH participating studies are not publicly available. Access to data needs to be requested from individual studies.
Conflict of Interest
None declared.
References
- 1. Suzuki K. The developing world of DOHaD. J Dev Orig Health Dis 2018;9:266–69. [DOI] [PubMed] [Google Scholar]
- 2. Lawlor DA, Andersen A-MN, Batty GD.. Birth cohort studies: past, present and future. Int J Epidemiol 2009;38:897–902. [DOI] [PubMed] [Google Scholar]
- 3. Jaddoe VWV, Felix JF, Andersen A-MN. et al. The LifeCycle Project-EU Child Cohort Network: a federated analysis infrastructure and harmonized data of more than 250,000 children and parents. Eur J Epidemiol 2020;35:709–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joly M-P, Boivin M, Junker A, Bocking A, Kramer MS, Atkinson SA.. An inventory of Canadian pregnancy and birth cohort studies: research in progress. BMC Pregnancy Childbirth 2012;12:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vrijheid M, Casas M, Bergström A. et al. European birth cohorts for environmental health research. Environ Health Perspect 2012;120:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Myatt L, Roberts JM, Redman CWG.. Availability of COLLECT, a database for pregnancy and placental research studies worldwide. Placenta 2017;57:223–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maelstrom Research team. Maelstrom Research [Internet]. 2012. https://www.maelstrom-research.org/ (14 December 2019, date last accessed).
- 8. Bergeron J, Doiron D, Marcon Y, Ferretti V, Fortier I.. Fostering population-based cohort data discovery: The Maelstrom Research cataloguing toolkit. PLoS One 2018;13:e0200926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doiron D, Marcon Y, Fortier I, Burton P, Ferretti V.. Software Application Profile: Opal and Mica: open-source software solutions for epidemiological data management, harmonization and dissemination. Int J Epidemiol 2017;46:1372–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fortier I, Raina P, Van den Heuvel ER. et al. Maelstrom Research guidelines for rigorous retrospective data harmonization. Int J Epidemiol 2017;46:103–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaye A, Marcon Y, Isaeva J. et al. DataSHIELD: taking the analysis to the data, not the data to the analysis. Int J Epidemiol 2014;43:1929–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HeLTI Consortium. Healthy Life Trajectories Initiative (HeLTI) [Internet]. 2019. https://helti-net.org/ (11 July 2020, date last accessed).
- 13.EUCAN-Connect Consortium. EUCAN-Connect [Internet]. 2019. https://www.eucanconnect.eu/ (14 December 2019, date last accessed).
- 14. Poole N, , Schmidt RA, , Bocking A, , Bergeron J, , Fortier I. The potential for fetal alcohol spectrum disorder prevention of a harmonized approach to data collection about alcohol use in pregnancy cohort studies. Int J Environ Res Public Health 2019;16:2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maelstrom Research team. ReACH- Research Advancement through Cohort Cataloguing and Harmonization [Internet]. Maelstrom Res. 2016. https://www.maelstrom-research.org/mica/network/reach (14 December 2019, date last accessed). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the ReACH participating studies are not publicly available. Access to data needs to be requested from individual studies.