Abstract
Patients with left main, left main equivalent, and three-vessel coronary artery disease (CAD) represent an overlapping spectrum of patients with advanced CAD that is associated with an adverse prognosis. Guideline-directed medical therapy is a necessary but often insufficient treatment option, as such patients frequently need mechanical revascularization by either coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention (PCI). In patients with advanced CAD presenting with acute myocardial infarction, PCI, of course, is the preferred treatment option. For stable patients with advanced CAD, CABG surgery remains the standard of care. However, observations from the SYNergy between Percutaneous Coronary Intervention with TAXus and Cardiac Surgery (SYNTAX) trial suggest that PCI may be a useful alternative in patients with three-vessel disease with a low SYNTAX score as well as in patients with left main disease and a low or intermediate SYNTAX score. In the subset of patients with diabetes mellitus, the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease trial unequivocally demonstrated the superiority of CABG surgery in improving outcomes. The findings of the recently published Everolimus-Eluting Stent System versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization and Nordic–Baltic–British Left Main Revascularization study trials point to a favorable role for PCI in certain low-to-moderate risk patients with left main stem disease.
Keywords: coronary artery disease, coronary artery bypass graft surgery, percutaneous coronary intervention, drug-eluting stents
Left main, left main equivalent, and three-vessel coronary artery disease (CAD) comprise an often-overlapping spectrum of advanced disease states. For clarity, these various anatomic subsets are defined as follows:
A significant left main coronary stenosis is defined as an angiographically estimated stenosis >50% or a fractional flow reserve <0.80 in the left main coronary artery ostium, mid-shaft, or distal bifurcation. 1 2 The majority (80%) of these critical stenoses are located in the distal bifurcation. 1 2 3 4 A critical left main stem stenosis is found in ∼4% of all patients undergoing coronary angiography. 4
Left main equivalent CAD is defined as combined stenoses of ≥70% reduction in the luminal diameter of the proximal left anterior descending (LAD) coronary artery before the first septal perforator and the proximal circumflex coronary artery before the first obtuse marginal branch. 5
Three-vessel disease is defined by index vessels; the LAD, the circumflex and the right coronary arteries (or distal circumflex in left dominance). A 70% reduction in luminal diameter is required in the proximal or middle segment of all three arteries to qualify as three-vessel disease. The middle segments include major branches arising from middle segments of an index vessel, such as a diagonal, an obtuse marginal, or a posterior descending artery (or distal segment in the special case of the LAD). 6
Of note, among patients with significant left main stem disease, 38% have concomitant three-vessel disease. 3 4 Accordingly, left main, left main equivalent, and three-vessel disease represent an overlapping spectrum of severe CAD.
Medical Management of Severe Coronary Artery Disease: A Necessity Not an Option
Severe CAD is associated with an adverse prognosis and, in many cases, mechanical revascularization by either coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention (PCI) needs to be considered to relieve symptoms and improve prognosis. However, it is equally important to be aware of the considerable salutary effect of medical therapy in the treatment of patients with left main and multivessel CAD. 7 8 In such patients, the use of guideline-directed secondary prevention and lifestyle interventions should be implemented, irrespective of whether or not they eventually undergo revascularization. 9 Intensive statin therapy reduces cardiovascular events and atherosclerotic disease progression compared with standard therapy and therefore should be considered the standard of care in patients with severe CAD. 10 In addition to reduction of low-density lipoprotein cholesterol, intensive statin therapy reduces inflammation which may, in turn, result in further long-term risk reduction. 10 Other disease-modifying pharmacological interventions which reduce adverse cardiovascular events, such as inhibitors of the renin–angiotensin–aldosterone system, should be routinely used in these patients. 11 More effective antiplatelet agents such as P2Y12 inhibitors have been shown to further reduce the risk of major adverse cardiac and cerebral events in patients with advanced CAD. 12
Coronary Artery Bypass Graft Surgery: An Historical Perspective
CABG surgery was introduced by René Favalaro, while working at the Cleveland Clinic, in 1968. 13 The ability of CABG surgery to improve survival in left main disease was soon recognized as was its ability to relieve anginal symptoms and improve quality of life in patients with advanced CAD. 14 Indeed, by 1981, CABG surgery had become the most commonly performed major operation in the United States (160,000 per year). 14 In 1994, Yusuf et al reported their analysis of seven randomized trials that compared a strategy of initial CABG surgery with one of initial medical therapy to assess the effects of mortality in patients with CAD. 15 This analysis studied 1,324 patients assigned to CABG surgery and 1,325 to medical management between 1972 and 1984. The study findings confirmed the significantly reduced mortality in left main stem disease conferred by CABG surgery. In addition, it was noted that this surgery also reduced mortality in patients with three-vessel CAD. 15 In 1995, Caracciolo et al reported on the long-term outcome of 912 patients with left main equivalent disease in the Coronary Artery Surgery Study Registry. 5 Median survival in the surgical group was 13.1 years compared with only 6.2 years in the medical group ( p < 0.0001). Further improvements in the technique of CABG (such as use of arterial grafts, 16 smaller incisions, off-pump CABG) as well as enhanced myocardial preservation and improved postoperative care led to even more successful outcomes. 17
Indeed, more than 50 years after its introduction, CABG remains the standard of care for symptomatic patients with advanced CAD. 18
Coronary Angioplasty: The Alternative Mechanical Revascularization Technique
On September 16, 1977, Andreas Grüntzig, working at the University Hospital, Zurich, performed the first coronary balloon angioplasty (percutaneous transluminal coronary angioplasty [PTCA]) procedure in man. 19 This procedure was initially performed predominantly in patients with single-vessel CAD. Later, the procedure was extended to patients with more advanced CAD. PTCA of critical left main stenosis was singularly unsuccessful, with a reported 9.1% procedural mortality and a 36% 3-year survival rate. 20 In contrast, the outcome of patients with multivessel disease undergoing PTCA compared quite favorably with the outcome of patients undergoing CABG surgery. In the Bypass Angioplasty Revascularization Investigation trial, 1,829 patients with multivessel coronary disease were randomized to treatment with PTCA (915 patients) or CABG (914 patients). 21 Three-vessel disease was present in 41% of patients in both groups and 70% of patients randomized to PTCA underwent multivessel intervention. While there was no difference in the primary outcome of death or myocardial infarction at 5 years, the need for repeat revascularization was higher in the PTCA group. An important observation made in this study was the significant survival advantage for diabetic patients undergoing CABG who received a left internal thoracic artery graft.
Coronary Stents: Improving Angioplasty Technology
Implantation of a coronary stent was first performed by Jacques Puel on March 28, 1986, in Toulouse, France. 22 This technology, championed by Ulrich Sigwart in Switzerland, gradually became a major competitor to CABG surgery for advanced CAD. In particular, the ability of stents to reduce restenosis rates and to treat balloon-induced coronary dissections considerably improved patient outcomes. As with PTCA, results with early stents (now called “bare metal stents”) were less favorable for left main stem stenosis than for multivessel disease. Treatment of the former with stents was characterized by high restenosis and repeat revascularization rates, particularly with bifurcation stenoses. Of great concern, restenosis of stents in the left main sometimes presented as sudden cardiac death. 23 Several large-scale trials have compared bare metal stenting with CABG surgery in patients with multivessel disease. The 5-year death and myocardial infarction rates were similar in both groups but need for revascularization was considerably greater in the stent group. 24 25 26
Of course, in the treatment of acute myocardial infarction, PCI (initially with PTCA and later with stents) has long been proven to be the safest mode of revascularization. 18
Drug-Eluting Stents: A Revolutionary Treatment
Drug-eluting stents (DESs) were introduced in 2002 and their use was found to result in dramatic reduction in rates of repeat revascularization. 27 Quickly realizing that DES might well prove a true game-changer in the treatment of patients with advanced coronary disease, Serruys et al undertook a large randomized trial comparing the relative efficacy of DES and CABG in such patients. 2 This trial, the SYNergy between Percutaneous Coronary Intervention with TAXus and Cardiac Surgery (SYNTAX) trial, has had a major influence on current day practice of PCI.
The SYNTAX Trial: Unique Features
The SYNTAX trial is the most important trial comparing CABG and PCI ever undertaken. In this study, 1,800 patients with three-vessel or left main CAD were randomly assigned (in a 1:1 ratio) to undergo CABG or PCI. Before detailing the results of this trial, several unique features of SYNTAX should be noted.
During the design of the study, a detailed anatomical description of the coronary arteries—known as the SYNTAX score—was developed. This scoring system takes into account for each lesion (up to a total of 12 lesions) features such as location, length, tortuosity, calcification, and per cent diameter stenosis. Weighting is also given for such characteristics as bifurcation lesions and total occlusion. 28 An online algorithm automatically summates each of these features to calculate the SYNTAX score. 29 SYNTAX scores fall into tertiles (of progressive anatomical complexity), described by Serruys et al, as low (≤22), intermediate (22–32), and high (≥33). 30
Numerous validation studies have confirmed the clinical validity of the SYNTAX score to identify high-risk subjects and aid decision-making between CABG and PCI in a broad range of patient types. 31 Indeed, the SYNTAX score has now entered everyday parlance in the interventional cardiology community. Moreover, the U.S. Food and Drug Association has mandated the SYNTAX score as entry criteria in ongoing contemporary coronary stent trials such as the Everolimus-Eluting Stent System versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial. 31 In addition, this scoring system has been adopted into both European and U.S. guidelines on revascularization for CAD. 32 33
Another feature of this trial was the requirement that, for every patient being considered for randomization, there had to be agreement between a local cardiologist and cardiac surgeon at each site as to the appropriateness for randomization. This so-called Heart Team approach has now become a class I indication in the treatment of patients with advanced CAD, according to both European and U.S. guidelines. 32 33 Calculation of the SYNTAX score for each individual patient is an integral part of the Heart Team approach. This calculation forces the interventional cardiologist and cardiac surgeon to systematically analyze the coronary angiogram and to specify the number of coronary artery lesions that require treatment and assess their angiographic location and anatomical complexity. 31
A third unique feature of the SYNTAX trial was its “all-comers” design. Prior to SYNTAX, studies comparing PCI and CABG in multivessel CAD had profound selection bias in the enrollment of patients prior to randomization. Specifically, only 2 to 12% of screened patients were randomized in these trials. Accordingly, extrapolation of the results of these trials to routine clinical practice was problematic. In contrast, in the SYNTAX trial, there was consecutive enrollment of all eligible patients with three-vessel or left main CAD. 31
The SYNTAX Trial: Methods and Design
The trial set out to determine if PCI was noninferior to CABG in left main and three-vessel CAD. The primary clinical end point was a composite of major adverse cardiac and cerebrovascular events (MACCEs) (i.e., death from any cause, stroke, myocardial infarction, or repeat revascularization) throughout the 12-month period after randomization.
A total of 4,337 patients were assessed for eligibility. Of these, 1,800 were randomized to treatment by either CABG ( n = 897) or PCI ( n = 903) using the Taxus Express paclitaxel-eluting stent (Boston Scientific). A further 1,275 patients were deemed ineligible for randomization because their CAD was either thought to be too complex for PCI (1,077 who underwent CABG) or too high risk for CABG (198 who underwent PCI). These patients were followed up in a nested CABG and nested PCI registry.
The SYNTAX Trial: Results
The rates of MACCE at 12 months were significantly higher in the PCI group (17.8 vs. 12.4% for CABG; p = 0.002). As a result, the criterion for noninferiority was not met and the authors concluded that CABG remained the standard of care for patients with three-vessel and left main CAD. 2
At 5-year follow-up, MACCE rated were again significantly lower in the CABG group (26.9 vs. 37.3% in the PCI group; p < 0.001). 34 The statistical design of the SYNTAX study was such that because noninferiority of PCI at 1 year was not proven, any comparison between subgroups or between individual MACCE components could only be regarded as observational and hypothesis generating. Accepting these limitations, it was observed at 5-year follow-up that the relative efficacy of CABG and PCI depended on the complexity of CAD, as calculated by the SYNTAX score. For patients with the highest complexity CAD (SYNTAX score >33), mortality was greater for PCI than with CABG, whether left main stem or three-vessel disease. For patients with three-vessel disease and low SYNTAX score (<23) or patients with left main stem disease and low and intermediate SYNTAX scores, 5-year outcome did not differ between CABG and PCI. Based on their observations at 5 years, the SYNTAX investigators suggested that approximately two-thirds of all patients with complex coronary disease are best treated with CABG. For the remaining patients, PCI is an excellent alternative to surgery.
Recently, 10-year follow-up data from the SYNTAX trial have been published. 35 CABG provided a significant survival benefit in patients with three-vessel disease but not in patients with left main CAD.
Lessons Learned since SYNTAX: FREEDOM, EXCEL, and NOBLE
For some, the findings of the SYNTAX trials at 5-year follow-up brought to an end the debate regarding the relative roles of CABG and PCI in the treatment of advanced CAD. 36 Indeed, these findings have had a profound influence on the way cardiologists and cardiac surgeons currently treat patients with advanced CAD. However, SYNTAX was not sufficiently powered to make definitive statements regarding patient subgroups. Two such subgroups, patients with diabetes mellitus and patients with left main coronary disease, have been the subject of more recent trials. These trials were sufficiently powered to allow definitive conclusions regarding optimal treatment of CAD in these subgroups. These key trials have confirmed and extended the observations made in SYNTAX. In particular, the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel disease (FREEDOM) trial demonstrated that CABG should be the preferred mode of revascularization in patients with advanced CAD and diabetes mellitus. 37 The intermediate term results of two studies comparing CABG with PCI (using newer generation DES) have been published within the last year. These trials, the Nordic–Baltic–British Left Main Revascularization study (NOBLE) 1 and the EXCEL trials, 3 have provided considerable new data regarding treatment of the specific subgroup of patients with left main coronary disease.
Revascularization Strategies in Patients with Diabetes Mellitus and Multivessel Disease
CAD in patients with diabetes, when compared with nondiabetic patients, tends to be more diffuse and complex. 38 Approximately 25% of patients with advanced CAD undergoing mechanical revascularization have diabetes mellitus. 2 In the FREEDOM trial, 1,900 patients with diabetes and multivessel disease (three-vessel disease in 81%) were randomized to CABG or to PCI using first-generation DES. 37 Patients in the CABG group had significantly lower rates of the composite end point of all-cause death, cerebrovascular accident, or myocardial infarction (18.7 vs. 26.6% in the PCI group; p < 0.01). 37 Esper et al retrospectively analyzed the coronary angiograms of the patients in the FREEDOM trial. 38 The SYNTAX score for each patient was calculated. There was a higher incidence of MACCE in PCI patients with low, intermediate, and high SYNTAX scores compared with those who underwent CABG. 39 Based on current data, the recent 2018 European Society of Cardiology/European Association for Cardiothoracic guidelines on myocardial revascularization recommend CABG for people with diabetes and multivessel disease irrespective of the SYNTAX score. 40
DES versus CABG in the Treatment of Left Main Coronary Artery Disease
In the EXCEL trial, 1,905 patients with left main CAD of low-to-intermediate complexity (mean SYNTAX score = 26.5 ± 9.3) were randomly assigned to PCI or CABG. 3 PCI was performed with an everolimus-eluting stent (Xience; Abbott Vascular, Minneapolis, MN). The primary end point was a composite of MACCE—death, stroke, or myocardial infarction. At 5 years, a primary outcome event had occurred in more patients in the PCI group (22.0%) than in patients in the CABG group (19.2%). However, this difference was not statistically significant, and PCI was judged as noninferior to CABG.
A somewhat different conclusion was drawn by the NOBLE trial investigators. The NOBLE trial was a prospective, randomized, noninferiority trial with enrollment at 36 hospitals in Denmark, Estonia, Finland, Germany, Latvia, Lithuania, Norway, Sweden, and the United Kingdom. Patients were randomly assigned (1:1) to receive PCI (predominantly with the biolimus-eluting stent) or CABG. The primary end point was a composite of MACCE—death, stroke, nonprocedural myocardial infarction, or repeat revascularization. At 5-year follow-up, MACCE rates were significantly greater for PCI (28.9%) than for CABG (19.1%, p = 0.0066). The investigators concluded that in revascularization of left main CAD, PCI was associated with inferior clinical outcome at 5 years compared with CABG. Mortality was similar after the two procedures, but patients treated with PCI had higher rates of nonprocedural myocardial infarction or need for revascularization. In NOBLE, the superiority of CABG was maintained regardless of the severity of CAD, as assessed by the SYNTAX score. Stroke rates in the CABG group were higher early on but, intriguingly, at 5-year follow-up, stroke rates were higher in the PCI group.
Some of the discrepancies may be ascribed to several important differences between the two trials. First, repeat revascularization was not included in the primary end point in EXCEL. If this particular MACCE had been included, the results of EXCEL would have come closer to the results in NOBLE. 41 Second, EXCEL included periprocedural myocardial infarction in their definition of myocardial infarction, whereas NOBLE did not. It has been argued that the EXCEL investigators used a new untested definition of periprocedural myocardial infarction that clearly penalized surgery and that was the key driver of the composite outcome that claim no difference in the two treatment strategies. 42 Third, patients with high SYNTAX score were excluded from EXCEL but not from NOBLE.
Accordingly, while in general CABG remains the standard of care in the treatment of left main disease, PCI may be a reasonable alternative in selected patients, such as those with less complex anatomy (SYNTAX score <22) or with ostial or shaft lesions in the absence of multivessel disease 43 ( Fig. 1 ).
Fig. 1.
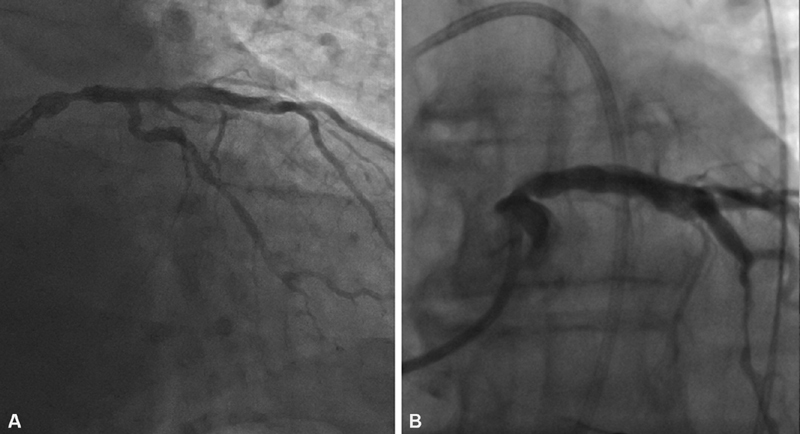
( A ) Coronary angiogram of a symptomatic patient showing a >50% stenosis (fractional flow reserve = 0.78) of the left main coronary artery (mid-shaft location). ( B ) Following discussion with the Heart Team, patient elected for PCI. Coronary angiogram following successful treatment of the stenosis with a drug-eluting stent.
Selection of Mode of Mechanical Revascularization: Beyond the SYNTAX Score
In patients with advanced CAD presenting with acute myocardial infarction, PCI is the preferred treatment option. In stable patients, selection of the mode of mechanical revascularization requires careful review of the patients' clinical as well as angiographic data (as exemplified in the Heart Team approach). Other crucial considerations are local expertise with PCI and CABG and, of course, patient preference. The importance of clinical variables in predicting adverse outcome following cardiac surgery is well recognized. The Society of Thoracic Surgery score predicts the risk of operative mortality and morbidity after cardiac surgery and may be obtained by using an online calculator. 44 This score is based on only three angiographic variables (presence of left main stem disease, number of vessels diseased, and proximal LAD location) but on 40 clinical variables. Notable adverse clinical factors include advanced age, chronic lung disease, renal disease, and peripheral vascular disease. Accordingly, while a patient may have a SYNTAX score that suggests the need for CABG, the presence of multiple comorbidities may make the patient too high risk for surgery. In such cases, PCI becomes the preferred option ( Fig. 2 ).
Fig. 2.
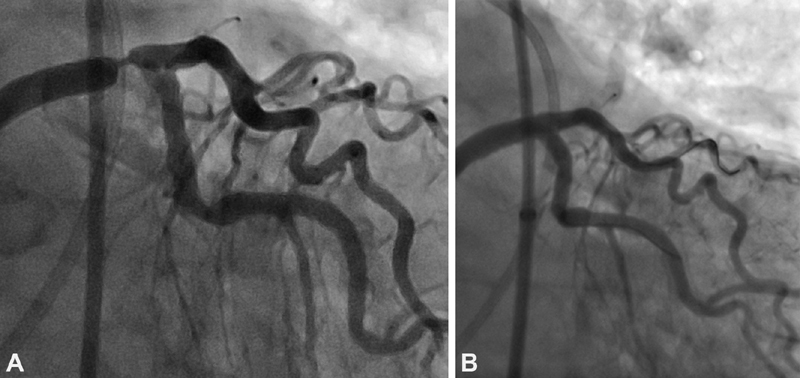
( A ) Severe stenosis of the distal left main coronary artery in an elderly patient with multiple comorbidities, including severe lung disease. The patient was deemed too high risk for CABG. ( B ) Angiogram following successful placement of a drug-eluting stent in the distal left main coronary artery.
Ranucci et al developed and subsequentially validated a simple risk score for mortality after elective CABG comprising only three clinical variable—age, preoperative creatinine value, and left ventricular ejection fraction. This is the so-called age, creatinine, and ejection fraction (ACEF) score. 45 Serruys et al combined the ACEF score with the SYNTAX score to produce the clinical SYNTAX score. 46 The latter score was shown to improve the mortality predictions following PCI in patients with complex CAD. These various tools provide valuable information both to the patient and the Heart Team to enhance decision-making.
In formulating therapeutic options for individual patients with advanced CAD, it is also important to be cognizant of local expertise with CABG and PCI. Several recent studies have demonstrated that high-volume operators (performing > 15 left main PCIs per year) have significantly better outcomes than lower volume operators. 47 48 The interventional cardiologist performing left main PCI must possess advanced skill sets, including the ability to treat competently aorto-ostial, heavily calcified and bifurcation lesions, as well as the ability to perform rotational atherectomy (to debulk lesions) and to implant mechanical support devices, such as the Impella device. 49 50
Conclusion
Patients with left main, left main equivalent, and three-vessel CAD represent an overlapping spectrum of patients with advanced CAD that is associated with an adverse prognosis. Guideline directed medical therapy is a necessary but often insufficient treatment option, as such patients frequently also need mechanical revascularization by either CABG or PCI. In patients with advanced CAD presenting with acute myocardial infarction, PCI, of course, is the preferred treatment option. For stable patients with advanced CAD, CABG remains the standard of care. However, observations from the SYNTAX trial suggest that PCI may be a useful alternative in patients with three-vessel disease with a low SYNTAX score as well as in patients with left main disease and a low or intermediate SYNTAX score. In the subset of patients with diabetes mellitus, the FREEDOM trial unequivocally demonstrated the superiority of CABG in improving outcomes. The findings of the recently published EXCEL and NOBLE trials point to a favorable role for PCI in certain low-to-moderate risk patients with left main stem disease.
Funding Statement
Funding None.
Conflict of Interest None declared.
Authorship
All authors had access to the data and a role in writing the manuscript.
References
- 1.NOBLE investigators Holm N R, Mäkikallio T, Lindsay M M.Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: updated 5-year outcomes from the randomised, non-inferiority NOBLE trial Lancet 2020395(10219):191–199. [DOI] [PubMed] [Google Scholar]
- 2.SYNTAX Investigators . Serruys P W, Morice M C, Kappetein A P. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 3.EXCEL Trial Investigators . Stone G W, Kappetein A P, Sabik J F. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381(19):1820–1830. doi: 10.1056/NEJMoa1909406. [DOI] [PubMed] [Google Scholar]
- 4.Ragosta M, Dee S, Sarembock I J, Lipson L C, Gimple L W, Powers E R. Prevalence of unfavorable angiographic characteristics for percutaneous intervention in patients with unprotected left main coronary artery disease. Catheter Cardiovasc Interv. 2006;68(03):357–362. doi: 10.1002/ccd.20709. [DOI] [PubMed] [Google Scholar]
- 5.Caracciolo E A, Davis K B, Sopko G. Comparison of surgical and medical group survival in patients with left main equivalent coronary artery disease. Long-term CASS experience. Circulation. 1995;91(09):2335–2344. doi: 10.1161/01.cir.91.9.2335. [DOI] [PubMed] [Google Scholar]
- 6.Myers W O, Gersh B J, Fisher L D. Medical versus early surgical therapy in patients with triple-vessel disease and mild angina pectoris: a CASS registry study of survival. Ann Thorac Surg. 1987;44(05):471–486. doi: 10.1016/s0003-4975(10)62104-2. [DOI] [PubMed] [Google Scholar]
- 7.COURAGE Trial Research Group . Boden W E, O'Rourke R A, Teo K K. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 8.Ramadan R, Boden W E, Kinlay S. Management of left main coronary artery disease. J Am Heart Assoc. 2018;7(07):e008151. doi: 10.1161/JAHA.117.008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundy S M, Stone N J, Bailey A L. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 10.American College of Cardiology/American Heart Association Task Force on Practice Guidelines Eckel R H, Jakicic J M, Ard J D.2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines J Am Coll Cardiol 201463(25 Pt B):2960–2984. [DOI] [PubMed] [Google Scholar]
- 11.GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell'infarto Miocardico Lancet 1994343(8906):1115–1122. [PubMed] [Google Scholar]
- 12.PEGASUS-TIMI 54 Steering Committee and Investigators . Bonaca M P, Bhatt D L, Cohen M. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 13.Favaloro R G. Saphenous vein autograft replacement of severe segmental coronary artery occlusion: operative technique. Ann Thorac Surg. 1968;5(04):334–339. doi: 10.1016/s0003-4975(10)66351-5. [DOI] [PubMed] [Google Scholar]
- 14.Braunwald E. Effects of coronary-artery bypass grafting on survival. Implications of the randomized coronary-artery surgery study. N Engl J Med. 1983;309(19):1181–1184. doi: 10.1056/NEJM198311103091911. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Zucker D, Peduzzi P.Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration Lancet 1994344(8922):563–570. [DOI] [PubMed] [Google Scholar]
- 16.Loop F D, Lytle B W, Cosgrove D M. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(01):1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 17.Head S J, Milojevic M, Taggart D P, Puskas J D. Current practice of state-of-the-art surgical coronary revascularization. Circulation. 2017;136(14):1331–1345. doi: 10.1161/CIRCULATIONAHA.116.022572. [DOI] [PubMed] [Google Scholar]
- 18.Farina P, Gaudino M FL, Taggart D P. The eternal debate with a consistent answer: CABG vs PCI. Semin Thorac Cardiovasc Surg. 2020;32(01):14–20. doi: 10.1053/j.semtcvs.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Gruntzig A.Transluminal dilatation of coronary-artery stenosis Lancet 19781(8058):263. [DOI] [PubMed] [Google Scholar]
- 20.O'Keefe J H, Jr, Hartzler G O, Rutherford B D. Left main coronary angioplasty: early and late results of 127 acute and elective procedures. Am J Cardiol. 1989;64(03):144–147. doi: 10.1016/0002-9149(89)90447-5. [DOI] [PubMed] [Google Scholar]
- 21.Bypass Angioplasty Revascularization Investigation (BARI) Investigators . Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med. 1996;335(04):217–225. doi: 10.1056/NEJM199607253350401. [DOI] [PubMed] [Google Scholar]
- 22.Sigwart U. The Stent Story: how it all started…. Eur Heart J. 2017;38(28):2171–2172. doi: 10.1093/eurheartj/ehx339. [DOI] [PubMed] [Google Scholar]
- 23.Ragosta M. Left main coronary artery disease: importance, diagnosis, assessment, and management. Curr Probl Cardiol. 2015;40(03):93–126. doi: 10.1016/j.cpcardiol.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Serruys P W, Ong A TL, van Herwerden L A. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol. 2005;46(04):575–581. doi: 10.1016/j.jacc.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 25.ERACI II Investigators . Rodriguez A E, Baldi J, Fernández Pereira C. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II) J Am Coll Cardiol. 2005;46(04):582–588. doi: 10.1016/j.jacc.2004.12.081. [DOI] [PubMed] [Google Scholar]
- 26.Hueb W, Lopes N H, Gersh B J. Five-year follow-up of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2007;115(09):1082–1089. doi: 10.1161/CIRCULATIONAHA.106.625475. [DOI] [PubMed] [Google Scholar]
- 27.Russo F D, Rao S V. Philadelphia: Wolters Kluwer; 2018. Coronary stents; pp. 187–195. [Google Scholar]
- 28.Sianos G, Morel M A, Kappetein A P. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(02):219–227. [PubMed] [Google Scholar]
- 29.SYNTAX score calculatorSYNTAX working-group, launched May 19, 2009 at: http://www.syntaxscore.com. Accessed September 10, 2020
- 30.Serruys P W, Onuma Y, Garg S. Assessment of the SYNTAX score in the SYNTAX study. EuroIntervention. 2009;5(01):50–56. doi: 10.4244/eijv5i1a9. [DOI] [PubMed] [Google Scholar]
- 31.Farooq V, Garg S, Serruys P W. Philadelphia: Elsevier; 2016. Individualized assessment for percutaneous or surgical revascularization; pp. 1–31. [Google Scholar]
- 32.Authors/Task Force members . Windecker S, Kolh P, Alfonso F. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35(37):2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 33.Fihn S D, Blankenship J C, Alexander K P. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64(18):1929–1949. doi: 10.1016/j.jacc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Mohr F W, Morice M C, Kappetein A P.Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial Lancet 2013381(9867):629–638. [DOI] [PubMed] [Google Scholar]
- 35.SYNTAX Extended Survival Investigators Thuijs D JFM, Kappetein A P, Serruys P W.Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial Lancet 2019394(10206):1325–1334. [DOI] [PubMed] [Google Scholar]
- 36.Taggart D P.CABG or stents in coronary artery disease: end of the debate? Lancet 2013381(9867):605–607. [DOI] [PubMed] [Google Scholar]
- 37.FREEDOM Trial Investigators . Farkouh M E, Domanski M, Sleeper L A. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 38.Esper R B, Farkouh M E, Ribeiro E E.SYNTAX score in patients with diabetes undergoing coronary revascularization in the FREEDOM trial J Am Coll Cardiol 201872(23 Pt A):2826–2837. [DOI] [PubMed] [Google Scholar]
- 39.Fedak P WM, Bhatt D L, Verma S.Coronary bypass surgery for diabetes and multivessel disease: forget the SYNTAX J Am Coll Cardiol 201872(23 Pt A):2838–2840. [DOI] [PubMed] [Google Scholar]
- 40.ESC Scientific Document Group Neumann F J, Sousa-Uva M, Ahlsson A.2018 ESC/EACTS guidelines on myocardial revascularization Eur Heart J 2019400287–165.30165437 [Google Scholar]
- 41.Ragosta M.Revascularization of Left Main Disease: Do we EXCEL at Stenting?. Or Is It More NOBLE to Treat With Surgery? Expert Analysis American College of Cardiology; 2020. Accessed September 20, 2020 at:https://www.acc.org/latest-in-cardiology/articles/2017/03/08/08/05/revascularization-of-left-main-disease
- 42.Taggart D P, Gaudino M. PCI or CABG for left main coronary artery disease. N Engl J Med. 2020;383(03):290. doi: 10.1056/NEJMc2000645. [DOI] [PubMed] [Google Scholar]
- 43.Lee M S, Manthripragada G. Philadelphia: Wolters Kluwer; 2018. Left mainstem Intervention; pp. 187–195. [Google Scholar]
- 44.STS Short-Term Risk CalculatorThe Society of Thoracic Surgeons, launched November 15, 2018 at: http://riskcalc.sts.org/stswebriskcalc/calculate. Accessed September 10, 2020
- 45.Ranucci M, Castelvecchio S, Conte M. The easier, the better: age, creatinine, ejection fraction score for operative mortality risk stratification in a series of 29,659 patients undergoing elective cardiac surgery. J Thorac Cardiovasc Surg. 2011;142(03):581–586. doi: 10.1016/j.jtcvs.2010.11.064. [DOI] [PubMed] [Google Scholar]
- 46.ARTS-II Investigators . Garg S, Sarno G, Garcia-Garcia H M. A new tool for the risk stratification of patients with complex coronary artery disease: the clinical SYNTAX score. Circ Cardiovasc Interv. 2010;3(04):317–326. doi: 10.1161/CIRCINTERVENTIONS.109.914051. [DOI] [PubMed] [Google Scholar]
- 47.Kinnaird T, Gallagher S, Anderson R. Are higher operator volumes for unprotected left main stem percutaneous coronary intervention associated with improved patient outcomes? A survival analysis of 6724 procedures from the British Cardiovascular Intervention Society National Database. Circ Cardiovasc Interv. 2020;13(06):e008782. doi: 10.1161/CIRCINTERVENTIONS.119.008782. [DOI] [PubMed] [Google Scholar]
- 48.Xu B, Redfors B, Yang Y. Impact of operator experience and volume on outcomes after left main coronary artery percutaneous coronary intervention. JACC Cardiovasc Interv. 2016;9(20):2086–2093. doi: 10.1016/j.jcin.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Glazier J J, Kaki A. The Impella device: historical background, clinical applications and future directions. Int J Angiol. 2019;28(02):118–123. doi: 10.1055/s-0038-1676369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collet C, Capodanno D, Onuma Y. Left main coronary artery disease: pathophysiology, diagnosis, and treatment. Nat Rev Cardiol. 2018;15(06):321–331. doi: 10.1038/s41569-018-0001-4. [DOI] [PubMed] [Google Scholar]


