Abstract
BACKGROUND AND PURPOSE: Internal carotid artery (ICA) aneurysms may present with cranial nerve dysfunction. Therapeutic ICA occlusion, when tolerated, is an effective treatment resulting in improvement or cure of symptoms in most patients. When ICA occlusion is not tolerated, selective endovascular aneurysm occlusion can be considered. We compare recovery of cranial nerve dysfunction in patients treated with selective coil occlusion and with therapeutic ICA occlusion.
MATERIALS AND METHODS: In 16 patients with 17 large or giant (11–45 mm) unruptured ICA aneurysms presenting with dysfunction of cranial nerves (CN) II, III, IV, or VI, selective coil occlusion was performed. From a cohort of 39 patients with ICA aneurysms treated with ICA occlusion and long-term follow-up, we selected 31 patients with aneurysms presenting with cranial nerve dysfunction. Clinical recovery at follow-up from oculomotor dysfunction and visual symptoms was compared for both treatment modalities.
RESULTS: Of 17 aneurysms treated with selective coiling, symptoms of cranial nerve dysfunction resolved in 3, improved in 10, and remained unchanged in 4. In 9 of 17 patients, additional coiling during follow-up was required. Of 31 aneurysms treated with carotid artery occlusion, cranial nerve dysfunction resolved in 19, improved in 9, and remained unchanged in 3. These differences were not significant. There were no complications of treatment.
CONCLUSION: Recovery of ICA aneurysm-induced cranial nerve dysfunction occurs in most patients, both after ICA occlusion and after selective coiling. In patients who cannot tolerate ICA occlusion, selective aneurysmal occlusion with coils is a valuable alternative.
Large and giant aneurysms of the cavernous, ophthalmic, and hypophyseal segments of the internal carotid artery (ICA) frequently present with symptoms of mass effect on the cranial nerves. Compression of the oculomotor nerve (CN III), trochlear nerve (CN IV), or abducens nerve (CN VI) results in ophthalmoplegia and is frequently associated with cavernous sinus aneurysms. Compression of the optic nerve (CN II) results in decreased visual acuity and visual field deficits and is mostly associated with carotid ophthalmic or hypophyseal aneurysms. In general, treatment in unruptured but symptomatic intradural located hypophyseal and ophthalmic aneurysms is indicated to prevent rupture and to alleviate symptoms of mass effect. Treatment of unruptured symptomatic cavernous sinus aneurysms may be indicated to improve cranial nerve function or to discontinue progression of dysfunction.
In patients who can tolerate therapeutic ICA occlusion, this therapy is effective in excluding the aneurysm from the circulation. Symptoms of mass effect resolve or improve in most patients. In addition, endovascular balloon occlusion of the ICA is simple to perform, safe, and inexpensive.1–8 When ICA occlusion is not tolerated, alternative treatments are selective aneurysm occlusion with coils or liquid embolic agent9,10 and bypass surgery preceding occlusion.11 The effect on cranial nerve dysfunction of selective occlusion with coils of ICA aneurysms in general and large and giant aneurysms specifically is poorly understood.12–18 In this study, we assess the clinical evolution of aneurysm-induced cranial nerve dysfunction after selective coil occlusion in 16 patients with 17 unruptured large and giant ICA aneurysms and compare the results with historical data of patients with similar aneurysms and symptoms treated with ICA occlusion.
Patients and Methods
General
At our institution, patients with unruptured large or giant ICA aneurysms presenting with cranial nerve dysfunction are preferably treated with endovascular ICA balloon occlusion. Only in patients who cannot tolerate permanent ICA occlusion, selective coiling or bypass surgery is offered.
Assessment of Cranial Nerve Dysfunction
Oculomotor dysfunction was defined as paresis of CN III, IV, or VI or a combination with diplopia in single or multiple gazes with or without ptosis and pupillary dysfunction.
Optic nerve dysfunction was defined as decreased visual acuity with visual field deficits as assessed with perimetric or confrontation testing.
The criteria for complete recovery of oculomotor dysfunction were no diplopia in all direction of gazes, complete resolution of ptosis, and partial or complete recovery of pupillary reaction. Partial recovery of oculomotor dysfunction was defined as residual diplopia in upward, downward, lateral, or medial gaze with or without normal primary gaze, residual ptosis, and pupillary dysfunction.
The criteria for complete recovery of optic nerve dysfunction were restoration of normal visual acuity and visual field. Residual visual field deficits indicated partial recovery.
Aneurysms Treated With Selective Coiling
Between January 1995 and March 2007, a total of 16 patients with 17 large or giant unruptured ICA aneurysms presenting with dysfunction of CN II, III, IV, or VI were treated with selective occlusion of the aneurysm with coils. There were 14 of 16 patients who did not tolerate ICA test occlusion and 2 patients who had bilateral giant ICA aneurysms. One patient had 1 ophthalmic and 1 cavernous sinus aneurysm both presenting with mass effect and both treated with selective coiling (Fig 1). One patient had bilateral cavernous sinus aneurysms, of which the 1 symptomatic aneurysm was coiled. There were 2 men and 14 women with a mean age of 60.0 years (median age, 57 years; range, 38–81 years). The location of the aneurysm was the carotid cavernous sinus in 7, carotid hypophyseal in 4, and carotid ophthalmic in 6. The size of the aneurysm was a mean of 25.6 mm (median, 25 mm; range, 11–45 mm). Of 17 aneurysms, 9 contained an intraluminal thrombus. All 7 cavernous sinus aneurysms presented with ophthalmoplegia or isolated CN III palsy in 1 patient with associated CN II dysfunction. All ophthalmic aneurysms presented with CN II dysfunction in 1 patient with associated CN III palsy. Of 4 hypophyseal aneurysms, 3 presented with CN II dysfunction and 1 with ipsilateral ophthalmoplegia and contralateral partial CN III palsy (Fig 2).
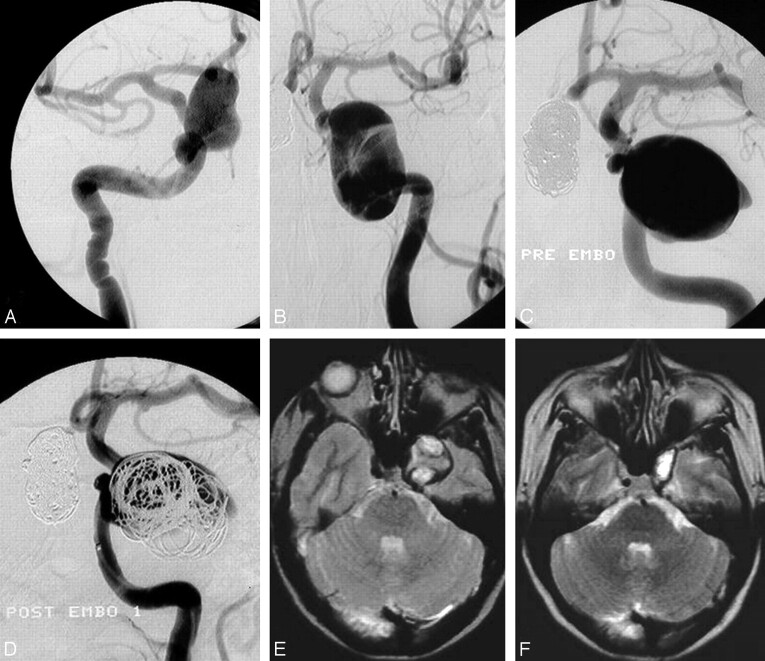
Fig 1.
A 48-year-old woman presenting in 1994 with visual field deficit from a right giant carotid ophthalmic aneurysm and acute left ophthalmoplegia from a left-sided carotid cavernous sinus aneurysm. A, Right ophthalmic aneurysm completely occluded with coils (see coil mesh in C and D). B, Left cavernous sinus aneurysm with intraluminal thrombus. Note additional small middle cerebral artery aneurysm. C, Angiogram 7 days after B shows enlargement of the aneurysm by resolution of intraluminal thrombus. D, Loose coil packing after embolization. Follow-up angiograms 6 to 72 months later showed progressive aneurysmal thrombosis with complete occlusion. E and F, MR imaging after 3 weeks (E) and 18 months (F). At 18 months, the aneurysm is almost completely obliterated. Visual field deficit remained unchanged, and ophthalmoplegia improved to isolated abducens palsy.
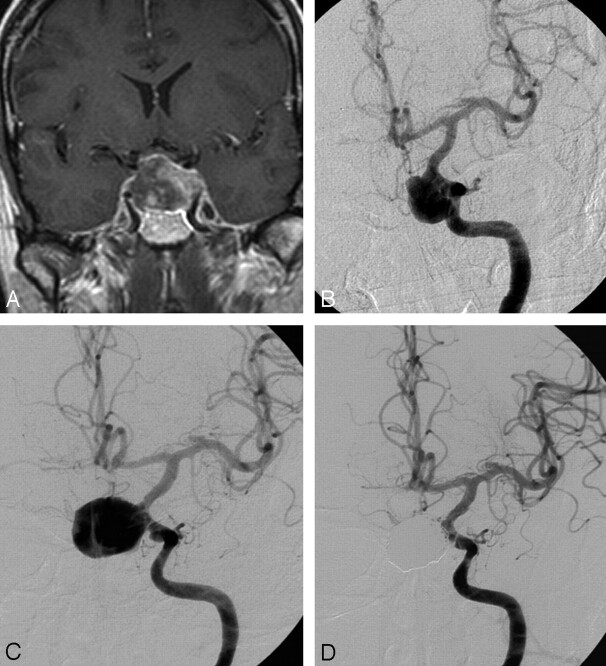
Fig 2.
A 52-year-old man presenting with acute ipsilateral ophthalmoplegia and contralateral CN III palsy with intact vision. A and B, Contrast-enhanced MR imaging (A) and angiography (B) shows giant left carotid hypophyseal aneurysm with intraluminal thrombus. Angiographic test occlusion was not tolerated. C, Angiogram 2 days after B shows enlargement of the aneurysmal lumen due to resolution of thrombus. D, Complete occlusion after stent-assisted coiling. One year later, right ophthalmoplegia was resolved. Residual upper gaze diplopia and 1- to 2-mm residual ptosis on the left side.
Five aneurysms were coiled with balloon assistance and 3 after stent placement in primary or additional coiling (Enterprise; Cordis Neurovascular, Miami Lakes, Fla). Coiling was performed with 50-cm long straight coils (Detach 18; Cook, Copenhagen, Denmark) or Guglielmi detachable 18 helical coils (Boston Scientific, Fremont, Calif).
Aneurysms Treated With ICA Occlusion
The protocol for therapeutic ICA occlusion has been described previously.3,6 In short, during balloon test occlusion in the awake patient, angiography of the contralateral ICA or vertebral artery, or both, was performed. Apart from clinical tolerance, synchronous opacification of the cerebral veins in the examined and occluded vascular territory indicated tolerance to permanent occlusion. After tolerance was determined, the ICA was permanently occluded with detachable balloons (Goldvalve #16 balloon; Nycomed, Paris, France or Balt, Montmorency, France). Imaging follow-up consisted of serial MR imaging to evaluate thrombosis and shrinkage of the aneurysm with time.
From a previously published cohort of 39 patients with ICA aneurysms treated with ICA occlusion and long-term clinical and imaging follow-up,4,5 we selected 31 patients with unruptured large and giant aneurysms symptomatic by cranial nerve dysfunction and located in the cavernous sinus (n = 26), and hypophyseal (n = 4) or ophthalmic (n = 1) segment. There were 4 men and 27 women with a mean age of 57.3 years (median age, 55 years; range, 26–78 years). Of 26 patients with cavernous sinus aneurysms, the presenting symptom was ophthalmoplegia in 23 (with additional trigeminal neuralgia in 1) and isolated CN VI palsy in 3. Of 4 patients with hypophyseal aneurysms, 3 presented with CN II dysfunction and 1 with ophthalmoplegia and trigeminal neuralgia. The 1 patient with an ophthalmic aneurysm presented with CN II dysfunction.
Follow-up Assessment
For patients treated with selective coiling, follow-up angiograms were scheduled at 6 months, and additional imaging follow-up (angiography, MR imaging, or MR angiography) was tailored as deemed necessary. When reopening of the aneurysm was apparent at follow-up, additional coiling was performed. For patients treated with ICA occlusion, follow-up imaging consisted of MR imaging at 3 months and at various intervals thereafter.
Recovery from cranial nerve dysfunction was assessed at various intervals during follow-up in the outpatient clinic by neurosurgeons, neurologists, or ophthalmologists. Evolution of aneurysmal size during follow-up after treatment was assessed for patients with serial MR imaging follow-up.
Statistical Analysis
The number of patients with resolved or reduced symptoms of cranial nerve dysfunction for both treatment modalities was compared with patients with unchanged symptoms with the Fisher exact test. The same was done for aneurysms with visual field deficit or oculomotor function as the major presenting symptom.
Results
Aneurysms Treated With Selective Coiling
Initial aneurysmal occlusion after coiling was complete or near complete in all 17 aneurysms. During angiographic follow-up in all patients at a mean of 29.8 months (median, 19 months; range, 6–102 months), 9 aneurysms reopened and were additionally coiled, and 4 of those were coiled for a third time. Final aneurysmal occlusion at last angiographic follow-up was complete or near complete in 16 aneurysms and incomplete in 1 aneurysm. There were no procedural complications leading to permanent morbidity or mortality, neither from primary nor from additional coiling. There were 7 of 16 patients (with 8 aneurysms) who had initial MR imaging and serial MR imaging follow-up, with intervals ranging from 6 to 36 months. Aneurysmal size was unchanged in 6, increased from 19 to 24 mm in 1 (Fig 3), and decreased from 35 to10 mm in 1.
Fig 3.
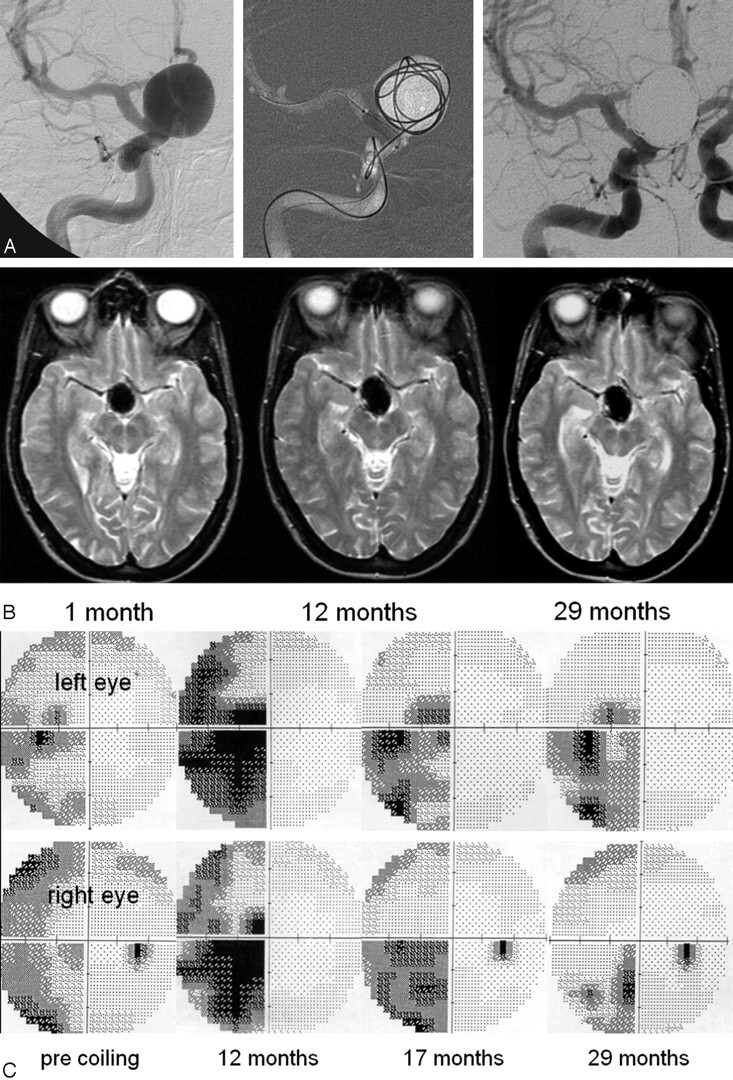
A 38-year-old woman presenting with progressive visual field deficit. A, Giant carotid hypophyseal aneurysm compressing the chiasm treated with balloon-assisted coiling. Angiographic test occlusion was not tolerated. B, MR imaging at 3 intervals after coiling. At 12 months, the aneurysm has increased in size from 24 to 31 mm. Size is stable during an interval of 12 to 24 months. C, Perimetric results before coiling and different intervals thereafter. Incomplete hemianopsia before coiling. At 12 months, increased aneurysmal size caused progressive hemianopsia that gradually decreased at 17 and 29 months. Final result was classified as unchanged.
Clinical follow-up was available in all patients at a mean of 35.3 months (median, 24 months; range, 6–108 months). At last clinical follow-up, on a per aneurysm basis, symptoms of cranial nerve dysfunction resolved in 3, improved in 10, and remained unchanged in 4 aneurysms. Of 9 aneurysms with visual field deficit as the major presenting symptom, this was reduced in 5 and remained unchanged in 4. Of 8 aneurysms with oculomotor dysfunction as the major presenting symptom, this resolved in 3 and was reduced in 5. All 5 patients with incomplete recovery had remaining diplopia in upward or medial gaze, not in primary gaze. Ptosis resolved in all patients.
Aneurysms Treated With Internal Carotid Artery Occlusion
There were no early or late complications of therapeutic ICA occlusion.4–6 At last clinical and MR imaging follow-up at a mean of 31 months (median, 19 months; range, 6–107 months), symptoms of mass effect in 31 patients were resolved in 19, improved in 8, and remained unchanged in 3. Of 4 patients with visual field deficit as the major presenting symptom, this deficit improved in all 4. Of 27 patients with oculomotor dysfunction as the major presenting symptom, this dysfunction resolved in 19, improved in 5, and remained unchanged in 3. All 5 patients with incomplete recovery had remaining diplopia in upward or medial gaze only, not in primary gaze. Ptosis resolved in all patients. In 2 patients with associated trigeminal neuralgia, this resolved in both. For most patients with clinical improvement or resolution, this was obvious in the first year after ICA occlusion. At last MR imaging follow-up at a mean of 31 months, 22 of 31 aneurysms were obliterated completely, 2 aneurysms decreased to 25% of their original diameter; 2 aneurysms, to 50% of their original diameter; and 5 aneurysms, to 75%.
Comparison of Recovery of Cranial Nerve Function in Patients Treated With Selective Coiling Versus ICA Occlusion
Of 17 aneurysms treated with selective coiling, symptoms of cranial nerve dysfunction resolved in 3, improved in 10, and remained unchanged in 4. Of 31 aneurysms treated with carotid artery occlusion, cranial nerve dysfunction resolved in 19, improved in 9, and remained unchanged in 3. These differences were not significant.
Results of recovery of visual field deficit and oculomotor symptoms for both treatment modalities are summarized in the Table. There was no significant difference in improvement or resolution of both types of presenting symptoms, though improvement of visual field deficits occurred more often after ICA occlusion. Complete normalization of visual field did not occur with both treatment modalities.
Recovery of ICA aneurysm-induced oculomotor dysfunction, visual symptoms, and all symptoms after selective coil occlusion in and after ICA occlusion
| Treatment | Oculomotor Dysfunction | P Value Resolved and Improved vs Unchanged | |||
|---|---|---|---|---|---|
| Resolved | Improved | Unchanged | |||
| ICA occlusion | 27 | 19 (70%) | 5 (19%) | 3 (11%) | 1.0 |
| Selective coiling | 8 | 3 (38%) | 5 (62%) | 0 | |
| Visual symptoms | Resolved | Improved | Unchanged | 0.23 | |
| ICA occlusion | 4 | 0 | 4 (100%) | 0 | |
| Selective coiling | 9 | 0 | 5 (56%) | 4 (44%) | |
| All symptoms | Resolved | Improved | Unchanged | 0.23 | |
| ICA occlusion | 31 | 19 (61%) | 9 (29%) | 3 (10%) | |
| Selective coiling | 17 | 3 (18%) | 10 (59%) | 4 (23%) |
Note:—ICA indicates internal carotid artery.
Discussion
In our study, we found that recovery of cranial nerve dysfunction induced by large or giant unruptured ICA aneurysms occurs in most patients, both after ICA occlusion and after selective coiling. In only 7 of 48 aneurysms, symptoms of cranial nerve deficits remained unchanged after therapy, and aggravation of symptoms did not occur. In our limited patient groups, we were unable to demonstrate a significant difference in alleviation of mass effect between the 2 treatment modalities. This may be explained by the diversity of factors involved in the recovery process: duration of symptoms before treatment; acute or gradual onset of symptoms; degree of cranial nerve dysfunction (complete vs partial); and presence of microvascular risk factors such as hypertension, diabetes, and advanced age. In our patients, the presence and magnitude of these possible factors were not always known. Both coiling and ICA occlusion were safe; complications did not occur. Also, with additional coiling in 9 of 17 aneurysms, there were no complications. The safety of coiling of large and giant aneurysms is supported by previous studies.19–21 In general, in patients with unruptured large or giant ICA aneurysms symptomatic by mass effect, therapy is indicated to reduce symptoms of mass effect and to eliminate the risk for rupture in aneurysms with an intradural location. Both ICA occlusion and selective coiling provide sufficient protection against rupture, with a high chance of improvement of symptoms of mass effect. ICA occlusion is, in our opinion, the treatment of choice because it is effective, safe, definitive, simple to perform, and inexpensive. In patients who cannot tolerate permanent ICA occlusion, alternative treatment should be considered. These alternatives consist of selective occlusion with coils or a liquid embolic agent (Onyx; ev3, Irvine, Calif) or bypass surgery preceding ICA occlusion. The first alternative, selective occlusion with coils, is technically more challenging than ICA occlusion, especially when balloon- or stent-assisted treatment is necessary.22,23 With stent-assisted treatment, prolonged antiplatelet medication is necessary. With currently available easy-to-place stents, feasibility and safety of the stent-assisted technique are enhanced. Imaging follow-up is mandatory because reopening with time occurs in a substantial proportion of patients with large and giant aneurysms, and additional coiling is frequently needed. Recently, filling the aneurysmal lumen with Onyx has been proposed as an alternative treatment.9,10 With this treatment, the neck of the aneurysm is sealed with a balloon in the carotid artery during Onyx injection. In a multicenter study by Molyneux,9 procedure- or device-related permanent neurologic morbidity at final follow-up was present in 8 (8.3%) of 97 patients, with 2 procedural deaths. In large and giant aneurysms, procedural time was lengthy (up to 6 hours). Delayed occlusion of the carotid artery occurred in 9 (9%) of 100 patients. At 12-month follow-up of 53 patients, 38 (72%) large and giant aneurysms were completely occluded. Retreatment was performed in 9 of 79 large and giant aneurysms. Sixteen patients had oculomotor palsies at presentation. At 12-month follow-up, the oculomotor palsy had improved or resolved in 14 patients, remained unchanged in 1 patient, and had worsened in 1. Fourteen patients had optic nerve compression before treatment. At 12-month follow-up, the nerve compression had improved in 6 patients, remained unchanged in 7, and worsened in 1. In our opinion, the high complication rate and high rate of delayed carotid artery occlusion do not justify this treatment in patients with unruptured aneurysms who cannot tolerate carotid artery occlusion. The third alternative to primary carotid artery occlusion is bypass surgery preceding permanent occlusion. High-flow bypass surgery with the excimer laser-assisted anastomosis technique has the advantage of not involving temporary occlusion of the donor or recipient vessel with inherent risks of inducing ischemia. In a study by Brilstra et al,11 short-term outcomes of this technique were evaluated in 77 patients. Of these 77 patients, 10 (13%) had operative complications resulting in dependency in 7 and death in 3. The authors concluded that, in view of this high risk for complications, this technique should be reserved for patients with intradural large or giant aneurysms with a high chance of rupture who cannot tolerate carotid artery occlusion.
Conclusion
For patients with unruptured large or giant carotid artery aneurysms presenting with cranial nerve dysfunction, therapeutic carotid artery occlusion, when tolerated, is the treatment of choice. Clinical results are excellent and complications are exceptional. In patients who cannot tolerate carotid artery occlusion or who have bilateral aneurysms, selective coiling, with or without balloon or stent assistance, is the best alternative with comparable clinical results. Onyx treatment is not justified in view of the high complication rate and high rate of delayed carotid artery occlusion. Bypass surgery should only be considered in patients with intradurally located aneurysms with a high chance of rupture.
References
- 1.van der Schaaf IC, Brilstra EH, Buskens E, et al. Endovascular treatment of aneurysms in the cavernous sinus: a systematic review on balloon occlusion of the parent vessel and embolization with coils. Stroke 2002;33:313–18 [DOI] [PubMed] [Google Scholar]
- 2.Larson JJ, Tew JM Jr., Tomsick TA, et al. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery 1990;36:23–30 [PubMed] [Google Scholar]
- 3.van Rooij WJ, Sluzewski M, Slob MJ, et al. Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol 2005;1:175–78 [PMC free article] [PubMed] [Google Scholar]
- 4.de Gast AN, Sprengers ME, van Rooij WJ, et al. Long term 3T-MRA follow-up after therapeutic occlusion of the internal carotid artery to detect possible de novo aneurysm formation. AJNR Am J Neuroradiol 2007;28:508–10 [PMC free article] [PubMed] [Google Scholar]
- 5.de Gast AN, Sprengers ME, van Rooij WJ, et al. Midterm clinical and magnetic resonance imaging follow-up of large and giant carotid artery aneurysms after therapeutic carotid artery occlusion. Neurosurgery 2007;60:1025–29 [DOI] [PubMed] [Google Scholar]
- 6.van Rooij WJ, Sluzewski M, Metz NH, et al. Carotid balloon occlusion for large and giant aneurysms: evaluation of a new test occlusion protocol. Neurosurgery 2000;47:116–21 [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg-Cohen N, Curry C, Miller NR, et al. Long term visual and neurological prognosis in patients with treated and untreated cavernous sinus aneurysms. J Neurol Neurosurg Psychiatry 2004;75:863–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lubicz B, Gauvrit JY, Leclerc X, et al. Giant aneurysms of the internal carotid artery: endovascular treatment and long-term follow-up. Neuroradiology 2003;45:650–55 [DOI] [PubMed] [Google Scholar]
- 9.Molyneux AJ, Cekirge S, Saatci I, et al. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol 2004;25:39–51 [PMC free article] [PubMed] [Google Scholar]
- 10.Weber W, Siekmann R, Kis B, et al. Treatment and follow-up of 22 unruptured wide-necked intracranial aneurysms of the internal carotid artery with Onyx HD 500. AJNR Am J Neuroradiol 2005;26:1909–15 [PMC free article] [PubMed] [Google Scholar]
- 11.Brilstra EH, Rinkel GJ, Klijn CJ, et al. Excimer laser-assisted bypass in aneurysm treatment: short-term outcomes. J Neurosurg 2002;97:1029–35 [DOI] [PubMed] [Google Scholar]
- 12.Mansour N, Kamel MH, Kelleher M, et al. Resolution of cranial nerve paresis after endovascular management of cerebral aneurysms. Surg Neurol 2007;68:500–04 [DOI] [PubMed] [Google Scholar]
- 13.Stiebel-Kalish H, Maimon S, Amsalem J, et al. Evolution of oculomotor nerve paresis after endovascular coiling of posterior communicating artery aneurysms: a neuro-ophthalmological perspective. Neurosurgery 2003;53:1268–74 [DOI] [PubMed] [Google Scholar]
- 14.Birchall D, Khangure MS, McAuliffe W. Resolution of third nerve paresis after endovascular management of aneurysms of the posterior communicating artery. AJNR Am J Neuroradiol 1999;20:411–13 [PMC free article] [PubMed] [Google Scholar]
- 15.Mavilio N, Pisani R, Rivano C, et al. Recovery of third nerve palsy after endovascular packing of internal carotid-posterior communicating artery aneurysms. Intervent Neuroradiol 2000;6:203–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn JY, Han IB, Yoon PH, et al. Clipping vs coiling of posterior communicating artery aneurysms with third nerve palsy. Neurology 2006;66:121–23 [DOI] [PubMed] [Google Scholar]
- 17.Wong GK, Ng SC, Tsang PK, et al. Clipping vs coiling of posterior communicating artery aneurysms with third nerve palsy. Neurology 2006;66:1959–60 [DOI] [PubMed] [Google Scholar]
- 18.Chen PR, Amin-Hanjani S, Albuquerque FC, et al. Outcome of oculomotor nerve palsy from posterior communicating artery aneurysms: comparison of clipping and coiling. Neurosurgery 2006;58:1040–46 [DOI] [PubMed] [Google Scholar]
- 19.van Rooij WJ, Sluzewski M. Coiling of very large and giant basilar tip aneurysms: midterm clinical and angiographic results. AJNR Am J Neuroradiol 2007;28:1405–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sluzewski M, Menovsky T, van Rooij WJ, et al. Coiling of very large or giant cerebral aneurysms: long-term clinical and serial angiographic results. AJNR Am J Neuroradiol 2003;24:257–62 [PMC free article] [PubMed] [Google Scholar]
- 21.Gruber A, Killer M, Bavinzski G, et al. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999;45:793–803 [DOI] [PubMed] [Google Scholar]
- 22.Kessler IM, Mounayer C, Piotin M, et al. The use of balloon-expandable stents in the management of intracranial arterial diseases: a 5-year single-center experience. AJNR Am J Neuroradiol 2005;26:2342–48 [PMC free article] [PubMed] [Google Scholar]
- 23.Saatci I, Cekirge HS, Ozturk MH, et al. Treatment of internal carotid artery aneurysms with a covered stent: experience in 24 patients with mid-term follow-up results. AJNR Am J Neuroradiol 2004;25:1742–49 [PMC free article] [PubMed] [Google Scholar]