Abstract
SUMMARY: The subarachnoid space around the optic nerve can be detected by fat-saturated T2-weighted MR imaging of the orbit, and dilation of this space reflects increased intracranial pressure. We examined 3 patients with CSF hypovolemia with MR imaging of the orbit and measured the optic nerve sheath diameter before and after treatment. We showed that the subarachnoid space is decreased in patients with CSF hypovolemia and the usefulness of this finding.
The subarachnoid space (SAS) around the optic nerve in the orbit can be detected by the fat saturation pulse sequence adapted for T2-weighted imaging.1,2 The CSF in the SAS around the optic nerve can be differentiated from the surrounding fat tissue and appears as round high-intensity circles on the coronal images. The optic nerve sheath (ONS) diameter can be estimated by measuring the outer diameter of the SAS. The SAS can be observed in normal subjects, and dilation of the SAS is associated with idiopathic intracranial hypertension or hydrocephalus presenting with increased intracranial pressure.2–5 Because increased intracranial pressure causes dilation of the SAS around the optic nerve, the outer diameter of the SAS may decrease in patients with CSF hypovolemia or intracranial hypotension. Observation of such collapse of the SAS provides suggestion of decreased CSF volume or pressure in CSF hypovolemia. Here, we report 3 cases of CSF hypovolemia in which the diameters of the SAS around the optic nerves were measured by MR imaging before and after epidural blood patch treatment.
Case Reports
Case 1
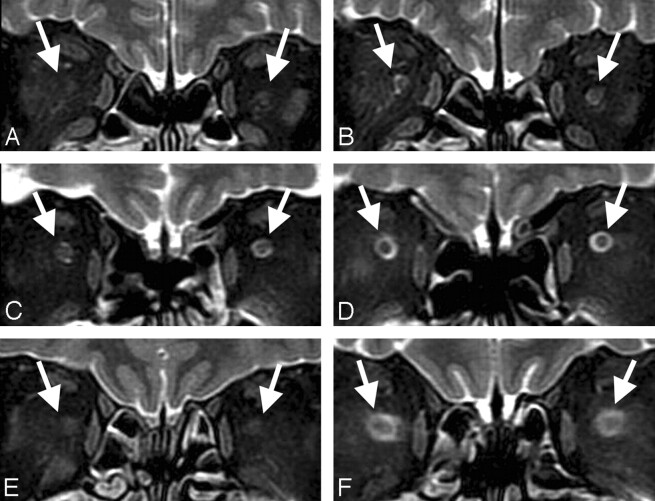
A 64-year-old man complaining of nonorthostatic persistent headache and plugged ear persisting for 2 weeks was referred to our hospital. Brain MR imaging demonstrated bilateral subdural fluid collections with diffuse pachymeningeal enhancement, descent of the brain, enlargement of the pituitary gland, and intracranial venous enlargement.6 Fat-saturated T2-weighted fast spin-echo MR images of the orbit were obtained using a 1.5T MR scanner (GE Medical Systems, Milwaukee, Wis) with TR at 3500 ms, TE at 100 ms, flip angle at 90°, section thickness at 3.5 mm, section gap at 0.3 mm, matrix at 320 × 224, FOV at 16 × 16 cm, echo-train length at 12, and band width at 31.2 kHz. Orbital thin-section fat-saturated MR imaging showed marked reduction of high signal intensity CSF within the SAS surrounding the optic nerves bilaterally (Fig 1A). Radioisotope cisternography revealed rapid excretion of tracer into the urine and CSF leakage at the upper thoracic spine. Opening pressure at lumbar puncture was 100 mm H2O. The diagnosis of CSF hypovolemia was determined according to the criteria for “headache attributed to spontaneous low CSF pressure” in the International Classification of Headache Disorder, 2nd edition.7 Epidural blood patch treatment was performed at the upper thoracic spine, and his headache and plugged ear resolved. Brain MR imaging 1 month after the treatment demonstrated disappearance of the MR findings related to CSF hypovolemia and marked improvement in the visualization of the SAS around the optic nerves. The ONS diameter was 5.5 mm on the right and 5.8 mm on the left (Fig 1B).
Fig 1.
Coronal fat-saturated T2-weighted orbital MR images of cases 1, 2, and 3. In case 1, MR images showing the subarachnoid space (arrow) had collapsed and the ONS could not be detected before treatment (A) but became visible with diameter of 5.5 mm on the right and 5.8 mm on the left after treatment (B). In case 2, MR images showing the ONS diameter just behind the optic globe before treatment were 5.0 mm on the right and 4.7 mm on the left (C) and became 5.9 mm and 5.3 mm, respectively, after treatment (D). In case 3, MR images showing the subarachnoid space had collapsed, and the ONS could not be detected before treatment (E) but became visible with a diameter of 6.2 mm on the right and 6.2 mm on the left after treatment (F).
Case 2
A 59-year-old man complained of typical orthostatic headache and plugged ear. Brain MR imaging demonstrated pachymeningeal enhancement and enlargement of the pituitary gland and ONS diameter just behind the optic globe of 5.0 mm on the right and 4.7 mm on the left (Fig 1C). Radioisotope cisternography showed CSF leakage at the thoracic spine. Opening pressure at lumbar puncture was below zero. His headache resolved within a few days of an epidural blood patch performed at the cervical and the lumbar spine. Follow-up MR imaging showed that the abnormal findings of the brain resolved, and the ONS diameter had increased to 5.9 mm on the right and 5.3 mm on the left (Fig 1D).
Case 3
A 52-year-old woman complained of orthostatic headache persisting for 1 month. Brain MR imaging showed diffuse pachymeningeal enhancement, enlargement of the pituitary gland, and bilateral subdural fluid collections. The SAS surrounding the optic nerves could not be detected on MR imaging of the orbit (Fig 1E). Radioisotope cisternography showed CSF leakage at the thoracic spine. Opening pressure at lumbar puncture was 30 mm H2O. Epidural blood patch treatment was performed at the lumbar spine, and her symptom disappeared. Follow-up MR imaging showed that the abnormal findings of the brain had disappeared, and the high signal intensity of CSF in the ONS returned, with a diameter of 6.2 mm on the right and 6.2 mm on the left (Fig 1F).
Discussion
In our case 1, the SAS of the optic nerve had collapsed even at a CSF opening pressure of 100 mm H2O, which can be considered as normal. In our case 2, the SAS was visible, though the CSF pressure was negative. In case 3, the SAS had collapsed with a low CSF opening pressure. Such discrepancies may be due to variations between individual subjects. A normally wide SAS may be visible even under negative intracranial pressure, whereas a narrower SAS may easily disappear with a mild decrease in pressure.
The SAS around the optic nerve can be observed by fat-saturated T2-weighted MR imaging in normal subjects. The ONS diameter measured just behind the optic globe has been reported as 5.52 ± 1.11 mm in normal patient volunteers.2 The diameter did not change significantly with the eyeball position or patient's position.1,8
We measured the SAS just behind the eyeball based on the previous method4 and confirmed widening of the SAS after successful treatment of spontaneous intracranial hypotension in our 3 patients. This change reflects the increased CSF volume, as well as increased intracranial pressure after occlusion of the CSF fistula. Therefore, changes in the diameter of the SAS of the ONS reflect alterations in the CSF pressure or volume. In contrast to the cranial dura mater, the ONS is surrounded by soft tissue of the orbit and may easily expand or collapse with changes in SAS pressure. Furthermore, the ONS is located in the uppermost part of the CSF space of the body in the supine position, in which MR images are usually obtained. Therefore, we speculate that the SAS of the optic nerve may collapse earlier than other parts of the CSF space in response to CSF volume decreases because of the pressure gradient caused by gravity.
The diagnostic value of the ONS diameter measurement may be limited because of individual variations, but absence or narrowing of the SAS strongly suggests decreased CSF volume or pressure. Therefore, such measurement would be valuable in the diagnosis of CSF hypovolemia. We speculate the diagnostic possibility of this finding, especially in patients with no obvious typical MR findings for CSF hypovolemia, such as diffuse pachymeningeal enhancement or brain sagging, because some patients with CSF hypovolemia do not show classic MR findings, except for typical orthostatic headache and low CSF pressure. Further experience is necessary to establish the diagnostic value of fat-saturated MR imaging in the evaluation of patients with suspected CSF hypovolemia.
Acknowledgments
We thank Hiroshi Kumagai and Satoshi Ikenaga for their assistance in collecting MR imaging data.
References
- 1.Lam BL, Glasier CM, Feuer WJ. Subarachnoid fluid of the optic nerve in normal adults. Ophthalmology 1997;104:1629–33 [DOI] [PubMed] [Google Scholar]
- 2.Seitz J, Held P, Strotzer M, et al. Magnetic resonance imaging in patients diagnosed with papilledema: a comparison of 6 different high-resolution T1- and T2(*)-weighted 3-dimensional and 2-dimensional sequences. J Neuroimaging 2002;12:164–71 [DOI] [PubMed] [Google Scholar]
- 3.Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology 1998;105:1686–93 [DOI] [PubMed] [Google Scholar]
- 4.Gass A, Barker GJ, Riordan-Eva P, et al. MRI of the optic nerve in benign intracranial hypertension. Neuroradiology 1996;38:769–73 [DOI] [PubMed] [Google Scholar]
- 5.Imamura Y, Mashima Y, Oshitari K, et al. Detection of dilated subarachnoid space around the optic nerve in patients with papilloedema using T2 weighted fast spin echo imaging. J Neurol Neurosurg Psychiatry 1996;60:108–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farb RI, Forghani R, Lee SK, et al. The venous distension sign: a diagnostic sign of intracranial hypotension at MR imaging of the brain. AJNR Am J Neuroradiol 2007;28:1489–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 (suppl 1):9–160 [DOI] [PubMed] [Google Scholar]
- 8.Romagnuolo L, Tayal V, Tomaszewski C, et al. Optic nerve sheath diameter does not change with patient position. Am J Emerg Med 2005;23:686–88 [DOI] [PubMed] [Google Scholar]