Abstract
BACKGROUND AND PURPOSE: How early spinal cord injury (SCI) lesions evolve in patients after injury is unknown. The purpose of this study was to characterize the early evolution of spinal cord edema and hemorrhage on MR imaging after acute traumatic SCI.
MATERIALS AND METHODS: We performed a retrospective analysis of 48 patients with clinically complete cervical spine injury. Inclusion criteria were the clear documentation of the time of injury and MR imaging before surgical intervention within 72 hours of injury. The length of intramedullary spinal cord edema and hemorrhage was assessed. The correlation between time to imaging and lesion size was determined by multiple regression analysis. Short-interval follow-up MR imaging was also available for a few patients (n = 5), which allowed the direct visualization of changes in spinal cord edema.
RESULTS: MR imaging demonstrated cord edema in 100% of patients and cord hemorrhage in 67% of patients. The mean longitudinal length of cord edema was 10.3 ± 4.0 U, and the mean length of cord hemorrhage was 2.6 ± 2.0 U. Increased time to MR imaging correlated to increased spinal cord edema length (P = .002), even after accounting for the influence of other variables. A difference in time to MR imaging of 1.2 days corresponded to an average increase in cord edema by 1 full vertebral level. Hemorrhage length was not affected by time to imaging (P = .825). A temporal increase in the length of spinal cord edema was confirmed in patients with short-interval follow-up MR imaging (P = .003).
CONCLUSION: Spinal cord edema increases significantly during the early time period after injury, whereas intramedullary hemorrhage is comparatively static.
Acute traumatic spinal cord injury (SCI) is a devastating event with an incidence of approximately 11,000 injuries in the United States each year.1 MR imaging is critical to the assessment of acute cervical SCI because it clearly depicts lesion location, extent, and severity. Spinal cord intramedullary edema and hemorrhage are readily appreciated2,3 and, to some extent, correlate with the clinical neurologic deficit.4–10 Prior studies have also revealed that both the presence of hemorrhage and increased hematoma length at MR imaging are associated with decreased motor recovery.7–10
Following the immediate structural and neurovascular insult, acute SCI sets into motion a cascade of secondary injuries.11,12 Work in animals has shown that intramedullary spinal cord hemorrhage and edema are dynamic, whether assessed by histopathology or MR imaging.13,14
The extent to which cord lesions evolve in patients during the early phase of SCI (ie, the first hours and days postinjury) is unknown; therefore, given similar clinical deficits, it is unclear to what extent variability in lesion size reflects differences in time to imaging after trauma. Similarly, it is not clear whether lesion expansion when observed on a short-interval follow-up MR imaging study is an ominous sign or a usual feature in the natural evolution of SCI. This fundamental lack of knowledge is particularly limiting with regard to research aimed at preventing the secondary injury cascade. With an increasing number of therapies for SCI coming to trial, MR imaging can be expected to continue to be increasingly incorporated into research protocols for these agents. An understanding of the acute evolution of SCI lesions on MR imaging is essential if imaging is to be used effectively in these protocols. Additionally, the variability of SCI lesions with time, if demonstrated, would likely have a significant impact on efforts to correlate spinal cord lesion size and location to the neurologic level of injury, because prior studies have not systematically accounted for differences in time to imaging. Such variability currently limits the reliability of MR imaging to serve as an accurate predictor of the patient's neurologic level and prognosis. This limitation is unfortunate in situations in which the MR imaging findings might be of particular utility, such as in the assessment of the obtunded patients or in patients not undergoing clinical evaluation at a specialized SCI center.
To better understand the evolution of SCI lesions, we retrospectively studied how the time interval between trauma and MR imaging affects spinal cord lesion size in patients with similar neurologic deficits. We also directly measured cord edema changes in a small number of patients for whom short-term MR imaging follow-up was available.
Methods
Study Cohort
Subjects (n = 48) were admitted to a regional SCI center between January 1998 and November 2002. Each patient underwent an acute clinical evaluation according to the International Standards for Neurologic and Functional Classification of Spinal Cord Injury,15 and the American Spinal Injury Association (ASIA) impairment grade and neurologic level of injury were determined. All patients were included in the study if they sustained an isolated cervical SCI from nonpenetrating trauma with a resulting neurologic level of C4, C5, or C6 and an ASIA A impairment score (complete loss of distal motor and sensory function), unless they met 1 of the following exclusion criteria. Subjects were excluded if there was not clear documentation of the time of injury in the medical record. This information was presumed accurate because the time of injury must have been clearly established to make the decision of whether to administer methylprednisolone (MPS), which must be given within 8 hours of injury, according to established protocol.16 To avoid any confounding effect of lack of MPS administration on lesion evolution,17 we excluded subjects if they did not receive MPS or if they did not receive MPS within 8 hours of injury. Subjects were also excluded if they did not have a diagnostic-quality MR imaging evaluation within 72 hours of injury.
MR Imaging
MR imaging was performed before surgical intervention. All images were obtained on the same 1.5T MR imaging unit (excluding upgrades) by using a similar protocol and were read by the same reviewer who was blinded to the interval between injury time and time to MR imaging. The MR imaging pulse sequences used for imaging included sagittal T1-weighted sequences (TR/TE, 600/20), sagittal T2-weighted sequences (fast spin-echo [FSE]: TR/TE/echo-train length, 2000/80/8), as well as gradient-echo sequences in the sagittal (TR/TE/flip angle, 350/20/15°) and axial (TR/TE/flip angle, 45/15/5°) planes.
The longitudinal extents of spinal cord edema and hemorrhage, if present, were retrospectively quantified by using methods defined in prior studies.7 Briefly, each spinal level was divided into 3 parts (arbitrary units) that corresponded to the upper (segment 1) and lower (segment 2) halves of each vertebral body and the intervertebral disk below each vertebral body (segment 3). Thus, 1 spinal level measures 3 U of length. The total length of hemorrhage, which is hypointense on gradient-echo images, and length of edema, which is hyperintense on T2-weighted images, were scored by relating the rostral and caudal boundaries of the spinal cord hemorrhage and edema to the nearest adjacent spinal vertebral landmark. Hemorrhage length was quantified using the gradient-echo images in all cases.
A subset of patients (n = 5) also underwent a short-interval (<5 days) MR imaging follow-up study (Table 1). Reasons for follow-up given in the patients' medical charts included difficult-to-follow neurologic examination (eg, due to patient sedation, delirium, or effort), questioned new neurologic abnormality, or, in 1 patient, transient hemodynamic instability after physical transfer. In no case was there documented ascension of the patient's neurologic level of injury. In the immediate days following the second MR imaging examination, the questioned abnormalities either resolved or were attributed to other causes (including medications and supratentorial infarction). According to clinical imaging protocols, postoperative studies did not include a gradient-echo imaging sequence, due to artifacts generated from metallic hardware. Thus, although the length of cord edema was quantified on follow-up studies and was directly comparable with the first MR image, direct follow-up comparisons of hemorrhage size were not possible.
Table 1:
Length of cord edema in patients with short-interval follow-up MR imaging
| Patient | Age | Time (h) |
Edema Length (U) |
Change in Length* | Intervening Procedure | ||
|---|---|---|---|---|---|---|---|
| Injury to MRI 1 | MRI 1 to MRI 2 | MRI 1 | MRI 2 | ||||
| 1 | 46 | 23.5 | 14 | 12 | 14 | +2 | Closed reduction |
| 2 | 83 | 8.25 | 40 | 12 | 16 | +4 | Closed reduction |
| 3 | 67 | 9.5 | 55 | 6 | 12 | +6 | Open reduction |
| 4 | 58 | 37.5 | 60 | 10 | 15 | +5 | None |
| 5 | 84 | 18.75 | 110 | 5 | 10 | +5 | Open reduction |
Paired t test demonstrates a significant increase (t = −6.5, P = .003) in edema length. Mean change in edema length was 4.4 U (95% CI, 2.5–6.3).
Statistical Analysis
To determine the relationship between the time interval between injury and MR imaging to the size of spinal cord lesions observed on MR imaging, we performed multiple regression analyses. Edema length and hemorrhage length were the dependent variables. Time to MR imaging was the independent variable. To control for factors that may have a significant effect on cord edema and hemorrhage independent of time to MR imaging, we performed multiple regression analysis initially by using variables such as age at injury, level of injury, and sex, but without the inclusion of time to MR imaging as a regressor. Level of injury had no significant effect in the initial analysis (P = .11) and, therefore, was not included in the final regression. Variables with a suggested significant effect, which were age and sex, were included in the final regression analyses, which also included the variable of interest, time to MR imaging, as a regressor. In patients who had a short-interval follow-up MR imaging study, a paired t test was used to evaluate for significant differences in edema length between the 2 MR imaging studies. Tests of statistical significance and the calculation of correlation coefficients were performed using Systat (Systat Software, Point Richmond, Calif).
Results
The time interval between injury and MR imaging (time to MR imaging) ranged from 2.5 to 66.25 hours, with a mean time of 18.8 ± 14.9 hours. These findings and other demographic/clinical data are summarized in Table 2.
Table 2:
Demographic and clinical characteristics of 48 ASIA A patients
| Characteristic | |
|---|---|
| Mean age (yr) | 47.1 ± 21.7 |
| Sex | |
| Male | 39 |
| Female | 9 |
| Neurologic level of injury | |
| C4 | 22 |
| C5 | 17 |
| C6 | 9 |
| Time from injury to MR imaging (h) | |
| Range | 2.5–66.25 |
| Mean | 18.8 ± 14.9 |
All 48 subjects were found to have spinal cord edema at MR imaging (Table 3). To examine the relationship of time to MR imaging to spinal cord edema length, we performed multiple regression analysis. This analysis demonstrated a correlation between increased time to MR imaging and increased cord edema length (R2 = 32%, F3,44 = 6.9, P = .002; Table 4). The magnitude of the effect of time to MR imaging on edema length is large, in that the regression coefficient for time to MR imaging is 0.108 (95% confidence interval [CI], 0.04–0.176). Stated differently, during the acute timeframe in this severely injured cohort, spinal cord edema length is calculated to increase by an average of 0.108 U of length per hour; given that there are 3 U per vertebral level, this rate corresponds to a 1-vertebral-level increase in edema for every additional 1.2 days between injury and MR imaging.
Table 3:
Spinal cord lesion characteristics on MR imaging
| Characteristic | |
|---|---|
| Patients with spinal cord | |
| Edema | 48/48 (100%) |
| Hemorrhage | 32/48 (67%) |
| Mean length | |
| Cord edema (units) | 10.3 ± 4.0 |
| Cord hemorrhage (units) | 2.6 ± 2.0 |
Table 4:
Results of multiple regression analysis to examine the effect of time to imaging on edema length
| Variable | Coefficient | Standard Error* | P value |
|---|---|---|---|
| Intercept | 10.9 | 1.4 | .001 |
| Time to MR imaging | 0.108 | 0.034 | .002 |
| Age | −0.065 | 0.023 | .008 |
| Sex | 2.5 | 1.3 | .06 |
Standard error of the estimated coefficient.
Because the size of spinal cord lesions is known to be affected by a number of clinical factors such as age,9,17 other available clinical factors were included in the regression analysis described previously to control for their potentially confounding effects. This analysis confirmed a significant effect of age on spinal cord edema. The regression coefficient for age is −0.065 (P = .008, 95% CI, −0.019 to −0.111). Thus, in this cohort, an additional 10 years of patient age corresponds to a decrease in spinal cord edema of 0.65 U, or approximately one fifth (21.7%) of a vertebral level. The effect of sex on cord edema was not significant in the final analysis, but there remained a trend (P = .06; regression coefficient, 2.5; 95% CI, 0.1–4.9) toward significance, with women exhibiting greater overall lengths of spinal cord edema. However, the number of women included in the study was comparatively small (9 of 48 patients).
Intramedullary spinal cord hemorrhage was demonstrated in 67% of patients. In contrast to the results for edema length, a relationship between time to MR imaging and spinal cord hemorrhage was not demonstrated by multiple regression analysis (P = .825). In the exploratory analysis, there was a trend toward decreased hemorrhage with increasing age (P = .13). Other studies have reported a significant effect of age on hemorrhage length.9,17
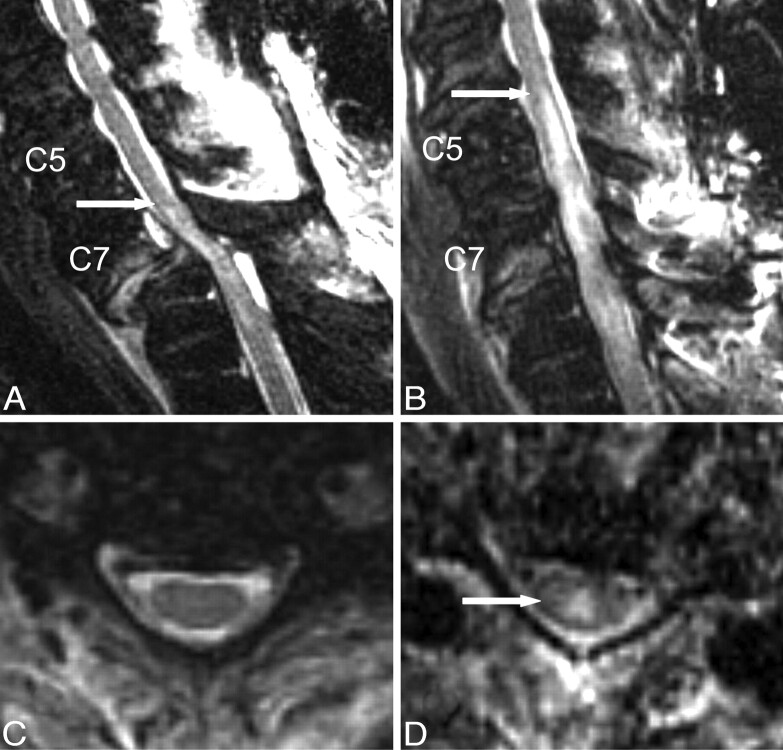
In 5 of the patients, a short-interval follow-up MR image was available (Table 1). All of these patients had undergone a follow-up study within 5 days, with an average time between MR imaging studies of 2.3 days. In the intervening time, 2 patients underwent open surgical reduction, 2 patients underwent closed reduction, and 1 patient had no intervention. As described in “Methods,” the reasons given for follow-up MR imaging were variable, but in no case was there an ascension of the neurologic level of injury. An example of a follow-up scan is shown in Fig 1.
Fig 1.
Short-interval follow-up cervical MR imaging of a 67-year-old male ASIA A patient with SCI. T2-weighted FSE images were obtained from an initial MR imaging examination performed 9.5 hours after injury (A and C), as well as from a follow-up postreduction examination performed 55 hours after the first MR imaging (B and D). Demonstrated on the sagittal images (A and B) is a large increase in T2-hyperintense spinal cord edema (arrows demonstrate cranial-most extent), whereas representative axial images from the C5/C6 interspace (C and D) confirm the absence of cord edema at that level on the initial study, with the development of cord edema (arrow) on the second study.
In all 5 patients, an increase in cord edema was observed between the 2 MR imaging examinations, which was statistically significant (t = −6.5, P = .003). The average increase in cord edema between studies was 4.4 U. Given 3 U per level, the average rate of increase was 1 vertebral level every 1.6 days. Thus, the results parallel the increase in edema predicted from the prior correlation of time to imaging with edema length. The magnitude of the increase was less than that calculated in the multiple regression analysis (ie, less than 1 vertebral level per 1.2 days). However, this diminished rate is not surprising, given that the multiple regression analysis was restricted to studies performed within 72 hours of injury, whereas in some of the follow-up patients, the second MR imaging examination was performed outside of the 72 hour timeframe. Animal data13 have shown that increases in edema eventually plateau.
Discussion
MR imaging is rapidly performed in patients presenting with spinal trauma and a concomitant neurologic deficit because of its unparalleled ability to depict spinal cord hemorrhage and edema. Although most patients with SCI are imaged early after injury, no study to date has examined the relationship between time to imaging and lesion extent on MR imaging in the acute setting. Likewise, prior studies relating neurologic status to MR imaging findings have not accounted for the potential effects of time to MR imaging, despite the fact that animal studies clearly show that early SCI lesions are dynamic.13,14 Because most patients are imaged early, an understanding of the early evolution of SCI lesions would be important in the initial diagnosis of SCI, both for the accurate determination of functional prognosis and for the performance of clinical research, particularly with regard to studies designed to test novel therapies that use MR imaging findings as selection criteria or as surrogate outcome measures.
Animal studies have correlated the histopathology of acute SCI with MR imaging findings. Weirich et al13 studied rats at time points ranging from 2 hours to 1 week postinjury. The study describes an initial very early pattern (t = 2 hours) of scattered focal gray matter hemorrhages, which rapidly evolve to diffuse hemorrhage throughout the white and gray matter, maximum at 12 hours. The acute spinal cord hemorrhage had specific signal-intensity characteristics, even at the earliest time point (t = 2 hours) appearing T2 hypointense. In contrast, T2-hyperintense signal intensity correlated to areas of edema formation, white matter myelin degeneration surrounding the hemorrhage, and necrotic and inflammatory changes. Although it was not quantified, the investigators reported that surrounding white matter edema became most severe by 48–72 hours after injury in lower severity contusions, with little additional change in sections obtained at 72 and 96 hours. Most interesting, in more severe contusions, the necrotic and infiltrative changes continued to progress in sections obtained at 72 and 96 hours, suggesting that the time course of lesion evolution is dependent on injury severity.
For a serial in vivo assessment of the evolution of cord signal-intensity changes, MR imaging has also been used to repeatedly scan the same animal. Bilgen et al14 studied hemorrhage in the very early phase (9–400 minutes postcontusion) in rats and reported that the volume of hemorrhage increased linearly with time at a rate of approximately 0.15% per minute, relative to the initial volume. In the same study, edema was observed at the gray matter/white matter junction as early as 12 minutes postinjury but was initially “scattered and focal,” making quantification difficult at early time points. Edema was readily apparent in both the gray matter and white matter compartments within 3 hours. Taken together, these animal studies suggest that hemorrhage, represented by T2 hypointensity, expands rapidly in the very early phase but plateaus comparatively early. Edema initially appears patchy but gradually becomes more extensive and confluent as the secondary injury cascade continues, expanding for a greater length of time than hemorrhage.
The results of the present study parallel prior animal studies. Using multiple regression analysis in a cohort of clinically complete spinal cord injuries (ASIA A), we found a significant correlation between time to MR imaging and the magnitude of spinal cord edema. The correlation was then confirmed in a subset of patients for whom short-term follow-up imaging was available. By contrast, no significant correlation was found between time to MR imaging and hemorrhage length. However, the detection of changes might have been limited by the small number of patients imaged within the very early phase (<6 hours), when changes in hematoma size would most likely be observed. Only 4 patients in this study were imaged within the first 6 hours after injury. Expansion of hematoma size with increased time to MR imaging might have been discernable if a larger number of patients were imaged immediately after injury. It is also possible that emergent MPS administration, which all study subjects received, may have played a role in limiting hemorrhage in the acute phase.17–20
The finding of dynamic cord edema on MR imaging has implications for its correlation with clinical neurologic status. Although an inverse relationship between the severity of the neurologic deficit and edema length has been reported, the relationship is less robust than is the association with hemorrhage.7–10 Additionally, the association of spinal cord edema location with the neurologic level of injury remains imprecise. However, the few available studies that have specifically differentiated between acute and subacute timeframes in their inclusion criteria did not factor the time interval from injury to MR imaging into their data analysis.7,9 However, on the basis of the current evidence, one can speculate that if time to MR imaging was taken into account, spinal cord edema would exhibit an improved association with neurologic level of injury, grade of functional motor and/or sensory impairment, and prognosis. Such associations, if demonstrated, would be of significant interest, in light of the fact that in many patients with lower grade SCI, edema is the only cord pathology seen on MR imaging (ie, no macroscopically detectable hemorrhage is present).
Besides time to MR imaging, other factors have an effect on edema length. Patient age was found to have an inverse correlation to cord edema length. Prior studies have also demonstrated a significant correlation between increased patient age and decreased spinal cord edema17 and hemorrhage.9,17 A correlation of female sex with increased edema was suggested in the exploratory analysis, though the trend did not reach statistical significance (P = .06) in the final analysis. Recent articles on the sex effects on clinical and neurologic outcomes after SCI suggested no difference21 or slight differences22 between sexes. However, it remains possible that for a given neurologic deficit, women may exhibit comparatively more edema than men, perhaps on the basis of injury pattern or hormonal/neurovascular differences. Given that only 9 of the 48 patients in the study were women, this suggestion would obviously require further investigation.
Consistent with the results of animal studies,13,14 we did not observe the presence of T2 hyperintense oxyhemoglobin even in the patients imaged most rapidly after injury (n = 4 patients imaged at <6 hours, the earliest imaged at t = 2.5 hours). Bilgen et al14 studied animals 9–400 minutes postinjury and reported that hemorrhage appears hypointense on T2-weighted images, even at the earliest times. Thus, the rate at which hematoma signal-intensity characteristics evolve in the spinal cord appears to differ significantly from that of parenchymal hemorrhage in the brain, which is classically characterized by a predictable and evolving MR imaging signal-intensity pattern for hours and days that corresponds to the progression of oxyhemoglobin to deoxyhemoglobin to methemoglobin.23 Caution is, therefore, indicated when applying observations in the brain to SCI hemorrhage. We observed that the predominant species of hemorrhage during the very early phase of SCI is deoxyhemoglobin rather than oxyhemoglobin, most likely due to the local hypoxic state of the injured segment.
There are limitations to the study. Although large-magnitude statistically significant increases in cord edema were predicted by using retrospective multiple regression analysis and directly confirmed in a small number of patients with short-interval follow-up imaging, the study was retrospective. Future prospective studies using a large number of subjects and serial imaging are needed to confirm retrospective findings and improve modeling of the behavior of spinal cord lesions postinjury. Because lesion size in experimental studies13 has been shown to depend on injury severity, an attempt was made to normalize injury severity by including only ASIA A patients. However, injury severity within this group will still vary. Additionally, because only patients with clinically complete cervical injuries were studied, it is unclear if patients with lower grades of injury severity also exhibit changes in spinal cord edema with time. As reviewed previously, there are data from animal work13 that suggest that the timing of MR imaging signal-intensity changes varies with injury severity.
MR imaging in the setting of traumatic SCI currently provides an invaluable and rapid assessment of cord pathology, augments the clinical examination (particularly in patients who cannot fully comply with a neurologic examination), and helps predict prognosis for functional recovery. Here we demonstrate that spinal cord edema increases significantly during the early time period after injury, whereas intramedullary hemorrhage is comparatively static. Because most patients are imaged rapidly after injury, understanding how cord lesions evolve during the early phase should assist in efforts to refine the correlation of neurologic findings with those on MR imaging, as well as to help better guide the use of MR imaging in clinical trials to assess novel therapies.
References
- 1.Spinal Cord Injury Information Network. Information and statistics about SCI. Available at: www.spinalcord.uab.edu. Accessed November 15,2006
- 2.Hackney DB, Asato R, Joseph PM, et al. Hemorrhage and edema in acute spinal cord compression: demonstration by MR imaging. Radiology 1986;161:387–90 [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni MV, McArdle CB, Kopanicky D, et al. Acute spinal cord injury: MR imaging at 1.5 T. Radiology 1987;164:837–43 [DOI] [PubMed] [Google Scholar]
- 4.Schaefer DM, Flanders A, Northrup BE, et al. Magnetic resonance imaging of acute cervical spine trauma: correlation with severity of neurologic injury. Spine 1989;14:1090–95 [DOI] [PubMed] [Google Scholar]
- 5.Bondurant FJ, Cotler HB, Kulkarni MV, et al. Acute spinal cord injury: a study using physical examination and magnetic resonance imaging. Spine 1990;15:161–68 [PubMed] [Google Scholar]
- 6.Flanders AE, Schaefer DM, Doan HT, et al. Acute cervical spine trauma: correlation of MR imaging findings with degree of neurologic deficit. Radiology 1990;177:25–33 [DOI] [PubMed] [Google Scholar]
- 7.Flanders AE, Spettell CM, Tartaglino LM, et al. Forecasting motor recovery after cervical spinal cord injury: value of MR imaging. Radiology 1996;201:649–55 [DOI] [PubMed] [Google Scholar]
- 8.Flanders AE, Spettell CM, Friedman DP, et al. The relationship between the functional abilities of patients with cervical spinal cord injury and the severity of damage revealed by MR imaging. AJNR Am J Neuroradiol 1999;20:926–34 [PMC free article] [PubMed] [Google Scholar]
- 9.Boldin C, Raith J, Fankhauser F, et al. Predicting neurologic recovery in cervical spinal cord injury with postoperative MR imaging. Spine 2006;31:554–59 [DOI] [PubMed] [Google Scholar]
- 10.Miyanji F, Furlan JC, Aarabi B, et al. Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome—prospective study with 100 consecutive patients. Radiology 2007;243:820–27 [DOI] [PubMed] [Google Scholar]
- 11.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 1991;75:15–26 [DOI] [PubMed] [Google Scholar]
- 12.Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery 1999;44:1027–39 [DOI] [PubMed] [Google Scholar]
- 13.Weirich SD, Cotler HB, Narayana PA, et al. Histopathologic correlation of magnetic resonance imaging signal patterns in a spinal cord injury model. Spine 1990;15:630–38 [DOI] [PubMed] [Google Scholar]
- 14.Bilgen M, Abbe R, Liu SJ, et al. Spatial and temporal evolution of hemorrhage in the hyperacute phase of experimental spinal cord injury: in vivo magnetic resonance imaging. Magn Reson Med. 2000;43:594–600 [DOI] [PubMed] [Google Scholar]
- 15.American Spinal Injury Association. International Standards for Neurological Classification for Spinal Cord Injury. Chicago: American Spinal Injury Association;2002
- 16.Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial—National Acute Spinal Cord Injury Study. JAMA 1997;277:1597–604 [PubMed] [Google Scholar]
- 17.Leypold BG, Flanders AE, Schwartz ED, et al. The impact of methylprednisolone on lesion severity following spinal cord injury. Spine 2007;32:373–78 [DOI] [PubMed] [Google Scholar]
- 18.Taoka Y, Okajima K, Uchiba M, et al. Methylprednisolone reduces spinal cord injury in rats without affecting tumor necrosis factor-alpha production. J Neurotrauma 2001;18:533–43 [DOI] [PubMed] [Google Scholar]
- 19.Means ED, Anderson DK, Waters TR, et al. Effect of methylprednisolone in compression trauma to the feline spinal cord. J Neurosurg 1981;55:200–08 [DOI] [PubMed] [Google Scholar]
- 20.Campbell JB, DeCrescito V, Tomasula JJ, et al. Effects of antifibrinolytic and steroid therapy on the contused spinal cord of cats. J Neurosurg 1974;40:726–33 [DOI] [PubMed] [Google Scholar]
- 21.Furlan JC, Krassioukov AV, Fehlings MG. The effects of gender on clinical and neurological outcomes after acute cervical spinal cord injury. J Neurotrauma 2005;22:368–81 [DOI] [PubMed] [Google Scholar]
- 22.Sipski ML, Jackson AB, Gomez-Marin O, et al. Effects of gender on neurologic and functional recovery after spinal cord injury. Arch Phys Med Rehabil 2004;85:1826–36 [DOI] [PubMed] [Google Scholar]
- 23.Gomori JM, Grossman RI, Goldberg HI, et al. Intracranial hematomas: imaging by high-field MR. Radiology 1985;157:87–93 [DOI] [PubMed] [Google Scholar]