Abstract
BACKGROUND AND PURPOSE: In some patients with nonperimesencephalic nontraumatic subarachnoid hemorrhage (aneurysmal SAH), no aneurysm can be found on digital subtraction angiography (DSA), and repeat DSA is advocated. 3D rotational angiography (3DRA) is considered superior to DSA in the detection of small intracranial aneurysms. In this study, we assessed the additional diagnostic value of 3DRA in detecting DSA-occult aneurysms in 23 patients with aneurysmal SAH.
MATERIALS AND METHODS: Between January 2006 and September 2007, 298 patients with suggested ruptured intracranial aneurysm were referred for DSA, and in 98 patients, DSA was negative. Of these 98 patients, 28 had aneurysmal SAH, and in 23 of these additional 3DRA was performed in the same or in a repeat angiographic procedure.
RESULTS: In 18 of 23 patients (78%), a ruptured small aneurysm was diagnosed on additional 3DRA. The location of 18 aneurysms was the anterior communicating artery (n = 11), the middle cerebral artery (n = 3), the posterior communicating artery (n = 2), the ophthalmic artery (n = 1), and the posterior inferior cerebellar artery (n = 1). Aneurysm size was 3 mm in 4, 2 mm in 9, and 1 mm in 5. Of 18 aneurysms, 9 were treated with coil placement; 7 with surgical clipping; and 2 were not treated.
CONCLUSION: In this study, 18 of 23 (78%) patients with negative findings on DSA had a small ruptured aneurysm when studied with 3DRA. These were most commonly located on the anterior communicating artery.
In 15%–20% of patients with a spontaneous subarachnoid hemorrhage (SAH), no aneurysm is found on the first digital subtraction angiography (DSA).1 In two thirds of these patients, CT shows a perimesencephalic pattern of hemorrhage (blood confined to the cisterns around the midbrain); these patients invariably have a good prognosis, which obviates additional angiography.2-4 Patients with a nonperimesencephalic pattern of hemorrhage on CT (aneurysmal SAH) are at risk of rebleeding. In most of these patients, the source of the hemorrhage is an occult aneurysm, but intracranial artery dissections, dural arteriovenous malformations, trauma, bleeding disorders, substance abuse, or other causes should also be considered.3 Repeat DSA5-7 or even exploratory surgery8,9 is generally advocated to detect an angiographically occult ruptured aneurysm, and in approximately one third of patients, an aneurysm is eventually detected during surgery or on the second or third DSA. In other studies,10,11 repeat angiography is considered justified only when the initial examination is technically inadequate, when vasospasm is present, or if further bleeding occurs. In an estimated 4%–5% of patients with aneurysmal SAH, no source of hemorrhage can be identified. These patients are at risk of vascular complications and poor outcome.4
Since its introduction more than a decade ago, 3D rotational angiography (3DRA) is considered superior to DSA both in the detection of intracranial aneurysms and in pretreatment evaluation.12-14 3DRA has several advantages over DSA: the possibility of free rotation of images, the lack of overprojecting bony structures, and extensive postprocessing capabilities that allow better detection of small aneurysms and better evaluation of local anatomy. To date, no studies are available using 3DRA in the detection of small ruptured aneurysms in patients with DSA-negative aneurysmal SAH. In this study, we assessed the additional diagnostic value of 3DRA performed in 23 patients with angiographically negative aneurysmal SAH.
Patients and Methods
This retrospective study was compliant with institutional privacy policy. The institutional review board gave exempt status for approval and informed consent.
In our tertiary referral center, in all patients with suggested ruptured intracranial aneurysms, native CT (without CTA) was immediately followed by 3- or 4-vessel cerebral DSA. In intubated patients in poor clinical condition, DSA was performed with the patients under deep sedation or general anesthesia. In drowsy and uncooperative patients, we preferably performed DSA with the patients under general anesthesia, but this was not always available. When an intracranial aneurysm was apparent or suggested on DSA in 2 or 4 projections, additional 3DRA was performed of the vessel harboring the aneurysm to confirm or refute its presence and to evaluate local anatomy for treatment planning.
In general, in patients with aneurysmal SAH on CT and negative DSA including the external carotid arteries, additional 3DRA was performed to detect a possible DSA-occult aneurysm. 3DRA acquisition requires patient cooperation because patient motion during the 8-second rotational run degrades image quality. If adequate 3D imaging could not be performed in the first DSA because of insufficient patient cooperation, DSA was later repeated with additional 3DRA with the patient under deep sedation or general anesthesia. Vessel selection for 3DRA was dependent on blood distribution on CT. When blood was evenly distributed throughout the basal cisterns, 3DRA was started with the carotid artery filling both A2s, as was apparent on the previous 2D DSA, to evaluate the anterior communicating artery complex. When a maximal amount of blood was present in a Sylvian fissure, 3DRA was started with the ipsilateral carotid artery. In the presence of additional hematomas, 3DRA was started with the vessel supplying the territory of the hematoma. In most patients, 3DRA of 1 or 2 vessels was sufficient; only in exceptional cases and in patients in whom no aneurysm was found was 3DRA of 3 vessels needed.
In patients with clinically suggested SAH, negative CT, xanthochromic spinal fluid, and negative findings on DSA, additional 2D or 3D angiographic imaging was generally not performed. Also in patients with intraventricular, intraparenchymal, or subdural hemorrhage only and in patients with trauma and SAH, no additional angiography was performed.
Angiography
Angiography was performed on a biplane neuroangiographic unit (Integris BN 3000 Neuro; Philips Medical Systems, Best, the Netherlands). DSA was performed with a 1024 × 1024 matrix with a 17- to 20-cm FOV and injection of 8–10 mL of contrast material in the internal carotid and vertebral arteries in 2 or 4 projections. After postprocessing when needed, relevant images were sent to the PACS. 3DRA was performed with an 8-second 180° rotational run with acquisition of 200 images and with injection of 3–4 mL of contrast material per second in the internal carotid or vertebral artery. On a dedicated workstation, 3D reconstructions were made in a matrix of 1283–5123. Relevant images were sent to a PACS. All raw 3D datasets were stored on the hard disk of the workstation and on a compact disk. Raw datasets stored on compact disks can be reloaded in the workstation for real-time evaluation and new high-resolution reconstructions.
Patient Selection
To determine whether additional 3DRA detected DSA-occult intracranial aneurysms in patients with aneurysmal SAH, we reviewed our PACS data base from January 2006 to September 2007. In this time period, 298 patients with suggested ruptured intracranial aneurysm were referred for cerebral DSA (Fig 1). All patients underwent complete 3- or 4-vessel DSA if indicated, including the external carotid arteries. Of 298 patients, 196 had at least 1 aneurysm visible on DSA, and 4 patients had an intradural vertebral dissection. Of these 200, 166 were treated with coils; 26 with surgical clipping; and 8 were not treated.
Fig 1.
Flow chart of 298 patients with suggested ruptured intracranial aneurysm referred for cerebral angiography. Asterisk indicates no additional imaging because of advanced age in 4 and death soon after admission in 1. PMH indicates perimesencephalic hemorrhage; IPH, intraparenchymal hemorrhage; IVH, intraventricular hemorrhage; SDH, subdural hemorrhage.
Of the 298 initial patients with suggested ruptured intracranial aneurysm, 98 had negative findings on DSA. In 75 of these 98 patients, no additional angiography was performed, in 70 of 75 patients because of low clinical suggestion of the presence of a ruptured aneurysm (perimesencephalic hemorrhage pattern on CT in 24, xanthochromic CSF in 30, intraventricular hemorrhage in 4, intraparenchymal hemorrhage in 8, subdural hematoma in 1, and traumatic subarachnoid hemorrhage in 3). In 5 of 75 patients with aneurysmal SAH, additional 3DRA was not performed because of advanced age in 4 and death soon after admission in 1.
The remaining 23 patients with aneurysmal SAH, initial negative findings on DSA, and additional 3D angiography were the subjects of this study.
Results
Of 23 selected patients, 10 were men and 13 were women with a mean age of 57.6 years (median, 54 years; range, 30–76 years). Besides the presence of subarachnoid blood, 6 patients had intraventricular blood and 4 other patients had an intraparenchymal hematoma. Clinical grading on admission was Hunt and Hess (HH) grade I–II in 8, HH III in 10, and HH IV–V in 5. Two patients had a recurrent hemorrhage in the interval between the first and second angiograms.
Additional angiography consisted of 3D angiography of 1–3 vessels in all patients, for a total of 32 vessels in 12 patients during initial DSA and in 11 patients during repeat DSA, depending on patient cooperation or availability of general anesthesia at the first DSA. On these additional 3DRA studies, in 18 of 23 patients, a ruptured aneurysm was diagnosed (Figs 2 and 3). The location of 18 aneurysms was the anterior communicating artery (n = 11), the middle cerebral artery (n =3), the posterior communicating artery (n = 2), the ophthalmic artery (n = 1), and the posterior inferior cerebellar artery (n = 1). Aneurysm size was 3 mm in 4, 2 mm in 9, and 1 mm in 5. Of 18 aneurysms, 9 were treated with coil placement; 7 with surgical clipping; and 2 were not treated.
Fig 2.
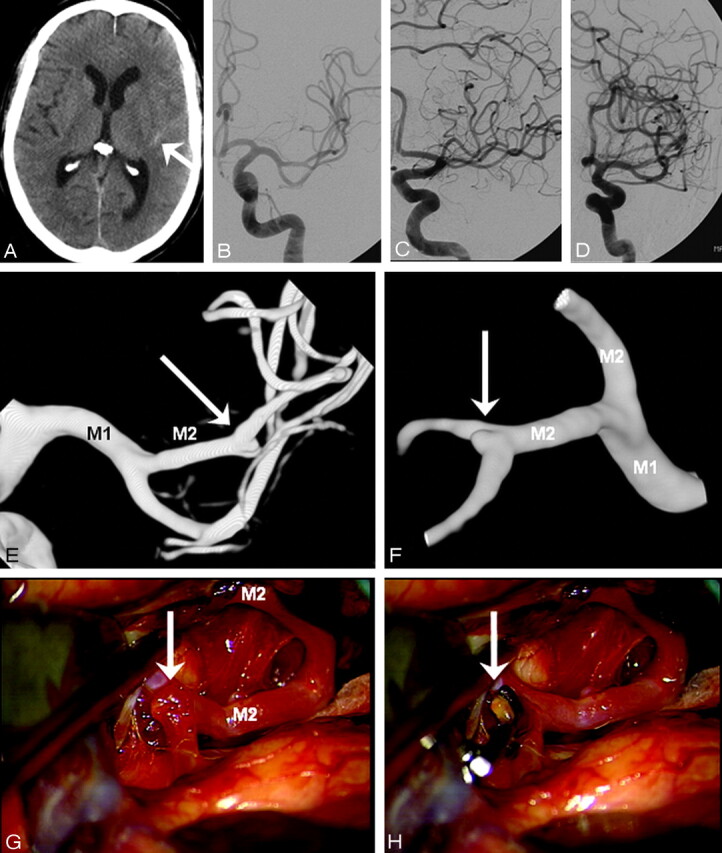
A 48-year-old man presenting 4 days after headache of sudden onset in good clinical condition. A, CT scan shows a small amount of blood in the left Sylvian fissure (arrow). B–D, DSA in 3 projections fails to show a middle cerebral artery aneurysm. E and F, 3DRA depicts a 1-mm aneurysm on a M2-M3 junction (arrow). G and H, Operative view (compare with F) before (G) and after (H) clipping. Blood remnants are proof of rupture of the small aneurysm.
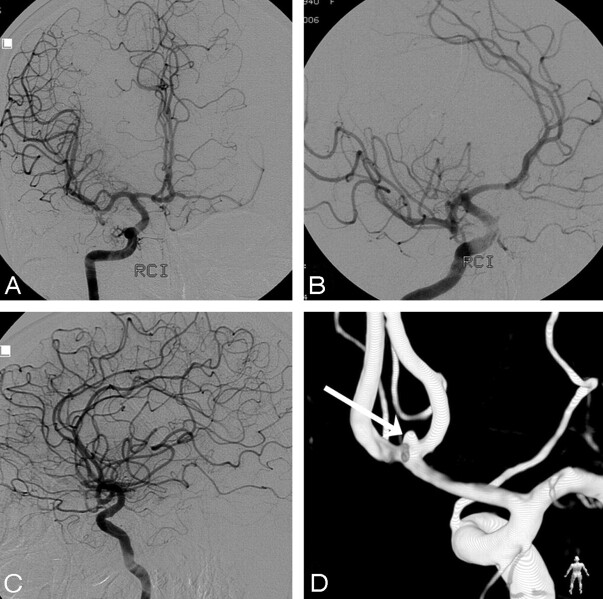
Fig 3.
A 67-year-old woman admitted with grade IV SAH, comatose and ventilated. CT scan showed diffuse subarachnoid blood. A–C, Right internal carotid artery angiogram in 3 projections fails to demonstrate an aneurysm. Note filling of both A2s and A1s. D, Posterior view of 3DRA depicts a 1.6-mm anterior communicating artery aneurysm (arrow). This aneurysm was clipped 1 week later.
Discussion
In this study, we found that additional 3D angiography on initial or repeat angiography detected a DSA-occult ruptured intracranial aneurysm in 18 of 23 patients (78%) with aneurysmal SAH, and in 16 of 18 patients, this aneurysm was subsequently treated. Most DSA-occult aneurysms (11 of 18) were located on the anterior communicating artery.
Comparison of our findings with diagnostic yield of studies using repeat DSA without 3DRA is hampered by differences in patient selection and the definition of an angiographically negative SAH. In earlier studies, perimesencephalic and aneurysmal SAH were not considered separate entities, leading to a lower proportion of false-negative initial angiograms: Du Mesnil de Rochemont et al11 collected data from 10 studies between 1978 and 1997 comprising 430 patients with angiographically negative SAH, and in 38 (8.8%) of these patients, repeat angiography revealed an aneurysm. In more recent studies, a distinction was made between perimesencephalic and aneurysmal SAH. In a study by Topcuoglu et al7 comprising 770 patients with aneurysmal SAH, in 36 of 41 patients, angiography was repeated and revealed aneurysms in 4 (11.1%). In a previous study from our institution,6 angiography was repeated in 25 of 36 patients with aneurysmal SAH and negative findings on initial angiograms, and repeat studies demonstrated aneurysms in 9 (36%). In a study by Jung et al,15 17 of 37 (46%) initial findings on angiograms in patients with aneurysmal SAH proved to be false-negative on repeat angiography. In only 1 study was the additional value of 3DRA in patients with angiographically negative aneurysmal SAH evaluated: Ishihara et al16 compared 2 historical groups of patients with angiographically negative SAH, 1 before and 1 after the introduction of 3DRA. The incidence of angiographically negative SAH was 9/105 (8.6%) in the DSA-only group and 6/142 (4.2%) in the 3DRA group.
These figures, in combination with other studies that assessed the additional value of 3DRA in the general detection of aneurysms,12-14 indicate that 3DRA probably depicts more DSA-occult ruptured aneurysms than repeat DSA only. Another advantage of using 3DRA as an additional imaging technique in patients with angiographically negative SAH is the possibility of a 3D acquisition in the same session as the first DSA in selected cooperative patients, thus obviating a second angiographic procedure. If a DSA-occult aneurysm is detected, therapy can be immediately instigated, and the risk of recurrent hemorrhage is reduced. In uncooperative patients, a second procedure cannot always be avoided because additional 3DRA requires general anesthesia or deep sedation to ensure no patient motion during the acquisition of the 8-second rotational run. In most cases, the acquisition of 3DRA can be limited to 1 or 2 vessels, depending on the distribution of subarachnoid blood on CT. For example, when a maximal amount of blood is present in a Sylvian fissure, 3DRA of the ipsilateral carotid artery is sufficient to confirm or exclude a middle cerebral artery aneurysm (Fig 2). When blood is evenly distributed in the basal cisterns, this may be indicative of an anterior communicating artery aneurysm, and 3DRA may be restricted to the 1 carotid artery filling both A2s, as is apparent on DSA (Fig 3). For the most part, 3DRA of 3 or even 4 vessels is needed only in cases in which no aneurysm will be found.
Our findings indicate that in most patients with angiographically negative aneurysmal SAH, a small ruptured treatable aneurysm is present, and this can be demonstrated by 3DRA. The advantage of 3DRA over DSA is obvious: free rotation of high-resolution images in any projection without overprojecting bony structures. Complicated anatomy such as in the anterior communicating artery complex can be unraveled easily, and small aneurysms are depicted readily.17 With 2D imaging of DSA, a small aneurysm may be obscured by overprojecting adjacent vessels in the limited number of available projections.
In our institution, CTA is not performed in patients with suggested ruptured intracranial aneurysms. In our opinion, supported by others,18 CTA is redundant because angiography should follow both negative and positive findings on CTA, either to detect a CTA-occult aneurysm or for triage between endovascular and surgical therapy. In comparative studies of CTA and DSA, aneurysms of 3 mm and smaller are commonly missed with CTA.19,20 In a recent study,21 it was shown that 3DRA depicts considerably more small additional aneurysms than DSA, which suggests that 3DRA should be the gold standard for detection of aneurysms instead of DSA. This gives further evidence that CTA has no value for the depiction of very small DSA-occult ruptured aneurysms.
A limitation of our retrospective study is that we defined angiographically negative DSA on the basis of the radiology report. Acquisition of additional 3DRA during the first angiography to detect a possible occult aneurysm was decided by the performing radiologist. We did not systematically re-evaluate these DSAs. However, in patients with repeat angiographic procedures performed with the patients under general anesthesia or deep sedation, indication was assessed after a review of all DSA images by at least 2 neuroradiologists. Another limitation is that we do not know how many initially missed aneurysms would have been detected if only DSA (without 3DRA) had been repeated. Therefore, assessment of the additional value of 3DRA over repeat DSA remains to some extent speculative.
Conclusion
In most patients with angiographically negative aneurysmal SAH, additional 3DRA detects a small treatable ruptured DSA-occult aneurysm, mostly located on the anterior communicating artery.
References
- 1.Rinkel GJ, van Gijn J, Wijdicks EF. Subarachnoid hemorrhage without detectable aneurysm: a review of the causes. Stroke 1993;24:1403–09 [DOI] [PubMed] [Google Scholar]
- 2.Rinkel GJ, Wijdicks EF, Hasan D, et al. Outcome in patients with subarachnoid haemorrhage and negative angiography according to pattern of haemorrhage on computed tomography. Lancet 1991;338:964–68 [DOI] [PubMed] [Google Scholar]
- 3.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;369:306–18 [DOI] [PubMed] [Google Scholar]
- 4.Ruigrok YM, Rinkel GJ, Van Gijn J. CT patterns and long-term outcome in patients with an aneurysmal type of subarachnoid hemorrhage and repeatedly negative angiograms. Cerebrovasc Dis 2002;14:221–27 [DOI] [PubMed] [Google Scholar]
- 5.Inamasu J, Nakamura Y, Saito R, et al. “Occult” ruptured cerebral aneurysms revealed by repeat angiography: result from a large retrospective study. Clin Neurol Neurosurg 2003;106:33–37 [DOI] [PubMed] [Google Scholar]
- 6.Houben MP, van Rooij WJ, Sluzewski M, et al. Subarachnoid hemorrhage without aneurysm on the angiogram: the value of repeat angiography [in Dutch]. Ned Tijdschr Geneeskd 2002;146:804–08 [PubMed] [Google Scholar]
- 7.Topcuoglu MA, Ogilvy CS, Carter BS, et al. Subarachnoid hemorrhage without evident cause on initial angiography studies: diagnostic yield of subsequent angiography and other neuroimaging tests. J Neurosurg 2003;98:1235–40 [DOI] [PubMed] [Google Scholar]
- 8.Jafar JJ, Weiner HL. Surgery for angiographically occult cerebral aneurysms. J Neurosurg 1993;79:674–79 [DOI] [PubMed] [Google Scholar]
- 9.Tatter SB, Crowell RM, Ogilvy CS. Aneurysmal and microaneurysmal “angiogram-negative” subarachnoid hemorrhage. Neurosurgery 1995;37:48–55 [DOI] [PubMed] [Google Scholar]
- 10.Gilbert JW, Lee C, Young B. Repeat cerebral pan-angiography in subarachnoid hemorrhage of unknown etiology. Surg Neurol 1990;33:19–21 [DOI] [PubMed] [Google Scholar]
- 11.du Mesnil de Rochemont R, Heindel W, Wesselmann C, et al. Nontraumatic subarachnoid hemorrhage: value of repeat angiography. Radiology 1997;202:798–800 [DOI] [PubMed] [Google Scholar]
- 12.Hirai T, Korogi Y, Suginohara K, et al. Clinical usefulness of unsubtracted 3D digital angiography compared with rotational digital angiography in the pre-treatment evaluation of intracranial aneurysms. AJNR Am J Neuroradiol 2003;24:1067–74 [PMC free article] [PubMed] [Google Scholar]
- 13.Sugahara T, Korogi Y, Nakashima K, et al. Comparison of 2D and 3D digital subtraction angiography in evaluation of intracranial aneurysms. AJNR Am J Neuroradiol 2002;23:1545–52 [PMC free article] [PubMed] [Google Scholar]
- 14.Hochmuth A, Spetzger U, Schumacher M. Comparison of three-dimensional rotational angiography with digital subtraction angiography in the assessment of ruptured cerebral aneurysms. AJNR Am J Neuroradiol 2002;23:1199–205 [PMC free article] [PubMed] [Google Scholar]
- 15.Jung JY, Kim YB, Lee JW, et al. Spontaneous subarachnoid haemorrhage with negative initial angiography: a review of 143 cases. J Clin Neurosci 2006;13:1011–17. Epub 2006 Aug 23 [DOI] [PubMed] [Google Scholar]
- 16.Ishihara H, Kato S, Akimura T, et al. Angiogram-negative subarachnoid hemorrhage in the era of three dimensional rotational angiography. J Clin Neurosci 2007;14:252–55 [DOI] [PubMed] [Google Scholar]
- 17.de Gast AN, van Rooij WJ, Sluzewski M. Fenestrations of the anterior communicating artery: incidence on 3D angiography and relationship to aneurysms. AJNR Am J Neuroradiol 2008;29:296–8. Epub 2007 Nov 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallmes DF, Layton K, Marx WF, et al. Death by nondiagnosis: why emergent CT angiography should not be done for patients with subarachnoid hemorrhage. AJNR Am J Neuroradiol 2007;28:1837–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romijn M, Gratama van Andel HA, van Walderveen MA, et al. Diagnostic accuracy of CT angiography with matched mask bone elimination for detection of intracranial aneurysms: comparison with digital subtraction angiography and 3D rotational angiography. AJNR Am J Neuroradiol 2008;29:134–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agid R, Lee SK, Willinsky RA, et al. Acute subarachnoid hemorrhage: using 64-slice multidetector CT angiography to “triage” patients' treatment. Neuroradiology 2006;48:787–94 [DOI] [PubMed] [Google Scholar]
- 21.van Rooij WJ, Sprengers ME, de Gast AN, et al. 3D rotational angiography: the new gold standard in detection of additional intracranial aneurysms. AJNR Am J Neuroradiol 2008;29:976–79 [DOI] [PMC free article] [PubMed] [Google Scholar]