Abstract
BACKGROUND AND PURPOSE: During surgery of symptomatic aneurysms, additional small angiographic occult aneurysms are commonly found. With 3D rotational angiography (3DRA) small aneurysms are more easily depicted than with digital subtraction angiography (DSA). In this study we compare 3DRA with DSA in the depiction of small additional aneurysms.
MATERIALS AND METHODS: Three hundred fifty 3D datasets of 1 vascular tree of 350 patients with at least 1 intracranial aneurysm on the dataset were re-evaluated for the presence of additional aneurysms by 2 observers in consensus. Two other observers, blinded to the 3D images, re-evaluated DSA images of the same 350 vascular trees for these additional aneurysms. Results were compared.
RESULTS: In 350 3D datasets, 350 target aneurysms and 94 additional aneurysms were detected. The mean size of 94 additional aneurysms was 3.54 mm (median, 3; range, 0.5–17 mm). The proportion of aneurysms ≤3 mm was significantly higher in additional aneurysms (61 of 94, 65%) than in the target aneurysms (61 of 350, 17%) (χ2, P < .0001). Of 94 additional aneurysms, 27 (29%) were missed on DSA by both observers. The mean size of the missed aneurysms was 1.94 mm (median, 2; range, 0.5–4 mm). The proportion of aneurysms ≤3 mm in missed additional aneurysms (26 of 27, 96%) was significantly higher than that in all additional aneurysms (61 of 94, 65%) (χ2, P = .0035). The location of missed additional aneurysms was not different from the location of all additional aneurysms.
CONCLUSION: 3DRA depicts considerably more small (≤3 mm) additional aneurysms than DSA. In selected patients, accurate detection of these aneurysms may have consequences for the choice of treatment technique and for the frequency and duration of imaging follow-up.
Digital subtraction angiography (DSA) is generally used as the gold standard in detection of intracranial aneurysms.1–3 However, during surgery of symptomatic aneurysms, additional small angiographic occult aneurysms are commonly found with an incidence of 3.7%–12.2%.4–6 Most neurosurgeons clip or wrap these additional microaneurysms to prevent their growth and rupture. Because the operative view during surgical exploration is restricted to part of the cerebral vasculature, it is likely that the incidence of these small additional aneurysms is higher than that reported in surgical series. Thus, apparently an unknown number of microaneurysms are commonly missed on cerebral DSA.
Patients and Methods
This study was compliant with institutional privacy policy. The institutional review board gave exempt status for approval and informed consent for this retrospective study.
2D and 3D Angiography
In our practice, in all patients with suggestion of intracranial aneurysms in whom treatment is considered, a complete cerebral 3D rotational angiography (3DSA) is performed on a biplane system with 2 or 4 projections per vessel. When an aneurysm is apparent or suggested on DSA of any of the imaged vascular trees, additional 3DRA is performed of the same vessel harboring the aneurysm to evaluate its anatomy and to determine the need for and type of treatment (coiling, surgery, or parent vessel occlusion). In patients with aneurysms allocated to coiling, 3DRA of the vessel harboring the aneurysm is repeated, with the patient under general anesthesia, immediately before coiling to find out the best working projection. Thus, 3DRA was performed of every vascular tree with apparent aneurysms on DSA.
Angiography was performed on a biplane neuroangiographic unit (Integris BN 3000 Neuro; Philips Medical Systems, Best, the Netherlands). DSA was performed with a 1024 × 1024 matrix with a 17- to 20-cm FOV and injection of 8- to 10-mL contrast material in the internal carotid and vertebral arteries in 2 or 4 projections. After postprocessing when needed, relevant images were sent to the PACS. 3DRA was performed with an 8-second 180° rotational run, with acquisition of 200 images and with injection of 3- to 4-mL contrast material per second in the internal carotid or vertebral artery. On a dedicated workstation, 3D reconstructions were made in a matrix of 1283–512.3 Relevant images were sent to a PACS. All raw 3D datasets were stored on the hard disk of the workstation and later on a compact disk. Raw datasets stored on compact disks can be reloaded in the workstation for real-time evaluation and new high-resolution reconstructions.
Patients
We included three hundred fifty 3D datasets of 1 vascular tree of 350 patients with at least 1 intracranial aneurysm on the 3D dataset and a complete cerebral DSA performed between March 2004 and May 2007. This timeframe was chosen because all DSA images could be evaluated on a PACS system that was implemented in November 2003. A complete DSA consisted of 3-vessel angiography in 273 patients and 4-vessel angiography in 77 patients, for a total of 1127 vessels. The three hundred fifty 3D datasets were re-evaluated on the workstation by 2 of the authors in consensus (A.N.d.G. and W.J.v.R.). In particular, next to the location and size of the target aneurysm for which 3DRA had been performed, the presence, location, and size of additional aneurysms were assessed. Size was defined as the maximal diameter as measured on 3DRA. Results of re-evaluation of 3DRA were entered in a data base that served as a reference. Aneurysm locations were further classified into carotid artery, middle cerebral artery, anterior cerebral artery, and posterior circulation.
Subsequently, 2 experienced neuroradiologists (M.S. and J.P.P.P.), blinded to 3DRA PACS images and radiologic reports, reviewed the DSA images of the corresponding 350 vascular trees in 2 or 4 projections on a PACS workstation for the presence and location of additional aneurysms in consensus. Relevant DSA images were rearranged in dedicated monitor hangings. Special DSA projections derived from 3D angiography that had served as working projections during coiling were not included in the hangings. Results were compared with the findings in the reference data base. Number, location, and size of false-negative and false-positive additional aneurysms were recorded. Aneurysm location was further classified as carotid artery, middle cerebral artery, anterior cerebral artery, and posterior circulation.
Statistical Analysis
A χ2 test (2-tailed) was used for comparison of the following data: the proportion of aneurysms ≤3 mm in additional aneurysms and in target aneurysms, the proportion of aneurysms ≤3 mm in additional aneurysms missed on DSA and in all additional aneurysms as detected on 3DRA, and the location (classified as carotid artery, middle cerebral artery, anterior cerebral artery, and posterior circulation) of additional aneurysms missed on DSA versus the location of all additional aneurysms.
Results
Number and Sizes of Target and Additional Aneurysms
In three hundred fifty 3D datasets, 350 target aneurysms and 94 additional aneurysms were detected for a total of 444 aneurysms.
The mean size of 350 target aneurysms was 7.4 mm (median, 6; range, 1–32 mm). The mean size of 94 additional aneurysms was 3.54 mm (median, 3; range, 0.5–17 mm). Of 94 additional aneurysms, 61 (65%) were ≤3 mm; and of 350 target aneurysms, 61 (17%) were ≤3 mm. The proportion of aneurysms ≤3 mm was significantly higher in additional an-eurysms (61 of 94, 65%) than in target aneurysms (61 of 350, 17%) (χ2, P < .0001).
The location of 94 additional aneurysms was the anterior cerebral artery in 15 (16%), the carotid artery in 36 (38%), the middle cerebral artery in 33 (35%), and the posterior circulation 10 (11%).
Results of Observers
Of 94 additional aneurysms, 27 (29%) were missed on DSA by both observers (Figs 1–5). The mean size of the missed aneurysms was 1.94 mm (median, 2; range, 0.5–4 mm). Of 27 missed aneurysms, 26 (96%) were ≤3 mm. The proportion of aneurysms ≤3 mm in missed additional aneurysms (26 of 27, 96%) was significantly higher than that in all additional aneurysms (61 of 94, 65%) (χ2, P = .0035).
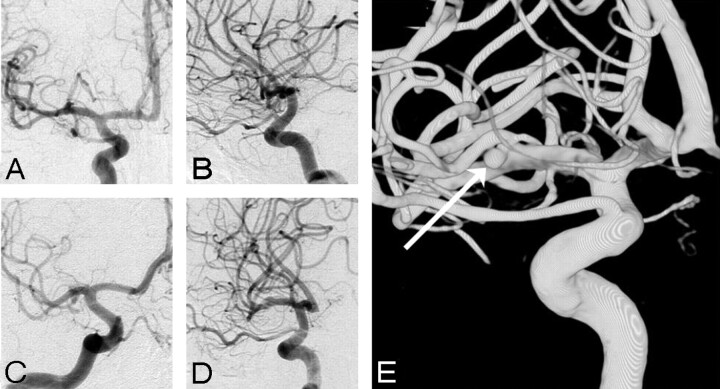
Fig 1.
A 2-mm middle cerebral artery aneurysm missed on DSA in 45-year-old man. A–D, DSA in 4 projections fails to depict an aneurysm. E, Demonstration of an aneurysm on 3DRA (arrow).
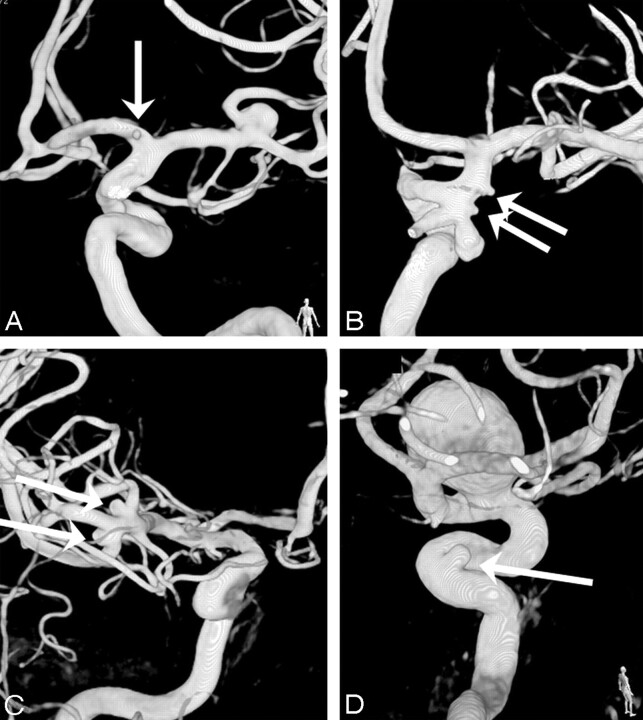
Fig 5.
Four more examples of missed additional aneurysms on DSA. A, A small middle cerebral artery aneurysm (arrow) in a patient with a ruptured anterior communicating artery aneurysm. B, Two small additional aneurysms on the anterior communicating and middle cerebral arteries (arrows) in a patient with a ruptured anterior communicating artery aneurysm. C, A very small (0.5 mm) A1 aneurysm (arrow) in a patient with a ruptured posterior communicating artery aneurysm. D, A small additional anterior communicating artery aneurysm (arrow) in a patient with a ruptured middle cerebral artery aneurysm.
The location of 27 missed additional aneurysms was the anterior cerebral artery in 6 (22%), the carotid artery in 12 (44%), the middle cerebral artery in 7 (26%), and the posterior circulation in 2 (7%). With the χ2 test, the location of missed additional aneurysms was not different from the location of all additional aneurysms.
Two false-positive aneurysms were observed on DSA, 1 on the ophthalmic artery and 1 in the middle cerebral artery.
Discussion
In this study, we found that 27 of 94 small additional aneurysms that were apparent on 3DRA of 350 vascular trees in 350 patients with a target aneurysm were missed on DSA. All except 1 of the missed aneurysms were 3 mm or smaller, and the smallest aneurysm was 0.5 mm. In addition, we found no significant difference in distribution of missed aneurysms compared with all additional aneurysms. Intuitively, one could presume that in complex vascular areas such as the anterior communicating artery complex and middle cerebral artery bifurcation or trifurcation, aneurysms would be more easily missed on 2D images than aneurysms at other locations. However, this intuition could not be confirmed.
The phenomenon of angiographically occult additional aneurysms found during surgery of symptomatic aneurysms is well known in the surgical literature: Yaşargil6 found, in his series of 1012 symptomatic aneurysms, 377 additional aneurysms, of which 169 (12.2% of all aneurysms) were 2 mm or smaller. Karasawa et al5 and Inamasu et al4 reported an incidence of 3.7% and 4.9% of angiographically occult microaneurysms of 2 mm or smaller. If technically possible, these microaneurysms were clipped or wrapped to prevent their growth and rupture.
Although the clinical significance of the presence of additional small aneurysms may be subject to debate,7 in our opinion, accurate detection of these small aneurysms may have consequences in the selection of patients for choice of therapy (coiling or clipping) and in the frequency and duration of follow-up. For example, in patients with target aneurysms suitable for both coiling and clipping, the presence of additional aneurysms that cannot be coiled may direct the therapy of choice to clipping if these additional aneurysms can be clipped in the same surgical procedure (Figs 2–4A–C, 5A–D). In patients who are treated for the target aneurysm but are left with untreated small additional aneurysms, imaging follow-up strategy may be more frequent and more prolonged to detect growth of these small aneurysms in a timely manner. If small additional aneurysms remain undetected, patients may be wrongly considered to have a single instead of multiple aneurysms. In epidemiologic studies concerning multiplicity of aneurysms, this may have consequences for determination of risk factors and outcome.8,9
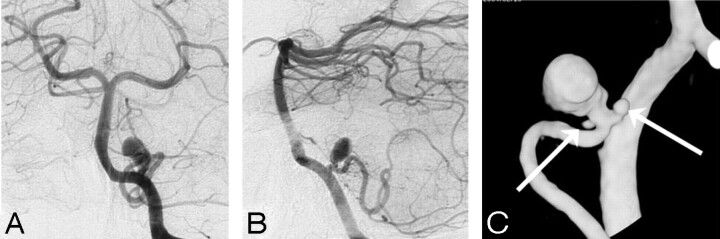
Fig 2.
Two angiographically occult additional microaneurysms adjacent to a ruptured posterior inferior cerebellar artery aneurysm in a 53-year-old woman. A and B, DSA in 2 projections demonstrates a posterior inferior cerebellar artery aneurysm. C, 3DRA detects 2 additional microaneurysms (arrows).
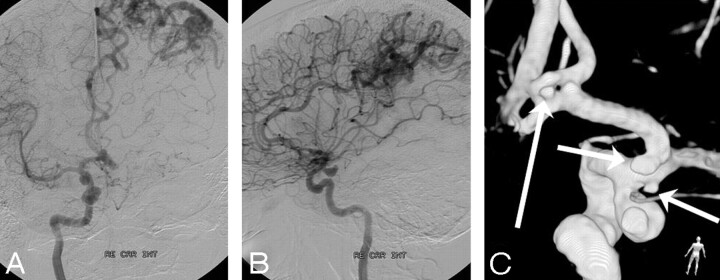
Fig 3.
A 44-year-old woman with subarachnoid hemorrhage. A and B, DSA in 2 projections reveals a posterior communicating artery aneurysm and an asymptomatic left parietal arteriovenous malformation. C, 3DRA shows, besides the posterior communicating artery aneurysm, 3 additional small aneurysms on the supraclinoidal carotid and proximal A1 arteries (short arrows) and on a fenestrated anterior communicating artery (long arrow).
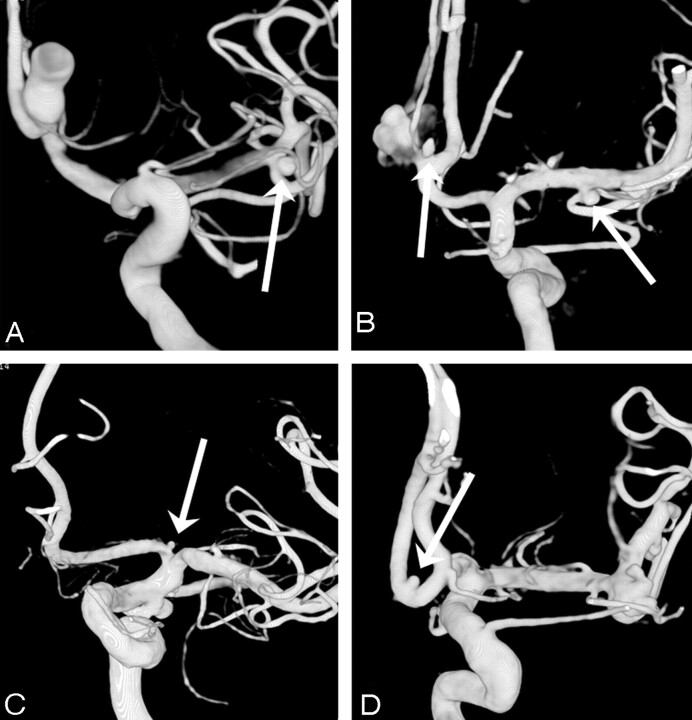
Fig 4.
Four examples of small additional aneurysms missed on DSA. A, A very small (0.5 mm) A1 aneurysm (arrow) in a patient with a ruptured middle cerebral artery aneurysm. B, Two supraclinoidal aneurysms (arrows) in a patient with a ruptured posterior communicating artery aneurysm. C, Two middle cerebral artery aneurysms (arrows) in a patient with a ruptured pericallosal artery aneurysm (not shown). D, An intracavernous carotid artery aneurysm (arrow) in a patient with a large ophthalmic artery aneurysm, symptomatic by mass effect.
In our experience, 3DRA is a major step forward in the detection and evaluation of intracranial aneurysms. The postprocessing capabilities of a 3D dataset allow viewing in any desired projection in high resolution without hindering overprojecting bony structures. This makes small aneurysms more obvious than those on the limited number of projections of DSA.10–14 In addition, complex vascular areas such as the anterior communicating artery complex can easily be unraveled and evaluated for the presence of aneurysms or vascular variations such as fenestrations.15 Measurement of aneurysm diameter and aneurysm volume can be performed accurately without the need for correction for magnification.11 Another advantage of 3DRA over DSA is its relative operator independency: After catheterization of the desired vessel, acquisition of the rotational run is standard procedure. Extensive postprocessing can be performed easily for scientific purposes, even many years after acquisition if the dataset is exported from the workstation to an external data-storage medium. On the other hand, DSA of intracranial vessels requires more experience and skill of the operator with respect to the decision of whether and which additional projections should be made. Image postprocessing is limited to window and width adjustment and pixel shifting, and storage of raw data is usually limited in time.
A disadvantage of 3DRA with respect to DSA is the higher contrast load per acquisitioned run (18–24 versus 6–8 mL), longer acquisition time (6–8 seconds), and increased patient radiation dose. In uncooperative patients, such as some patients with acute subarachnoid hemorrhage, patient movement may degrade image quality. In our study, most 3DRA datasets were acquired in patients under general anesthesia before coiling of the target aneurysm. Total patient contrast load and radiation dose can be decreased when only 3DRA acquisitions are obtained of all vessels, without preceding DSA runs. In this way, not only are the total contrast load and radiation dose roughly the same as those for DSA, but aneurysm imaging is also optimized. Currently, this is our protocol in cooperative patients with suspected intracranial aneurysms.
Our findings indicate that DSA may no longer be considered the gold standard for detection of intracranial aneurysms in studies that evaluate aneurysm-detection rates of noninvasive image techniques such as CTA and MRA. Both of these imaging techniques are inaccurate and unreliable in the detection of aneurysms of 3 mm and smaller.1–3 In our three hundred fifty 3D datasets of single vascular trees, 444 aneurysms were detected and 122 of these (27%) were 3 mm or smaller. Thus, a considerable proportion of intracranial aneurysms can potentially be missed with CTA and MRA. Future studies concerning CTA and MRA in intracranial aneurysms should be compared with 3DRA instead of DSA.
Conclusion
3DRA depicts considerably more small additional aneurysms than DSA. In selected patients, accurate detection of these aneurysms may have consequences for choice of treatment technique and for the frequency and duration of imaging follow-up. In cooperative patients with suggestion of intracranial aneurysms, 3- to 4-vessel 3DRA only (without preceding DSA runs) should be recommended as the optimal image strategy.
References
- 1.Kouskouras C, Charitanti A, Giavroglou C, et al. Intracranial aneurysms: evaluation using CTA and MRA—correlation with DSA and intraoperative findings. Neuroradiology 2004;46:842–50 [DOI] [PubMed] [Google Scholar]
- 2.Yoon DY, Lim KJ, Choi CS, et al. Detection and characterization of intracranial aneurysms with 16-channel multi-detector row CT angiography: a prospective comparison of volume-rendered images and digital subtraction angiography. AJNR Am J Neuroradiol 2007;28:60–67 [PMC free article] [PubMed] [Google Scholar]
- 3.Jayaraman MV, Mayo-Smith WW, Tung GA, et al. Detection of intracranial aneurysms: multi-detector row CT angiography compared with DSA. Radiology 2004;230:510–18 [DOI] [PubMed] [Google Scholar]
- 4.Inamasu J, Suga S, Horiguchi T, et al. Cerebral micro aneurysms found incidentally during aneurysm surgery. Neurol Res 2001;23:304–08 [DOI] [PubMed] [Google Scholar]
- 5.Karasawa H, Matsumoto H, Naito H, et al. Angiographically unrecognized microaneurysms: intraoperative observation and operative technique. Acta Neurochir (Wien) 1997;139:416–19, discussion 419–20 [DOI] [PubMed] [Google Scholar]
- 6.Yaşargil MG. Microneurosurgery. Vol. 1. Stuttgart, Germany: Georg Thieme Verlag;1984. :301–03
- 7.Wermer MJ, van der Schaaf IC, Algra A, et al. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke 2007;38:1404–10 [DOI] [PubMed] [Google Scholar]
- 8.Kaminogo M, Yonekura M, Shibata S. Incidence and outcome of multiple intracranial aneurysms in a defined population. Stroke 2003;34:16–21 [DOI] [PubMed] [Google Scholar]
- 9.Juvela S. Risk factors for multiple intracranial aneurysms. Stroke 2000;31:392–97 [DOI] [PubMed] [Google Scholar]
- 10.Anxionnat R, Bracard S, Ducrocq X, et al. Intracranial aneurysms: clinical value of 3D digital subtraction angiography in the therapeutic decision and endovascular treatment. Radiology 2001;218:799–808 [DOI] [PubMed] [Google Scholar]
- 11.Tanoue S, Kiyosue H, Kenai H, et al. Three-dimensional reconstructed images after rotational angiography in the evaluation of intracranial aneurysms: surgical correlation. Neurosurgery 2000;47:866–71 [DOI] [PubMed] [Google Scholar]
- 12.Sugahara T, Korogi Y, Nakashima K, et al. Comparison of 2D and 3D digital subtraction angiography in evaluation of intracranial aneurysms. AJNR Am J Neuroradiol 2002;23:1545–52 [PMC free article] [PubMed] [Google Scholar]
- 13.Hochmuth A, Spetzger U, Schumacher M. Comparison of three-dimensional rotational angiography with digital subtraction angiography in the assessment of ruptured cerebral aneurysms. AJNR Am J Neuroradiol 2002;23:1199–205 [PMC free article] [PubMed] [Google Scholar]
- 14.Beck J, Rohde S, Berkefeld J, et al. Size and location of ruptured and unruptured intracranial aneurysms measured by 3-dimensional rotational angiography. Surg Neurol 2006;65:18–27 [DOI] [PubMed] [Google Scholar]
- 15.de Gast AN, van Rooij WJ, Sluzewski M. Fenestrations of the anterior communicating artery: incidence on 3D angiography and relation with aneurysms. AJNR Am J Neuroradiol 2008;29:296–8. Epub 2007 Nov 16 [DOI] [PMC free article] [PubMed] [Google Scholar]