Abstract
Perilla frutescens (L.) Britton is a classic herbal plant used widely against asthma in China. But its mechanism of beneficial effect remains undermined. In the study, the antiallergic asthma effects of Perilla leaf extract (PLE) were investigated, and the underlying mechanism was also explored. Results showed that PLE treatment significantly attenuated airway inflammation in OVA-induced asthma mice, by ameliorating lung pathological changes, inhibiting recruitment of inflammatory cells in lung tissues and bronchoalveolar lavage fluid (BALF), decreasing the production of inflammatory cytokines in the BALF, and reducing the level of immunoglobulin in serum. PLE treatment suppressed inflammatory response in antigen-induced rat basophilic leukemia 2H3 (RBL-2H3) cells as well as in OVA-induced human peripheral blood mononuclear cells (PBMCs). Furthermore, PLE markedly inhibited the expression and phosphorylation of Syk, NF-κB, PKC, and cPLA2 both in vivo and in vitro. By cotreating with inhibitors (BAY61-3606, Rottlerin, BAY11-7082, and arachidonyl trifluoromethyl ketone) in vitro, results revealed that PLE's antiallergic inflammatory effects were associated with the inhibition of Syk and its downstream signals NF-κB, PKC, and cPLA2. Collectively, the present results suggested that PLE could attenuate allergic inflammation, and its mechanism might be partly mediated through inhibiting the Syk pathway.
1. Introduction
Asthma is one of the most common respiratory diseases characterized by varying degrees of chronic airway inflammation [1]. With increasing prevalence, it causes 250,000 deaths annually and will affect approximately 400 million individuals globally by 2025 [1, 2]. The most effective anti-inflammatory drugs used in asthma are inhaled corticosteroids (ICS) to suppress airway inflammation [3]. Unfortunately, even with the maximal medical therapy, over half of asthmatic patients do not achieve adequate medical control [4, 5]. The intake of ICS is also associated with numerous adverse effects, including impaired growth in children, suppressed hypothalamic-pituitary axis, and increased risk of infections [6]. Thus, there is an urgent need to develop novel anti-inflammatory drugs for asthma treatment.
Traditional Chinese medicine has been widely used in treating asthma in clinic for many years due to the ability to inhibit allergic hyperreactivity and regulate immune balance [7]. Perilla frutescens (L.) Britton, an annual classic herb that belongs to the Lamiaceae mint family [8], exerts great anti-inflammation and immune-regulation effects [9]. However, the therapeutic effects and potential mechanism of Perilla leaves on asthma have not yet been fully elucidated.
Spleen tyrosine kinase (Syk) is a 72 kD cytoplasmic nonreceptor tyrosine kinase, which plays key roles in allergic asthma inflammatory responses [10] through promoting IgE activation, degranulation mediator release, eicosanoid production, and cytokine synthesis [11, 12]. Syk activation occurs primarily through SH2 domains binding to FcεRI-ITAMs (immunoreceptor tyrosine-based activation motifs) [13] and then phosphorylating its own activation loop in tyrosine 525/526 to fully activate itself and transduce FcεRI signaling in mast cells and basophils [14]. As an upstream signaling molecule, activated Syk regulates multiple signaling molecules and amplifies inflammatory signals [15], including nuclear factor-κB (NF-κB), protein kinase C (PKC), and cytosolic phospholipase A2 (cPLA2) [16]. Recently, Syk inhibitors have been considered to constitute a new anti-inflammatory treatment strategy for asthma [14]. Therapies by inhibiting Syk may be more effective than treatments that inhibit a single downstream event.
In the present study, by using OVA-induced asthma mouse model, OVA-induced human PBMC inflammation model, and DNP-IgE/BSA-induced RBL-2H3 cell model, the anti-inflammatory effects of PLE on asthma were investigated. In addition, its mechanism of targeting Syk and downstream signals was explored.
2. Materials and Methods
2.1. Materials and Reagents
OVA, DNP-IgE, PIPES, 4-nitrophenyl-N-acetyl-β-D-glucosamide, BAY61-3606, Rottlerin, and BAY11-7082 were purchased from Sigma-Aldrich (MO, USA). Arachidonyl trifluoromethyl ketone (ATK) was from Cayman Chemical (MI, USA). Dexamethasone sodium phosphate (Dex) was purchased from Rongsheng Pharmaceutical Co., Ltd. (Jiaozuo, China). Aluminum hydroxide gel was from Meihua chemical industry limited company (Shanghai, China). All enzyme-linked immunosorbent assay (ELISA) kits were from BioLegend (CA, USA). ABC kit was obtained from Zhongshan Golden Bridge Biotechnology Co, Ltd. (Beijing, China). Lymphocyte Separation Medium was from Haoyang Biological Manufacture Co., Ltd. (Tianjin, China). RPMI-1640 medium, DMEM medium, and FBS were from Gibco (NY, USA). DNP-BSA was from Biosearch (CA, USA). Toluidine blue was from Amresco (Ohio, USA). Antibodies against Syk, p-Syk (Y525/526), PKC, p-PKC, NF-κB p65, p-NF-κB p65 (Ser536), cPLA2, p-cPLA2 (Ser505), and β-actin were obtained from Cell Signaling Technology (MA, USA). The hemocytometer was from Mindray BC-5000 vet (Shenzhen, China). The inverted research microscope was from Nikon Eclipse Ti2 (Japan). PLE was prepared as described in previous study, and the major compounds in PLE were roseoside, vicenin-2, and rosmarinic acid [17].
2.2. Animals
Male Balb/c wild-type mice (18-20 g, 6-8 weeks old, Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) were raised under controlled temperature (24 ± 2°C), humidity (60 ± 5%), and photoperiod (12 h light/dark cycle). Standard laboratory chow diet and water were provided ad libitum.
2.3. Ethics Declarations and Approval for Animal Experiments
All the animal experiments were carried out according to the Standard Operating Procedure for Animal Experimental Center, Institute of Materia Medica (IMM), Chinese Academy of Medical Sciences & Peking Union Medical College (CAMS & PUMC), approved by the Animal Care &Welfare Committee of IMM, CAMS & PUMC, and conformed to internationally accepted ethical standards.
2.4. OVA-Induced Allergic Airway Inflammation and Treatment
The OVA-induced asthma mouse model was established according to a previously described method [17]. Briefly, animals were divided randomly into 6 groups: control (Con) group, model (Mod) group, PLE 100 mg/kg group, PLE 200 mg/kg group, PLE 400 mg/kg group, and Dex 0.5 mg/kg [18] group. On days 1, 7, and 14, mice in the model group and experimental groups were sensitized with intraperitoneal injection of 30 μg OVA adsorbed in 1 mg aluminum hydroxide gel [19]. Mice were intragastrically administrated with vehicle or varying treatments on days 15-28 and challenged with intratracheal instillation of 80 μg OVA on days 26, 27, and 28. Animals were euthanized on day 29.
2.5. Measurement of Cell Counts in Bronchoalveolar Lavage Fluid (BALF)
Mice were sacrificed by cervical dislocation 24 h after the last OVA challenge, and BALF was obtained by intratracheal instillation with 700 μl PBS triply. The supernatant was collected after centrifugation at 4°C for the determination of TNF-α, IL-4, IL-6, and IFN-γ by ELISA kits. The cell pellets were resuspended in 500 μl PBS for enumeration of total white blood cells (WBC), lymphocytes (LYM), monocytes (MONO), neutrophils (NEU), and eosinophils (EOS) using a hemocytometer.
2.6. Histologic Assessment and Immunohistochemical Analysis of Lung Tissues
Mouse lung tissues were fixed, paraffin-embedded, cut, and stained with hematoxylin-eosin (H&E) for analysis of inflammatory cell infiltration as described previously [17]. In another experiment, lung sections were dewaxed and rehydrated. Antigen retrieval was performed by heated citrate. Sections were then incubated with anti-Syk and anti-p-Syk antibodies at 4°C overnight and incubated with HRP-conjugated secondary antibody. After staining with an ABC kit, positive staining was observed under a microscope and percentage of positively stained cells was evaluated, and the mean integrated optical density (MOD) was quantified by the Image-Pro Plus 7.0 software.
2.7. Human PBMC Isolation, Culture, Stimulation, and Treatment
Whole blood samples of healthy volunteers were obtained from the Clinical Laboratory Center, Beijing Friendship Hospital. Human PBMCs were isolated by gradient centrifugation over Lymphocyte Separation Medium and cultured in RPMI-1640 complete medium. The cells were then aliquoted into a 96-well culture plate at 2.5 × 105/well and pretreated with different concentrations of PLE (25 μg/ml, 50 μg/ml, and 100 μg/ml) and Dex (1 μM). The cells were then stimulated twice with OVA [20] (0.5 μg/ml) at 1 h and 12 h, respectively. The supernatants were then collected at 24 h to detect the levels of IL-6 and IL-8 production.
2.8. Approval for Human Experiments
The study involved with human sample was conducted in line with the guidelines outlined in the Declaration of Helsinki and had full ethical approval from the Institutional Ethics Committee of the Clinical Laboratory, Beijing Friendship Hospital. Informed consent was received from the volunteers.
2.9. RBL-2H3 Cell Culture, Stimulation, and Treatment
RBL-2H3 cells were cultured in DMEM medium supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. The plated cells were sensitized with anti-DNP IgE (50 ng/ml) [21] for 12 h before being pretreated with different concentrations of treatments for 1 h. Then, the cells were induced with DNP-BSA (25 ng/ml) in PIPES buffer for 45 min, cells were used for toluidine blue staining, and cell culture supernatants were collected for β-Hex detection. Or the cells were induced with DNP-BSA (25 ng/ml) in complete DMEM medium for 4 h (cells were used for immunofluorescence (IF) staining and western blotting (WB) analysis) or 12 h (supernatants were collected for TNF-α detection).
2.10. β-Hexosaminidase Secretion Assay
After DNP-BSA stimulation, 0.05 ml cell supernatant was added to equal volume of p-nitrophenyl-N-acetyl-β-D-glucosaminide solution (2 mM, pH = 4.5) and incubated for 1 h at 37°C. Then, 0.2 ml stop solution (100 μM NaHCO3/Na2CO3, pH = 10.0) was added to stop the reaction. The optical density value was read immediately at 405 nm. The release of β-Hex (%) was calculated as β‐Hex release (%) = the level of β‐Hex in each test well/the average level of β‐Hex in the model group × 100%.
2.11. Toluidine Blue Staining, IF Staining, and WB Analysis
Toluidine blue staining was used to visualize morphological changes of cells, and IF staining and WB were employed to determine the protein levels of Syk, p-Syk, PKC, p-PKC, NF-κB p65, p-NF-κB p65, cPLA2, and p-cPLA2. These experiments were performed as described previously [17].
2.12. ELISA Analysis
The concentrations of cytokines in BALF, culture supernatants from PBMCs and RBL-2H3 cells, and the levels of immunoglobulins in serum were detected by ELISA kits.
2.13. Statistical Analysis
Data were all expressed as mean ± standard error (SEM). As the normality test by Kolmogorov-Smirnov test (K-S test) was passed, data were analyzed by the Student t-test for comparison between two groups and one-way ANOVA for multiple groups followed by Fisher's least significant difference (LSD) test or otherwise by using the Kruskal-Wallis H test. P values less than 0.05 were considered as significant. The above analyses were conducted with GraphPad Prism (6.0) and IBM SPSS (19.0) statistical software.
3. Results
3.1. PLE Exerted Anti-Inflammatory Effect on Allergic Asthma In Vivo
PLE significantly attenuated the allergic airway inflammation in asthmatic mice induced by OVA. By using H&E staining (Figures 1(a) and 1(b)), the results of lung histologic changes showed that PLE treatment markedly attenuated OVA-induced extensive accumulation of inflammatory cells into bronchi and vein regions in a dose-dependent manner (P < 0.01). In agreement with the histologic appearance, PLE treatment significantly decreased the number of total leukocytes, lymphocytes, monocytes, neutrophils, and eosinophils in BALF of asthma mice (P < 0.05 or 0.01, Figure 1(c)). Furthermore, PLE treatment significantly reduced the production of IL-6 (Figure 1(d)), TNF-α (Figure 1(e)), and IL-4 (Figure 1(f)) in BALF as well as the level of IgE (Figure 1(h)), IgG1 (Figure 1(i)), IgG2a (Figure 1(j)), and IgG2b (Figure 1(k)) in serum (P < 0.05 or 0.01). Importantly, PLE treatment significantly decreased the level of IL-4, but had no effect on the level of IFN-γ (data not shown), resulting in a significant restoration of the IL-4/IFN-γ ratio imbalance (Figure 1(g)). This suggests that PLE can inhibit Th2 responses.
Figure 1.
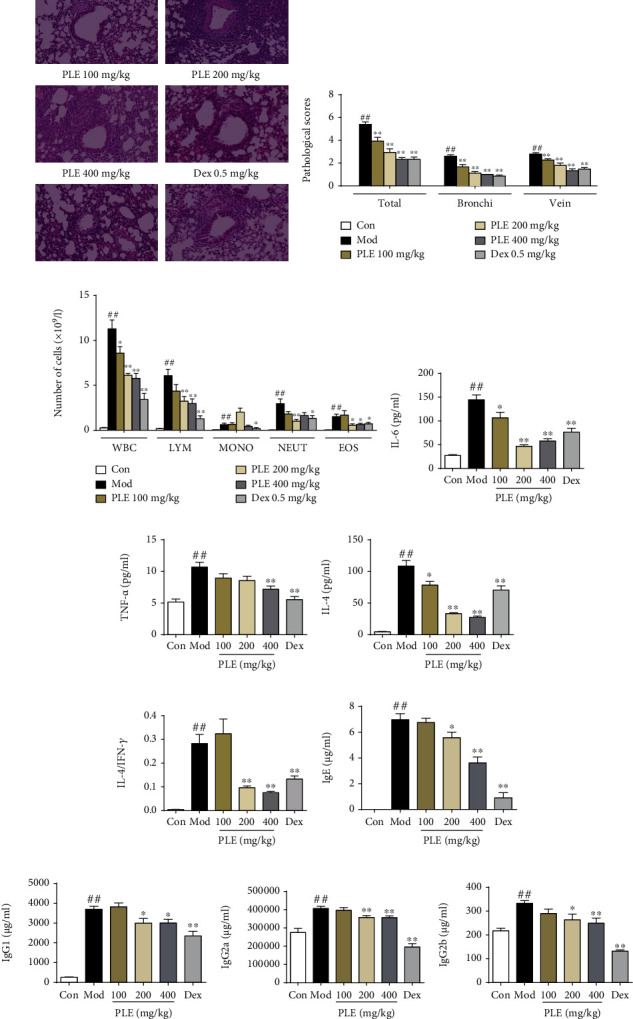
PLE exerted anti-inflammation effect in allergic asthma in vivo. (a) Representative images showing pathological changes in lung tissues determined by H&E staining (magnification, ×100). (b) The scores of inflammatory cell infiltration in lung tissues (n = 15 independent slices from three mice of each group). (c) WBC, LYM, MONO, NEUT, and EOS counts in BALF (n = 8). (d–g) The level of IL-6, TNF-α, and IL-4 and the ratio of IL-4/IFN-γ in BALF (n = 8). (h–k) The concentration of IgE, IgG1, IgG2a, and IgG2b in serum (n = 8). ##P < 0.01vs. the control group; ∗P < 0.05 and ∗∗P < 0.01vs. the model group.
3.2. PLE Exerted Anti-Inflammatory Effect on Allergic Asthma In Vitro
PLE treatment also significantly suppressed the inflammatory responses in OVA-induced human PBMC allergic model and DNP-IgE/BSA-induced RBL-2H3 allergic cell model. The production of IL-6 (Figure 2(a)) and IL-8 (Figure 2(b)) was significantly increased in OVA-induced human PBMCs, which was significantly inhibited by PLE treatment (P < 0.01). In DNP-IgE/BSA-induced RBL-2H3 cells, PLE treatment significantly suppressed the production of TNF-α (P < 0.05 or 0.01, Figure 2(c)) and the release of β-Hex (P < 0.01, Figure 2(d)). In addition, by using toluidine blue staining, DNP-IgE/BSA induced the cellular morphological changes (DNP-IgE/BSA-induced cells became round or irregular shape, and some cells were vacuolated with diminished cytoplasm, in contrast to cells in the control group with spindle-shaped and closely packed secretory granules in the cytoplasm) and degranulation in RBL-2H3 cells, which were significantly inhibited with PLE treatment (P < 0.01, Figures 2(e) and 2(f)).
Figure 2.
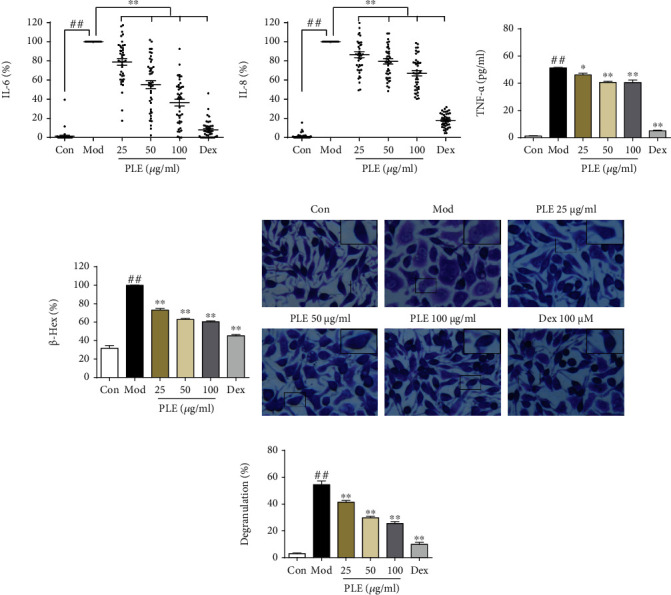
PLE exerted anti-inflammation effect in allergic asthma in vitro. (a, b) The levels of IL-6 and IL-8 production (%) in OVA-induced human PBMCs (n = 42). (c) The concentrations of TNF-α in DNP-IgE/BSA-induced RBL-2H3 cells (n = 3 independent experiments) measured by ELISA. (d) The level of β-Hex (%) in DNP-IgE/BSA-induced RBL-2H3 cells (n = 3 independent experiments). (e) Images of RBL-2H3 cells stained by toluidine blue (magnification, ×400) with (f) its degranulation rate (%) (n = 3). ##P < 0.01vs. the control group; ∗P < 0.05 and ∗∗P < 0.01vs. the model group.
3.3. PLE Attenuated Allergic Airway Inflammation In Vivo by Inhibiting Syk and Its Downstream Mediators
The activation of Syk and its downstream mediators in lung tissues of asthma mice was significantly inhibited with PLE treatment. The positively stained cells and MOD of Syk and p-Syk in lung tissues were significantly decreased with PLE treatment (P < 0.01, Figures 3(a)–3(f)) as determined by immunohistochemical analysis, which were consistent with WB, showing significant inhibition in the expression of Syk and p-Syk (Figures 3(g) and 3(h)). Furthermore, PLE treatment inhibited the expression of PKC (Figure 3(i)), p-PKC (Figure 3(j)), p65 (Figure 3(k)), p-p65 (Figure 3(l)), cPLA2 (Figure 3(m)), and p-cPLA2 (Figure 3(n)) (P < 0.05 or 0.01) significantly in OVA-induced asthma mouse lung tissues.
Figure 3.
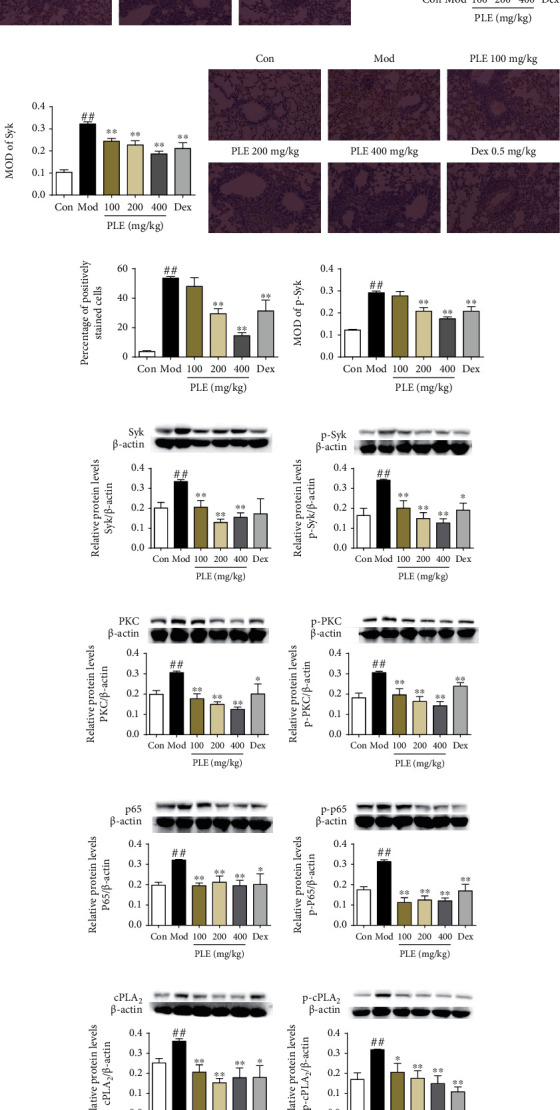
Effects of PLE on the expressions and phosphorylation of Syk and its downstream signaling molecules in vivo. (a) The representative photomicrographs (×200), (b) percentage of positively stained cells, and (c) mean integrated optical density (MOD) of Syk staining (n = 15 independent slices from three mice in each group). (d) Representative photomicrographs (×200), (e) percentage of positively stained cells, and (f) MOD of p-Syk staining (n = 15 independent slices from three mice in each group). The expression of (g) Syk, (h) p-Syk, (i) PKC, (j) p-PKC, (k) p65, (l) p-p65, (m) cPLA2, and (n) p-cPLA2 in OVA-induced asthma mouse lung tissues was determined by WB (n = 5). ##P < 0.01vs. the control group; ∗P < 0.05 and ∗∗P < 0.01vs. the model group.
3.4. PLE Attenuated Allergic Airway Inflammation In Vitro via Inhibiting Syk and Its Downstream Mediators
PLE treatment also significantly inhibited the expression and activation of Syk in DNP-IgE/BSA-induced RBL-2H3 cells. The expression of Syk, as determined by IF labeling, was significantly inhibited by PLE treatment in a dose-dependent manner (P < 0.01, Figure 4(a)). A similar result was observed for p-Syk antibody labeling (Figure 4(b)).
Figure 4.
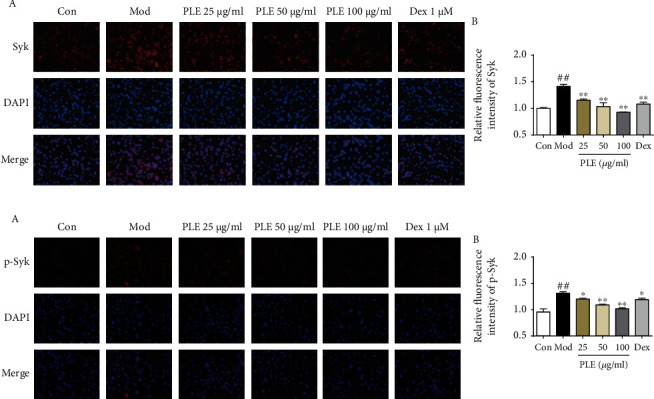
Effects of PLE on the expressions and phosphorylation of Syk in RBL-2H3 cells in vitro. (a) (A) The representative photomicrographs (magnification, ×200) of Syk and (B) their relative fluorescence intensity investigated by IF staining (n = 3). (b) (A) The representative photomicrographs (magnification, ×200) of p-Syk and (B) their relative fluorescence intensity investigated by IF staining (n = 3). ##P < 0.01vs. control group; ∗P < 0.05 and ∗∗P < 0.01vs. model group.
3.5. PLE Attenuated Allergic Airway Inflammation Associated with Syk and Its Downstream Signals PKC, NF-κB, and cPLA2
To examine the role of Syk by which PLE attenuates allergic airway inflammation, inhibitors of Syk (BAY61-3606) and its downstream signals PKC (Rottlerin), NF-κB (BAY11-7082), and cPLA2 (ATK) were employed. Treatment with BAY61-3606 significantly inhibited the degranulation and TNF-α release in DNP-IgE/BSA-induced RBL-2H3 cells, which were collaboratively inhibited by cotreating with PLE (Figure 5(a)). In addition, PLE cotreatment with Rottlerin, BAY11-7082, and ATK collaboratively inhibited β-Hex (Figure 5(b)) along with TNF-α (Figure 5(c)) production in DNP-IgE/BSA-induced RBL-2H3 cells, respectively.
Figure 5.
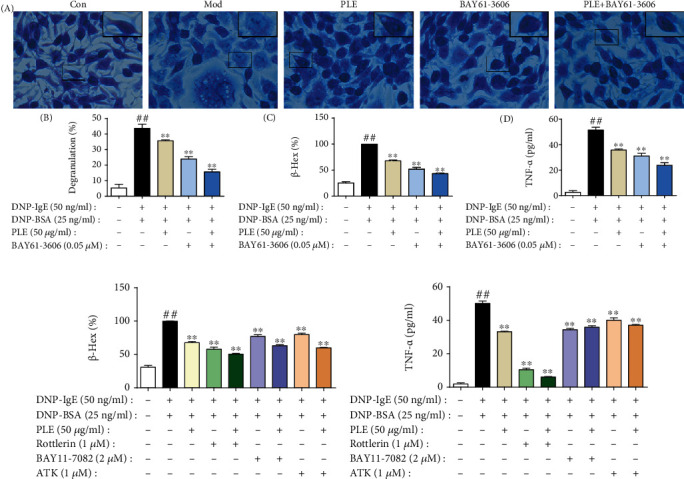
Verification that Syk, PKC, NF-κB, and cPLA2 were related with PLE attenuated inflammation in DNP-IgE/BSA-induced RBL-2H3 cells. (a) (A) Images of RBL-2H3 cells stained by toluidine blue (magnification, ×400), (B) the percent of degranulation (%) (n = 3), (C) the release rate of β-Hex (%), and (D) the concentration of TNF-α in cells cotreated with PLE and Syk inhibitor (n = 3 independent experiments). (b) The release rate of β-Hex and (c) production of TNF-α in cells cotreated with PLE and PKC, NF-κB, or cPLA2 inhibitor (n = 3 independent experiments). ##P < 0.01vs. the control group; ∗P < 0.05 and ∗∗P < 0.01vs. the model group.
3.6. PLE Attenuated Allergic Airway Inflammation by Targeting Syk
PLE cotreatment with BAY61-3606 almost collaboratively abolished the expression of Syk (Figure 6(a)) and p-Syk (Figure 6(c)) determined by IF staining, which were further confirmed with WB (Figures 6(b) and 6(d)). Interestingly, PLE decreased the expression of PKC, NF-κB, and cPLA2, which were superimposed by cotreating with BAY61-3606 (Figures 6(e)–6(j)). However, except Rottlerin, BAY11-7082 and ATK did not inhibit the protein expression of Syk and p-Syk (Figures 6(k)–6(p)). The data collectively revealed that Syk was the important target through which PLE mediated allergic inflammatory response.
Figure 6.
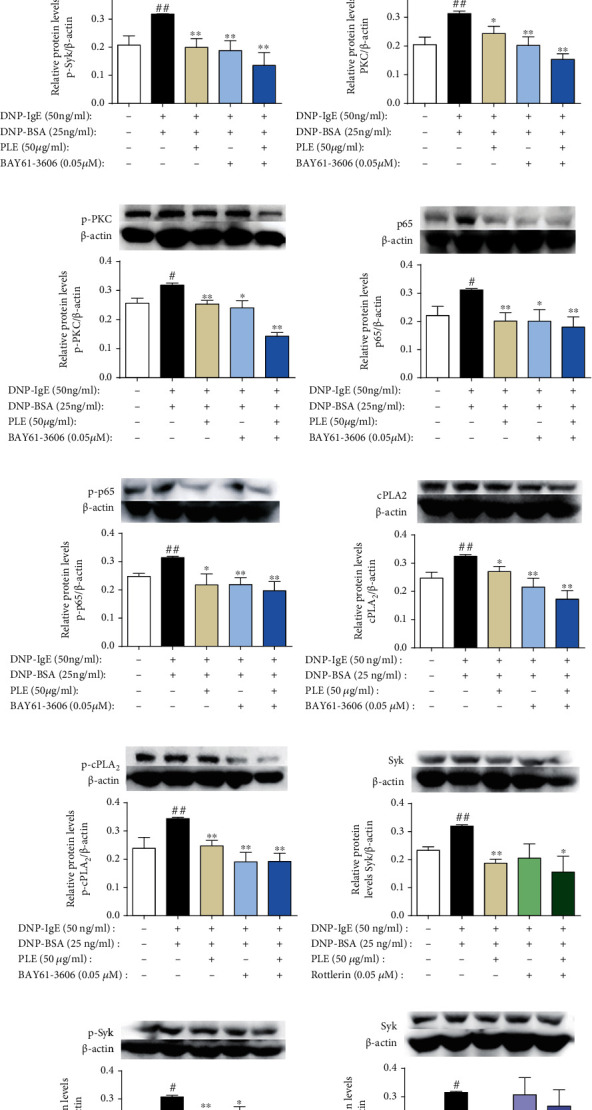
PLE attenuated allergic inflammation by targeting Syk in DNP-IgE/BSA-induced RBL-2H3 cell in vitro. (a) (A) Images (magnification, ×200) of Syk expression and (B) their relative fluorescence intensities determined by IF staining (n = 3). (b) The expression of Syk cotreating with PLE and BAY61-3606 detected by WB (n = 5). (c) (A) Images of (magnification, ×200) p-Syk expression and (B) their relative fluorescence intensities determined by IF staining (n = 3). (d) The expression of p-Syk cotreating with PLE and BAY61-3606 detected by WB (n = 5). (e–j) The expression of PKC, p-PKC, p65, p-p65, cPLA2, and p-cPLA2 cotreating with PLE and BAY61-3606 detected by WB (n = 5). (k–p) The expression of Syk and p-Syk cotreating with PLE and Rottlerin, BAY11-7082, and ATK detected by WB (n = 5). ##P < 0.01vs. the control group; ∗P < 0.05 and ∗∗P < 0.01vs. the model group.
4. Discussion
Allergic asthma is a chronic inflammatory lung disease with a steadily increasing incidence and poses a health challenge to people worldwide. Owing to the drug resistance and adverse reactions of current available anti-inflammation treatment, new managements are still needed for airway inflammation of allergic asthma. The results of the present study demonstrate that PLE, an extract from Perilla leaves, has a significant antiallergic airway inflammation property in vivo and in vitro.
Importantly, data in this study suggested PLE might notably reduce inflammatory mediators and alleviate immediate allergic response. PLE regulated OVA-induced mouse allergic inflammation responses, including inhibiting infiltration of inflammatory cells in lung tissues, reducing the accumulation of inflammatory cells (lymphocytes, macrophages, neutrophils, and eosinophils) in the BALF, and decreasing the production of inflammatory cytokines (IL-6, TNF-α, and IL-4) in the BALF and the secretion immunoglobulin (IgE, IgG1, IgG2a, and IgG2b) in serum. Meanwhile, PLE treatment decreased the inflammatory cytokine (IL-6 and IL-8) production in OVA-induced human PBMCs and attenuated degranulation and TNF-α secretion in antigen-induced RBL-2H3 cells. And PLE treatment also significantly decreased the level of IL-4 and the ratio of IL-4/IFN-γ, suggesting a regulatory effect on Th1/Th2 imbalance which causes the pathogenesis of asthma [22].
Further, we investigated potential target and mechanism by which PLE regulated the allergic airway inflammation. Asthma occurs as type I hypersensitivity reactions when antigens bind to the IgE-FcεRI complex and then recruit and activate Syk [23], which is known to involve in regulation of allergic inflammation response [24] and has been recognized as a potential target for the treatment of immune-mediated disorders such as asthma [25]. In respiratory allergies, upregulated Syk results in the activation of downstream signaling molecules, including PKC, NF-κB, and cPLA2, leading to the release of cytokines and inflammatory mediators [16, 26]. Thus, Syk can be an attractive target for therapeutic intervention in asthma. The results of our study showed that PLE treatment significantly suppressed the expression of Syk and p-Syk both in vivo and in vitro. This indicated that PLE exerted anti-inflammatory effects possibly by inhibiting the phosphorylation and expression of Syk.
Also, PKC, NF-κB, and cPLA2 levels were detected in vivo and in vitro. PKC is a well-known family of homologous serine/threonine kinases associated with asthma pathogenesis including airway inflammation, tissue injury, and remodeling [27–29]. NF-κB has a viral role in the inflammatory networks of asthma by regulating the expression of cytokines, chemokines, adhesion molecules, and infiltrating inflammatory cells [30, 31]. cPLA2 is responsible for the process of asthma through producing arachidonic acid, which is subsequently metabolized into inflammatory mediators such as prostaglandins, thromboxanes, and leukotrienes, resulting in airway eosinophilia and bronchoconstriction [32]. Our data showed that PLE suppressed the expression and phosphorylation of PKC, NF-κB, and cPLA2in vivo and in vitro, suggesting that PLE may exert its protective effects partly through mediating PKC, NF-κB, and cPLA2.
Furthermore, by cotreating PLE with BAY61-3606 (Syk inhibitor), Rottlerin (PKC inhibitor), BAY11-7082 (NF-κB inhibitor), and ATK (cPLA2 inhibitor) in DNP-IgE/BSA-stimulated RBL-2H3 cells, respectively, it was demonstrated that Syk might be a potential target through which PLE could alleviate the inflammatory response via modulating its downstream signaling molecules PKC, p65, and cPLA2. By cotreating PLE with BAY61-3606, a further extent of inhibition in the expression and phosphorylation of Syk, PKC, NF-κB, and cPLA2 was observed, while BAY11-7082 and ATK did not impact the expression of Syk and p-Syk. Cotreating Rottlerin with PLE suggested a mutual interaction between Syk and PKC with PLE treatment.
5. Conclusion
In conclusion, PLE can significantly prevent allergic airway inflammation in vivo and in vitro by targeting Syk and then regulating its downstream signals PKC, p65, and cPLA2.
Acknowledgments
The authors acknowledge the Clinical Laboratory Center of Beijing Friendship Hospital for the provision of healthy volunteers' blood sample. We also acknowledge Dr. Chunsuo Yao for the provision of PLE. This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 81973539, 81473398, and 81803810), the Beijing Natural Science Foundation Program (Grant no. 7182116), the CAMS Initiative for Innovative Medicine (Grant no. 2016-I2M-2-006), the Drug Innovation Major Project of China (Grant no. 2018ZX09711001-003-001), and the PUMC Graduate Innovation Fund (Grant no. 2019-1007-14).
Abbreviations
- PLE:
Perilla leaf extract
- ATK:
Arachidonyl trifluoromethyl ketone.
Contributor Information
Ming-bao Lin, Email: mingbaolin@imm.cams.cn.
Qi Hou, Email: houq@imm.ac.cn.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
There are no conflicts of interest concerning this work.
Authors' Contributions
HY performed most experiments, analyzed the data, wrote the main manuscript, and prepared figures. WS organized the healthy volunteers and collected the blood samples. YNF, SYL, JQY, ZQZ, and XYL participated in some experiments. MBL and QH designed experiments and gave final approval of the version to be submitted. All authors reviewed the manuscript. Hui Yang and Wei Sun contributed equally and are listed as co-first authors.
References
- 1.Vuolo F., Abreu S. C., Michels M., et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. European Journal of Pharmacology. 2019;843:251–259. doi: 10.1016/j.ejphar.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 2.Olin J. T., Wechsler M. E. Asthma: pathogenesis and novel drugs for treatment. BMJ. 2014;349(nov24 8):p. g5517. doi: 10.1136/bmj.g5517. [DOI] [PubMed] [Google Scholar]
- 3.Hossny E., Rosario N., Lee B. W., et al. The use of inhaled corticosteroids in pediatric asthma: update. World Allergy Organization Journal. 2016;9:p. 26. doi: 10.1186/s40413-016-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agbetile J., Green R. New therapies and management strategies in the treatment of asthma: patient-focused developments. Journal of Asthma and Allergy. 2011;4:1–12. doi: 10.2147/JAA.S8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes P. J. New therapies for asthma: is there any progress? Trends in Pharmacological Sciences. 2010;31(7):335–343. doi: 10.1016/j.tips.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Heffler E., Madeira L. N. G., Ferrando M., et al. Inhaled corticosteroids safety and adverse effects in patients with asthma. The Journal of Allergy and Clinical Immunology: In Practice. 2018;6(3):776–781. doi: 10.1016/j.jaip.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Li J., Zhang F., Li J. The immunoregulatory effects of traditional Chinese medicine on treatment of asthma or asthmatic inflammation. The American Journal of Chinese Medicine. 2015;43(6):1059–1081. doi: 10.1142/S0192415X15500615. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed H. M. Ethnomedicinal, phytochemical and pharmacological investigations of Perilla frutescens (L.) Britt. Molecules. 2019;24(1) doi: 10.3390/molecules24010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon I. H., Kim H. S., Kang H. J., et al. Anti-inflammatory and antipruritic effects of luteolin from perilla (P. frutescens L.) leaves. Molecules. 2014;19(6):6941–6951. doi: 10.3390/molecules19066941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moy L. Y., Jia Y., Caniga M., et al. Inhibition of spleen tyrosine kinase attenuates allergen-mediated airway constriction. American Journal of Respiratory Cell and Molecular Biology. 2013;49(6):1085–1092. doi: 10.1165/rcmb.2013-0200OC. [DOI] [PubMed] [Google Scholar]
- 11.Kovarova M., Rivera J. A molecular understanding of mast cell activation and the promise of anti-allergic therapeutics. Current Medicinal Chemistry. 2004;11(15):2083–2091. doi: 10.2174/0929867043364801. [DOI] [PubMed] [Google Scholar]
- 12.Matsubara S., Koya T., Takeda K., et al. Syk activation in dendritic cells is essential for airway hyperresponsiveness and inflammation. American Journal of Respiratory Cell and Molecular Biology. 2006;34(4):426–433. doi: 10.1165/rcmb.2005-0298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travers T., Kanagy W. K., Mansbach R. A., et al. Combinatorial diversity of Syk recruitment driven by its multivalent engagement with FcεRIγ. Molecular Biology of the Cell. 2019;30(17):2331–2347. doi: 10.1091/mbc.E18-11-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramis I., Otal R., Carreno C., et al. A novel inhaled Syk inhibitor blocks mast cell degranulation and early asthmatic response. Pharmacological Research. 2015;99:116–124. doi: 10.1016/j.phrs.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Yi Y. S., Son Y. J., Ryou C., Sung G. H., Kim J. H., Cho J. Y. Functional roles of Syk in macrophage-mediated inflammatory responses. Mediators of Inflammation. 2014;2014:12. doi: 10.1155/2014/270302.270302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin D., Park S. H., Choi Y. J., et al. Dietary compound kaempferol inhibits airway thickening induced by allergic reaction in a bovine serum albumin-induced model of asthma. International Journal of Molecular Sciences. 2015;16(12):29980–29995. doi: 10.3390/ijms161226218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H., Sun W., Ma P., et al. Multiple components rapidly screened from perilla leaves attenuate asthma airway inflammation by synergistic targeting on Syk. Journal of Inflammation Research. 2020;13(13):897–911. doi: 10.2147/JIR.S281393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang Y. H., Kim S. J., Kim H., Yee S. T. The protective effects of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside in the OVA-induced asthma mice model. International Journal of Molecular Sciences. 2018;19(12):p. 4013. doi: 10.3390/ijms19124013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J., Li F. S., Pang N. N., et al. Inhibition of Asthma in OVA Sensitized Mice Model by a Traditional Uygur Herb Nepeta bracteata Benth. Evidence-based Complementary and Alternative Medicine. 2016;2016:8. doi: 10.1155/2016/5769897.5769897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakazato J., Kishida M., Kuroiwa R., Fujiwara J., Shimoda M., Shinomiya N. Serum levels of Th2 chemokines, CCL17, CCL22, and CCL27, were the important markers of severity in infantile atopic dermatitis. Pediatric Allergy and Immunology. 2008;19(7):605–613. doi: 10.1111/j.1399-3038.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 21.Cerqua I., Terlizzi M., Bilancia R., et al. 5α-Dihydrotestosterone abrogates sex bias in asthma like features in the mouse. Pharmacological Research. 2020;158, article 104905 doi: 10.1016/j.phrs.2020.104905. [DOI] [PubMed] [Google Scholar]
- 22.Zou X. L., Chen Z. G., Zhang T. T., Feng D. Y., Li H. T., Yang H. L. Th17/Treg homeostasis, but not Th1/Th2 homeostasis, is implicated in exacerbation of human bronchial asthma. Therapeutics and Clinical Risk Management. 2018;14:1627–1636. doi: 10.2147/TCRM.S172262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geahlen R. L. Getting Syk: spleen tyrosine kinase as a therapeutic target. Trends in Pharmacological Sciences. 2014;35(8):414–422. doi: 10.1016/j.tips.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahar N., Bibi S., Masood N., Faryal R. Status of serine tyrosine kinase at germline and expressional levels in asthma patients. Molecular Biology Research Communications. 2019;8(2):69–77. doi: 10.22099/mbrc.2019.33040.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Z. Y., Kim M. K., Kim-Han T. H., Indik Z. K., Schreiber A. D. Effect of locally administered Syk siRNA on allergen-induced arthritis and asthma. Molecular Immunology. 2013;53(1-2):52–59. doi: 10.1016/j.molimm.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Barnes P. J. Kinases as novel therapeutic targets in asthma and chronic obstructive pulmonary disease. Pharmacological Reviews. 2016;68(3):788–815. doi: 10.1124/pr.116.012518. [DOI] [PubMed] [Google Scholar]
- 27.Kim H., Zamel R., Bai X. H., Liu M. PKC activation induces inflammatory response and cell death in human bronchial epithelial cells. PLoS One. 2013;8(5, article e64182) doi: 10.1371/journal.pone.0064182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S., Wang L., Dorf M. E. PKC phosphorylation of TRAF2 mediates IKKalpha/beta recruitment and K63-linked polyubiquitination. Molecular Cell. 2009;33(1):30–42. doi: 10.1016/j.molcel.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang Q., Mao L., Kobayashi T., et al. PKCdelta mediates thrombin-augmented fibroblast-mediated collagen gel contraction. Biochemical and Biophysical Research Communications. 2008;369(4):1199–1203. doi: 10.1016/j.bbrc.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q., Wang L., Chen B., Zhuo Q., Bao C., Lin L. Propofol inhibits NF-κB activation to ameliorate airway inflammation in ovalbumin (OVA)-induced allergic asthma mice. International Immunopharmacology. 2017;51:158–164. doi: 10.1016/j.intimp.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Schuliga M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules. 2015;5(3):1266–1283. doi: 10.3390/biom5031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira G. P., de Abreu M. G., Pelosi P., Rocco P. R. Exogenous glutamine in respiratory diseases: myth or reality? Nutrients. 2016;8(2):p. 76. doi: 10.3390/nu8020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


