Abstract
Background.
Maintaining balance in response to perturbations during walking often requires the use of corrective responses to keep the center of mass within the base of support. The relationship between center of mass and base of support is often quantified using the margin of stability. Although people post-stroke increase the margin of stability following perturbations, control deficits may lead to asymmetries in regulation of margins of stability, which may also cause maladaptive coupling between the sagittal and frontal planes during balance-correcting responses.
Methods.
We assessed how paretic and non-paretic margins of stability are controlled during recovery from forward perturbations and determined how stroke-related impairments influence the coupling between the anteroposterior and mediolateral margins of stability. Twenty-one participants with post-stroke hemiparesis walked on a treadmill while receiving slip-like perturbations on both limbs at foot-strike. We assessed anteroposterior and mediolateral margins of stability before perturbations and during perturbation recovery.
Findings.
Participants walked with a smaller anteroposterior and larger mediolateral margin of stability on the paretic versus non-paretic sides. When responding to perturbations, participants increased the anteroposterior margin of stability bilaterally by extending the base of support and reducing the excursion of the extrapolated center of mass. The anteroposterior and mediolateral margin of stability in the paretic limb negatively covaried during reactive steps such that increases in anteroposterior and reductions in mediolateral margins of stability were associated.
Interpretation.
Balance training interventions to reduce fall risk post-stroke may benefit from incorporating strategies to reduce maladaptive coupling of frontal and sagittal plane stability.
Keywords: Gait, Stroke, Margin of Stability, Balance, Stability, Reactive control
1. Introduction
People post-stroke often have trouble maintaining dynamic balance while walking1, leading to an increased risk of falls2,3. When responding to unexpected perturbations, dynamic balance is primarily maintained by reactive control strategies such as stepping responses. Reactive stepping may be impaired post-stroke, due to weakness in the paretic leg4,5, delays in intra- and inter-limb reflexes6, abnormal coordination7–11, and impaired initiation of successful stepping responses with the paretic leg12. Reactive control of dynamic balance can be quantified by the Margin of Stability (MoS)13, a variable that incorporates Center of Mass (CoM) position and velocity in the Extrapolated CoM (XCoM), and the anteroposterior (AP) or mediolateral (ML) edge of the base of support. Although improving reactive dynamic balance control is an important objective in post-stroke gait rehabilitation, we do not fully understand how post-stroke impairments influence the regulation of the MoS during reactive stepping responses.
In response to perturbations during walking, non-disabled people increase the ML and AP MoS by making a stepping response that accounts for the direction and magnitude of the perturbation14–17. People post-stroke regulate both their AP and ML MoS in a manner that differs from non-disabled persons during unperturbed walking. For example, they have smaller bilateral AP MoS18,19 and tend to balance their CoM over the non-paretic leg, which results in a larger paretic than non-paretic AP and ML MoS20. It, therefore, seems that people post-stroke unburden their paretic leg, while they stabilize and propel themselves with the non-paretic leg4,5,21. Control of AP and ML MoS during reactive balance responses in people post-stroke has been studied both during stance and during walking. When fore-aft perturbations occur while standing, people post-stroke have smaller paretic than non-paretic compensatory step length responses22,23. In response to lateral perturbations during walking19, they increase the paretic and non-paretic ML MoS and decrease the AP MoS, regardless of whether the perturbation occurs during the paretic or non-paretic step19. While it is known that there are asymmetries in regulation of the AP and ML MoS, the underlying mechanisms that drive this asymmetry have not yet been determined.
The MoS can be regulated in two ways; (i) By changing the XCoM position and (ii) by changing the base of support, i.e., step length or step width. Control deficits in the paretic limb may lead to asymmetries in how these strategies are combined. While the non-paretic MoS could be controlled more via foot placement strategies, the paretic MoS might be preferentially controlled by using the non-paretic limb to modulate the position of the XCoM since foot placement control using the paretic leg may be impaired4,5,7–10,12. To understand how people post-stroke change their AP and ML MoS in reaction to forward perturbations during walking, it is necessary to understand how changes in each MoS are influenced by changes in XCoM position and changes in the base of support via foot placement strategies.
In addition to understanding the role of foot placement strategies and the control of the center of mass on AP and ML MoS, respectively, there is evidence that AP and ML stability may covary systematically. For example, to increase step length in response to a forward perturbation, a transverse rotation of the pelvis is often necessary24. However, this rotation may result in reduced step width25, and could, therefore lead to a decrease in the ML MoS26, which could lead to a fall. The impact of this covariation may be particularly severe for people with post-stroke hemiparesis because paretic stepping responses are impaired12. This can be empirically verified by assessing whether an increase in AP MoS in the recovery step following a forward perturbation is associated with a decrease in ML MoS in the same step, due to decreased step width26, and whether this relation differs between the paretic and non-paretic leg. Indeed, when people post-stroke increase their ML MoS in response to a lateral perturbation, they simultaneously decrease their AP MoS19. However, the strength of this relationship during AP perturbations has yet to be investigated.
Here, we examine the covariation between AP MoS and ML MoS in response to forward perturbations during walking in people post-stroke. Our first aim was to assess how paretic and non-paretic AP and ML MoS are controlled during unperturbed walking and the recovery steps following a forward perturbation. We hypothesized that people post-stroke would walk with asymmetric ML and AP MoS during steps before the perturbation18–20. Furthermore, we hypothesized that people post-stroke would increase their AP MoS and decrease their ML MoS in the recovery step following a perturbation. In addition, we hypothesized that people post-stroke will show less of an increase in AP MoS on the paretic than the non-paretic leg. Our second aim was to determine whether the AP MoS and ML MoS covary during the recovery step after a perturbation and to determine whether this covariation differs between the paretic and non-paretic leg. We hypothesized that the increase in AP MoS in response to a forward perturbation would negatively covary with the ML MoS. Furthermore, we hypothesized that this covariation would be stronger for the paretic than the non-paretic leg.
2. Methods
2.1. Participants and ethics statement
Twenty-one chronic stroke survivors (Table 1) participated in this study. Inclusion criteria were (i) a sustained unilateral stroke more than six months prior to the experiment, (ii) paresis confined to one side, (ii) ability to provide informed consent and communicate with the experimenters, (iv) the ability to walk at least five minutes on a treadmill without assistance or walking aids. The use of an ankle-foot orthosis was permitted during the experiment, however only two out of twenty-one participants wore an ankle-foot orthosis and these two participants were not identified as outliers in any of our analyses. The procedures of this study were approved by the University of Southern California Institutional Review Board (Los Angeles, CA, USA) and were consistent with the Declaration of Helsinki27. All participants provided written informed consent before the experiment.
Table 1 -.
Participant demographics and clinical assessments (N=21).
| Metric | Value (SD) |
|---|---|
| Female / male | 6 / 15 |
| Left / right side hemiparesis | 8 / 13 |
| Age (years) | 59.4 (12.6) |
| Activity-specific Balance Confidence Scale | 55.0 (36.7) |
| Berg Balance Scale | 51.3 (3.7) |
| Functional Gait Assessment | 23.3 (4.8) |
| 10-Meter Walk Test (m s−1) | 0.87 (0.23) |
| Falls Efficacy Scale | 30.4 (12.1) |
| Fugl-Meyer lower extremity | 28.2 (3.2) |
| Self-selected walking speed (m s−1) | 0.60 (0.18) |
| Step length asymmetry | −0.004 (0.112) |
2.2. Experimental protocol
Before participants walked on the treadmill, we performed a set of clinical assessments. We evaluated balance using the Berg Balance Scale28, balance self-efficacy using the Activity-specific Balance Confidence Scale29, walking performance using the Functional Gait Assessment30 and 10-Meter Walk Test at self-selected speed, fear of falling using the Falls Efficacy Scale31, and motor impairment using the lower extremity motor domain of the Fugl-Meyer assessment32. Step length asymmetry (SLA) was calculated with Eqn. 1, in which a positive asymmetry indicates a larger non-paretic than paretic step length and a negative asymmetry indicates a smaller non-paretic than paretic step length.
| (1) |
After the clinical assessments, participants walked on an instrumented dual-belt treadmill (Bertec, Columbus, OH, USA) to determine their self-selected walking speed. They started with 70 % of the speed measured during the 10-Meter Walk Test, and speed was adjusted by increments or decrements of 0.05 m s−1 until they verbally indicated that their preferred walking speed was reached. Participants then completed a familiarization trial with at least two slip-like perturbations on each side33.
Participants walked for three minutes at their self-selected speed to accommodate to treadmill walking, after which they completed two trials of three minutes at their self-selected speed during which they received perturbations. We used rapid accelerations of one of the treadmill’s belts to act as perturbations, resulting in a slip-like perturbation with a forward loss of balance34. During each trial, we applied six perturbations to each leg. Perturbations were triggered using Python code that predicted foot-strike timing using ground reaction forces recorded by the treadmill’s embedded force plates. Each perturbation consisted of a 0.2 m s−1 increase in speed at an acceleration of 3.0 m s−2, lasting 0.7 s and accelerating back to the self-selected speed during the swing phase of the perturbed leg. We chose to use a fixed increase in belt speed for two reasons. First, we wanted to avoid the possibility that any observed differences in perturbation responses between participants were due to differences in perturbation size. Second, a fixed perturbation size also mimics the characteristics of real-world perturbations that are independent of walking speed, such as an unexpected change in height of the walking surface. Perturbations occurred within 18 to 24 steps after the previous perturbation to let participants re-establish their normal walking pattern and minimize anticipatory responses to the perturbations. Participants had breaks of at least three minutes in between each trial to minimize fatigue. Participants did not hold on to handrails while walking on the treadmill, but they wore a harness to prevent them from falling. All participants were able to stay upright in response to the perturbations. After the selection of correctly timed perturbations, we included 19 (SD 5) perturbations per participant.
2.3. Data acquisition
A 10-camera motion capture system (Qualisys AB, Göteborg, Sweden) recorded 3D kinematics at 100 Hz, and the treadmill embedded force plates recorded ground reaction forces at 1000 Hz. Retroreflective markers (14 mm) were placed at anatomical landmarks to create a 12-segment, full-body model. This model was based on a 13-segment model35,36, but the pelvis segment was assumed to be rigidly connected to the trunk because the harness blocked the markers necessary to track the pelvis accurately. We validated this 12-segment model with data from a set of non-disabled participants that included the necessary markers to track the pelvis segment. The root mean square error between the 12-segment and 13-segment model was low for the CoM position (AP: 0.0041 (SD 0.0034) m, ML: 0.0017 (SD 0.0012) m, vertical: 0.0095 (SD 0.0051) m) and CoM velocity (AP: 0.0052 (SD 0.0015) m s−1, ML: 0.0025 (SD 0.0008) m s−1, vertical: 0.0030 (SD 0.0015) m s−1). We placed marker clusters on the upper arms, forearms, thighs, shanks, and the back of heels. At the beginning of each trial, marker positions were calibrated during a five-second standing trial. All joint markers were removed after standing calibration.
2.4. Data analysis
Kinematic and kinetic data were processed in Visual3D (C-Motion, Rockville, MD, USA) and analyzed in MATLAB (version r2018b; The MathWorks Inc., Natick, MA, USA). Marker positions and ground reaction forces were low-pass filtered with a 4th order Butterworth filter, at a 6 Hz and 20 Hz cutoff respectively37–39. The timing of perturbations relative to foot-strike was re-examined post-hoc. We removed the perturbations that occurred more than 150 ms after the foot-strike. We also removed perturbations for which deceleration began before the toe-off of the perturbed leg. Load cell measured the vertical force on the harness (Litegait, Tempe, AZ, USA), and we excluded steps in which we measured more than 30% of the participant’s body weight as this would indicate that the participant relied on the harness to remain upright23,40,41.
Step width (m) was defined as the ML distance between the 5th metatarsal marker and the contralateral foot’s 5th metatarsal marker at foot-strike. Step length was defined as the AP distance between the 1st distal phalanx marker of the leading foot and the 1st distal phalanx marker of the trailing foot at foot-strike. The AP and ML MoS were calculated for the paretic and non-paretic leg independently using a modified version of the MoS, which captures the nonlinearity in the CoM trajectory42 (Eqns. 2 & 3).
| (2) |
| (3) |
We calculated the AP MoS at foot-strike, with leg length (l), limb angle (θ) as measured by the angle of a vector extending from the CoM to the 1st distal phalanx marker in the sagittal plane, and limb angular velocity in the sagittal plane (, Eqn. 1). We calculated ML MoS at foot-strike, with leg length (l), limb angle (θ) as measured by the angle of a vector extending from the CoM to the 5th metatarsal marker in the frontal plane, and limb angular velocity in the frontal plane (, Eqn. 1). AP and ML XCoM positions (m) were analyzed at foot-strike.
2.5. Statistical analysis
We conducted a series of statistical tests to assess how people post-stroke control their paretic and non-paretic AP and ML MoS in response to a forward perturbation. All statistical analyses were performed in MATLAB (version r2018b; The MathWorks Inc., Natick, MA, USA). We analyzed the last step before each perturbation (Pre-perturbation), the step during which the participant was perturbed (Perturbation), and the three subsequent recovery steps (Recovery 1–3) for each participant, leg, and perturbation side independently. As such, if the paretic leg was perturbed, the Pre-perturbation, Recovery 1 and Recovery 3 steps were made with the non-paretic leg, and the Perturbation and Recovery 2 steps were made with the paretic leg and vice-versa if the non-paretic leg was perturbed. However, in our statistical analysis, comparisons between Pre-perturbation, Perturbation and Recovery 1–3 are always made within all paretic steps or all non-paretic steps, to be able to separately compare paretic with non-paretic control of the AP and ML MoS. Statistical significance was set at an alpha of 5%.
First, to establish how the paretic and non-paretic AP and ML MoS are modified in response to a slip-like perturbation, we determined how the MoS varied from pre-perturbation through perturbation and recovery steps using two Repeated Measures ANOVAs for AP MoS and ML MoS, respectively. In each of these analyses, we included step (Pre-perturbation, Perturbation, Recovery 1–3) and leg (paretic or non-paretic) as within-subject factors and an interaction between step and leg. If the main effects were significant, we performed post-hoc tests to (i) compare paretic with non-paretic MoS and (ii) determine if the MoS in the recovery steps following the perturbation (Recovery 1–3) differed from Pre-perturbation. If a step by leg interaction was significant, we performed post-hoc tests to (i) compare paretic with non-paretic MoS at Pre-perturbation and (ii) assess whether changes in the MoS from Pre-perturbation to Recovery 1–3 differed between the paretic and non-paretic leg. We used the Greenhouse-Geisser correction if the assumption of sphericity was violated and the Tukey-Kramer correction for multiple comparisons in all post-hoc testing.
Second, we wanted to understand the relative contributions of non-paretic/paretic differences in the XCoM position and base of support to pre-perturbation non-paretic/paretic differences in MoS. To this end we performed a multiple linear regression with predictors 1) difference in non-paretic and paretic XCoM position and 2) difference in non-paretic and paretic edges of the base of support (step length for AP and step width for ML). This analysis was performed for the AP and ML direction separately. We also used multiple linear regression to assess the relative contributions of changes in XCoM position and changes in base of support (step length for AP and step width for ML) to the change in MoS from Pre-perturbation to Recovery 1. This analysis was performed for the AP and ML direction, and paretic and non-paretic leg separately.
Finally, we determined whether the AP MoS and the ML MoS covaried during pre-perturbation steps and the first recovery step following a perturbation. We fit two linear mixed effect models to quantify the relationship between independent variables AP MoS and leg (paretic or non-paretic) and the dependent variable ML MoS. This model included main effects for AP MoS and leg, an AP MoS by leg interaction, and a random intercept for each participant. We expected to observe covariation between AP MoS and ML MoS in recovery steps but not during pre-perturbation steps. Therefore, the first model was fit with each participant’s paretic and non-paretic AP MoS during Pre-perturbation steps, and the second model was fit with each participant’s paretic and non-paretic AP MoS during Recovery 1 steps. Two participants were excluded from this analysis as they received less than five correctly-timed perturbations on either the paretic or non-paretic side.
3. Results
3.1. Paretic and non-paretic margins of stability throughout perturbation recovery
For the AP MoS (Fig. 1A), our repeated measures ANOVA revealed significant main effects for leg (F(1,20) = 12.503, p = 0.002) and step (F(4,20) = 24.562, p < 0.001), which indicates that the AP MoS differed between paretic and non-paretic leg and across steps. We found no interaction between leg and step (p = 0.199). Post-hoc comparisons showed a significantly smaller paretic than non-paretic AP MoS (p = 0.002), and a significant increase in AP MoS from Pre-perturbation to Recovery 1 (p < 0.001). This indicates that participants walked with a smaller paretic than non-paretic AP MoS during pre-perturbation, perturbation, and recovery steps and restored their pre-perturbation MoS after one step.
Fig. 1 -.
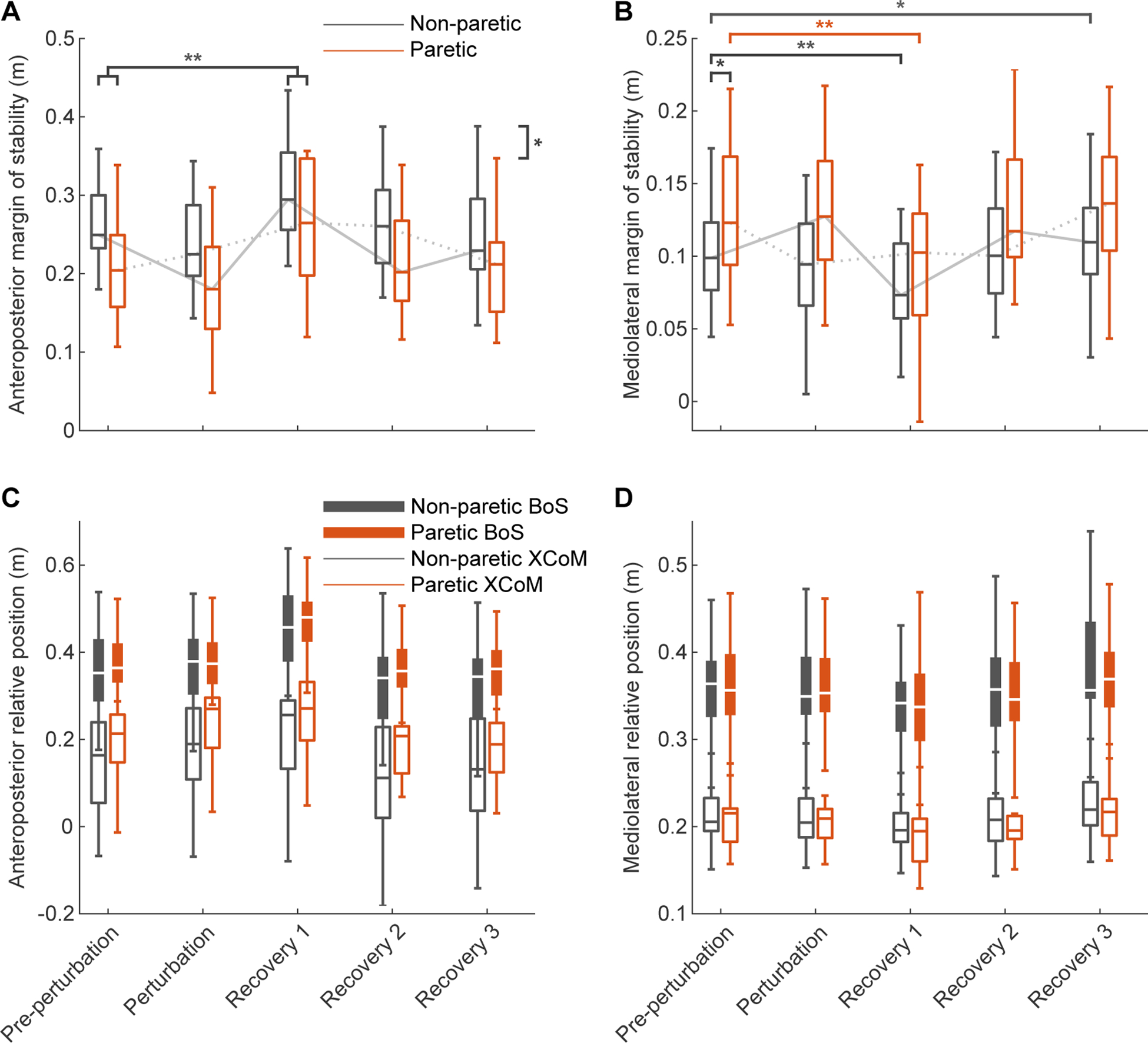
Group distribution of margins of stability for pre-perturbation, perturbation, and recovery steps (N=21). A) Paretic and non-paretic anteroposterior Margins of Stability (MoS). B) Paretic and non-paretic mediolateral MoS. C) Paretic and non-paretic anteroposterior edge of the Base of Support (BoS; leading leg 1st distal phalanx marker) and Extrapolated Center of Mass (XCoM) positions relative to the contralateral edge of the BoS (trailing leg 1st distal phalanx marker. D) Paretic and non-paretic mediolateral edge of the BoS (leading leg 5th metatarsal marker) and XCoM positions relative to the contralateral BoS (trailing leg 5th metatarsal marker). Asterisks indicates significant differences in MoS between limbs or phases (* p < 0.05, ** p < 0.001). The order of steps alternates between paretic and non-paretic, based on the side that was perturbed. The grey dotted lines indicate the series of steps in which the non-paretic leg was perturbed, the solid grey lines the series of steps in which the paretic leg was perturbed.
For the ML MoS (Fig. 1B), we found significant main effects for leg (F(1,20) = 8.464, p = 0.009) and step (F(4,20) = 45.118, p <0.001), and we found a significant interaction between leg and step (F(4,80) = 2.997, p = 0.040). Post-hoc comparisons showed a significantly larger paretic than non-paretic ML MoS during Pre-perturbation steps (p = 0.008), a significant decrease in ML MoS from Pre-perturbation to Recovery 1 in the paretic (p < 0.001) and non-paretic (p < 0.001) legs, and a significant increase in ML MoS from Pre-perturbation to Recovery 3 in the non-paretic leg (p = 0.008). This indicates that participants walked with larger paretic than non-paretic ML MoS before the perturbations. Furthermore, this shows that participants returned to pre-perturbation levels by the second recovery step when the perturbation occurred during non-paretic leg stance, but were still recovering until the third recovery step when the perturbation occurred during paretic leg stance.
3.2. Contributions to pre-perturbation differences between paretic and non-paretic margins of stability
The pre-perturbation difference between the non-paretic and paretic AP MoS was explained by a linear model including the difference between the non-paretic and paretic AP XCoM (β = −0.071, p < 0.001, Fig. 2A) and the difference between the non-paretic and paretic step length (β = 0.086, p <0.001, Fig. 2B). This model had an R2 of 0.675 (F(2,18) = 18.700, p < 0.001). This indicates that a smaller paretic than non-paretic AP MoS during pre-perturbation steps was explained by a more anterior paretic than non-paretic AP XCoM position and shorter paretic than non-paretic step length. The pre-perturbation difference between the paretic and non-paretic ML MoS could not be explained by a linear model, including the difference between the non-paretic and paretic ML XCoM and the difference between the non-paretic and paretic step width (p = 0.06, Fig. 2C, D). This suggests that pre-perturbation differences in paretic and non-paretic ML MoS may have different contributions between participants.
Fig. 2 -.
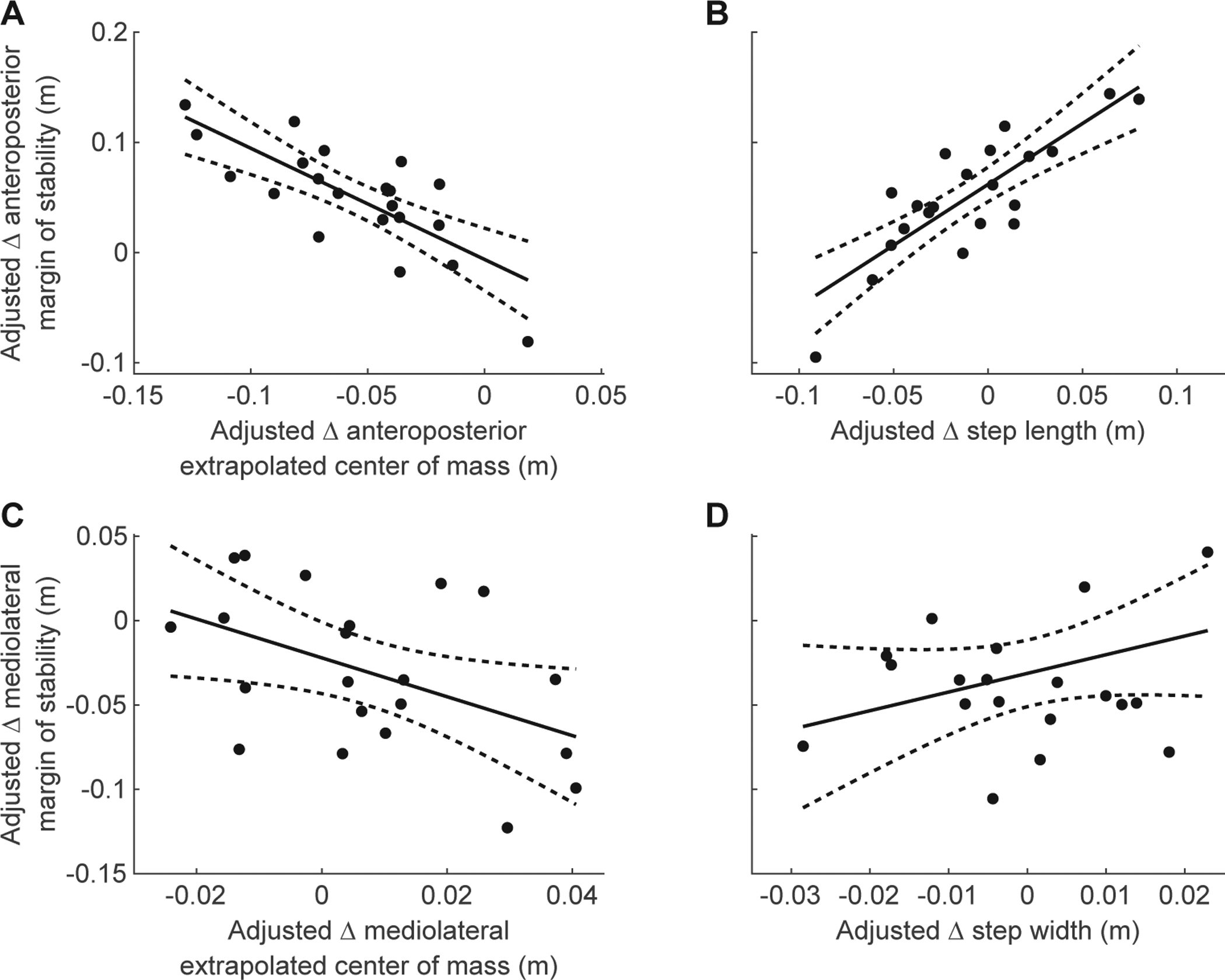
Partial regression plots of pre-perturbation difference in non-paretic and paretic (Δ) Margin of Stability (MoS), Extrapolated Center of Mass (XCoM), and Base of Support (BoS) (N=21). Δ Indicates difference between non-paretic and paretic leg at pre-perturbation. All data shown are adjusted values from the multiple regression analysis. A) Partial regression between Δ AnteroPosterior (AP) XCoM and Δ AP MoS. B) Partial regression between Δ step length and Δ AP MoS. C) Non-significant partial regression between Δ MedioLateral (ML) XCoM and Δ ML MoS. D) Non-significant partial regression between Δ step width and Δ ML MoS.
3.3. Contributions to changes margins of stability during perturbation recovery
The change from Pre-perturbation to Recovery 1 in paretic AP MoS was explained by a linear model including the change in paretic AP XCoM (β = −0.055, p < 0.001, Fig. 3A) and the change in paretic step length (β = 0.036, p < 0.001, Fig. 3B). This model had an R2 of 0.914 (F(2,18) = 95.1, p < 0.001). The change in non-paretic AP MoS was explained by a linear model including the change in non-paretic AP XCoM (β = −0.040, p < 0.001, Fig. 3C) and the change in non-paretic step length (β = 0.028, p < 0.001, Fig. 3D). This model had an R2 of 0.930 (F(2,18) = 120, p < 0.001). Therefore, both modulation of XCoM position by the trailing leg and increased step length by the leading leg contribute to increases in AP MoS during perturbation recovery.
Fig. 3 -.
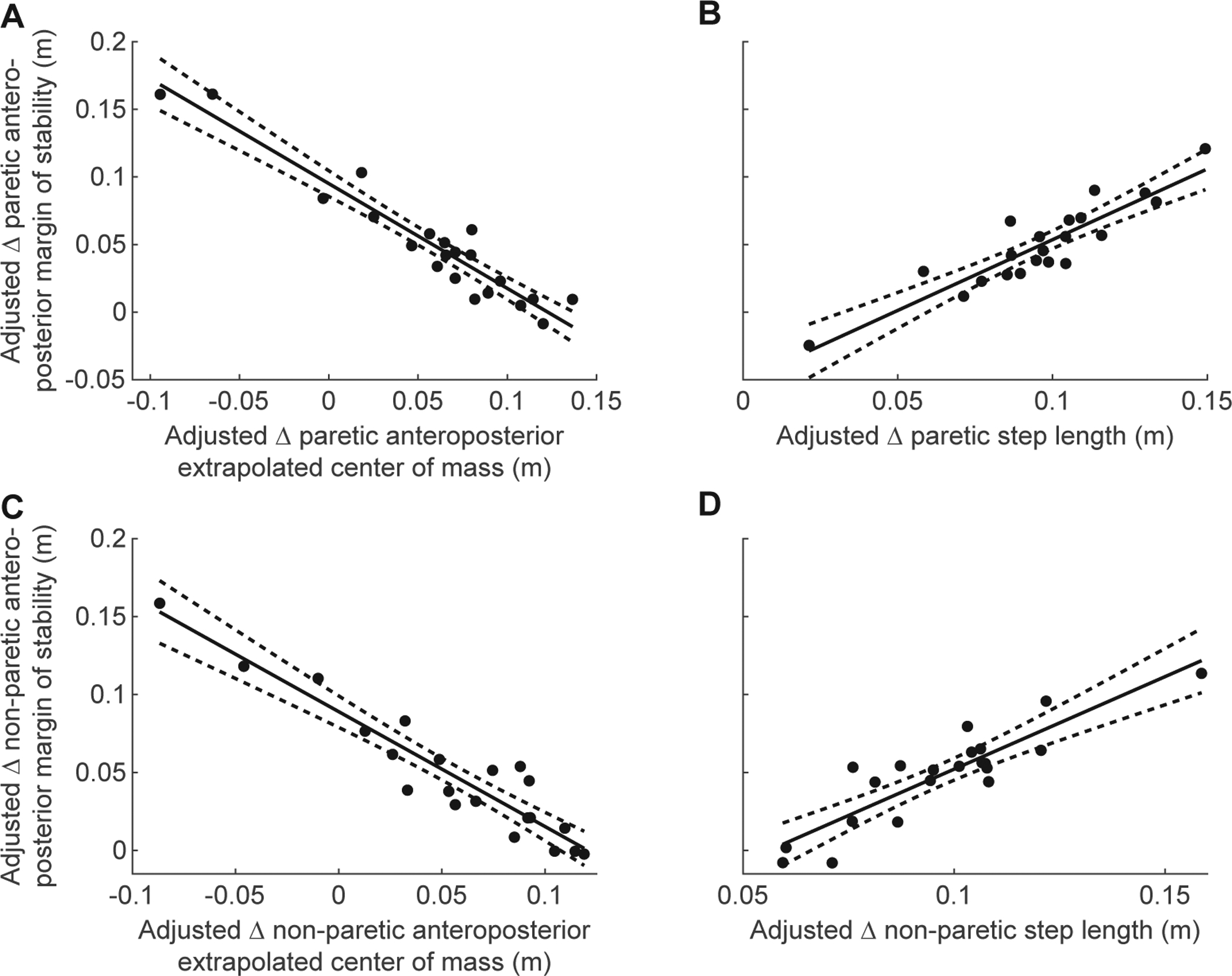
Partial regression plots of change from Pre-perturbation to Recovery 1 (Δ) in Extrapolated Center of Mass (XCoM) and Δ Base of Support (BoS) vs. Δ Margin of Stability (MoS) for the paretic and non-paretic leg in the AnteroPosterior (AP) direction (N=21). Δ Indicates change from Pre-perturbation to Recovery 1. All data shown are adjusted values from the multiple regression analysis. A) Partial regression between paretic Δ AP XCoM and Δ AP MoS. B) Partial regression between paretic Δ step length and Δ AP MoS. C) Partial regression between non-paretic Δ AP XCoM and Δ AP MoS. D) Partial regression between non-paretic Δ step length and Δ AP MoS.
The change from Pre-perturbation to Recovery 1 in paretic ML MoS was explained by a linear model including the change in paretic ML XCoM (β = −0.028, p = 0.008, Fig. 4A) and the change in paretic step width (β = 0.028, p = 0.009, Fig. 4B). This model had an R2 of 0.347 (F(2,18) = 4.78, p = 0.022). The change in non-paretic ML MoS could not be explained by a linear model that included the change in non-paretic ML XCoM and the change in non-paretic step width (p = 0.290, Fig. 4C, D). The model used to explain variance in paretic ML MoS had a relatively low R2 (0.347 versus 0.914 for the paretic AP MoS), and we were unable to fit a model to the non-paretic ML MoS. This indicates that although people post-stroke decrease their ML MoS in response to forward perturbations in gait, these changes are likely caused by participant-specific changes in ML XCoM position and step width.
Fig. 4 -.
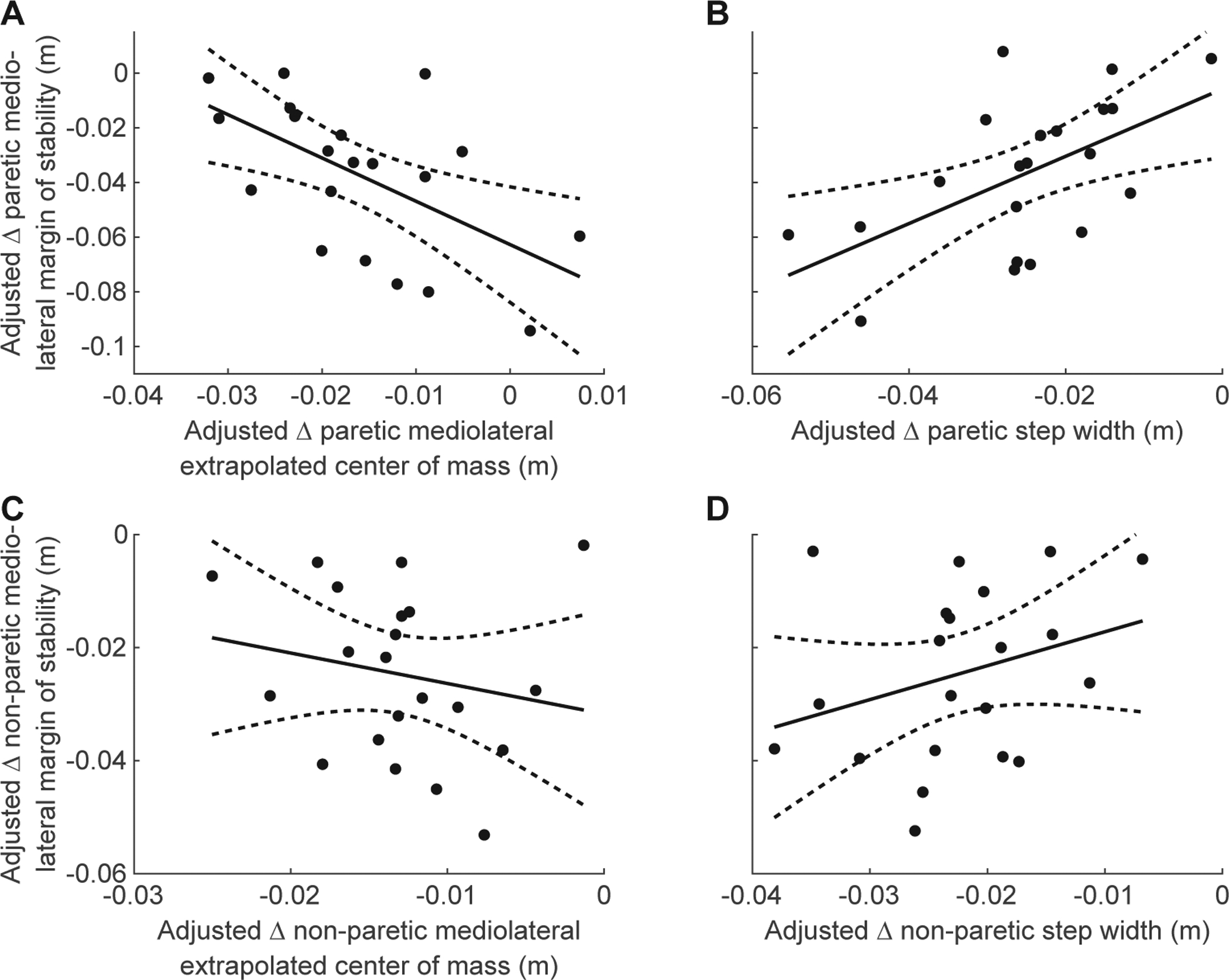
Partial regression plots of change from Pre-perturbation to Recovery 1 (Δ) in Extrapolated Center of Mass (XCoM) and Δ Base of Support (BoS) vs. Δ Margin of Stability (MoS) for the paretic and non-paretic leg in the Mediolateral (ML) direction (N=21). Δ Indicates change from Pre-perturbation to Recovery 1. All data shown are adjusted values from the multiple regression analysis. A) Partial regression between paretic Δ ML XCoM and Δ ML MoS. B) Partial regression between paretic Δ step width and Δ ML MoS. C) Non-significant partial regression between non-paretic Δ ML XCoM and Δ ML MoS. D) Non-significant partial regression between non-paretic Δ step width and Δ ML MoS.
3.4. Covariation between the anteroposterior and mediolateral margin of stability during perturbation recovery
There was no significant effect of AP MoS (p = 0.089), leg (p = 0.917) or interaction between AP MoS and leg (p = 0.060) on ML MoS during Pre-perturbation steps (Fig. 5A, B). This indicates that there is no covariation between AP MoS and ML MoS during unperturbed walking in people post-stroke. In contrast, there were significant main effects of AP MoS (β = −0.16, F(1,381) = 15.622, p < 0.001) and leg (β = −0.056, F(1,381) = 14.316, p < 0.001) as well as an interaction between AP MoS and leg (β = 0.14, F(1,381) = 8.727, p = 0.003) (Fig. 5C, D) on ML MoS at Recovery 1. This indicates that an increase in paretic AP MoS in response to a forward perturbation leads to a reduction in paretic ML MoS during the first recovery step (Fig. 5C). In contrast, there was no significant association between AP MoS and ML MoS during Recovery 1 for the non-paretic limb (p = 0.73, Fig. 5D).
Fig. 5 -.
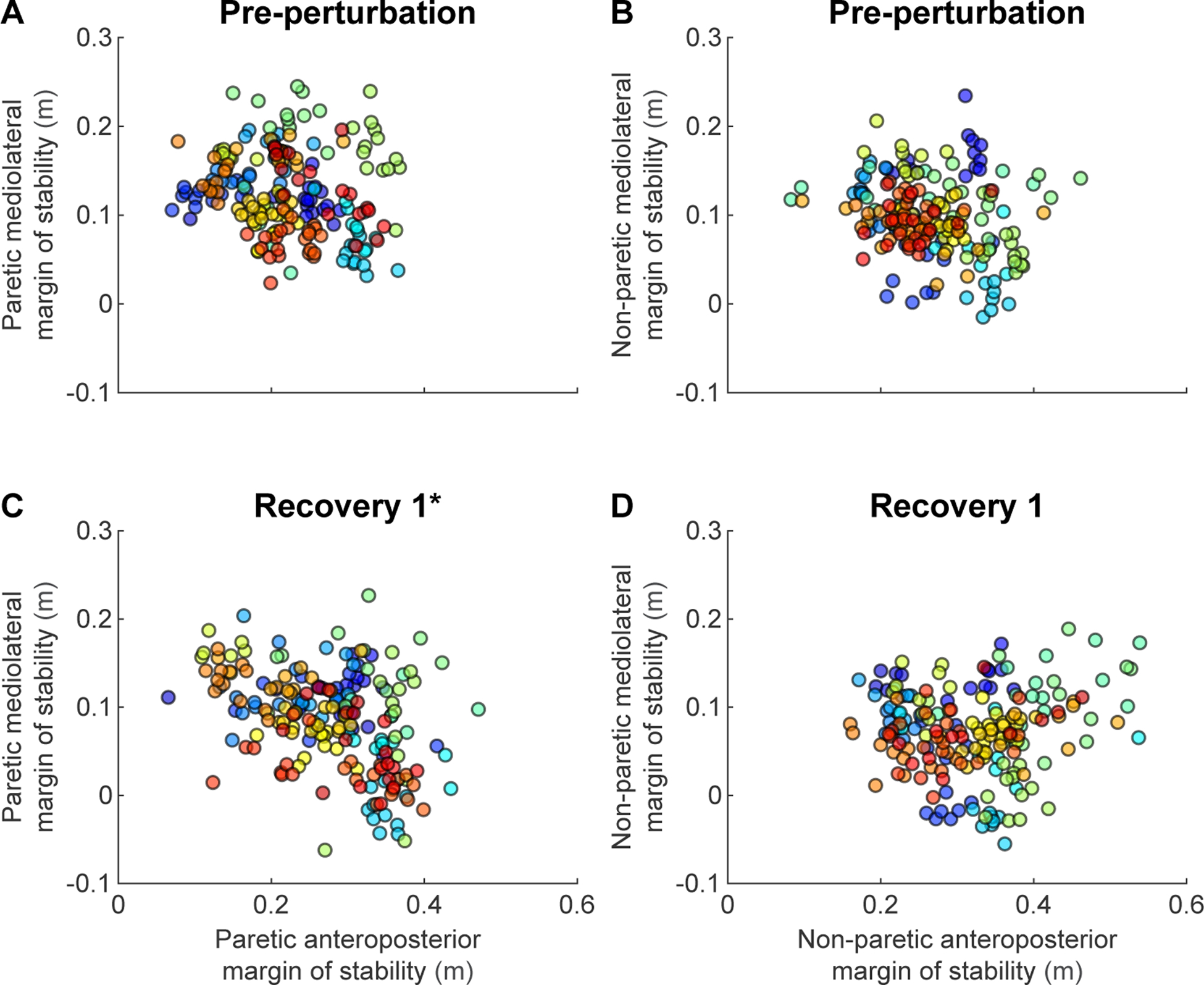
Covariation between anteroposterior Margin of Stability (MoS) and mediolateral MoS (N=19). Each color represents an individual participant. A) Paretic MoS at Pre-perturbation. B) Non-paretic MoS at Pre-perturbation. C) Paretic MoS at Recovery 1. D) Non-paretic MoS at Recovery 1. Participants who received less than five perturbations on each side were excluded from this analysis. Asterisks indicate panels that illustrate a significant covariation between the anteroposterior MoS and the mediolateral MoS.
4. Discussion
People with post-stroke hemiparesis are more susceptible to perturbations of dynamic balance during walking1. To increase our understanding of reactive balance control strategies in people post-stroke, we aimed to (i) to assess how the paretic and non-paretic AP and ML MoS are controlled in response to forward perturbations and (ii) to determine whether the AP MoS and the ML MoS during recovery steps covaried in response to a forward perturbation. We found that people post-stroke have smaller paretic than non-paretic AP MoS, and larger paretic than non-paretic ML MoS during unperturbed walking, consistent with previous studies18–20. In response to a forward perturbation during walking, people post-stroke increase their paretic and non-paretic AP MoS, while they simultaneously decrease their ML MoS. We also found that the paretic AP MoS and the paretic ML MoS covary during the first recovery step after a forward perturbation. This implies that improving sagittal plane balance may reduce frontal plane balance during reactive steps with the paretic leg.
4.1. People post-stroke walk with smaller anteroposterior and larger mediolateral margins of stability on the paretic side
We found that people post-stroke walk with smaller AP MoS and larger ML MoS on the paretic than the non-paretic side. The smaller paretic AP MoS at pre-perturbation was both due to a more anterior position of the XCoM relative to the trailing foot and reduced step length on the paretic side. The positive relationship between the difference in paretic and non-paretic AP MoS and the difference in paretic and non-paretic step length that was found in this study can be explained by step length asymmetry in people post-stroke. If people post-stroke walk with a shorter paretic than non-paretic step length, this leads to a smaller paretic than non-paretic AP MoS. In contrast, a longer paretic step leads to a larger paretic AP MoS4,43. The difference between paretic and non-paretic ML MoS may be due to both impaired sensation of the CoM state44 and stance time asymmetry4 in people post-stroke, which have been suggested to lead to a larger paretic ML MoS45,46. These findings indicate that the asymmetric paretic and non-paretic AP and ML MoS in people post-stroke may be a strategy to compensate for reduced sensation of CoM state44, unload the paretic leg during weight bearing20 and make the paretic side less sensitive to unexpected lateral perturbations by maintaining a larger paretic ML MoS26.
4.2. People post-stroke increase anteroposterior and decrease mediolateral margins of stability in response to a forward perturbation
We found that people post-stroke increase their paretic and non-paretic AP MoS in response to a forward perturbation, while they simultaneously decrease their paretic and non-paretic ML MoS. The increase in AP MoS during the recovery step was due to both less anterior displacement of the AP XCoM position and increased step length. This implies that the trailing limb reduces the forward momentum of the body in response to a forward perturbation, thereby achieving a larger AP MoS. Since this finding occurred in both the paretic and non-paretic leg, this suggests that people post-stroke are still capable of controlling the body’s momentum with the paretic leg during late stance, despite the paretic leg’s weakness4,5 and impaired coordination7–10.
The source of the reduction in ML MoS following a forward perturbation varied between participants, both due to more lateral displacement of the ML XCoM and reduced step width. This variation may be due to the heterogeneous nature of the post-stroke population. Differences between participants may have derived from differences in the ability to modulate stance time and thereby the ML XCoM excursion46. In addition, differences between participants may have derived from differences in the ability to modulate ML foot placement post-stroke44,47. Furthermore, all participants were exposed to the same perturbation belt speed and belt acceleration, while unperturbed treadmill belt speed was self-selected. Given the wide range of self-selected walking speeds in this population, the perturbation may have been more difficult for some participants than others, which may have contributed to the inter-subject variability. An alternative explanation is that there is only a small range of reduction in ML MoS between participants (3–6 cm, Fig. 4). Therefore, there may not have been enough variation between participants to get an accurate model of sources underlying the reduction in ML MoS. Previously, Hak et al. (2013) showed that in response to a lateral perturbation, people post-stroke increase their ML MoS19. This finding and the results of the present study are in line with previous work that suggests that people increase their base of support in the direction of the perturbation14–17, forward in the current study and lateral in Hak et al. (2013)19.
People post-stroke needed an extra recovery step to control their ML MoS when recovering from a perturbation of the paretic leg compared to a perturbation of the non-paretic leg. This means that when the non-paretic leg was perturbed, the first recovery step was made with the paretic leg and ML MoS was restored in the second recovery step, which was made with the non-paretic leg. In contrast, when the paretic leg was perturbed, the first recovery step was made with the non-paretic leg, the second recovery step was made with the paretic leg and a corrective third recovery step was needed with the non-paretic leg in which the ML MoS was slightly higher than during pre-perturbation. This suggests that a single step with the non-paretic leg after a perturbation during paretic stance is not sufficient and an additional recovery step with the non-paretic leg, after a paretic recovery step, is necessary to regain control over the body’s mediolateral center of mass displacement.
4.3. Covariation between sagittal and frontal plane balance during reactive stepping in people post-stroke
We also found that the AP MoS and the ML MoS covaried in the paretic limb during the first recovery step following a forward perturbation. On the contrary, we did not observe any associations between AP MoS and ML MoS during pre-perturbation steps or in non-paretic stepping responses. This is consistent with our hypothesis that covariation between the AP MoS and the ML MoS only occurs during reactive stepping responses, where people may have to rely more on transverse rotation of the pelvis to take larger steps than during unperturbed walking. Furthermore, this covariation was only observed in the paretic limb, which suggests that people post-stroke may rely on more transverse rotation of the pelvis to increase the paretic step length in reaction to a forward perturbation compared to when an increase of the non-paretic step length is desired. It should be noted that this is the first study to find covariation between mediolateral and fore-aft measures of balance in the paretic limb during reactive stepping post-stroke. It remains to be seen if this pattern of covariation extends to other perturbation magnitudes and perturbation types. Future studies should also determine if people post-stroke have the ability to decouple mediolateral and fore-aft balance control and, if so, determine if this decoupling can improve multi-step balance recovery.
4.4. Clinical implications of covariation in the reactive control of sagittal and frontal plane balance
Our findings have important implications for clinical practice. To prevent future falls, rehabilitation can be targeted at improving reactive stepping responses through perturbation-based training. However, to know at what to target rehabilitation practice, we must know which aspects of the stepping pattern will improve reactive balance responses in people post-stroke. We found that changes in AP MoS during reactive stepping were both due to changes in XCoM position and step length. The changes in the XCoM position during reactive stepping are most likely due to control of the body’s momentum by the trailing limb. Therefore, rehabilitation could be targeted at increasing the ability to bear weight on the paretic limb during perturbations of walking post-stroke to improve the paretic limb’s control of the body’s momentum.
Furthermore, we found covariation between the AP and ML MoS in paretic reactive stepping responses following a perturbation. This implies that an improvement in sagittal plane balance may compromise frontal plane balance in paretic stepping responses. In addition, this indicates that different relations hold during perturbed than unperturbed walking, which stresses the importance of training dynamic balance control in a task-specific fashion, e.g., by perturbation-based gait training to improve paretic stepping responses. Perturbation training could also be used to decouple the paretic AP and ML MoS in people post-stroke so that they can learn to maintain sagittal plane balance without compromising frontal plane balance. However, it remains to be investigated whether the here found paretic coupling does indeed put people post-stroke at risk for falling during walking. Finally, this study has shown that analysis of stability metrics during unperturbed walking may not predict how these measures change in response to perturbations. Therefore, the mechanisms that may lead to falls are likely best revealed when we challenge participants’ balance during walking.
While the present study brings novel information on balance control during reactive stepping in people post-stroke, it has some limitations. Here, we were interested in paretic versus non-paretic differences in control of the MoS and covariation between frontal and sagittal plane balance. However, to be able to assess abnormalities in control of the non-paretic MoS, a healthy control group is necessary, which was not included in this study. Furthermore, participants may have modified their gait in reaction to the expected perturbations33. We provided the participants with a familiarization trial before the experiment started to minimize first trial effects. However, the behavior during pre-perturbation steps in the current study could still differ from the participants’ normal walking pattern, as they may have exhibited a more cautious gait strategy.
5. Conclusion
In this study, we described how people with post-stroke hemiparesis control their AP and ML MoS in response to a forward perturbation during walking. People post-stroke walk with asymmetric AP and ML MoS during normal walking and in recovery from perturbations. These asymmetries in MoS may be the result of compensatory strategies to safeguard dynamic balance against perturbations on the paretic side. We found a systematic covariation between paretic sagittal and frontal plane balance measures, which implies that frontal plane balance is compromised when sagittal plane balance is improved during paretic stepping responses. This covariation during paretic stepping responses may be due to impairment in mechanical coupling, e.g., transverse rotation of the pelvis. Future rehabilitation efforts could focus on decoupling this covariation to improve dynamic balance in people post-stroke.
Acknowledgments
We thank Natalia Sanchez, Ph.D., for her help with Python coding.
Funding
This work was supported by St. Beatrixoord Noord-Nederland grant 210.180, NIH/NCATS grant UL1TR001855, and NIH NICHD grant R01HD091184.
Footnotes
Declarations of interest
None.
References
- 1.Den Otter AR, Geurts ACH, de Haart M, Mulder T, Duysens J. Step characteristics during obstacle avoidance in hemiplegic stroke. Exp Brain Res. 2005;161(2):180–192. doi: 10.1007/s00221-004-2057-0. [DOI] [PubMed] [Google Scholar]
- 2.Weerdesteyn V, de Niet M, van Duijnhoven HJR, Geurts ACH. Falls in individuals with stroke. J Rehabil Res Dev. 2009;45(8):1195–1214. doi: 10.1682/JRRD.2007.09.0145. [DOI] [PubMed] [Google Scholar]
- 3.Belgen B, Beninato M, Sullivan PE, Narielwalla K. The association of balance capacity and falls self-efficacy with history of falling in community-dwelling people with chronic stroke. Arch Phys Med Rehabil. 2006;87(4):554–561. doi: 10.1016/j.apmr.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005;22(1):51–56. doi: 10.1016/j.gaitpost.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Olney SJ, Richards C. Hemiparetic gait following stroke. part I: Characteristics. Gait Posture. 1996;4(2):136–148. doi: 10.1016/0966-6362(96)01063-6. [DOI] [Google Scholar]
- 6.Sharafi B, Hoffmann G, Tan AQ, Dhaher YY. Evidence of impaired neuromuscular responses in the support leg to a destabilizing swing phase perturbation in hemiparetic gait. Exp Brain Res. 2016;234(12):3497–3508. doi: 10.1007/s00221-016-4743-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finley JM, Perreault EJ, Dhaher YY. Stretch reflex coupling between the hip and knee: Implications for impaired gait following stroke. Exp Brain Res. 2008;188(4):529–540. doi: 10.1007/s00221-008-1383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes CT, Dhaher YY. Evidence of abnormal lower-limb torque coupling after stroke. Stroke. 2008;39(1):139–147. doi: 10.1161/STROKEAHA.107.492413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan AQ, Dhaher YY. Evaluation of lower limb cross planar kinetic connectivity signatures post-stroke. J Biomech. 2014;47(5):949–956. doi: 10.1016/j.jbiomech.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sánchez N, Acosta AM, Lopez-Rosado R, Stienen AHA, Dewald JPA. Lower extremity motor impairments in ambulatory chronic hemiparetic stroke: Evidence for lower extremity weakness and abnormal muscle and joint torque coupling patterns. Neurorehabil Neural Repair. 2017;31(9):814–826. doi: 10.1177/1545968317721974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Kam D, Geurts ACH, Weerdesteyn V, Torres-Oviedo G. Direction-specific instability poststroke is associated with deficient motor modules for balance control. Neurorehabil Neural Repair. 2018;32(6–7):655–666. doi: 10.1177/1545968318783884. [DOI] [PubMed] [Google Scholar]
- 12.Kajrolkar T, Bhatt T. Falls-risk post-stroke: Examining contributions from paretic versus non paretic limbs to unexpected forward gait slips. J Biomech. 2016;49(13):2702–2708. doi: 10.1016/j.jbiomech.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Hof AL, Gazendam MGJ, Sinke WE. The condition for dynamic stability. J Biomech. 2005;38(1):1–8. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Hak L, Houdijk H, Steenbrink F, et al. Speeding up or slowing down?: Gait adaptations to preserve gait stability in response to balance perturbations. Gait Posture. 2012;36(2):260–264. doi: 10.1016/j.gaitpost.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Hof AL, Vermerris SM, Gjaltema WA. Balance responses to lateral perturbations in human treadmill walking. J Exp Biol. 2010;213(15):2655–2664. doi: 10.1242/jeb.042572. [DOI] [PubMed] [Google Scholar]
- 16.Vlutters M, van Asseldonk EHF, van der Kooij H. Center of mass velocity-based predictions in balance recovery following pelvis perturbations during human walking. J Exp Biol. 2016;219(10):1514–1523. doi: 10.1242/jeb.129338. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Srinivasan M. Stepping in the direction of the fall: The next foot placement can be predicted from current upper body state in steady-state walking. Biol Lett. 2014;10(9):20140405. doi: 10.1098/rsbl.2014.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao P, Dingwell JB, Higginson JS, Binder-Macleod S. Dynamic instability during post-stroke hemiparetic walking. Gait Posture. 2014;40(3):457–463. doi: 10.1016/j.gaitpost.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hak L, Houdijk H, van der Wurff P, et al. Stepping strategies used by post-stroke individuals to maintain margins of stability during walking. Clin Biomech. 2013;28(9):1041–1048. doi: 10.1016/j.clinbiomech.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 20.van Meulen FB, Weenk D, van Asseldonk EHF, Schepers HM, Veltink PH, Buurke JH. Analysis of balance during functional walking in stroke survivors. PLos One. 2016;11(11):e0166789. doi: 10.1371/journal.pone.0166789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen JL, Kautz SA, Neptune RR. Forward propulsion asymmetry is indicative of changes in plantarflexor coordination during walking in individuals with post-stroke hemiparesis. Clin Biomech. 2014;29(7):780–786. doi: 10.1016/j.clinbiomech.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel PJ, Bhatt T. Modulation of reactive response to slip-like perturbations: Effect of explicit cues on paretic versus non-paretic side stepping and fall-risk. Exp Brain Res. 2015;233(11):3047–3058. doi: 10.1007/s00221-015-4367-9. [DOI] [PubMed] [Google Scholar]
- 23.Patel PJ, Bhatt T. Fall risk during opposing stance perturbations among healthy adults and chronic stroke survivors. Exp Brain Res. 2018;236(2):619–628. doi: 10.1007/s00221-017-5138-6. [DOI] [PubMed] [Google Scholar]
- 24.Whitcome KK, Miller EE, Burns JL. Pelvic rotation effect on human stride length: Releasing the constraint of obstetric selection. Anat Rec. 2017;300(4):752–763. doi: 10.1002/ar.23551. [DOI] [PubMed] [Google Scholar]
- 25.McAndrew Young PM, Dingwell JB. Voluntarily changing step length or step width affects dynamic stability of human walking. Gait Posture. 2012;35(3):472–477. doi: 10.1016/j.gaitpost.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hof AL, van Bockel RM, Schoppen T, Postema K. Control of lateral balance in walking. experimental findings in normal subjects and above-knee amputees. Gait Posture. 2007;25(2):250–258. doi: 10.1016/j.gaitpost.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 27.World Medical Association. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 28.Berg K, Wood-Dauphine S, Williams JI, Gayton D. Measuring balance in the elderly: Preliminary development of an instrument. Physiother Can. 1989;41(6):304–311. doi: 10.3138/ptc.41.6.304. [DOI] [Google Scholar]
- 29.Powels LE, Myers AM. The activities-specific balance confidence (ABC) scale. J Gerentol Med Sci. 1995;50(1):M28–34. [DOI] [PubMed] [Google Scholar]
- 30.Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. 2004;84(10):906–918. doi: 10.1093/ptj/84.10.906. [DOI] [PubMed] [Google Scholar]
- 31.Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the falls efficacy scale-international (FES-I). Age Ageing. 2005;34(6):614–619. doi: 10.1093/ageing/afi196. [DOI] [PubMed] [Google Scholar]
- 32.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 33.Bhatt T, Wening JD, Pai Y-C. Adaptive control of gait stability in reducing slip-related backward loss of balance. Exp Brain Res. 2006;170(1):61–73. doi: 10.1007/s00221-005-0189-5. [DOI] [PubMed] [Google Scholar]
- 34.Liu C, De Macedo L, Finley JM. Conservation of reactive stabilization strategies in the presence of step length asymmetries during walking. Front Hum Neurosci. 2018;12:251. doi: 10.3389/fnhum.2018.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Havens KL, Mukherjee T, Finley JM. Analysis of biases in dynamic margins of stability introduced by the use of simplified center of mass estimates during walking and turning. Gait Posture. 2018;59:162–167. doi: 10.1016/j.gaitpost.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song J, Sigward S, Fisher B, Salem GJ. Altered dynamic postural control during step turning in persons with early-stage parkinson’s disease. Parkinsons Dis. 2012;2012:386962. doi: 10.1155/2012/386962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winter DA. Biomechanics and motor control of human movement. 4th ed. New Jersey, NJ: John Wiley & Sons, Inc.; 2009:384. [Google Scholar]
- 38.Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair. 2009;23(7):735–744. doi: 10.1177/1545968309332880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurz MJ, Arpin DJ, Corr B. Differences in the dynamic gait stability of children with cerebral palsy and typically developing children. Gait Posture. 2012;36(3):600–604. doi: 10.1016/j.gaitpost.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 40.Salot P, Patel P, Bhatt T. Reactive balance in individuals with chronic stroke: Biomechanical factors related to perturbation-induced backward falling. Phys Ther. 2016;96(3):338–347. doi: 10.2522/ptj.20150197. [DOI] [PubMed] [Google Scholar]
- 41.Yang F, Bhatt T, Pai Y. Role of stability and limb support in recovery against a fall following a novel slip induced in different daily activities. J Biomech. 2009;42(12):1903–1908. doi: 10.1016/j.jbiomech.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park S, Finley JM. Characterizing dynamic balance during adaptive locomotor learning. EMBC. 2017:50–53. doi: 10.1109/EMBC.2017.8036760. [DOI] [PubMed] [Google Scholar]
- 43.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88(1):43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Dean JC, Kautz SA. Foot placement control and gait instability among people with stroke. J Rehabil Res Dev. 2015;52(5):577–590. doi: 10.1682/JRRD.2014.09.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stimpson KH, Heitkamp LN, Embry AE, Dean JC. Post-stroke deficits in the step-by-step control of paretic step width. Gait Posture. 2019;70:136–140. doi: 10.1016/j.gaitpost.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buurke TJW, Lamoth CJC, van der Woude LHV, Hof AL, den Otter R. Bilateral temporal control determines mediolateral margins of stability in symmetric and asymmetric human walking. Sci Rep. 2019;9:12494. doi: 10.1038/s41598-019-49033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dean JC, Embry AE, Stimpson KH, Perry LA, Kautz SA. Effects of hip abduction and adduction accuracy on post-stroke gait. Clin Biomech. 2017;44:14–20. doi: 10.1016/j.clinbiomech.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


