Abstract
Introduction
Postthrombotic syndrome (PTS) is a form of secondary chronic venous insufficiency (CVI) that occurs after deep vein thrombosis (DVT). Effective treatments for PTS are lacking. Micronized purified flavonoid fraction (MPFF) is a venoactive drug used in the treatment of CVI.
Objective
To determine whether MPFF is a good candidate to explore as a therapeutic agent for PTS.
Methods
We performed a narrative review in which we identified 14 systematic reviews, 33 randomized controlled trials, and 19 observational studies that discussed the use of MPFF in CVI, as well as studies that reported on the mechanistic action of MPFF in relation to the pathophysiology of PTS.
Results
MPFF targets a number of pathophysiologic components of PTS. Based on animal models and human studies investigating objective vascular and lymphatic measures, MPFF promotes venous recanalization after DVT, decreases venous remodeling and reflux, inhibits inflammatory processes, improves venous tone and stasis, improves lymphatic circulation, improves capillary hyperpermeability, and decreases tissue hypoxia. Furthermore, MPFF shows promise in improving clinical manifestations, quality of life, and objective venous parameters of CVI. Studies suggest good patient acceptability and tolerability with the use of MPFF in CVI.
Conclusion
MPFF is a good candidate to explore as a potential therapy for PTS. Confirmatory high‐quality studies are still needed to reinforce the evidence supporting the use of MPFF in CVI. Double‐blind randomized controlled trials with clinical endpoints are needed to assess the clinical efficacy of MPFF in the treatment of PTS.
Keywords: diosmin, flavonoids, hesperidin, postthrombotic syndrome, venous insufficiency, venous thrombosis
Essentials.
Postthrombotic syndrome (PTS) is venous insufficiency occurring after deep vein thrombosis.
Effective therapies for the PTS are lacking.
Micronized purified flavonoid fraction (MPFF) targets its pathophysiological components.
MPFF should be explored as a potential therapy for PTS.
1. INTRODUCTION
Postthrombotic syndrome (PTS) refers to the clinical manifestations of chronic venous insufficiency (CVI) that occur after deep vein thrombosis (DVT). PTS is the most frequent complication of DVT. 1 Its clinical manifestations are variable, ranging from mild symptoms and signs such as mild pain, swelling, and hyperpigmentation, to more severe manifestations such as intractable pain, venous claudication, and leg ulceration. 2 Though it is not a lethal condition, PTS is burdensome. It is the main determinant of quality of life (QOL) after DVT. 3 Management options for PTS, preventive or therapeutic, are limited. 4 Recent large randomized controlled trials (RCTs) showed that the use of catheter‐directed thrombolysis to prevent PTS after DVT was generally ineffective.5, 6, 7 A 2017 Cochrane systematic review with meta‐analysis concluded that the use of elastic compression stockings reduced the overall incidence of PTS following DVT (relative risk [RR], 0.62; 95% confidence interval [CI], 0.38‐1.01; P = .05). 8 The evidence, however, remains of low quality considering the methodological limitations of the trials included, such as the lack of adequate blinding. Furthermore, there is very low‐certainty evidence supporting the use of elastic compression stockings in the treatment of PTS. 9 New therapeutic targets are thus needed for the treatment of established PTS. 10
One of the therapeutic options to explore is the use of venoactive drugs or phlebotonics. 11 Venoactive drugs comprise a heterogeneous group of medicinal products of plant or synthetic origin. While rarely used in North America, they are commonly used in Europe for the treatment of CVI.11, 12 A Cochrane meta‐analysis, including 53 RCTs (n = 6013 participants), of the effectiveness of venoactive medications in the treatment of CVI reported a beneficial effect by venoactive drugs on edema (RR, 0.70; 95% CI, 0.63‐0.78) and on some CVI symptoms, when compared with placebo. 13 However, as underlined by authors of this review, there is a lack of strong evidence supporting the use of venoactive drugs for the treatment of CVI in general and in established PTS.
Among venoactive drugs that could be tested in a high‐quality trial for the treatment of PTS is micronized purified flavonoid fraction (MPFF), which appears to have a particularly favorable profile. MPFF is composed of micronized diosmin and flavonoids. Its flavonoid fraction can take many forms, including that of hesperidin, diosmetin, and linarin. 14 The proportion of micronized diosmin to flavonoids varies, with 9:1 being a commonly used ratio. MPFF acts on improving venous obstruction, valvular reflux, and inflammatory venous damage, which are key components contributing to the pathogenesis of PTS. 15 In this article, we review the mechanism of action of MPFF and its relevance in the treatment of the pathophysiologic components of PTS. Given that PTS presents as CVI after DVT, we review the clinical efficacy, tolerability, and acceptability of the use of MPFF in CVI, with a focus on PTS. Finally, we establish whether MPFF should be further explored as a potential new therapeutic agent for PTS.
2. METHODS
We conducted a scientific literature search using PubMed, MEDLINE, Cochrane libraries (Cochrane Database of Systematic Reviews, Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, and National Health Service Economic Evaluation Database), and ClinicalTrials.gov databases from inception to November 28, 2018, with the latest updated PubMed search on July 25, 2020, to retrieve the relevant articles reporting the use of MPFF in the treatment of CVI and PTS. We identified additional literature from reference lists of relevant articles, conference proceedings, abstracts, reports, presentations, online theses, journals, and books. We performed manual searches on the latter to identify potential articles missing from initial electronic searches. Given the heterogeneous nature of MPFF, we used a broad range of search terms to identify a wide array of studies that may be of relevance. The terms include registered commercial names of MPFF, drug categories such as phlebotonics under which MPFF falls, and individual components of MPFF such as diosmin and flavonoids. We performed updated PubMed searches using terms including Alvenor, Ardium, Arvenum, Capiven, Daflon, Detralex, diosmetin, diosmin, diosmiplex, Elatec, Flavonoids, Flebotropin, hesperidin, isorhoifolin, linarin, micronized purified flavonoid fraction, phlebotonics, Variton, Venarus, Venitol, and veno‐active drugs in combinations with the following: chronic venous disease, chronic venous insufficiency, phlebothrombosis, post‐phlebitic disease, post‐thrombotic syndrome, venous stasis and venous ulcer.
An overview of our study selection is depicted in Figure 1. The studies retrieved and reviewed were restricted to systematic reviews, RCTs, observational studies, and articles discussing the mechanism of action of MPFF. First, we included studies describing the use of MPFF, which is composed of diosmin and an additional flavonoid fraction, in CVI or PTS. In these studies, MPFF treatment was compared with placebo, conventional treatment, baseline status in observational studies, or other agents such as aminaphthone, coumarin‐toxerutin combination, or diosmin alone (Tables 1, 2, 3). Studies discussing an agent other than MPFF alone, without comparing it to MPFF, were excluded. Second, we also included studies on patients with venous thrombosis who did not have CVI at onset, assessing for MPFF’s effect on clinical manifestations and objective venous changes related to CVI and PTS. Third, we included studies discussing the mechanistic action of MPFF on pathophysiological mechanisms underlying PTS, including those without clinical endpoints and those performed in animal models. All other studies included were performed in human subjects. Studies published in the English language and those translated to English were included. We excluded duplicate studies, studies not discussing venous disease, and studies discussing other types of venous disease. Among the systematic reviews, several were duplicates in that the same group of studies were meta‐analyzed and identical results were presented, with minor addendums at times. They were thus considered as a single systematic review for the purposes of this narrative review to minimize multiple publication bias, unless a noticeable addition in content was noted. Similarly, we also treated duplications of RCTs and observational studies as a single study. RCT publications presenting the results of multiple trials within the same publication were considered a single RCT‐type publication.
FIGURE 1.
Flow diagram of study selection. CDSR: Cochrane Database of Systematic Reviews; CENTRAL: Central Register of Controlled Trials; DARE: Database of Abstracts of Reviews of Effects; EMBASE: Excerpta Medica Database; NHS EED: National Health Service Economic Evaluation Database
TABLE 1.
Systematic reviews discussing the use of micronized purified flavonoid fraction in chronic venous insufficiency
|
First author Year of publication |
Type of CVI |
Study design Number of participants Dosage and duration of MPFF |
Duration of follow‐up | Main results and interpretations |
|---|---|---|---|---|
| Boada, 1999 91 | Any CVI |
Systematic review and meta‐analysis 21 studies included (n=817) Included 3 RCTs with phlebotonics: |
At least 4 wk |
Efficacy: Venoactive drugs, including MPFF:
Acceptability and tolerability not reported in abstract |
| Lyseng‐Williamson, 2003 90 | Any CVI |
Systematic review Included 7 RCTs on CVI23, 24, 39, 40, 45, 64, 78 10 to 170 participants in included RCTs Treatment duration from 2 to 12 months |
2‐12 months |
Efficacy: MPFF:
Acceptability not reported Tolerability:
|
| Martinez, 2005 94 | Any CVI |
Systematic review and meta‐analysis Included 44 RCTs of oral phlebotonics: 4413 participants in total 2417 participants received a phlebotonic agent and 1996 received placebo Treatment duration from 2 to 6 months |
2‐6 months |
Efficacy: Phlebotonics:
MPFF:
Acceptability not reported Tolerability: Most frequently reported side effects were gastrointestinal disorders |
| Ramelet, 2005 93 | Any CVI |
Systematic review Included 4 RCTs on MPFF33, 77, 78, 83 40‐160 participants in included studies |
2‐6 months |
Efficacy: Grade A level of evidence for the use of MPFF Acceptability not reported Tolerability not reported |
| Coleridge‐Smith, 200531, 104 | Venous ulcers |
Systematic review and meta‐analysis Included 5 RCTs: 723 participants in total MPFF (Daflon) 500 mg BID for 2‐6 months and conventional treatment (compression and local care)
|
2‐6 months |
Efficacy: MPFF was associated with:
MPFF may be a useful adjunct to conventional therapy in large and long‐standing ulcers Acceptability not reported Tolerability: Adverse effects such as gastrointestinal disturbances were present in 10% of people |
|
Nelson, 2008 98 Nelson, 2011 32 |
Venous ulcers |
Systematic review Part on flavonoids included 5 RCTs and 1 systematic review on these same RCTs31, 104 on which present review comments MPFF (Daflon) 500 mg BID for 2‐6 months and conventional treatment (compression and local care)
723 participants in total |
2‐6 months |
Efficacy:
Commenting on previous systematic review 31, 104 :
Review excluded 2 unpublished RCTs from the meta‐analysis because of missing data at baseline, intermediate time points, or study incompletion; it is not clear what impact these RCTs would have had on the meta‐analysis Acceptability not reported Tolerability: 10% of people reported gastrointestinal disturbances |
| Allaert, 2012 92 | Any CVI |
Systematic review and meta‐analysis 10 double‐blind RCTs (1010 patients): |
2‐6 months |
Efficacy:
Acceptability and tolerability not reported |
| Scallon, 2013 97 | Venous ulcers |
Cochrane systematic review and meta‐analysis 5 RCTs (723 participants) on MPFF: Same RCTs as Nelson’s32, 98 and Coleridge‐Smith’s31, 104 systematic reviews MPFF (Daflon) 500 mg BID for 2 months 77 or |
2‐6 months |
Efficacy:
Acceptability not reported Tolerability: More side effects as compared to placebo (RR, 1.52; 95% CI, 1.01‐2.3): 47/218 patients in the MPFF group and 30/213 patients in the control group Most commonly skin changes (including eczema), gastrointestinal disturbances (including diarrhea), and hypertension |
| Rabe, 2013 96 | Any CVI |
Systematic review Included 3 double‐blind RCTs On flavonoids (with MPFF)77, 83, 105 101‐105 participants in RCTs MPFF 500 mg BID for 2 months or hidrosmin 200 mg TID for 45 d vs placebo |
45‐60 d |
Efficacy:
Acceptability and tolerability not reported |
| Martinez, 2016 13 | Any CVI |
Systematic review and meta‐analysis 53 RCTs (6013 participants): |
Up to 12 months |
Efficacy: No pooled results for MPFF Acceptability not reported Tolerability:
|
| Varatharajan, 201695, 103 | Any CVI |
Systematic review and meta‐analysis 1 systematic review on flavonoids including MPFF 97 with 723 participants. MPFF (Daflon) 500 mg at the usual dosage for 2‐6 months |
2‐6 months |
Efficacy: Flavonoids (including MPFF) may be effective adjuncts but methodological shortcomings and issues with bias limit the validity of results Acceptability and not reported Tolerability: Side effects of flavonoids: skin changes, gastrointestinal disturbances such as diarrhea, hypertension |
| Bush, 201789, 110 | Any CVI |
Systematic review 10 papers including a Cochrane review13, 23, 39, 47, 48, 71, 75, 77, 78, 80, 106 34‐160 participants in each included study MPFF (Daflon) 500 mg BID for 4 wk to 6 months |
4 wk to 6 months |
Efficacy:
Acceptability not reported Tolerability: The risk of adverse effects appears minimal:
|
| Kakkos, 2018 88 | Any CVI |
Systematic review and meta‐analysis 7 double‐blind RCTs (1692 patients)24, 71, 72, 78, 79, 80, 83 with copublications23, 24; MPFF compared with placebo |
4 wk to 4 months |
Efficacy: MPFF, compared to placebo, improved:
According to authors, MPFF is highly effective in patients with CVI when it comes to improving leg symptoms, edema, and QOL. This is based on high‐quality evidence, in their opinion Acceptability and tolerability not reported |
| Mansilha, 2019 87 | Varicose veins, with endovenous, sclerotherapy, or surgical treatment |
Systematic review 5 open‐label studies67, 73, 74, 81, 82 60‐245 patients in studies MPFF 1000‐3000 mg daily |
2 wk preop to 30 days postop, up to 90 days total |
Efficacy:
Acceptability and tolerability not reported |
Not reported: Information not reported in the full text of the manuscript. Not reported in abstract: Information not reported in the abstract and the full text of the manuscript is not available.
Abbreviations: BID: twice daily; CI: confidence interval; CVI: chronic venous insufficiency; d: day(s); months: month(s); MPFF: micronized purified flavonoid fraction; NNT: number needed to treat; OR: odds ratio; QOL: quality of life; RCT: randomized controlled trial; RR: relative risk; RRR: relative risk reduction; SMD: standardized mean difference; TID: 3 times a day; wk: week(s).
TABLE 2.
Randomized controlled trials discussing the use of micronized purified flavonoid fraction in chronic venous insufficiency
|
First author Year of publication |
Type of CVI |
Study design Number of participants Dosage and duration of MPFF |
Duration of follow‐up | Main results and interpretations |
|---|---|---|---|---|
| Biland, 1982 80 | Not available |
Double‐blind RCT 70 patients |
4 wk |
Efficacy: Objective improvement of venous disease as assessed by physician in the form of improvement of leg redness, edema, and skin changes Acceptability and tolerability not available |
| Amiel, 1987 99 | Includes patients with PTS |
Double‐blind RCT MPFF 500‐mg 2 tablets daily Included in narrative review 111 |
Not available |
Efficacy: Positive effect on venous tone measured by plethysmography starting 1 h after administration Acceptability and tolerability not available |
| Chassignolle, 1987 24 | Any CVI |
Double‐blind RCT 40 patients MPFF (Daflon) 500‐mg 2 tablets daily vs placebo for 2 months |
2 mo |
Efficacy:
Patient’s satisfaction
Clinician’s satisfaction
Tolerability: Well tolerated, with no side effects reported by the 18 patients who completed the trial |
| Laurent, 1988 47 | Organic or functional CVI |
2 double‐blind RCTs 200 patients MPFF (Daflon) 1000 mg daily for 2 months vs placebo |
2 months |
Efficacy: MPFF:
Acceptability: Good acceptability Tolerability not reported in abstract |
|
Cospite, 1989 45 Potential duplicate of Amato, 1994 46 |
Lower‐limb CVI, functional CVI, varicose veins, PTS |
Multicenter, double‐blind, RCT 90 outpatients (including functional CVI, 39 patients; varicose veins, 32 patients; PTS, 17 patients) MPFF (5682 SE, Daflon) two 500‐mg tablets a day vs single diosmin (900 mg) for 2 months Include in systematic review 90 |
Up to 2 months |
Efficacy:
Acceptability
Tolerability:
|
| Tsouderos, 1989 and 199123, 41 |
Crossover phase II trial PTS Pharmacoclinical trial CVI without varicose, during pregnancy and PTS Phase III clinical trial Functional CVI |
3 double‐blind RCTs (including that of Chassignolle, at al 1987 24 ) Crossover phase II trial 20 patients with PTS Single dose of MPFF (Daflon) 1000 mg vs placebo Pharmacoclinical trial 3 groups of 10 women each Daflon 500 mg two tablets daily for 1 wk Phase III clinical trial 2 parallel groups of 20 patients each MPFF (Daflon) 500 mg 2 tablets daily vs placebo for 2 months Included in meta‐analyses13, 88, 94 and systematic review 90 |
2 h 1 wk 2 months |
Efficacy: Crossover phase II trial MPFF decreased:
Modifications were observed 2 h after administration. No significant change observed in T50 outflow, cardiac index, capillary filtration index, blood pressure, cardiac or respiratory rate Pharmacoclinical trial
Phase III clinical trial MPFF, after 1 and 2 months of treatment:
Acceptability not reported in abstract Tolerability: Crossover phase II trial 2 h after administration: no significant change in cardiac index, blood pressure, cardiac or respiratory rate Pharmacoclinical trial: Not reported in abstract Phase III clinical trial: Not reported in abstract |
| Planchon, 1990 79 | Any CVI |
RCT 110 patients |
2 months |
Efficacy: MPFF significantly reduced:
Cramps Acceptability and tolerability not available |
|
Barbe, 1992 25 |
Including PTS, functional CVI, pregnancy‐related CVI |
3 double‐blind RCTs RCT 1: Patients with PTS RCT 2: Women with CVI −10 with functional CVI, −10 with pregnancy‐related CVI −10 with postthrombotic CVI RCT 3: patients with functional CVI |
Not available |
Efficacy: MPFF led to:
Acceptability and tolerability not available |
|
Chassignolle, 1994 and 199933, 44 Potential duplicate of Chassignolle, 1987 24 and of trial by Tsouderos23, 41 |
Functional CVI |
Double‐blind RCT 40 women (22‐49) MPFF (Daflon) vs placebo for 2 months Plethysmographic and clinical outcomes included in meta‐analyses13, 94 |
2 months |
Efficacy: As compared with placebo, MPFF improved:
Acceptability:
Tolerability:
|
| Menyhei, 1994 70 | Any CVI including PTS, primary varicose veins |
Multicenter, double‐blind RCT 320 patients MPFF (Daflon) 500 mg BID vs 1000 mg once in the morning vs 1000 mg once in the evening for 2 months |
2 months |
Efficacy: As compared with baseline:
No difference between groups Acceptability and tolerability not reported in abstract |
| Gilly, 199442, 43, 78 | Symptomatic disturbance of veno‐lymphatic system with or without CVI, including PTS |
Two‐center, double‐blind RCT 160 outpatients MPFF (Daflon) 500 mg vs placebo for 8 wk Included in meta‐analyses13, 88, 94 and systematic reviews90, 93 |
2 months |
Efficacy: Significant improvement with MPFF in terms of:
Acceptability: Good acceptability. Tolerability:
|
|
Amato, 1994 46 Potential duplicate of Cospite, 1989 45 |
Any CVI stable for 1 y |
Multicenter, double‐blind RCT 90 patients MPFF (Daflon) 500 mg 2 tablets vs diosmin equivalent dose for 2 months |
2 months |
Efficacy: Statistically greater improvements in terms of clinical symptoms and plethysmographic parameters with:
Acceptability: Satisfaction: 95% in the MPFF group vs 80% in the diosmin group (P < .01) Tolerability:
|
| Ibegbuna, 1997 26 | Symptomatic varicose veins in one leg and abnormal elastic modulus without varicosities in other leg |
Open‐label RCT 25 patients MPFF (Daflon) 500 mg 2 tablets daily vs no treatment for 4 wk |
4 wk |
Efficacy: MPFF significantly improved elastic modulus and venous tone in patients at risk of developing varicose veins when compared to no treatment Acceptability and tolerability not reported |
| Guilhou, 199740, 77 | Any venous ulcer, including PTS related |
Multicenter, double‐blind, RCT 105 patients MPFF (Daflon) 500 mg 2 tablets daily and compression therapy vs placebo and compression for 2 months Included in meta‐analyses13, 94, 97, 104 and systematic reviews90, 93, 96 |
2 months |
Efficacy: As compared with placebo, MPFF led to: ‐ Among patients with ulcer size ≤10 cm, greater and faster rate of ulcer healing (32% vs 13%; P = .03) and shorter duration of healing (P = .04)
Acceptability: 2 patients withdrew consent in MPFF group (vs 4 in placebo group) for reasons unrelated to side effects Tolerability:
|
| Glinski, 1999 and 200139, 76 | Any venous leg ulcer, including PTS related |
Multicenter, open‐label RCT 140 patients Standard treatment (including compression) plus MPFF (Daflon) 2 tablets daily for 6 months vs standard treatment alone |
6 months |
Effectiveness: As compared with standard treatment alone, addition of MPFF was associated with:
Acceptability not reported Tolerability: Slightly more adverse effects in the control group, although no statistical difference in adverse effects between the groups; adverse effects not specified |
| Danielsson, 2002 83 | Symptomatic CVI |
Double‐blind RCT 101 patients MPFF 500 mg BID vs placebo for 60 d Included in meta‐analyses13, 88, 93, 94 and systematic review 96 |
60 d |
Efficacy: MPFF did not significantly improve:
Results might be more favorable to MPFF in subgroup of patients with edema:
Acceptability not reported Tolerability:
|
|
Belcaro, 2002 85 |
Severe CVI |
RCT Group I 90 patients MPFF (Daflon) 500 mg every 8 h vs rutoside (0‐[beta‐hydroxyethyl]‐rutosides, Venoruton) 2 g/d for 8 wk Group II (included additionally in Cesarone’s publication) 38 : 122 patients: Comparable patients included in a registry following the same study format |
8 wk |
Efficacy: Rutoside significantly:
Acceptability not reported Tolerability: No side effects and no dropouts observed; good compliance |
| Maruszynski, 200436, 84 | Symptomatic CVI, CEAP C0‐3 |
Multicenter, double‐blind RCT 119 patients MPFF 500 mg BID vs diosmin 600 mg BID |
28 d |
Efficacy:
Acceptability not reported Tolerability: Both MPFF and diosmin were safe and well‐tolerated:
|
| Roztocil, 2003 75 | Venous ulcers, including PTS related |
RCT 150 patients MPFF (Daflon) 2 tablets daily and standard treatment including compression vs standard treatment alone for 6 months |
6 months |
Efficacy: MPFF significantly:
Acceptability: MPFF considered excellent by 85% of patients Tolerability: No treatment‐related side effects reported; 99%‐100% compliance during course of study |
| Cesarone, 2006 86 | Severe CVI, venous ulceration |
RCT 86 patients MPFF (Daflon) 1000 mg daily vs pycnogenol 50 mg TID vs Pycnogenol 300 mg daily for 8 wk |
8 wk |
Efficacy: Pycnogenol significantly superior to MPFF in:
Acceptability not reported Tolerability: Treatments well tolerated in all groups, no side effects reported, no dropouts |
| Veverková, 2005 and 200635, 74 | Patients who underwent a stripping procedure of the great saphenous vein |
Open‐label, multicenter RCT 181 patients MPFF (Daflon) 500 mg 2 tablets daily from 14 d before to 14 d after stripping surgery (1 months total) vs control (not treated with MPFF) |
14 d before to 14 d after procedure |
Efficacy: MPFF significantly:
Acceptability not reported Tolerability:
|
|
Pokrovsky, 2007 73 Saveljev, 2008 34 |
Varicose veins, undergoing phlebectomy |
RCT 245 patients MPFF (Detralex) 1000 mg daily (n=200) vs no agent (n=45) 2 weeks before phlebectomy for varicose veins until 30 d after procedure Included in systematic review 87 |
2 wk before to 30 d after the procedure |
Efficacy: MPFF significantly decreased:
MPFF improved orthostatic and exercise tolerance in the early postoperative period; it had no impact on QOL 30 d postoperatively Acceptability not reported Tolerability: Minor adverse effects (gastric irritation) of MPFF appeared in 4 cases (1.6%) during the first 2 wk of administration and resolved spontaneously |
| Bogachev, 2012 67 | CEAP C2‐4 s, undergoing endovascular treatment |
Open‐label RCT 230 patients MPFF (Detralex) 1000 mg daily (n=126) vs compression therapy (n=104) from 14 d before procedure to 30 d after procedure Included in systematic review 87 |
2 wk before to 30 d after procedure |
Efficacy: MPFF:
|
| Belczak, 2014 72 | CEAP C2‐5 |
Double‐blind, RCT 136 patients MPFF vs aminaphthone vs coumarin and troxerutin vs placebo (starch) for 30 d Included in meta‐analysis 88 |
Until 30 d after treatment |
Efficacy:
Acceptability not reported Tolerability: 9 patient dropouts:
|
| Stoiko, 2015 82 | CVI undergoing endovenous thermal ablation |
Open‐label RCT 60 patients MPFF 3000 mg on days 1‐4, 2000 mg on days 5‐7 vs control Included in systematic review 87 |
7 d after procedure |
Efficacy:
Acceptability and tolerability not reported in abstract |
| Rabe, 2015 71 | CEAP C3‐4 |
Multinational, parallel group, double‐blind RCT 1137 participants MPFF (Daflon) 500 mg 2 tablets administered at 1 dosing at lunchtime for 4 months vs placebo Included in meta‐analysis 88 |
6 months |
Efficacy: MPFF significantly:
Acceptability not reported Tolerability:
|
| Kirienko, 2016 69 | CEAP C0‐4 |
International, parallel‐group, double‐blind RCT 174 patients MPFF 1000 mg once daily vs MPFF 500 mg BID for 8 wk |
8 wk |
Efficacy:
Acceptability not reported in abstract Tolerability:
|
| Carpentier, 2017 68 | CEAP C0 s−4 s |
International, parallel‐group, double‐blind RCT 1139 patients MPFF 1000 mg once daily vs MPFF 500 mg BID for 8 wk |
8 wk |
Efficacy:
Acceptability not reported Tolerability:
|
| Katseni, 2017 27 | CVI and lower‐extremity venous ulcer |
RCT 60 patients Group A: Elastic compression stockings Group B: MPFF 500 mg BID and elastic compression stockings Group C: MPFF 500 mg BID, antibiotics, and elastic compression stockings |
40 d |
Efficacy:
Acceptability and tolerability not reported |
| Toledo, 2017 66 | Venous ulcers |
Longitudinal prospective RCT 30 patients Group 1 (n=15): Pycnogenol 50 mg orally TID Group 2 (n=15): MPFF (diosmin/hesperidin) 450/50 mg orally BID for 90 d |
90 d |
Efficacy: Pycnogenol and MPFF:
Acceptability and tolerability not reported |
| Ignat’ev, 2018 65 | PTS of the lower extremities |
Open prospective RCT 80 patients MPFF (Venarus) and conservative treatment (n=40) vs conservative treatment (n=40) alone |
Not reported in abstract |
Efficacy:
Acceptability and tolerability not reported in abstract |
| Lobastov, 2019 102 | Femoropopliteal DVT, investigating for development of PTS |
Open‐label RCT 60 patients MPFF 1000 mg daily and rivaroxaban vs rivaroxaban alone |
6 months |
Efficacy: In the MPFF group:
Acceptability not reported Tolerability:
|
| Saveliev, unpublished | CVI, including trophic ulcers |
Open RCT: multicenter trial involving 3 centers in Russia 124 patients Group 1: MPFF (Detralex) 500 mg 2 tablets daily and standard local therapy with compression bandaging Group 2: standard therapy with elastic compression and local treatment alone Included in meta‐analyses 97 |
6 months |
Efficacy:
Acceptability not available Tolerability: Adverse events:
|
Not reported: Information not reported in the full text of the manuscript. Not reported in abstract: Information not reported in the abstract and the full text of the manuscript is not available. Not available: Neither the abstract nor the full text of the manuscript are available, and information not reported in other reviews.
Abbreviations: BID: twice daily; CEAP: Clinical‐Etiology‐Anatomy‐Pathophysiology Comprehensive Classification System for Chronic Venous Disorders; CIVIQ: Chronic Venous Insufficiency Questionnaire; CVI: chronic venous insufficiency; d: day; DVT: deep vein thrombosis; h: hour(s); months: month(s); MPFF: micronized purified flavonoid fraction; PTS: postthrombotic syndrome; QOL: quality of life; RCT, randomized controlled trial; TID: 3 times a day; VCSS: Venous Clinical Severity Score; Ve‐QOL: Venous Quality of Life Score; wk: week; y: year.
TABLE 3.
Observational studies discussing the use of micronized purified flavonoid fraction in chronic venous insufficiency
|
First author Year of publication |
Type of CVI |
Study design Number of participants Dosage and duration of MPFF |
Duration of follow‐up | Main results and interpretations |
|---|---|---|---|---|
| Guillot, 1989 64 | Any CVI |
Multicenter study 170 outpatients MPFF (Daflon) 2 tablets daily vs baseline Included in systematic review 90 |
12 months |
Efficacy: With MPFF, significant improvement in:
Benefits started from second month of treatment and increased with time Acceptability: Excellent or useful in 91% of cases (58% excellent, 33% useful, 9% nil) Tolerance: Mainly epigastric pain (4.1%, n=7) |
| Blume, 1992 63 | Any CVI, including PTS and varicose veins |
20 patients: 9 PTS, 11 varicose veins |
Every 2 wk |
Efficacy: MPFF was associated with significant decrease in leg volume by optoelectronic method of the more affected lower leg of 263 mL (8%) in all patients and 392 mL (12%) in patients with varicose veins Acceptability and tolerability not available |
| Iablokov, 1996 62 | Severe CVI |
76 patients MPFF (Detralex) 500 mg BID for 2 months |
Not reported in abstract |
Efficacy: MPFF relieved CVI symptoms in most cases Acceptability not reported in abstract Tolerability: Well tolerated |
| Jantet, 2000 68and 200248, 49 | Symptomatic CVI, CEAP C1‐4 |
Prospective, controlled, multicenter, international study First consolidated data 48 Worldwide results 49 Intention‐to‐treat analysis (confirmed to have taken 2 tablets of MPFF): 3075 patients 48 4527 patients 49 Per‐protocol (adhered to all protocol conditions): 2395 patients 48 3174 patients 49 MPFF (Daflon) 500 mg 2 tablets daily for 6 months. Instructions to not change their habits as to the wearing or not of compression stockings |
6 months |
Efficacy: MPFF significantly:
Acceptability:
Tolerability not reported |
| Ting, 2001 61 | Mild to moderate CVI |
Prospective study 28 patients MPFF (Daflon) 500 mg oral BID for 6 months |
6 months |
Efficacy: MPFF significantly:
Improvement in cramps was not statistically significant. No significant change in venous filling index, ejection fraction, or residual volume fraction; clinical improvement without associated changes in venous hemodynamics as measured by air plethysmography Acceptability not reported Tolerability: No side effects encountered |
| Sirotin, 2003 60 | CEAP C0‐4 |
14 patients MPFF (Detralex) |
Not reported in abstract |
Efficacy: MPFF led to:
Acceptability and tolerability not reported in abstract |
| Navratilova, 2010 59 | Symptomatic CVI with edema |
Observational study 213 patients included 196 patients completed study in accordance with protocol MPFF (Daflon) 500 mg 2 tablets daily for 6 months |
6 months |
Efficacy: As compared with baseline, MPFF significantly:
Acceptability:
Tolerability:
|
| Pitsch, 2011 101 | Telangiectasia, with or without varicose veins and edema, undergoing sclerotherapy |
Observational study 3202 patients MPFF (Daflon) 500 mg 2 tablets daily in patients undergoing sclerotherapy (microsclerosis with foam, liquid sclerosing agents or laser) from the first session to the last session of sclerotherapy, for 2 months |
2 months |
Efficacy: MPFF and sclerotherapy significantly:
81% of patients satisfied or very satisfied with the combination of sclerotherapy and MPFF Tolerability: Side effects in 2.4% of patients: Mainly hematomas (0.4%), postprocedure pain (0.3%), and inflammation (0.3%) |
| Lenkovic, 2012 58 |
Any CVI with at least 3 symptoms (pain, heaviness, swelling, night cramps) CEAP C0‐C6 |
Prospective study 1212 patients MPFF (Daflon) 500 mg 2 tablets daily for 6 months |
6 months |
Effectiveness: As compared to baseline, regardless of stage of disease, MPFF significantly improved (P < .05):
Acceptability and tolerability not reported |
| Gudymovich, 2013 100 | Any CVI |
Observational study MPFF (Venarus) |
At least 4 wk |
Efficacy: MPFF:
Acceptability and tolerability not reported in abstract |
| Zubarev, 2014 57 | CEAP C2 |
Observationnal study 19 patients MPFF (Detralex) 1000 mg daily for 3 months |
3 months |
Effectiveness:
Acceptability and tolerability not reported in abstract |
| Yanushko, 2014 56 | Symptomatic CVI |
Prospective observational study 557 patients MPFF (Daflon) 500 mg 2 tablets daily for 2 months |
6 months |
Efficacy: Strong significant (P < .01) decrease in the number of patients with the following symptoms at the end of the second month of treatment:
6% reduction of patients with edema In terms of QOL improvement, the Global Index Score (GIS) decreased from 32.9±21.0 at baseline to 14.6±14.7 (P < .0001) after 2 months of treatment Acceptability: 94% of patients and 96% of physicians estimated efficacy of MPFF to be high or very high Tolerability: 4.2% of cases had adverse events on MPFF: Mostly gastrointestinal, appearing after 2‐3 d and disappearing at the end of treatment; 1 case of urticaria reported |
| Son’kin, 2014 53 | PTS |
Open multicenter retrospective study 110 patients MPFF (Venarus) with conventional PTS treatment or conventional treatment alone for 3 months at least |
At least 3 months |
Efficacy: MPFF lead to:
Greatest improvements occurred when MPFF was administered for at least 3 months Acceptability not reported in abstract Tolerability: No side effects noted during study |
| Zudin, 2014 30 | DVT without varicose disease, investigating for development of PTS |
Prospective study 66 patients Group I (n=22): Angiotropic and metabolic infusion therapy, direct and indirect anticoagulant and elastic compression Group II (n=22): Same as Group I, with MPFF (Venarus) 1000 mg daily for 2 months every half year Group III (n=22): Same as Group I, with MPFF (Venarus) 1000 mg daily uninterrupted |
18 months |
Efficacy:
Acceptability and tolerability not reported in abstract |
| Tsoukanov, 2015 28 |
Subjective CVI, CEAP C0 |
Open‐label prospective study 41 women MPFF (Daflon) 1000 mg once daily in the morning for 2 months |
2 months |
Efficacy: Significant improvement in:
MPFF led to reduction of greater saphenous vein reflux in most treated patients and decrease in vein diameter Acceptability and tolerability not reported |
| Tsukanov, 2016 55 | PTS secondary to iliac thrombosis with small varicose pelvic veins, with impaired urination |
Observational study 70 patients with acute iliac thrombosis, among which 24 patients received MPFF: those suffering most from the disease, that is, with urination impairment MPFF 1000 mg once daily for 1 months |
1 months |
Efficacy: MPFF:
Acceptability not reported Tolerability: No side effects noticed |
| Tsukanov, 2017 54 | Telangiectasia, reticular varices |
Observational study 96 patients MPFF (Daflon) 1000 mg for 90 d |
90 d |
Efficacy: MPFF:
Acceptability and tolerability not reported |
| Bogachev, 2018 52 | CEAP C1 with dilated intradermal veins, undergoing sclerotherapy |
Multicenter observational study 1150 patients: 905 took MPFF, remainder had sclerotherapy alone MPFF 1000 mg daily for 6 wk, beginning 2 wk before sclerotherapy |
2 wk before sclerotherapy to 4 wk after sclerotherapy |
Efficacy:
Acceptability: Outcomes of treatment exceeded patient expectations, by Darvall questionnaire. Tolerability: No adverse events with MPFF; fewer sclerotherapy‐induced hyperpigmentation with adjunctive MPFF compared to sclerotherapy alone |
| Mazzaccaro, 2018 81 | Varicose veins treated with radiofrequency ablation, stripping, crossectomy, or phlebectomy |
Observational study, case‐controlled, comparing those who complied to venoactive drug vs those who did not 132 patients Compression therapy with venoactive drug (MPFF 500 mg BID or sulodexide 250 mg BID) for 90 d following procedure Included in systematic review 87 |
90 d |
Efficacy: No significant difference between patients who took a venoactive drug (MPFF or sulodexide) and those who did not in terms of:
Acceptability not reported Tolerability:
|
Not reported: Information not reported in the full text of the manuscript. Not reported in abstract: Information not reported in the abstract and the full text of the manuscript is not available. Not available: Neither the abstract nor the full text of the manuscript are available, and information not reported in other reviews.
Abbreviations: BID: twice daily; CEAP: Clinical‐Etiology‐Anatomy‐Pathophysiology Comprehensive Classification System for Chronic Venous Disorders; CIVIQ: Chronic Venous Insufficiency Questionnaire; CVI: chronic venous insufficiency; d: day(s); DVT: deep vein thrombosis; months: month(s); MPFF: micronized purified flavonoid fraction; PTS: postthrombotic syndrome; QOL: quality of life; wk: week(s).
Given the scarcity of the manuscripts accessible for detailed review of older studies and substantial heterogeneity between studies in terms of design, population, duration of treatment, and outcomes assessed, conducting a meta‐analysis as part of a systematic review with statistical pooling was neither suitable nor feasible. Instead, a narrative review and descriptive presentation of findings was deemed methodologically appropriate. When the information was retrievable, each article was described in terms of the type of CVI investigated, study design, number of participants, dosage, composition and duration of pharmacotherapy, duration of follow‐up, main results, and interpretations. This information was summarized in Tables 1, 2, 3. Studies were then discussed in terms of their reports of the efficacy, acceptability, and tolerability of MPFF in the treatment of CVI. When the full manuscript or the abstract of a study was inaccessible but key findings were presented in other review articles, we instead used this source of information.
3. RESULTS
3.1. Description of studies
We identified 14 systematic reviews, 33 RCTs, and 19 observational study publications discussing the use of MPFF in CVI, including PTS (Figure 1). Tables 1, 2 through 3 summarize the studies by study type. Obvious duplicate publications were cited and grouped together within a single row. Fifteen studies were retained as relevant to the discussion of the mechanistic action of MPFF in relation to the pathophysiology of PTS.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30
Among studies we have identified on the use of MPFF in CVI, including PTS, there were two duplicate publications of systematic reviews.31, 32 One of these duplicates was a systematic review that was conducted in 2011, three years following the earlier review. The latter discussed the same RCTs when it comes to MPFF in CVI and differs only in the addition of a table presenting such studies. 32 There were 11 duplicate RCT‐type publications,33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 including one duplicate of a publication discussing three separate RCTs 41 and two duplicates of a single RCT,42, 43 with the remaining being duplicates of distinct RCTs. A total of 11 RCT studies were duplicated at least once. It is important to note that although these duplicate publications discussed trials that were presented in another publication, some of them presented additional study groups or discussed additional content, such as QOL.37, 38 Additionally, two RCT publications24, 25 and a third RCT publication that also has a duplicate publication33, 44 seemed to be presenting trials similar to the ones published by Tsouderos. 23 The more recent Chassignolle RCT publications33, 44 also bear a resemblance to the original Chassignolle 1987 RCT 24 . Two other RCTs also seemed to be potential duplicate publications of each other.45, 46 The distant date of publication and resulting inaccessibility of the full manuscripts of studies prevented us from confirming these suspicions. Three RCT publications presented the results of several trials within the same publication.23, 25, 47 There was a single duplicate publication of an observational study. 48 The preliminarily consolidated results of this study were first published in 2000, 48 whereas the worldwide results were subsequently published 2 years later. 49
3.2. Efficacy
3.2.1. MPFF’s mechanism of action and the pathophysiology of PTS
Following DVT, conventional treatment with anticoagulants prevents further thrombus extension without lysis of the thrombus, with the hope that the residual clot burden is cleared by endogenous thrombolysis. Unfortunately, residual thrombus often remains. 50 The pathogenesis of PTS begins with venous obstruction and valvular reflux resulting from acute, then residual venous thrombosis (Figure 2). Inflammation can delay thrombus resolution, worsening both obstruction and reflux. As a consequence of persistent venous occlusion and reflux, venous hypertension results. 50 Venous hypertension leads to tissue hypoperfusion and hyperpermeability, which together cause the clinical manifestations of PTS: edema, hyperpigmentation, ulceration, heaviness, and pain. Studies support the relevance of the mechanism of action of MPFF in treating pathophysiological components of PTS, making it an interesting candidate for the potential management of PTS.
FIGURE 2.
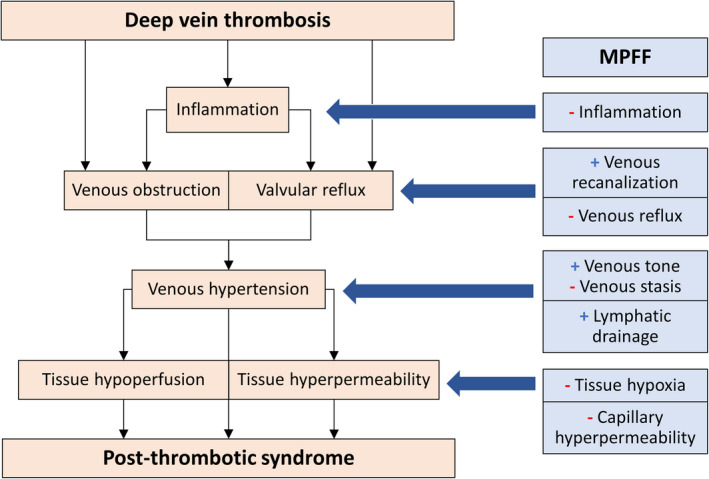
The pathophysiology of the postthrombotic syndrome (PTS) and the mechanism of action of micronized purified flavonoid fraction. The pathogenesis of PTS begins with venous obstruction and valvular reflux resulting from acute, then residual venous thrombosis. Inflammation can delay thrombus resolution, worsening both obstruction and reflux. As a consequence of persistent venous occlusion and reflux, venous hypertension results. Venous hypertension leads to tissue hypoperfusion and hyperpermeability, which together cause the clinical manifestations of PTS. MPFF improves venous recanalization following DVT and decreases venous reflux, acting on two key steps in the establishment of venous hypertension leading to PTS: venous obstruction and valvular reflux. MPFF has anti‐inflammatory effects, which may prevent further obstruction and reflux resulting from inflammatory responses following deep vein thrombosis. MPFF increases venous tone, decreases venous stasis, and improves lymphatic circulation, further relieving venous hypertension. Finally, MPFF acts on the deleterious outcomes of venous hypertension: it decreases tissue hypoxia and capillary hyperpermeability. MPFF: micronized purified flavonoid fraction
The pathophysiology of PTS and the relevance of the mechanism of action of MPFF are summarized in Figure 2. First, MPFF could favor venous recanalization after a DVT. In Zudin’s 30 observational study of patients with DVT, those treated with MPFF in addition to conventional anticoagulant treatment achieved better and 15% to 20% faster rates of recanalization compared to patients not treated with MPFF.
Second, MPFF protects the venous wall from remodeling and reflux. In a model for venous hypertension induced by femoral arterial‐venous fistula, male Wistar rats received MPFF 4 days before induction of the fistula, and venous reflux was measured by duplex ultrasound. 29 Rats who received MPFF had less venous reflux, valvular damage, and manifestations of leakage such as limb edema compared to their counterparts who did not receive the drug. 29 In a prospective observational study of female patients with CVI, MPFF decreased valvular reflux and vein diameter. 28
Third, MPFF protects venous and capillary systems by inhibiting inflammatory processes. MPFF decreased granulocyte and macrophage venous valvular infiltration in the previously discussed rat model of venous hypertension. 29 In an observational study of patients with CVI, MPFF decreased expression of adhesion molecules by neutrophils and monocytes, inhibited the leukocyte‐endothelium interaction, and decreased inflammatory mediator release. 51 In a RCT comparing MPFF to elastic compression stockings in patients with CVI, MPFF led to a decrease in white blood cell trapping around venous ulcers. 27
Fourth, MPFF improves venous tone and reduces stasis. Double‐blind placebo‐controlled trials in patients with CVI showed that MPFF reduces venous hypertension by increasing venous tone and reducing venous capacitance, distensibility, and stasis.23, 24, 25, 26
Fifth, MPFF improves lymphatic circulation, an important contributor to blood return, by increasing contractility of lymphatic capillaries. 22 In a prospective observational study of patients with severe CVI, MPFF improved lymphatic microangiopathy by increasing the number of functional lymphatic capillaries. 21
Sixth, MPFF has a protective effect on the microcirculation with improvement of capillary hyperpermeability and resistance. In a model of increased microvascular permeability induced in male hamster cheek pouches by histamine, bradykinin, and leukotriene, 20 10 days of MPFF decreased macromolecular permeability and reduced the number of leukocytes adhering to the venular endothelium. 20 In a double‐blind RCT of patients with idiopathic cyclic edema syndrome, MPFF was associated with significant improvement in capillary hyperpermeability, significant reduction in the sensation of swelling, and significant weight loss. 19 A RCT of patients with abnormal capillary fragility treated with MPFF showed that MPFF improved capillary resistance and relieved symptoms of capillary fragility such as spontaneous ecchymosis and epistaxis. 18
Finally, MPFF stimulates the microcirculation and prevents tissue hypoxia. This beneficial effect of MPFF was suggested by evidence of higher transcutaneous oximetry measurements in patients with CVI randomly assigned to MPFF when compared to placebo. 17 In an observational study of patients with CVI, MPFF improved venous microangiopathy and reduced capillary stasis by increasing red blood cell velocity in capillaries, compared to baseline. 16
MPFF acts on the main steps of the pathophysiology leading to PTS (Figure 2). MPFF improves venous recanalization following DVT and decreases venous reflux, acting on two key steps in the establishment of venous hypertension leading to PTS: venous obstruction and valvular reflux. MPFF has anti‐inflammatory effects, which may prevent further obstruction and reflux resulting from inflammatory responses following DVT. MPFF increases venous tone, decreases venous stasis, and improves lymphatic circulation, further relieving venous hypertension. Finally, MPFF acts on the deleterious outcomes of venous hypertension: It decreases tissue hypoxia and capillary hyperpermeability. Altogether, MPFF’s mechanism of action makes it a relevant therapeutic agent to explore as therapy for PTS.
3.2.2. Improvement of CVI signs and symptoms
A summary of the findings of efficacy, acceptability, and tolerability presented in systematic reviews, RCTs and observational studies are presented in Tables 1, 2, and 3, respectively. MPFF is a heterogeneous drug, and its manufacturing process varies. As a result, differences in the composition of the drug used may have led to variability of reported results. When available, we described the specific brand name and dose of MPFF used in studies (Tables 1, 2, 3). Among the studies we identified, 15 observational studies28, 64 totaling 8303 patients and 21 RCTs23, 24, 80 totaling 4817 patients showed a benefit of MPFF in improving the clinical manifestations of CVI, while 1 observational study 81 and 2 RCTs 82, 83 showed no improvement of CVI signs and symptoms with MPFF compared to no MPFF. Eight observational studies49, 54, 55, 56, 58, 59, 61, 64 totaling 6873 patients and 9 RCTs44, 69, 71, 73, 75, 77, 78, 79, 83 totaling 2222 patients showed a benefit of MPFF in improving sensory and functional symptoms of CVI, such as leg pain, paresthesia, and feeling of swelling. One observational study 81 and 2 RCTs 82 (including the unpublished Saveliev RCT) showed no significant differences in pain improvement with MPFF compared to no treatment with MPFF. Three RCTs68, 69, 70 totaling 1633 patients showed that there was no difference in symptom improvement among patients who took MPFF 1000 mg once daily compared to 500 mg twice daily and that both regimens improved clinical manifestations of CVI equally compared to baseline.
One RCT 72 found that MPFF was superior to aminaphthone and a coumarin‐toxerutin combination in terms of leg volume reduction. Two RCTs,45, 46 which are similar and may represent duplicate publication, found MPFF to be superior to diosmin in improving CVI clinical manifestations. The RCTs compared MPFF, composed of 90% diosmin and 10% hesperidin, with diosmin alone. Although sparse, the limited evidence suggests that the addition of hesperidin, the purification of such a flavonoid fraction, the micronization, or any other processing step to obtain MPFF may confer additional pharmacologic benefit over diosmin alone. This must be explored in further studies, as one RCT reported that diosmin and MPFF had similar effectiveness. 84 One RCT found MPFF to be similar to pycnogenol in improving venous ulcer healing and limb circumference. 66 One RCT 85 found rutoside to be superior and another found pycnogenol 86 to be superior to MPFF in improving signs and symptoms of CVI.
Six systematic reviews31, 87, 88, 89, 90, 91 concluded that MPFF showed benefit in improving signs and symptoms of CVI. Two systematic reviews suggested grade A evidence for the use of MPFF in CVI.92, 93 One systematic review concluded that MPFF’s effect on signs and symptoms of CVI were no different from placebo. 94 Five systematic reviews94, 95, 96, 97, 98 reported uncertain benefit or that further higher‐quality evidence was needed to support the use of MPFF in CVI.
3.2.3. Improvement in venous ulcer healing
Seven RCTs27, 65, 66, 75, 76, 77 (including the unpublished Saveliev RCT) totaling 689 patients showed a benefit of MPFF in CVI ulcer healing. One RCT 77 showed no difference with MPFF compared to placebo in healing ulcers >10 cm. Four systematic reviews31, 89, 90, 97 reported improvement of ulcer healing with MPFF. One systematic review 97 pointed out flaws in the current evidence of MPFF use in CVI ulcers, including inadequate reporting in RCTs and potential publication bias. One systematic review 98 reported that good‐quality evidence is lacking to show whether MPFF combined with compression therapy was superior to compression alone in improving ulcer healing.
3.2.4. Improvement in objective venous measures
Studies reported on the effect of MPFF on objective venous measures related to CVI, including limb perimeter, plethysmographic parameters, elastic modulus, capillary permeability, venous capacity, venous outflow, venous tone, venous distensibility, venous emptying time, venous reflux, and other venous hemodynamic measures. Seven observational studies28, 54, 55, 57, 60, 61, 63 totaling 288 patients reported improvement of objective venous measures with MPFF, while one observational study 61 reported no significant change in venous filling index, ejection fraction, residual volume fraction, or venous hemodynamics despite clinical improvement of CVI with MPFF.
Ten RCTs23, 24, 25, 26, 27, 99 totaling 690 patients (excluding two RCTs25, 99 where the number of patients was unavailable) reported a benefit of MPFF in improving objective venous measures related to CVI. Two RCTs,45, 46 which may be duplicate publications, reported that MPFF was superior to diosmin in improving objective venous measures. One RCT 72 showed that leg volume reductions of ≥100 mL were more frequent with MPFF when compared to aminaphthone, coumarin in combination with troxerutin, and placebo. One RCT 83 showed no significant improvement of foot‐volumetric or ultrasonographic parameters compared to placebo. One RCT 86 found pycnogenol to be superior, and one RCT 85 found rutoside to be superior to MPFF in improving objective venous measures of CVI.
Three systematic reviews88, 91, 92 reported a benefit of MPFF in improving objective venous measures in CVI, and one systematic review 92 found MPFF to be superior to ruscus extract, hydroxyethylrutoside, diosmin, and placebo in reducing ankle circumference.
3.2.5. Improvement in QOL
Eight observational studies28, 49, 52, 53, 54, 56, 100, 101 totaling 9683 patients (excluding one observational study 100 ) and four RCTs67, 68, 71, 74 totaling 2687 patients showed improvement in QOL with MPFF in CVI. One observational study 81 and two RCTs73, 82 showed no significant impact of MPFF on QOL. One RCT 37 found rutoside to be superior, and another 72 found aminaphthone to be superior for QOL improvement in CVI when compared to MPFF. One systematic review 89 reported improved QOL with MPFF, while another systematic review 88 concluded that the evidence on the effect of MPFF on QOL in patients with CVI was weak.
In terms of the use of validated QOL assessment tools, six RCTs67, 68, 71, 73, 74, 82 and five observational studies28, 49, 52, 54, 101 used the Chronic Venous Insufficiency Questionnaire, one RCT 37 used the Venous Quality of Life Score (Ve‐QOL), one observational study used the Short‐Form 12, 81 and one observational study 56 used the Global Index Score. The remaining studies either used an adapted QOL questionnaire 72 or did not report the method of measurement.53, 100
3.2.6. Improvement when MPFF was used with ancillary interventions
Two observational studies52, 101 found MPFF to be effective in improving symptoms and QOL in patients with CVI when combined with sclerotherapy. One observational study 30 found MPFF to be effective in decreasing CVI manifestations when added to angiotropic metabolic infusion therapy, anticoagulation, and elastic compression. Two RCTs73, 74 found that MPFF reduced signs and symptoms of CVI in patients undergoing venous surgery. One systematic review 90 concluded that MPFF could be used in combination with sclerotherapy, surgery, or compression therapy and could be considered as an alternative to surgery in CVI. Another systematic review 87 found promising results regarding the use of MPFF as an adjunct to sclerotherapy and surgical and endovenous therapy, but calls for the need for further placebo‐controlled studies to confirm the benefits.
3.2.7. Use of MPFF in patients with PTS
Three observational studies53, 55, 63 totaling 300 patients reported that MPFF improved clinical manifestations or objective venous measures in patients with PTS. One observational study 30 reported faster venous recanalization, improved objective venous measures, and decreased clinical manifestations with MPFF combination therapy in patients with DVT compared to angiotropic metabolic infusion, anticoagulation, and elastic compression therapies without MPFF. Two RCTs23, 99 on MPFF showed improvement of objective venous measures in patients with PTS. One RCT 102 found that MPFF combined with rivaroxaban in femoropopliteal DVT improved Villalta score and Venous Clinical Severity Score and decreased the incidence of PTS in patients with DVT compared to rivaroxaban alone. A single RCT reported improvement of PTS symptoms with MPFF treatment compared to conservative treatment alone. 65
3.3. Patient acceptability
Six observational studies49, 52, 56, 59, 64, 101 and three RCTs24, 75, 78 described good patient acceptability of MPFF, with patients reporting good to excellent effectiveness and satisfaction with MPFF. Two RCTs,45, 46 which are potential duplicate publications, found that more patients were satisfied with MPFF than with diosmin. Nonetheless, none of the systematic reviews we identified commented on patient acceptability, and very few RCTs and observational studies described patient opinion and perspectives on MPFF. Thus, when it comes to the description of patient acceptability, reporting bias cannot be excluded in individual observational studies and RCTs.
3.4. Adverse effects
One systematic review found MPFF to be well tolerated, 90 and three systematic reviews13, 89, 90 found MPFF’s tolerability to be similar to that of placebo. One observational study 62 and four RCTs24, 46, 84, 86 reported that MPFF was well tolerated. One observational study 59 and three RCTs71, 75, 85 reported good compliance with MPFF. Five observational studies52, 53, 59, 61, 81 and two RCTs85, 86 reported no side effects with MPFF use. Two RCTs71, 78 reported fewer adverse events with MPFF compared to placebo, and one RCT 77 reported similar tolerability of MPFF and placebo. One systematic review 97 and one RCT 83 found MPFF to have more side effects than placebo. One observational study 56 and six RCTs68, 69, 73, 78, 83, 84 reported mild or transient adverse effects with subsequent resolution. One RCT 71 reported serious adverse events, including erysipelas and hypertensive crisis, and four RCTs68, 71, 78, 83 reported adverse effects leading to treatment interruption, including nausea and hypotension.
In terms of specific adverse effects of MPFF, gastrointestinal side effects were the most common, as reported by two observational studies,56, 64 nine RCTs,45, 46, 68, 69, 71, 72, 73, 78, 102 and six systematic reviews.13, 31, 90, 94, 97, 98 Other reported adverse effects included autonomic effects (one RCT 78 and one systematic review 90 ), hypertension (the unpublished Saveliev RCT, one published RCT, 71 and one systematic review 97 ), mucocutaneous side effects (one observational trial, 56 three RCTs,69, 71, 84 and one systematic review 97 ), insomnia (one RCT 78 ), headaches (three RCTs68, 71, 78), urinary morbidity (one RCT 72 ), sinopulmonary morbidity (one RCT 71 ), bleeding (one observational study 101 and one RCT 102 ), weight loss (the unpublished Saveliev RCT), postprocedural pain and inflammation in patients who underwent sclerotherapy (one observational study 101 ) and depression (one RCT 71 ).
4. DISCUSSION
4.1. MPFF as a potential therapeutic agent for PTS
As presented in this review, MPFF is an excellent candidate for further study as a therapeutic agent to treat PTS because the mechanism of action of MPFF is directly relevant to its pathophysiology, the clinical efficacy of MPFF in the treatment of CVI is promising, and high‐quality studies directly investigating MPFF’s clinical efficacy in PTS are lacking.
First, our review demonstrates that MPFF’s mechanism of action is directly relevant to each stage of the PTS pathophysiology (Figure 2). MPFF helps relieve venous obstruction by promoting venous recanalization following DVT and decreases venous reflux. Its anti‐inflammatory effects could help prevent further worsening of venous obstruction and reflux resulting from DVT‐related inflammatory responses. By improving venous tone and stasis and promoting lymphatic drainage, MPFF can help relieve venous hypertension. Finally, MPFF can improve capillary hyperpermeability and tissue hypoxia, sequelae of venous hypertension following DVT. Thus, by the various elements of its mechanism of action, MPFF has potential as a therapeutic agent to treat PTS.
Second, MPFF has already shown promise in the treatment of clinical manifestations of CVI that are similar to that of PTS and include edema, hyperpigmentation, ulceration, leg cramps, limb heaviness, and pain. PTS and primary CVI also share objectifiable venous abnormalities such as abnormal venous tone, venous capacity, capillary permeability, and venous reflux. Common findings among studies of MPFF to treat CVI include improvement of signs and symptoms of CVI, objective venous measures, and QOL, leading many authors to recommend MPFF for the treatment of CVI.90, 92, 93 However, uncertainty around such benefit has led others to call for higher‐quality evidence.94, 95, 96, 97, 98 The available evidence suggests that MPFF treatment is associated with good patient acceptability, but the level of evidence remains low. In terms of adverse effects, MPFF seems to be generally well tolerated.13, 89, 90 Taken together, MPFF shows promise in its clinical efficacy, acceptability, and tolerability for the treatment of CVI, which supports its potential candidacy as a therapeutic agent for the treatment of PTS.
Third, although MPFF’s mechanism of action and efficacy in CVI support its potential use in PTS, the literature directly investigating its clinical efficacy in PTS is sparse. Thus, there is a need for well‐designed clinical trials studying MPFF, specifically in patients with PTS. While observational studies30, 53, 55, 63 described improvement in clinical manifestations and objective venous measures with the use of MPFF in patients with PTS, only a single RCT 65 used clinical endpoints to assess the use of MPFF as pharmacologic monotherapy in PTS.
Taken together, the relevance of MPFF’s mechanism of action in the pathophysiology of PTS, the clinical efficacy of MPFF in CVI and the lack of RCTs rigorously assessing MPFF’s clinical efficacy in PTS calls for a need for further research investigating the use of MPFF as therapy in PTS, namely, in well‐designed double‐blind RCTs with clinical endpoints.
4.2. Limitations of the evidence
Weaknesses in the quality of studies reviewed contributes to the suboptimal strength of the current evidence supporting the use of MPFF in CVI. First, studies potentially suffered from publication bias and poor methodology in reporting, randomization, allocation concealment, and blinding, 97 limiting the validity of results.95, 103 Second, many articles were duplicate publications, which potentially decreases the validity of published reviews, overestimating the magnitude of results presented. Third, several publications report funding from or author affiliation with the manufacturers of venoactive drugs or were published in journals overseen by pharmaceutical companies. This may have put certain studies at higher risk of bias stemming from potential conflicts of interest. Finally, language bias was apparent, with several studies identified published only in Russian, for example, which may have led to selection bias in reviews.
4.3. Limitations of the current review
Only the abstract was available for 1 of 14 systematic reviews, 91 for 8 of 33 RCTs,23, 41, 46, 47, 65, 67, 69, 70, 82 and for 7 of 19 observational studies,30, 53, 57, 60, 62, 64, 100 with associated duplicate publications cited. Neither the abstract nor the full manuscript were available for 6 RCTs25, 33, 44, 79, 80, 99 (including the unpublished Saveliev RCT) and 1 observational study, 63 with their respective duplicate publications cited. This limits the current review in several ways. First, the analysis of such studies often depended on the reporting of review articles that included them. The review articles themselves carry intrinsic methodological limitations and errata could have been carried over into the present publication. Second, a detailed perusal of the study design, methodology, patient population, interventions, and outcomes were at times not possible, which limited our ability to assess the quality of many studies. Third, our literature review yielded unpublished studies included in other reviews,97, 104 which did not describe them in detail. Their full manuscripts were unavailable. One unpublished RCT by Saveliev was described as reporting partly negative findings. Such studies may have contributed to publication bias favoring the efficacy of MPFF in CVI management. 97
5. CONCLUSION AND NEXT STEPS
To the best of our knowledge, no systematic reviews have focused on the use of MPFF in PTS. In the current review, we provided rationale that MPFF should be evaluated as a new therapeutic agent for PTS. To accomplish this, we described the relevance of the mechanism of action of MPFF in the pathophysiology of PTS. As PTS manifests as CVI following DVT, we reviewed in a narrative fashion systematic reviews, RCTs, and observational studies investigating the use of MPFF in CVI and highlighted the current lack of clinical evidence supporting the use of MPFF in PTS. We explained that MPFF shows promise in terms of clinical efficacy, patient acceptability, and tolerability in CVI and that its therapeutic potential in patients with PTS should therefore be investigated in high‐quality RCTs. For this purpose, we are conducting the Micronized Purified Flavonoid Fraction for the Treatment of Post‐Thrombotic Syndrome (MUFFIN‐PTS) trial (ClinicalTrials.gov identifier: NCT03833024), a double‐blind multicenter RCT that will compare the clinical efficacy of a 6‐month regimen of MPFF (Venixxa) 500 mg oral twice daily to that of placebo in conjunction with conventional PTS treatment in PTS patients (Table 4).
TABLE 4.
Overview of the Micronized Purified Flavonoid Fraction for the Treatment of Postthrombotic Syndrome (MUFFIN‐PTS) trial
| Micronized Purified Flavonoid Fraction for the Treatment of Post‐Thrombotic Syndrome (MUFFIN‐PTS) trial (NCT03833024) |
|---|
|
Study design
|
|
Objective
|
|
Participant characteristics
|
|
Study groups
|
|
Primary outcome
|
|
Secondary outcomes
|
Abbreviations: months: month(s); MPFF: micronized purified flavonoid fraction; PTS: postthrombotic syndrome; QOL: quality of life; RCT: randomized controlled trial.
RELATIONSHIP DISCLOSURE
J‐PG received consulting fees from Servier, outside of this review article. All other authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
KXL and GD performed the literature review and drafted the manuscript, tables, and Figure 1. KXL led the manuscript revisions and created Figure 2. J‐PG and SRK conceived of the project and guided the literature review, manuscript, tables, and figures. All authors revised and approved the final version of the manuscript.
ACKNOWLEDGMENTS
Dr Kahn is supported by a Tier 1 Canada Research Chair. Drs Kahn and Galanaud are investigators of the CanVECTOR Network, which receives grant funding from the Canadian Institutes of Health Research (Funding Reference: CDT‐142654).
Handling Editor: Suzanne Cannegieter
Funding information
The authors received no financial support for the publication of this review article
REFERENCES
- 1. Kahn SR, Pengo V. Special issue: Late consequences of venous thromboembolism. Thromb Res. 2018;164:99. [DOI] [PubMed] [Google Scholar]
- 2. Galanaud JP, Monreal M, Kahn SR. Epidemiology of the post‐thrombotic syndrome. Thromb Res. 2018;164:100‐109. [DOI] [PubMed] [Google Scholar]
- 3. Lubberts B, Paulino Pereira NR, Kabrhel C, Kuter DJ, DiGiovanni CW. What is the effect of venous thromboembolism and related complications on patient reported health‐related quality of life? A meta‐analysis. Thromb Haemost. 2016;116:417‐431. [DOI] [PubMed] [Google Scholar]
- 4. Prandoni P. Healthcare burden associated with the post‐thrombotic syndrome and potential impact of the new oral anticoagulants. Eur J Haematol. 2012;88:185‐194. [DOI] [PubMed] [Google Scholar]
- 5. Haig Y, Enden T, Grotta O et al. Post‐thrombotic syndrome after catheter‐directed thrombolysis for deep vein thrombosis (CaVenT): 5‐year follow‐up results of an open‐label, randomised controlled trial. Lancet Haematol. 2016;3:e64‐71. [DOI] [PubMed] [Google Scholar]
- 6. Vedantham S, Goldhaber SZ, Julian JA et al. Pharmacomechanical catheter‐directed thrombolysis for deep‐vein thrombosis. N Engl J Med. 2017;377:2240‐2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Notten P, Ten Cate‐Hoek AJ, Arnoldussen C et al. Ultrasound‐accelerated catheter‐directed thrombolysis versus anticoagulation for the prevention of post‐thrombotic syndrome (CAVA): a single‐blind, multicentre, randomised trial. Lancet Haematol. 2020;7:e40‐e49. [DOI] [PubMed] [Google Scholar]
- 8. Appelen D, van Loo E, Prins MH, Neumann MH, Kolbach DN. Compression therapy for prevention of post‐thrombotic syndrome. Cochrane Database Syst Rev. 2017;9:CD004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Azirar S, Appelen D, Prins MH, Neumann MH, de Feiter AN, Kolbach DN. Compression therapy for treating post‐thrombotic syndrome. Cochrane Database Syst Rev. 2019;9:CD004177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabinovich A, Kahn SR. How I treat the postthrombotic syndrome. Blood. 2018;131:2215‐2222. [DOI] [PubMed] [Google Scholar]
- 11. Belcaro G, Dugall M, Luzzi R et al. Management of varicose veins and chronic venous insufficiency in a comparative registry with nine venoactive products in comparison with stockings. Int J Angiol. 2017;26:170‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wittens C, Davies AH, Baekgaard N et al. Editor's choice ‐ management of chronic venous disease: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2015;49:678‐737. [DOI] [PubMed] [Google Scholar]
- 13. Martinez‐Zapata MJ, Vernooij RW, Uriona Tuma SM et al. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. 2016;4:CD003229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paysant J, Sansilvestri‐Morel P, Bouskela E, Verbeuren TJ. Different flavonoids present in the micronized purified flavonoid fraction (Daflon 500 mg) contribute to its anti‐hyperpermeability effect in the hamster cheek pouch microcirculation. Int Angiol. 2008;27:81‐85. [PubMed] [Google Scholar]
- 15. Moustafa A, Alim HM, Chowdhury MA, Eltahawy EA. Postthrombotic syndrome: long‐term sequela of deep venous thrombosis. Am J Med Sci. 2018;356(2):152‐158. [DOI] [PubMed] [Google Scholar]
- 16. Allegra C, Bartolo M Jr, Carioti B, Cassiani D. An original microhaemorheological approach to the pharmacological effects of Daflon 500 mg in severe chronic venous insufficiency. Int J Microcirc Clin Exp. 1995;15:50‐54. [DOI] [PubMed] [Google Scholar]
- 17. Belcaro G, Cesarone MR, de Sanctis MT et al. Laser Doppler and transcutaneous oximetry: modern investigations to assess drug efficacy in chronic venous insufficiency. Int J Microcirc Clin Exp. 1995;15(suppl 1):45‐49. [DOI] [PubMed] [Google Scholar]
- 18. Galley P, Thiollet M. A double‐blind, placebo‐controlled trial of a new veno‐active flavonoid fraction (S 5682) in the treatment of symptomatic capillary fragility. Int Angiol. 1993;12:69‐72. [PubMed] [Google Scholar]
- 19. Behar A, Lagrue G, Cohen‐Boulakia F, Baillet J. Study of capillary filtration by double labelling I131‐albumin and Tc99m red cells. Application to the pharmacodynamic activity of Daflon 500 mg. Int Angiol. 1988;7:35‐38. [PubMed] [Google Scholar]
- 20. Bouskela E, Donyo KA. Effects of oral administration of purified micronized flavonoid fraction on increased microvascular permeability induced by various agents and on ischemia/reperfusion in the hamster cheek pouch. Angiology. 1997;48:391‐399. [DOI] [PubMed] [Google Scholar]
- 21. Allegra C, Bartolo M Jr, Carioti B, Cassiani D, Besse Boffi MG. Microlymphography: assessment of Daflon 500 mg activity in patients with chronic venous insufficiency. Lymphology. 1997;31:12‐16. [Google Scholar]
- 22. Ramelet AA. Pharmacologic aspects of a phlebotropic drug in CVI‐associated edema. Angiology. 2000;51:19‐23. [DOI] [PubMed] [Google Scholar]
- 23. Tsouderos Y. Are the phlebotonic properties shown in clinical pharmacology predictive of a therapeutic benefit in chronic venous insufficiency? Our experience with Daflon 500 mg. Int Angiol. 1989;8:53‐59. [PubMed] [Google Scholar]
- 24. Chassignolle J‐F, Amiel M, Lanfranchi G et al. Activité thérapeutique de Daflon 500 mg dans l’insuffisance veineuse fonctionnelle. J Int Med. 1987;99:32‐37. [Google Scholar]
- 25. Barbe R, Amiel M. Pharmacodynamic properties and therapeutic efficacy of Daflon 500 mg. Phlebology. 1992;7:41‐44. [Google Scholar]
- 26. Ibegbuna V, Nicolaides AN, Sowade O, Leon M, Geroulakos G. Venous elasticity after treatment with Daflon 500 mg. Angiology. 1997;48:45‐49. [DOI] [PubMed] [Google Scholar]
- 27. Katseni K, Bramis K, Katsenis K. Beneficial effect of MPFF administration on the healing process of venous ulcer. Hell J Surg. 2017;89:196‐202. [Google Scholar]
- 28. Tsoukanov Y, Tsoukanov A, Nikolaychuk A. Great saphenous vein transitory reflux in patients with symptoms related to chronic venous disorders, but without visible signs (C0s), and its correction with MPFF treatment. Phlebolymphology. 2015;22:18‐24. [Google Scholar]
- 29. Pascarella L, Lulic D, Penn AH et al. Mechanisms in experimental venous valve failure and their modification by Daflon 500 mg. Eur J Vasc Endovasc Surg. 2008;35:102‐110. [DOI] [PubMed] [Google Scholar]
- 30. Zudin AM, Zasorina MA, Vikhert TA, Gonsales AK, a Tarkovskiĭ A. [Ultrasound assessment of alterations in venous haemodynamics in patients with post‐thrombotic disease permanently taking phlebotonics]. Angiol Sosud Khir. 2014;20:52‐57. [PubMed] [Google Scholar]
- 31. Coleridge‐Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta‐analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005;30:198‐208. [DOI] [PubMed] [Google Scholar]
- 32. Nelson EA. Venous leg ulcers. BMJ Clin Evid. 2011;2011:1902. [PMC free article] [PubMed] [Google Scholar]
- 33. Chassignolle JF, Amiel G, Lanfranchi G, Barbe R. Activité thérapeutique de Daflon 500 mg dans l’insuffisance veineuse fonctionnelle. JIM. 1999;99:32‐36. [Google Scholar]
- 34. Saveuev VS, Pokrovsky A, Ai K, Bogachev VY, Zolotukhin I, Sapelkin SV. Stripping of the great saphenous vein under micronized purified flavonoid fraction (MPFF) protection (results of the Russian multicenter controlled trial DEFANCE). Phlebolymphology. 2008;15:45‐51. [Google Scholar]
- 35. Veverková L, Jedlicka V, Wechsler J, Kalač J. Analysis of the various procedures used in great saphenous vein surgery in the Czech Republic and benefit of Daflon 500 mg to postoperative symptoms. Phlebolymphology. 2006;13:195‐201. [Google Scholar]
- 36.Maruszynski M. A double blind, randomized study evaluating the influence of semi‐synthetic diosmin, and a purified, micronized flavonoid fraction (diosmin and hesperidin), on symptoms of chronic venous insufficiency of the lower limb – a four‐week observation: http://sensoril.com/wp‐content/uploads/2012/11/Maruszynski‐clinical‐trial‐Poland‐2004‐English‐translation.pdf; 2004(accessed 15 December 2019).
- 37. Cesarone MR, Belcaro G, Pellegrini L et al. Venoruton vs Daflon: evaluation of effects on quality of life in chronic venous insufficiency. Angiology. 2006;57:131‐138. [DOI] [PubMed] [Google Scholar]
- 38. Cesarone MR, Belcaro G, Pellegrini L et al. HR, 0‐(beta‐hydroxyethyl)‐rutosides, in comparison with diosmin+hesperidin in chronic venous insufficiency and venous microangiopathy: an independent, prospective, comparative registry study. Angiology. 2005;56:1‐8. [DOI] [PubMed] [Google Scholar]
- 39. Gliñski W, Chodynicka B, Roszkiewicz J et al. The beneficial augmentative effect of micronised purified flavonoid fraction (MPFF) on the healing of leg ulcers: an open, multicentre, controlled, randomised study. Phlebology. 1999;14:151‐157. [Google Scholar]
- 40. Guilhou JJ, Février F, Debure C et al. Benefit of a 2‐month treatment with a micronized, purified flavonoidic fraction on venous ulcer healing. A randomized, double‐blind, controlled versus placebo trial. Int J Microcirc Clin Exp. 1997;17(suppl 1):21‐26. [DOI] [PubMed] [Google Scholar]
- 41. Tsouderos Y. Venous tone: are the phlebotonic properties predictive of a therapeutic benefit? A comprehensive view of our experience with Daflon 500 mg. Z Kardiol. 1991;80(suppl 7):95‐101. [PubMed] [Google Scholar]
- 42. Frileux C, Gilly R. Activité thérapeutique de Daflon 500 mg dans l’insuffisance veineuse chronique des membres inférieurs. J Int Méd. 1987;36‐39. [Google Scholar]
- 43. Thiollet M, Frileux C. Evaluation of a new micronized diosmin in the treatment of chronic venous incompetence: a double‐blind, placebo controlled trial. J Vasc Surg. 1992;15:447. [Google Scholar]
- 44. Chassignolle J, Amiel M, Lanfranchi G, Barbe R. Activité thérapeutique de Daflon 500 mg dans l'insuffisance veineuse fonctionnelle. J Int Med. 1994;99:32–35. [Google Scholar]
- 45. Cospite M, Dominici A. Double blind study of the pharmacodynamic and clinical activities of 5682 SE in venous insufficiency. Advantages of the new micronized form. Int Angiol. 1989;8:61‐65. [PubMed] [Google Scholar]
- 46. Amato C. Advantage of a micronized flavonoidic fraction (Daflon 500 mg) in comparison with a nonmicronized diosmin. Angiology. 1994;45:531‐536. [PubMed] [Google Scholar]
- 47. Laurent R, Gilly R, Frileux C. Clinical evaluation of a venotropic drug in man. Example of Daflon 500 mg. Int Angiol. 1988;7:39‐43. [PubMed] [Google Scholar]
- 48. Jantet G. RELIEF study: first consolidated European data. Reflux assEssment and quaLity of lIfe improvement with micronized Flavonoids. Angiology. 2000;51:31‐37. [DOI] [PubMed] [Google Scholar]
- 49. Jantet G. Chronic venous insufficiency: worldwide results of the RELIEF study. Reflux assEssment and quaLity of lIfe improvEment with micronized Flavonoids. Angiology. 2002;53:245‐256. [DOI] [PubMed] [Google Scholar]
- 50. Kahn SR. The post‐thrombotic syndrome. Hematol Am Soc Hematol Educ Program. 2016;2016:413‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shoab SS, Porter J, Scurr JH, Coleridge‐Smith PD. Endothelial activation response to oral micronised flavonoid therapy in patients with chronic venous disease–a prospective study. Eur J Vasc Endovasc Surg. 1999;17:313‐318. [DOI] [PubMed] [Google Scholar]
- 52. Bogachev VY, Boldin BV, Turkin PY. Administration of micronized purified flavonoid fraction during sclerotherapy of reticular veins and telangiectasias: results of the national, multicenter, observational program VEIN ACT PROLONGED‐C1. Adv Ther. 2018;35:1001‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Son'kin IN, Shaidakov EV, Krylov DV, Bulatov VL, Remizov AS, Rezvantsev MV. [Efficacy of Venarus in treatment of patients with post‐thrombotic disease of lower limbs]. Angiol Sosud Khir. 2014;20:77‐83. [PubMed] [Google Scholar]
- 54. Tsukanov YT, Nikolaichuk AI. Orthostatic‐loading‐induced transient venous refluxes (day orthostatic loading test), and remedial effect of micronized purified flavonoid fraction in patients with telangiectasia and reticular vein. Int Angiol. 2017;36:189‐196. [DOI] [PubMed] [Google Scholar]
- 55. Tsukanov YT, Tsukanov AY, Levdanskiy EG. Secondary varicose small pelvic veins and their treatment with micronized purified flavonoid fraction. Int J Angiol. 2016;25:121‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yanushko V, Bayeshko AA, S. S. Benefits of MPFF on primary chronic venous disease‐related symptoms and quality of life: the DELTA study. Phlebolymphology. 2014;21:146‐151. [Google Scholar]
- 57. Zubarev AR, Krivosheeva NV, a Demidova K, Rychkova IV. [Ultrasound elastography in assessing efficacy of treatment for lower limb varicose veins with a phlebotrophic drug containing a micronized purified flavonoid fraction]. Angiol Sosud Khir. 2014;20:90‐96. [PubMed] [Google Scholar]
- 58. Lenkovic M, Zgombic ZS, Blazic TM, Brajac I, Periša D. Benefit of Daflon 500 mg in the reduction of chronic venous disease‐related symptoms. Phlebolymphology. 2012;19:79‐83. [Google Scholar]
- 59. Navratilova Z. Efficacy of a 6‐month treatment with Daflon 500 mg* in patients with venous edema (Efficacy of Daflon 500 mg* in Edema Treatment. EDET). Phlebolymphology. 2010;17:137‐142. [Google Scholar]
- 60. Sirotin BZ, Zhmerenetski KV. [Microcirculation end effect of detralex in patients with chronic venous insufficiency of the lower extremities]. Angiol Sosud Khir. 2003;9:60‐65. [PubMed] [Google Scholar]
- 61. Ting AC, Cheng SW, Wu LL, Cheung GC. Clinical and hemodynamic outcomes in patients with chronic venous insufficiency after oral micronized flavonoid therapy. Vasc Surg. 2001;35:443‐447. [DOI] [PubMed] [Google Scholar]
- 62. Iablokov EG, Bogachev V, Domoradskaia AI. [The drug therapy of chronic venous insufficiency (a trial of the clinical use of the preparation Detralex)]. Ter Arkh. 1996;68:80‐81. [PubMed] [Google Scholar]
- 63. Blume J, Langenbahn H, M. DC. Quantification of oedema using the volometer technique: therapeutic application of Daflon® 500 mg in chronic venous insufficiency. Phlebology. 1992;37‐40. [Google Scholar]
- 64. Guillot B, Guilhou JJ, de Champvallins M, Mallet C, Moccatti D, Pointel JP. A long term treatment with a venotropic drug. Results on efficacy and safety of Daflon 500 mg in chronic venous insufficiency. Int Angiol. 1989;8:67‐71. [PubMed] [Google Scholar]
- 65. Ignat'ev IM. Open prospective randomized study of the results of using Venarus in postthrombotic disease. Angiol Sosud Khir. 2018;24:97‐101. [PubMed] [Google Scholar]
- 66. Toledo RR, Santos M, Schnaider TB. Effect of pycnogenol on the healing of venous ulcers. Ann Vasc Surg. 2017;38:212‐219. [DOI] [PubMed] [Google Scholar]
- 67. Bogachev V, Golovanova OV, Kuzhetsov AN, Shekoian AO. [On advisability of perioperative phleboprotection in endovascular treatment of lower in varicose disease: first initial results of the decision study]. Angiol Sosud Khir. 2012;18:90‐95. [PubMed] [Google Scholar]
- 68. Carpentier P, van Bellen B, Karetova D et al. Clinical efficacy and safety of a new 1000‐mg suspension versus twice‐daily 500‐mg tablets of MPFF in patients with symptomatic chronic venous disorders: a randomized controlled trial. Int Angiol. 2017;36:402‐409. [DOI] [PubMed] [Google Scholar]
- 69. Kirienko A, Radak D. Clinical acceptability study of once‐daily versus twice‐daily micronized purified flavonoid fraction in patients with symptomatic chronic venous disease: a randomized controlled trial. Int Angiol. 2016;35:399‐405. [PubMed] [Google Scholar]
- 70. Menyhei GAG, Hetenyi A et al. Chronobiology and clinical activity of Daflon 500 mg in chronic venous insufficiency. Phlebology. 1994;9:15‐18. [Google Scholar]
- 71. Rabe E, Agus GB, Roztocil K. Analysis of the effects of micronized purified flavonoid fraction versus placebo on symptoms and quality of life in patients suffering from chronic venous disease: from a prospective randomized trial. Int Angiol. 2015;34:428‐436. [PubMed] [Google Scholar]
- 72. Belczak SQ, Sincos IR, Campos W, Beserra J, Nering G, Aun R. Veno‐active drugs for chronic venous disease: a randomized, double‐blind, placebo‐controlled parallel‐design trial. Phlebology. 2014;29:454‐460. [DOI] [PubMed] [Google Scholar]
- 73. Pokrovsky AV, Saveljev VS, Kirienko AI et al. Surgical correction of varicose vein disease under micronized diosmin protection (results of the Russian multicenter controlled trial DEFANS). Angiol Sosud Khir. 2007;13:47‐55. [PubMed] [Google Scholar]
- 74. Veverkova L, Kalac J, Jedlicka V, Wechsler J. Analysis of surgical procedures on the vena saphena magna in the Czech Republic and an effect of Detralex during its stripping. Rozhl Chir. 2005;84(8):410‐412, 414‐416. [PubMed] [Google Scholar]
- 75. Roztocil K, Stvrtinová V, Strejcek J. Efficacy of a 6‐month treatment with Daflon 500 mg in patients with venous leg ulcers associated with chronic venous insufficiency. Int Angiol. 2003;22:24‐31. [PubMed] [Google Scholar]
- 76. Glinski W, Chodynicka B, Roszkiewicz J et al. Effectiveness of a micronized purified flavonoid fraction (MPFF) in the healing process of lower limb ulcers. An open multicentre study, controlled and randomized. Minerva Cardioangiol. 2001;49:107‐114. [PubMed] [Google Scholar]
- 77. Guilhou JJ, Dereure O, Marzin L et al. Efficacy of Daflon 500 mg in venous leg ulcer healing: a double‐blind, randomized, controlled versus placebo trial in 107 patients. Angiology. 1997;48:77‐85. [DOI] [PubMed] [Google Scholar]
- 78. Gilly RPG, Frileux C. Evaluation of a new venoactive micronized flavonoid fraction (S 5682) in symptomatic disturbances of the venolymphatic circulation of the lower limb: a double‐blind, placebo‐controlled study. Phlebology. 1994;9:67‐70. [Google Scholar]
- 79. Planchon B. Venous insufficiency and Daflon 500 mg [Insuffisance veineuse et Daflon 500 mg]. Artères Veines. 1990;IX:376‐380. [Google Scholar]
- 80. Biland L, Blättler P, Scheibler P, Studer S, Widmer LK. [On the therapy of so‐called leg pains. Controlled double‐blind study of the therapeutic efficacy of Daflon]. Vasa. 1982;11:53‐58. [PubMed] [Google Scholar]
- 81. Mazzaccaro D, Muzzarelli L, Modafferi A, Righini PC, Settembrini AM, Nano G. Use of venoactive drugs after surgery for varicose veins: a preliminary study. Int Angiol. 2018;37:79‐84. [DOI] [PubMed] [Google Scholar]
- 82. Stoiko YM, Mazaishvili KV, Khlevtova TV, Tsyplyashchuk AV, Kharitonova SE, Akimov SS. [Effect of pharmacotherapy on course of postoperative period after endovenous thermal ablation]. Angiol Sosud Khir. 2015;21:77‐81. [PubMed] [Google Scholar]
- 83. Danielsson G, Jungbeck C, Peterson K, Norgren L. A randomised controlled trial of micronised purified flavonoid fraction vs placebo in patients with chronic venous disease. Eur J Vasc Endovasc Surg. 2002;23:73‐76. [DOI] [PubMed] [Google Scholar]
- 84. Maruszynski MSW, Andziak P. A double blind, randomized study evaluating the influence of semisynthetic diosmin, and purified, micronized flavonoid fraction (diosmin and hesperidin), on symptoms of chronic venous insufficiency of lower limbs ‐ a four week observation. Przeglad Flebologiczny. 2004;12:89‐95. [Google Scholar]
- 85. Belcaro G, Cesarone MR, Bavera P et al. HR (Venoruton1000, Paroven, 0‐[beta‐hydroxyethyl]‐rutosides) vs. Daflon 500 in chronic venous disease and microangiopathy: an independent prospective, controlled, randomized trial. J Cardiovasc Pharmacol Ther. 2002;7:139‐145. [DOI] [PubMed] [Google Scholar]
- 86. Cesarone MR, Belcaro G, Rohdewald P et al. Comparison of Pycnogenol and Daflon in treating chronic venous insufficiency: a prospective, controlled study. Clin Appl Thromb Hemost. 2006;12:205‐212. [DOI] [PubMed] [Google Scholar]
- 87. Mansilha A, Sousa J. Benefits of venoactive drug therapy in surgical or endovenous treatment for varicose veins: a systematic review. Int Angiol. 2019;38:291‐298. [DOI] [PubMed] [Google Scholar]
- 88. Kakkos S, Nicolaides A. Efficacy of micronized purified flavonoid fraction (Daflon(R)) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: a systematic review and meta‐analysis of randomized double‐blind placebo‐controlled trials. Int Angiol. 2018;37(2):143‐154. [DOI] [PubMed] [Google Scholar]
- 89. Bush R, Comerota A, Meissner M, Raffetto JD, Hahn SR, Freeman K. Recommendations for the medical management of chronic venous disease: The role of micronized purified flavanoid fraction (MPFF). Phlebology. 2017;32:3‐19. [DOI] [PubMed] [Google Scholar]
- 90. Lyseng‐Williamson KA, Perry CM. Micronised purified flavonoid fraction: a review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63:71‐100. [DOI] [PubMed] [Google Scholar]
- 91. Boada JNN, Clin GJ. Therapeutic effect of venotonics in chronic venous insufficiency. A meta‐analysis. Drug Investig. 1999;18(6):413‐432. 10.2165/00044011-199918060-00001. [DOI] [Google Scholar]
- 92. Allaert FA. Meta‐analysis of the impact of the principal venoactive drugs agents on malleolar venous edema. Int Angiol. 2012;31:310‐315. [PubMed] [Google Scholar]
- 93. Ramelet AA, Boisseau MR, Allegra C et al. Veno‐active drugs in the management of chronic venous disease. An international consensus statement: current medical position, prospective views and final resolution. Clin Hemorheol Microcirc. 2005;33:309‐319. [PubMed] [Google Scholar]
- 94. Martinez MJ, Bonfill X, Moreno RM, Vargas E, Capellà D. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. 2005;4(4):CD003229. [DOI] [PubMed] [Google Scholar]
- 95. Varatharajan L, Thapar A, Lane T, Munster AB, Davies AH. Pharmacological adjuncts for chronic venous ulcer healing: a systematic review. Phlebology. 2016;31:356‐365. [DOI] [PubMed] [Google Scholar]
- 96. Rabe E, Guex JJ, Morrison N et al. Treatment of chronic venous disease with flavonoids: recommendations for treatment and further studies. Phlebology. 2013;28:308‐319. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006477.pub2/full [DOI] [PubMed] [Google Scholar]
- 97. Scallon C, Bell‐Syer SE, Aziz Z. Flavonoids for treating venous leg ulcers. Cochrane Database Syst Rev. 2013;(5):CD006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nelson EA, Jones J. Venous leg ulcers. BMJ Clin Evid. 2008;2008.17 [PMC free article] [PubMed] [Google Scholar]
- 99. Amiel MBR. Etude du délai et de la durée d’action de Daflon 500mg. J Int Med. 1987;88:22‐24. [Google Scholar]
- 100. Gudymovich VG, Stoiko IUM, Iakovleva NM, Nikitina AM. [Phlebotrophic therapy with venarus in patients suffering from lower limb chronic venous insufficiency]. Angiol Sosud Khir. 2013;19:88‐91. [PubMed] [Google Scholar]
- 101. Pitsch F. Benefit of Daflon 500 mg in combination with sclerotherapy of telangiectasias of the lower limbs: results from the SYNERGY and SATISFY surveys. Phlebolymphology. 2011;19:182‐187. [Google Scholar]
- 102. Lobastov K, Schastlivtsev I, Barinov V. Use of micronized purified flavonoid fraction together with rivaroxaban improves clinical and ultrasound outcomes in femoropopliteal venous thrombosis: results of a pilot clinical trial. Adv Ther. 2019;36:72‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Coccheri S, Bignamini AA. Pharmacological adjuncts for chronic venous ulcer healing. Phlebology. 2016;31:366‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Smith PC. Daflon 500 mg and venous leg ulcer: new results from a meta‐analysis. Angiology. 2005;56(suppl 1):S33‐S39. [DOI] [PubMed] [Google Scholar]
- 105. Domínguez C, Brautigam I, González E et al. Therapeutic effects of hidrosmin on chronic venous insufficiency of the lower limbs. Curr Med Res Opin. 1992;12:623‐630. [DOI] [PubMed] [Google Scholar]
- 106. Fermoso J, Legido AG, Del Pino J, Valiente R. Therapeutic value of hidrosmin in the treatment of venous disorders of the lower limbs. Curr Ther Res Clin Exp. 1992;52:124‐134. [Google Scholar]
- 107. Olszewski W. Clinical efficacy of micronized purified flavonoid fraction (MPFF) in edema. Angiology. 2000;51:25‐29. [DOI] [PubMed] [Google Scholar]
- 108. ISRCTN18841175. Effects of micronised purified flavonoic fraction on microcirculation in women suffering from chronic venous disease. http://www.isrctn.com/ISRCTN18841175. 2009 (accessed 28 October 2014)..
- 109. NCT01532882 . Efficacy and safety of Diosmin 600mg versus placebo on painful symptomatology in patients with chronic venous disease of lower limbs (EDEN). http://clinicaltrials.gov/show/NCT01532882. 2012 (accessed 28 October 2014).
- 110. Corrigendum. Phlebology. 2017;32:NP36. [DOI] [PubMed] [Google Scholar]
- 111. Pitsch F. The place of Daflon 500 mg in recent guidelines on the management of chronic venous disease. Medicographia. 2011;33:306‐318. [Google Scholar]
- 112. Struckmann JR. Clinical efficacy of micronized purified flavonoid fraction: an overview. J Vasc Res. 1999;36:37‐41. [DOI] [PubMed] [Google Scholar]
- 113. Ramelet A. Management of chronic venous disease: the example of Daflon 500 mg. Phlebolymphology. 2009;16:321‐330. [Google Scholar]


