Abstract
Background
Studies evaluating the effects of the COVID-19 pandemic on public healthcare systems are limited, particularly in cancer management. As no such studies have been carried out in Spain, our objective is to describe and quantify the impact of the COVID-19 pandemic on cancer patients in Spanish hospitals during the first wave of the pandemic.
Materials and methods
This retrospective, multicenter, nationwide study collected information from hospital departments treating oncology patients. An electronic questionnaire comparing outcomes and management of oncohematological patients for the March-June 2019 and March-June 2020 periods was used.
Results
Information from 78 departments (36 tertiary hospitals) was analyzed. Forty-four departments implemented adapted protocols during March 2020. Most of these (n = 38/44; 86.4%) carried out COVID-19 triage, while 26 of 44 (59.1%) carried out onsite polymerase chain reaction tests for clinically suspected cases. A shift from in-person to telephone visits was observed in 43 of 44 (97.7%) departments. Comparing the March-June 2019 and March-June 2020 periods, the number of new patients decreased by 20.8% (from 160.2 to 126.4). Decreases were also seen in the mean number of total (2858.2 versus 1686.1) and cancer (465.5 versus 367.2) biopsies, as well as the mean number of bone marrow biopsies (30.5 versus 18.6). Concerning the number of patients visiting specific cancer care departments, a decrease from 2019 to 2020 was seen for mean number of chemotherapy treatments (712.7 versus 643.8) and radiation therapy (2169.9 versus 2139.9). Finally, a reduction from 2019 to 2020 of 12.9% (from 8.6 to 7.4) in the mean number of patients included in clinical trials was noted.
Conclusions
This study provides the first comprehensive data concerning the impact of COVID-19 on cancer care in Spain. The pandemic caused a 20.8% decrease in newly diagnosed patients, which may impact future outcomes. Measures must be taken to ensure cancer management receives priority in times of healthcare emergencies.
Key words: COVID-19, pandemic, cancer, care, Spain
Highlights
-
•
The number of new cancer patients decreased 20.8%
-
•
Assistance protocols were adapted
-
•
Inclusion in clinical trials decreased by 12.9%
Introduction
Coronaviruses are one group of the most pathogenic agents affecting the human respiratory system.1 Coronavirus outbreaks were responsible for healthcare emergencies such as the severe acute respiratory syndrome (SARS)-CoV reported in 2003, and the Middle East respiratory syndrome (MERS)-CoV in 2012.2 In December 2019, a new coronavirus disease (COVID-19) emerging most likely from China has been responsible for causing an atypical form of pneumonia.3,4 Since then, the etiological agent, named SARS-CoV-2, has spread worldwide, with the World Health Organization declaring the current outbreak a public health emergency on 30 January 2020.5 In Spain, the first case of COVID-19 was reported on 31 January 2020.6 Between 1 March and 30 June 2020, a total of 240 978 individuals tested positive for COVID-19 in Spain and 27 134 deaths were reported.7 The disease exhibits a wide variety of clinical manifestations ranging from asymptomatic or mild forms to severe pneumonia with respiratory and systemic, multi-organ dysfunction (including acute respiratory distress syndrome, sepsis, and septic shock) requiring intensive care treatment.1,8 As a consequence of the COVID-19 pandemic, routine clinical practices in Spain were modified extensively to deal with such a critical healthcare scenario.9
Cancer is a major public health issue worldwide10 and the second most common cause of mortality, accounting for approximately one in six deaths. By 2030 its incidence is expected to increase by ∼45%,10 making it the world's leading cause of death. As cancer morbidity and mortality are directly related to the stage at diagnosis,11 earlier detection and treatment are therefore associated with better prognosis and survival outcomes.12,13 Cancer patients are a particularly at-risk population for developing severe COVID-19. This is particularly the case for patients receiving active treatment, those who have metastatic disease, and those who are affected by lung and hematological malignancies.14, 15, 16, 17
Given the impact of COVID-19 on healthcare resources, the redeployment of physicians (including oncologists) to COVID-19-specific tasks, and the possible reluctance of sick people to seek medical assistance at healthcare facilities, there is a heightened risk that the diagnosis and treatment of cancer patients during the pandemic will be delayed for unacceptably long periods. To this end, multicenter studies evaluating the effect of the pandemic on public healthcare systems are limited, particularly in relation to cancer management.18, 19, 20, 21 As no such studies have been carried out in Spain, the objective of the present work was to evaluate and quantify the impact of the COVID-19 pandemic on the hospital-based care of cancer patients during the first wave of the pandemic from March to June 2020 in Spain.
Materials and methods
Study design
This was a retrospective, multicenter, nationwide study in which data were collected from participating hospitals across four key departments involved in the management of oncohematological patients (hematology, medical oncology, radiation oncology, and pathology). Data were collected for the March to June period of 2019 and compared with the same period in 2020. Five national scientific societies in Spain [Spanish Society of Pathological Anatomy (SEAP-IAP); Spanish Society of Oncological Nursing (SEEO); Spanish Society of Hematology and Hemotherapy (SEHH); Spanish Society of Medical Oncology (SEOM); and Spanish Society of Radiation Oncology (SEOR)] together with the Spanish Association Against Cancer (AECC) collaborated in the study. An electronic (e-)questionnaire addressing a range of variables was sent to departments as indicated above in 37 public general hospitals. The questionnaire for each department is shown in Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2021.100157. All participating sites were public general hospitals providing tertiary level care, some of which were the only hospitals of this type in the concerned region. As Spain has fewer than a hundred public general tertiary care hospitals, the overall obtained data are considered representative of the national situation. The number of participating hospitals per region was chosen to be indicative of the COVID-19 incidence rate during the first wave, which was heterogeneous across the country. This study was approved by the Ethics Review Board of the 12 de Octubre University Hospital in Madrid (20/410, 28 July 2020).
Data collection and variables evaluated
The e-questionnaire was developed by physicians directly involved in the hospital care of cancer patients during the first wave of the pandemic in Spain (March to June 2020). Data were retrospectively collected between 30 July and 12 October 2020 from the mentioned departments. The questionnaire was composed of two main sections: (i) anti-COVID-19-specific measures taken in relation to patient care as measured by the implementation of specific action protocols and general measures taken in each department to adapt to the situation; and (ii) impact on cancer care, measuring changes in the number of new patient referrals, number of diagnostic procedures (such as solid tumors and bone marrow biopsies), number of treatments administered, and finally participation in clinical trials. It should be noted that between March and June 2020, polymerase chain reaction (PCR) tests were only carried out on clinically suspected cases. The e-questionnaire used to record responses to the different variables was sent to the heads of the participating departments' participants.
Data analysis
The impact of the pandemic was established by assessing absolute and relative changes during the considered period (March to June) in 2019 (control) versus the same period in 2020. Categorical variables were expressed as absolute and relative frequencies, whereas continuous variables as the mean and range (minimum and maximum values). All descriptive analyses were carried out with the statistical software package SAS 9.4 (SAS Institute, NC; 2013).22
Results
Participating hospitals
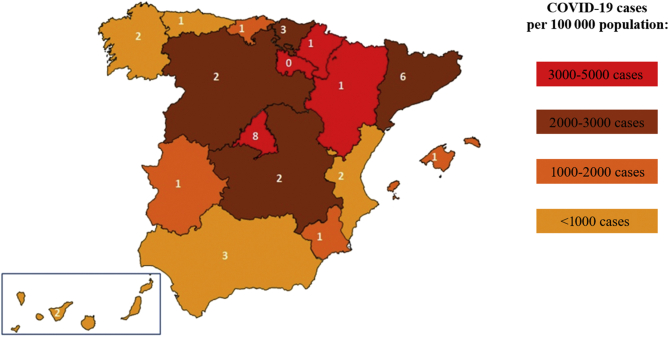
A total of 37 tertiary hospitals were invited to participate in the study (Figure 1), with 36 of the 37 (97.3%) sites providing data from at least one of the four departments. Overall, the participation of the departments was: hematology, 12 hospitals (32%); medical oncology, 13 hospitals (35%); radiation oncology: 19 hospitals (51%); and pathology: 34 hospitals (92%). Information from a total of 78 departments was available for analysis. The complete list of hospitals providing data from each department and the names of participating researchers are shown in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2021.100157.
Figure 1.
Distribution of participating hospitals in the autonomous regions of Spain and the number of COVID-19 cases treated by each.
The value in white indicates the number of participating hospitals in each region.
Specific anti-COVID-19 measurements
Forty-four departments initiated specific action protocols against COVID-19; 24 (54.5%) of these did so in the first half of March 2020, and the remainder in the second half of that same month (Table 1). Of the 44 departments, 38 (86.4%) implemented COVID-19 infection and contact triage procedures during routine clinical practice, and 26 departments (59.1%) carried out onsite PCR tests for clinically suspected COVID-19 cases. A shift from in-person to telephone/internet consultations was observed in 43 (97.7%) of the 44 departments, while physical assistance was maintained in 95.5% of departments for those patients whose condition could not be evaluated by telephone. Most departments (97.7%, n = 43/44) followed national guidelines (SEHH guidelines for hematology; SEOM guidelines for medical oncology; SEOR guidelines for radiation oncology) for the modification of therapeutic strategies. Overall, between March and June 2020, out of a mean of 34.3 patients (range: 0-215) per department, 77.5% (mean 26.6, range: 0-125) of patients who were clinically suspected of having COVID-19 tested positive. Among symptomatic patients, 62.1% (n = 21.3, range: 0-159) were admitted to hospital, and 19.7% (mean 6.8, range: 0-30) of them died thereafter.
Table 1.
Specific anti-COVID-19 measurements
| Total (n = 44) n (%) |
Hematology (n = 12) n (%) |
Medical oncology (n = 13) n (%) |
Radiation oncology (n = 19) n (%) |
|
|---|---|---|---|---|
| Specific action protocols | ||||
| Date of first implemented measures | ||||
| 1-15 March | 24 (54.5) | 9 (75.0) | 8 (61.5) | 7 (36.8) |
| 16-30 March | 20 (45.5) | 3 (25.0) | 5 (38.5) | 12 (63.2) |
| Separated circuits: non-contaminated/contaminated | 38 (86.4) | 12 (100.0) | 13 (100.0) | 13 (68.4) |
| Onsite PCR | 26 (59.1) | 10 (83.3) | 9 (69.2) | 7 (36.8) |
| Change from physical to telemedicine consultations | 43 (97.7) | 12 (100.0) | 13 (100.0) | 18 (94.7) |
| Ongoing physical visits in cases where necessary | 42 (95.5) | 12 (100.0) | 13 (100.0) | 17 (89.5) |
| Specific guidelines for the modification of therapeutic strategies | ||||
| From same department | 35 (79.5) | 10 (83.3) | 10 (76.9) | 15 (78.9) |
| National guidelines | 43 (97.7) | 12 (100.0) | 13 (100.0) | 18 (94.7) |
| International guidelines | 34 (77.3) | 8 (66.7) | 10 (76.9) | 16 (84.2) |
| Other guidelines | 12 (27.3) | 4 (33.3) | 4 (30.8) | 4 (21.1) |
PCR, polymerase chain reaction.
New and successive medical visits
Compared with the March-June period in 2019, the mean number of new patient visits decreased by 20.8% (from 160.2, range: 151.4-173.1, to 126.4, range: 105.3-144.3) for the same period in 2020. For successive consultations (including follow-up, treatment, and other kinds of visit), many were shifted from in-person to remote (telephone) visits, with the mean number of telephone visits increasing three-fold compared with the same period in 2019. On the other hand, the mean number of physical visits decreased by 22.3% (from 1224.4, range: 1184.7-1282.6, to 952.0, range: 758.6-1143.9) during the 2020 period compared with 2019 (Table 2).
Table 2.
COVID-19 impact on cancer care
| Percentage change (March-June 2019 versus 2020) | Mean data for March-June 2019 versus 2020 | |
|---|---|---|
| Medical consultationsa | ||
| New visits | −20.8 | 160.2 versus 126.4 |
| Successive consultations | ||
| Telephone | 298.5 | 118.0 versus 467.3 |
| Physical | −22.3 | 1224.4 versus 952.0 |
| Diagnosisb | ||
| Cytologies | −57.1 | 1950.9 versus 843.7 |
| Cytologies with cancer diagnosis | −30.2 | 57.7 versus 40.0 |
| Ratio cancer/cytologies | 52.2 | 4.1 versus 6.3 |
| Biopsies | −41.2 | 2858.2 versus 1686.1 |
| Biopsies with cancer diagnosis | −21.2 | 465.5 versus 367.2 |
| Ratio cancer/biopsies | 40.9 | 16.6 versus 23.4 |
| Specific diagnostic proceduresc | ||
| Bone marrow aspirates | −31.4 | 58.8 versus 39.9 |
| Bone marrow biopsies | −38.3 | 30.5 versus 18.6 |
| Flow cytometry tests | −12.5 | 220.7 versus 192.6 |
| Neoplastic genetic studies | −15.8 | 626.0 versus 524.0 |
| Treatments | ||
| Patients treated in day hospitals (ambulatory therapy)d | −14.3 | 980.0 versus 837.9 |
| Treatments in day hospitalsd | −7.7 | 1500.1 versus 1377.7 |
| Patients treated with chemotherapyd | −9.5 | 712.7 versus 643.8 |
| Patients treated with G-CSFd | 27.0 | 64.0 versus 81.2 |
| Patients treated with immune checkpoints inhibitorse | 11.0 | 121.4 versus 135.0 |
| Patients treated with tyrosine kinase inhibitorse | 2.1 | 189.3 versus 191.6 |
| Hematopoietic stem cell transplantsf | ||
| Autologous | −7.5 | 3.3 versus 2.9 |
| Allogenic | −27.2 | 3.4 versus 2.4 |
| Radiation therapiesg | ||
| Patients treated | −5.3 | 146.4 versus 138.4 |
| Treatments carried out (sessions) | −1.3 | 2169.9 versus 2139.9 |
| Clinical research activitiesh | ||
| Trials interrupted by the pandemic | 0.1 | – |
| Trial discontinuations due to the pandemic | 2.2 | – |
| Clinical trials recruitment | 32.1 | 30.4 versus 40.1 |
| Screening visits | −1.6 | 14.6 versus 13.9 |
| Enrolled patients | −12.9 | 8.6 versus 7.4 |
G-CSF, granulocyte colony-stimulating factors.
Data obtained from 37 departments (hematology, medical oncology, and radiation oncology) of 28 hospitals.
Pathology departments of 34 hospitals.
Hematology departments of nine hospitals.
Seven hematology and nine medical oncology departments.
Medical oncology departments of eight hospitals.
Hematology departments of six hospitals that carry out transplants.
The radiation oncology departments of 18 hospitals.
29 departments (hematology, medical oncology, and radiation oncology) of 22 hospitals.
Diagnostic procedures
For solid tumors, the mean number of total cytologies and biopsies decreased by 57.1% (1950.9 versus 843.7) and 41.2% (2858.2 versus 1686.1), respectively, in the 2020 period compared with the 2019 period. Considering specific cancer diagnoses, mean numbers were reduced by 30.2% (57.7 versus 40.0) for cytologies and 21.2% (465.5 versus 367.2) for biopsies. Regarding diagnostic procedures carried out on hematological patients in the 2020 period, a decrease of 31.4% (58.8 versus 39.9) was reported for bone marrow aspirates and 38.3% (30.5 versus 18.6) for bone marrow biopsies compared with 2019 figures. The reduction in the mean number of flow cytometry tests in 2020 versus 2019 was 12.5% (220.7 versus 192.6) and 15.8% (626.0 versus 524.0) for additional genetic studies on blood cancers (Table 2).
Treatment delivery
We observed a decrease in 2020 of 14.3% in the mean number of patients visiting day hospitals (980.0 versus 837.9), with a decrease of 9.5% in the mean number of patients receiving chemotherapy (712.7 versus 643.8). The use of granulocyte colony-stimulating factors increased by 27.0% in 2020 with respect to the same period in 2019 (64.0 versus 81.2). Interestingly, the use of immune checkpoint inhibitors (ICIs) in 2020 increased by 11.0% compared with 2019 figures (121.4 versus 135.0). We also observed an increase of 2.1% in the use of tyrosine kinase inhibitors (189.3 versus 191.6) in 2020 compared with 2019 data. The mean number of autologous hematopoietic stem cell transplants decreased by 7.5% (3.3 versus 2.9) in 2020 compared with the same period in 2019, while the mean number of allogeneic transplants decreased by 27.2% (3.4 versus 2.4). Finally, the mean number of patients visiting radiation oncology facilities and the mean number of treatments administered decreased by 5.3% (146.4 versus 138.4) and 1.3% (2169.9 versus 2139.9), respectively (Table 2).
Clinical research
The inclusion of patients in oncology-specific clinical trials decreased by 12.9% during the 2020 period compared with the 2019 period (mean 7.4, range: 4.1-11.0, versus mean 8.6, range: 8.0-9.9), whereas an increase of 32.1% in the 2020 period compared with 2019 was observed in the number of clinical trials in general (mean 40.1, range: 38.8-41.7, versus mean 30.4, range: 28.7-31.5), presumably due to COVID-19 research (Table 2).
Discussion
The COVID-19 pandemic has dramatically impacted most public healthcare systems around the world. The Spanish public healthcare system guarantees universal health coverage and free access for all Spanish individuals and eligible foreigners.23 During the first wave of the COVID-19 pandemic (March to June 2020), the Spanish healthcare system was forced to reorganize a large proportion of its resources toward the management of SARS-CoV-2-infected patients and to implement specific action protocols to avoid the infection of healthy healthcare professionals and patients admitted to or attending hospitals.24 The goal of the present study was to collect, for the first time in Spain, accurate data on the impact of the first wave of the pandemic on care provision and outcomes in oncology patients from a representative sample of 36 hospitals.17,25,26 Our study presents some major limitations, such as its retrospective nature and the exclusive hospital perspective of cancer management as neither primary care nor screening data were included (different sources for these data exceeded our operational capacity). Additionally, not all contacted departments responded to the questionnaire, which may limit the representation since data could be influenced by selection and measurement bias.
Our results reveal that routine practice in hematology, medical oncology, radiation oncology, and pathology departments in Spain was impacted early in the pandemic. Specific action protocols were implemented during the month of March 2020 in participating departments, including the development of clinical triage protocols and onsite COVID-19 PCR testing for patients attending hospitals. These measurements aimed to protect all patients from infection.
It is important to note that of all symptomatic patients, 62.1% were admitted, 77.5% were confirmed by PCR, and 19.7% died from COVID-19-related causes. It is also important to point out that PCR testing was not routinely available during the first wave of the pandemic in Spain unless there was solid clinical evidence of suspected infection. This could explain the high rate of positive results in tested patients. The 19.7% mortality rate for admitted cancer patients was similar to that reported for the general population who required hospital admission due to COVID-19 at that time.27
We also observed a shift from physical visits to telephone visits at most (97.7%) of the participating sites, particularly for follow-up consultations. However, only a 14.3% decrease was seen in the mean number of cancer patients being cared for in day hospitals. In solid tumors, the shift from intravenous to oral agents due to the pandemic situation has been very limited (although no measurements on this topic are available). For hematology malignancies, the reduction in the number of day hospital treatments was related to those therapies considered less relevant. However, others considered as more critical were less affected (the number of therapies with ICIs increased). In-person visits were also maintained for those cases where there was a need for patients to be physically examined. Given the above, it can be concluded that departments and healthcare professionals assisting cancer patients reacted early in the pandemic to protect their patients by implementing specific measurements such as clinical triage or PCR and by adapting practices to the given circumstances.18,20,21
We observed similar trends to changing and adapting care variables like successive visits to hospitals (including follow-up, review, and other visits that were not the first visit to the specialist), with a three-fold increase in the number of telephone visits and a 22.3% decrease of in-person visits. Regarding treatments, we identified a decrease of 9.5% in the mean number of chemotherapy treatments and 1.3% in radiation treatments, together with a clear increase in the use of colony-stimulating factors (increase of 27.0% when compared with the same period in 2019) and ICIs (11.0%). All of these results corroborate the effort made to adapt different treatment modalities and schedules to the healthcare situation at the time. Differences between the decrease in systemic and radiation treatments might reflect the use of radiotherapy as an alternative to systemic and/or surgical treatment. In addition, data on other systemic treatments (chemotherapy, ICIs, tyrosine kinase inhibitors) revealed similar efforts to adapt to the prevailing situation while maintaining and adapting treatments for patients with already diagnosed solid tumors. That being said, the most concerning data for us are those related to attention provided to newly diagnosed patients.18,20
For patients visiting an oncology or hematology department for the first time, a decrease of 20.8% in the mean number of new visits, considered as a surrogate of newly diagnosed cases, was identified. Similarly, for solid tumors, we observed a decrease (21.2%) in the mean number of cancer biopsies carried out during the studied period. The percentage differences between 2019 and 2020 with respect to total cytologies and biopsies could indicate a certain prioritization to perform these diagnostic procedures when a cancer was suspected. For hematological tumors, a decrease in the mean number of diagnostic procedures such as bone marrow aspirates (31.4%) and biopsies (38.3%) was reported, which likely reflects a reduction in the number of consecutive explorations in previously diagnosed patients (i.e. leukemia patients under therapeutic monitoring), as well as a reduction of new diagnoses of diseases where bone marrow examination is essential (myelodysplasia, leukemia, myeloma). In addition, a reduction in the mean number of allogeneic (27.2%) and autologous (7.5%) hematopoietic stem cell transplantations was also noted, probably reflecting a delay in the performing of these procedures as a strategy of physicians to avoid deep and long-term myelosuppression in these patients.
In our opinion, these data concerning the decrease in new visits, cancer biopsies, and hematology diagnoses are the most concerning of this study, but also potentially the most actionable. While acknowledging the study's limitations, our data provide a good idea of the quantitative impact of COVID-19 on cancer care and assistance. In addition, our data are consistent with previously published data from other countries,18,20,21 and individual institutions in our country.28 A survey carried out by Cancer Research UK showed that the pandemic impacted their testing (two in five patients), cancer care (two in three patients), and treatment (one in three patients).18 Dinmohamed et al.20 revealed a reduction in cancer diagnosis by 26% for all cancer sites (excluding skin cancer) and 60% for skin cancer (excluding basal cell carcinoma) in April 2020. Moreover, Jazieh et al.21 reported a reduction of services in more than half of all centers (55%) and the missing of at least one cycle of therapy by >10% of cancer patients (46%). The situation meant that patients were exposed to harm due to the interruption of cancer-specific care (36% of centers) and noncancer-related care (39%).
Our data highlight that one in five patients with cancer was either not diagnosed or diagnosed late during the first wave of the pandemic in Spain, where many (if not all) non-COVID-19 diagnosis-related procedures were stopped. This took place together with the collapse of Spain's primary healthcare system, which represents the most important entry to the health system for cancer patients. Moreover, and although this hypothesis cannot be quantified, we consider that patients' fear of being infected in hospitals and primary care clinics might also have contributed to the decrease of new cancer diagnoses. This ‘gap’ generated during the first wave has probably not been covered to the present time and might be even greater after the second and third waves of the pandemic. These data are important given their potential consequences, as it is well known that an early diagnosis and treatment are associated with better prognosis and survival outcomes in cancer patients.16,17 Delayed cancer treatment is related to increased mortality across surgical, systemic treatment, and radiotherapy indications.29 In this way, the delay in cancer diagnosis during the COVID-19 pandemic will probably result in an increase in the stage at which treatment is initiated for a large proportion of patients and may impact long-term survival, morbidity, and quality of life. Indeed, it has been estimated that delays in cancer diagnosis will be responsible for an additional 10 000 deaths from breast and colorectal cancer in the coming 10 years in the USA.30 In a national population-based modeling study in the UK, the impact on net survival at 1, 3, and 5 years after diagnosis was used to estimate the number of additional deaths that could be attributed to cancer compared with pre-pandemic data.31 The increased rate of mortality due to breast, colorectal, esophageal, and lung cancer could result in 3291-3621 additional deaths within 5 years.
Finally, the COVID-19 pandemic has also impacted clinical research. In our study, the inclusion of patients in cancer clinical trials was reduced by approximately 12.9%. The American Society of Clinical Oncology (ASCO) recently published recommendations for improving cancer research and care during the COVID-19 pandemic.32 Among specific goals, ASCO included the need to ‘recognize and address threats to clinician, provider, and patient well-being; and improve patient access to high-quality cancer care via telemedicine’. These recommendations are in agreement with the results identified in our study.32 Without doubt, the impact of COVID-19 on public healthcare systems in relation to cancer is global, and this concern extends to other sectors like the economy. This in turn may impact other issues associated with cancer, such as research, which in Spain is funded less than in some neighboring countries.21,31,33, 34, 35, 36
Conclusions
This study provides the first comprehensive dataset describing the negative impact of the COVID-19 pandemic on cancer care in Spain. Oncology patients are particularly vulnerable to this pandemic, presenting more frequent and severe forms of disease, often requiring hospitalized care, and with poorer evolution. In addition, we have documented a significant delay in cancer diagnosis and treatment that may potentially have consequences on outcomes: reduced survival time, less effective treatments, and increased morbidity and suffering. We have shared these results with Spain's health authorities and suggest cancer diagnosis and management should be prioritized in critical times such as the current pandemic scenario. A united approach is needed to address the shortfall in new diagnoses created by this situation. Indeed, the solution is likely to be complex and multidisciplinary (from evaluating current structures and human resources to ensure that the most vulnerable cancer patients are vaccinated in a timely manner). At the time of writing, (February 2021), Spain was experiencing a new wave of COVID-19, which in our opinion, will increase delays in the diagnosis and treatment of cancer patients if no concerted actions are taken, with the impact no doubt to be felt heavily by patients and the public healthcare system alike.
Acknowledgements
The authors express their gratitude to all the doctors/departments of the hospitals involved in the project and to Meisys for advice on the writing of this paper.
Funding
This work was supported by funding from the AECC, a non-profit organization, whose medical department funded the study and the medical writing (no grant number).
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui D.S., Azhar E.I., Memish Z.A., Zumla A. Human coronavirus infections—Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), and SARS-CoV-2. Reference Module in Biomedical Sciences. 2020 B978-0-12-801238-3.11634. [Google Scholar]
- 3.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tufan A., Avanoğlu Güler A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50(SI-1):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization COVID-19 Public Health Emergency of International Concern (PHEIC) Global research and innovation forum. https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum Available at:
- 6.Spiteri G., Fielding J., Diercke M. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25:2000178. doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spanish Health Ministry Situation of COVID-19 in Spain (05 June 2020) https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Actualizacion_127_COVID-19.pdf Available at:
- 8.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multi-organ response. Curr Probl Cardiol. 2020:100618. doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornton J. Covid-19: how coronavirus will change the face of general practice forever. BMJ. 2020;368:m1279. doi: 10.1136/bmj.m1279. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer Available at:
- 11.McPhail S., Johnson S., Greenberg D., Peake M., Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer. 2015;112:108–115. doi: 10.1038/bjc.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitaker K. Earlier diagnosis: the importance of cancer symptoms. Lancet Oncol. 2020;21:6–8. doi: 10.1016/S1470-2045(19)30658-8. [DOI] [PubMed] [Google Scholar]
- 13.Crosby D., Lyons N., Greenwood E. A roadmap for the early detection and diagnosis of cancer. Lancet Oncol. 2020;21(11):1397–1399. doi: 10.1016/S1470-2045(20)30593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn L., Garassino M. COVID-19 in patients with cancer: managing a pandemic within a pandemic. Nat Rev Clin Oncol. 2020;18:1–2. doi: 10.1038/s41571-020-00441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Ramón C., Hernandez-Rivas J.A., Rodríguez García J.A. Impact of Sars-CoV2 infection on 491 hematological patients: the Ecovidehe multicenter study. Blood. 2020;136(1):5–6. [Google Scholar]
- 16.Piñana J.L., Martino R., García-García I. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Suárez J., de la Cruz J., Cedillo Á. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. 2020;13(1):133. doi: 10.1186/s13045-020-00970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Research UK . 2020. Cancer Patient Experience Survey 2020: The impact of COVID-19 on cancer patients in the UK.https://www.cancerresearchuk.org/sites/default/files/pes-covid_2020.pdf Available at: [Google Scholar]
- 19.Greenwood E., Swanton C. Consequences of COVID-19 for cancer care – a CRUK perspective. Nat Rev Clin Oncol. 2020;18:3–4. doi: 10.1038/s41571-020-00446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinmohamed A.G., Visser O., Verhoeven R.H.A. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jazieh A.R., Akbulut H., Curigliano G. Impact of the COVID-19 pandemic on cancer care: a global collaborative study. JCO Glob Oncol. 2020;6:1428–1438. doi: 10.1200/GO.20.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SAS/ACCESS® 9.4 Interface to ADABAS: Reference. [computer software]. Version 9.4. SAS Institute Inc.; Cary, NC: 2013. [Google Scholar]
- 23.HealthManagement Overview of the Spanish Healthcare System. https://healthmanagement.org/c/hospital/issuearticle/overview-of-the-spanish-healthcare-system Available at:
- 24.Legido-Quigley H., Mateos-García J.T., Campos V.R., Gea-Sánchez M., Muntaner C., McKee M. The resilience of the Spanish health system against the COVID-19 pandemic. Lancet Public Health. 2020;5:e251–e252. doi: 10.1016/S2468-2667(20)30060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grupo español de cáncer de pulmón SOLID study. https://www.gecp.org/el-gecp-presenta-en-seom-el-mayor-estudio-de-seroprevalencia-del-sars-cov-2-del-mundo-en-enfermos-con-cancer/ Available at:
- 26.Pollán M., Pérez-Gómez B., Pastor-Barriuso R. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenthal N., Cao Z., Gundrum J., Sianis J., Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3(12):e2029058. doi: 10.1001/jamanetworkopen.2020.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogado J., Obispo B., Pangua C. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin Transl Oncol. 2020;22:1–5. doi: 10.1007/s12094-020-02381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanna T.P., King W.D., Thibodeau S. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpless N.E. COVID-19 and cancer. Science. 2020;368:1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- 31.Maringe C., Spicer J., Morris M. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pennell N.A., Dillmon M., Levit L.A. American Society of Clinical Oncology road to recovery report: learning from the COVID-19 experience to improve clinical research and cancer care. J Clin Oncol. 2021;39(2):155–169. doi: 10.1200/JCO.20.02953. [DOI] [PubMed] [Google Scholar]
- 33.Nicola M., Alsafi Z., Sohrabi C. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.OECD Key Biotechnology Indicators. http://oe.cd/kbi Available at:
- 35.OECD Main Science and Technology Indicators Database. www.oecd.org/sti/msti.htm Available at:
- 36.Huang C., Huang L., Wang Y. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.