ABSTRACT
Stunting is associated with cognitive impairment and later chronic disease. Previous trials to prevent stunting have had little effect, and no trials seem to have provided larger amounts of energy and high-quality proteins to already stunted children. We aimed to assess the effects of milk protein (MP) and whey permeate (WP) in large-quantity lipid-based nutrient supplements (LNS-LQ), among stunted children, on linear growth and child development. This was a randomized, double-blind, 2-by-2 factorial trial. Stunted children aged 12–59 mo from eastern Uganda (n = 750) were randomly assigned to receive 100 g LNS-LQ with or without MP and WP (n = 4 × 150) or no supplement (n = 150) for 3 mo. The primary outcomes were change in knee-heel and total length. Secondary outcomes included child development, body composition, anthropometry, and hemoglobin. Micronutrient status, intestinal function, and microbiota were also assessed. Our findings will contribute to an understanding of the role of milk ingredients and LNS in linear catch-up growth. This trial was registered at www.isrctn.com as ISRCTN13093195.
Keywords: stunting, linear growth, lipid-based nutrient supplement (LNS), milk protein, whey permeate, child development, body composition, gut
Will large quantity lipid-based nutrient supplements containing milk protein and whey permeate improve linear growth as well as body composition, gut function, and child development in stunted children?
Introduction
Globally, 144 million children under the age of 5 y are classified as stunted, having a length- or height-for-age z score (HAZ) of less than −2 (1). Stunting is associated with adverse short- and long-term health outcomes. It is associated with delayed cognitive development, increased morbidity and mortality (2), poor schooling performance (3, 4), and later with reduced economic productivity (4) and risk of chronic disease (5, 6). Stunting also contributes to an intergenerational cycle of malnutrition and poverty, whereby a child born to a stunted mother is more likely to be stunted themselves (7).
In the east African region, close to 1 in 3 children under the age of 5 are stunted (1). High stunting prevalence is experienced in many low- and middle-income countries and is indicative of exposure to environments of inadequate care, suboptimal nutrition, and recurrent infections (3, 5, 6). The majority of growth faltering occurs from 3 to 24 mo of age (8). Nutrition interventions to reduce the risk of stunting have therefore focused on prevention through optimizing maternal and early infant nutrition (9, 10). These interventions, however, have had little impact on linear growth. This was summarized in a recent meta-analysis, whereby complementary feeding interventions, in food-insecure settings, improved HAZ by a mere 0.08 overall (10). The lack of effect on linear growth has been attributed, at least in part, to environmental enteric dysfunction (EED) (11, 12). The premise is that frequent exposure to pathogens in environments with unsafe water and inadequate sanitation and hygiene [water, sanitation, and hygiene (WASH)] encourages a state of systemic and intestinal inflammation, as well as morphological and functional changes to the intestine, which can, in turn, exacerbate nutrient deficiencies. However, large trials combining comprehensive WASH interventions with small-quantity lipid-based nutrient supplements (LNS-SQ) reported no effects from the WASH interventions and only minimal effects from the LNS-SQ (13–17).
While there have been many studies aiming to prevent stunting in young children, or to improve linear growth in wasted children, this is to our knowledge, the first trial which provided large-quantity LNS (LNS-LQ) to children recruited on the basis of stunting. There have been concerns that supplementation in stunted children will lead to excessive accretion of fat rather than lean tissue, and therefore increase the subsequent risk of chronic disease. These concerns, however, are not substantiated by the evidence. Recent supplementation studies among children with moderate (18) and severe acute (19) malnutrition have shown that even those who are also stunted predominantly gain fat-free mass (20). There is a gap in the evidence, however, as to the extent that an LNS-LQ or one containing milk protein (MP) will encourage catch-up growth in already stunted children, and to what extent this impacts body composition (21–23), mitigates vulnerability to illness, and improves child development and other functional outcomes (23, 24). However, it is possible that nutritional support to stunted children could have beneficial effects even in the absence of linear catch-up growth. We now know that the co-existence of wasting (low weight-for-height) and linear growth faltering increases a child's risk of morbidity and mortality (25, 26). Moreover, new evidence from a large 40-y cohort study in The Gambia suggests that stunting not only develops as a chronic condition but also develops interactively with episodes of wasting as a short-term adaptation (27, 28).
Previous nutrition interventions may have been limited by an inadequate supply of energy and high-quality proteins. Considering this, and the recent evidence demonstrating that even short children with wasting predominantly gain fat-free mass (20), there is sufficient justification to assess the effects of an LNS-LQ among stunted children.
Milk intake has long been associated with linear growth (29, 30) and is suggested to have a stronger effect in low-income compared with high-income countries (29). However, a new review based on studies from predominantly high-income countries was not able to confirm an effect of milk intake on linear growth (31). Several studies have shown that the addition of milk in supplements to treat acute malnutrition has had positive effects on body composition, weight gain, recovery, and anemia (32–37), but limited (18) or no effect in encouraging linear catch-up growth (32, 35, 38). In studies from low- and high-income countries, milk intake in children has been associated with improved lean mass deposition (31), bone-mineral composition (39), and cognitive function (40, 41), benefits that may be experienced to a greater extent in children exposed to growth-deficient environments (31, 42). Furthermore, the different components of milk may provide unique health benefits (43). MPs have a complete amino acid profile (31) and are thought to promote growth by stimulating the growth factors insulin-like growth factor-I (IGF-I) and insulin (44). On the other hand, whey permeate (WP) is predominantly composed of lactose and bioavailable minerals, which may have prebiotic effects (45) as well as a role in bone mineralization and fat-free mass accretion (46).
In this study, we aimed to assess the individual and combined effects of MP and WP, provided as part of an LNS-LQ, using a 2 × 2 factorial design, among stunted children. The primary outcome was linear growth. Secondary outcomes were child development, body composition, HAZ, weight-for-age (WAZ), and weight-for-height z scores (WHZ), weight, midupper arm circumference (MUAC), head circumference, and hemoglobin. In addition, we assessed the main effect of LNS on these outcomes, irrespective of milk ingredients, as well as the role of the gut as mediator or modifier of effects.
Methods
The reporting of this protocol followed the Standard Protocol Items: Recommendations for International Trials (SPIRIT) 2013 checklist.
Trial overview and design
The MAGNUS study (Milk Affecting Growth, Cognition, and the Gut in Child Stunting) was a randomized, double-blind, 2-by-2 factorial trial testing the effects of MP and WP in LNS-LQ. An unsupplemented group was included as a reference. For a 12-wk period between February and December 2020, 750 Ugandan children classified as stunted received 1 of 4 formulations of LNS-LQ as a daily supplement (n = 4 × 150) or continued with the family diet (n = 150) (see Figure 1). All caregivers received individual nutrition counseling at baseline. All participants were followed up at the same intervals throughout the intervention period (see Figure 2). This design will allow us to assess the individual and combined effects of MP and WP among the 600 children allocated to LNS, based on the factorial design: If the effects are independent, then we can compare the 300 given LNS with MP to the 300 given LNS without milk. And likewise, we can compare the 300 given LNS with WP to the 300 given LNS without WP. If the effects are not independent, then we will compare each of the 4 combinations pairwise. In addition, we will be able to assess the effect of LNS by comparing the 600 given LNS, irrespective of milk ingredients, to the 150 given no supplements.
FIGURE 1.
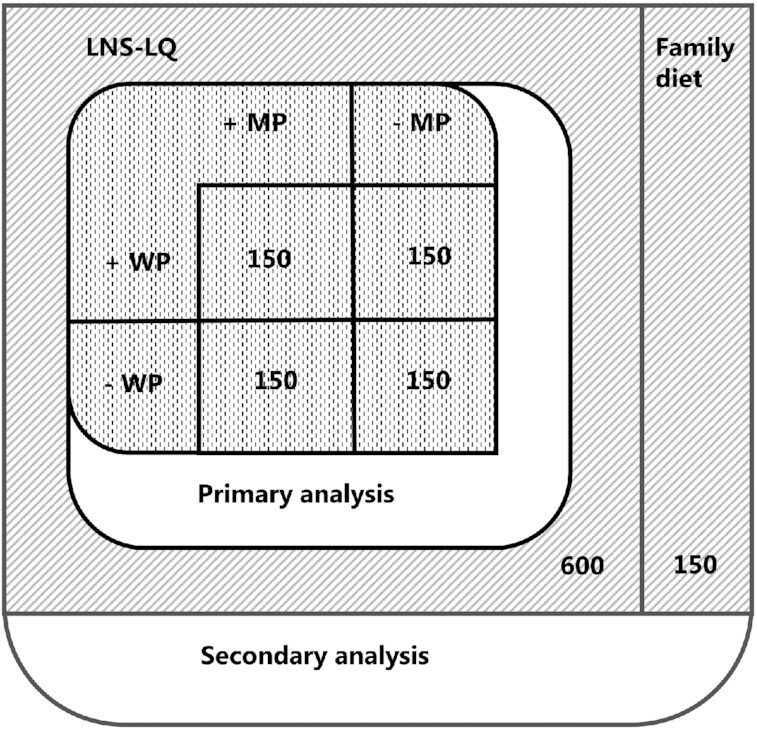
The primary analysis will compare LNS-LQ with and without MP and WP in a 2-by-2 factorial design with 150 participants in each given combination. Secondary analysis will compare all LNS-LQ interventions (n = 600) with the reference group (family diet, n = 150). LNS-LQ, large-quantity lipid-based nutrient supplements; MP, milk protein; WP, whey permeate.
FIGURE 2.
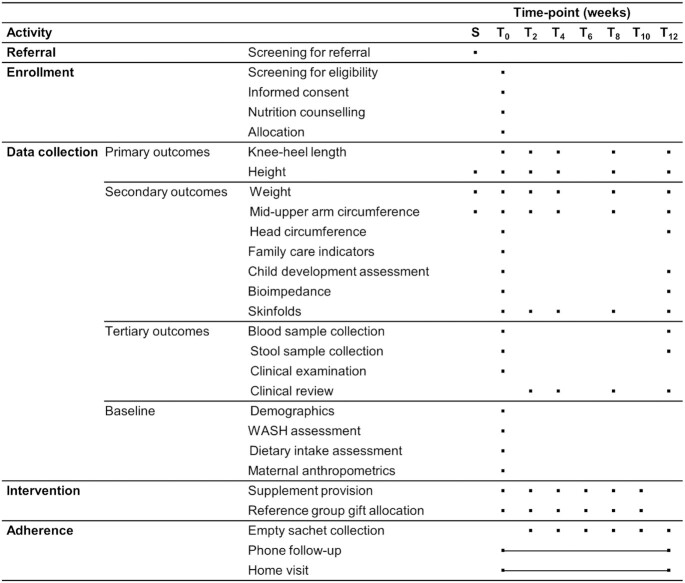
MAGNUS data collection time points and visits. Timeline and visit overview for participants enrolled in the study. Phone follow-up and home visits were carried out as required and thus were unfixed time points. All participants were invited to the same follow-up visits. Time points were considered valid if taken within ±7 d of baseline, week 2 and week 4, and ±14 d from all other time points. Hemoglobin was a secondary outcome. LNS-LQ, large-quantity lipid-based nutrient supplement; MAGNUS, Milk Affecting Growth, Cognition, and the Gut in Child Stunting; MUAC, midupper arm circumference; S, screening in village for referral; T, week of visit from baseline (T0) to discharge (T12); WASH, water, sanitation, and hygiene.
The intervention
LNS are fortified lipid-based pastes that are well adapted for use in resource-limited settings; they are produced to a high safety standard, do not require refrigeration or preparation, and are packaged in standard portion sizes.
Our LNS-LQ supplements, manufactured by Nutriset (Malaunay, France), varied with respect to the incorporation of WP and MP isolate (MPI). The MPI contained casein and whey proteins in the same proportions as milk; the lactose and mineral components were removed so that the MPI was close to 90% protein by weight with a Digestible Indispensable Amino Acid Score (DIAAS) of 120 (47). As a comparator to MPI, soy protein isolate, a high-quality plant protein with a DIAAS of 84, was used (47). WP contained 80–85% lactose and minerals (potassium, phosphorus, magnesium, calcium, sodium, and to a lesser extent zinc). As a comparator to WP, maltodextrin, a standard ingredient used in LNS products was used. All formulations were standardized to contain similar proportions of energy, protein, and carbohydrates. The supplements contained a mineral and vitamin mix to improve micronutrient content, and in 2 of the formulations the milk minerals provided by WP were in addition to the standard amount provided in all formulations (Table 1).
TABLE 1.
Nutrient composition for 4 formulations of LNS-LQ supplied to 1- to 5-y-old stunted children1
| Milk protein and whey permeate LNS-LQ | Milk protein and no whey permeate LNS-LQ | Soy protein and whey permeate LNS-LQ | Soy protein and no whey permeate LNS-LQ | |
|---|---|---|---|---|
| Macronutrients (components per 100 g) | ||||
| Energy, kcal | 531 | 535 | 530 | 534 |
| Carbohydrates, g | 42 | 43 | 42 | 43 |
| Lactose, g | 15.7 | 0.4 | 15.3 | 0 |
| Proteins, g | 13.9 | 13.5 | 13.9 | 13.5 |
| Milk proteins, g | 7.15 | 6.75 | 0.40 | 0 |
| Vegetable proteins, g | 6.75 | 6.75 | 13.50 | 13.50 |
| Lipids, g | 33.7 | 33.7 | 33.7 | 33.7 |
| Linoleic acid (C18:2), g | 3.0 | 3.0 | 3.0 | 3.0 |
| Linolenic acid (C18:3), g | 0.5 | 0.5 | 0.5 | 0.5 |
| Minerals | ||||
| Calcium, mg | 691 | 594 | 691 | 594 |
| Copper, mg | 1.65 | 1.65 | 1.65 | 1.65 |
| Iron, mg | 12 | 12 | 12 | 12 |
| Iodine, µg | 127 | 113 | 127 | 113 |
| Magnesium, mg | 199.2 | 175.8 | 199.2 | 175.8 |
| Manganese, mg | 1.8 | 1.8 | 1.8 | 1.8 |
| Phosphorus, mg | 661 | 539 | 661 | 539 |
| Potassium, mg | 1315 | 985 | 1315 | 985 |
| Sodium,2 mg | 84 | 7 | 156 | 79 |
| Selenium, µg | 30 | 30 | 30 | 30 |
| Zinc, mg | 12.5 | 12.5 | 12.5 | 12.5 |
| Vitamins | ||||
| Vitamin A, mg | 619 | 619 | 619 | 619 |
| Vitamin B-1, mg | 1.2 | 1.1 | 1.2 | 1.1 |
| Vitamin B-12, µg | 3.2 | 3.0 | 3.2 | 3.0 |
| Vitamin B-2, mg | 3.1 | 2.8 | 2.7 | 2.4 |
| Niacin, mg | 14.9 | 14.6 | 14.9 | 14.6 |
| Pantothenic acid, mg | 5.7 | 4.5 | 5.7 | 4.5 |
| Vitamin B-6, mg | 2.1 | 2.0 | 2.1 | 2.0 |
| Biotin, µg | 74.1 | 67.6 | 74.1 | 67.6 |
| Folic acid, µg | 223 | 223 | 223 | 223 |
| Vitamin C, mg | 67.9 | 67.6 | 67.9 | 67.6 |
| Vitamin D, µg | 16.9 | 16.9 | 16.9 | 16.9 |
| Vitamin E, mg | 18 | 18 | 18 | 18 |
| Vitamin K, µg | 30 | 30 | 30 | 30 |
The same amount of micronutrient premix was used in all formulations. The additional micronutrients provided are from the other ingredients used. LNS-LQ, large-quantity lipid-based nutrient supplement.
Soy protein isolate and whey permeate contribute additional sodium.
The 600 participants randomly assigned to LNS-LQ received one 100-g sachet (530–535 kcal)/d for 12 wk, distributed every 14 d. Those randomly assigned to the family diet received laundry soap at each visit.
Participant recruitment and enrollment
The study was conducted from 2 local community health centers in Walukuba and Buwenge. All participants were recruited from within the surrounding district of Jinja, in the Busoga Subregion, eastern Uganda. Here, the prevalence of child stunting is estimated to be 29%, similar to the national average (48). To identify stunted children, communities within the district of Jinja were mobilized by Village Health Teams for an initial screening for referral. Study staff screened children in the community for age, stunting, and severe acute malnutrition (SAM). All children identified as having SAM were referred for appropriate treatment; others who met the inclusion criteria for stunting and age were invited to one of the study sites for eligibility screening.
At the study sites, children were considered eligible if they were aged between 12 and 59 mo and had an HAZ of less than −2, according to the WHO growth standards (49). Children <12 mo old were not eligible to avoid interfering with breastfeeding. Caregivers had to be living in the catchment area and willing to return for follow-up visits, and able to provide written informed consent and agree to both phone follow-up (if a phone contact was available) and home visits. Children were excluded if they were identified with SAM according to the WHO classification (50), had medical complications requiring hospitalization, a history of allergy to peanuts or milk, obvious disability that impeded eating capacity, or a disability that impeded the measurement of length or height. Children were also excluded if they were participating in another study, if the family planned to move away from the catchment area within 6 mo, if previously enrolled in the MAGNUS study, or if another child from the same household was already included.
Informed consent
If all eligibility criteria were met, trained staff took the caregiver through the informed-consent information individually, using the most appropriate of 3 commonly spoken languages in the region (English, Lusoga, or Luganda). The same information was given verbally and in writing. Caregivers were also taken through a short verbal questionnaire to ensure that the information provided was adequately understood. After necessary clarifications were given, the caregiver consented on behalf of the participant. If illiterate, a literate witness was present during the informed-consent process. Consenting caregivers were asked for permission to store 1–2 mL of blood and stool samples from the participant for future use; this was independent of trial consent. The caregiver could opt to withdraw consent at any time.
Blinding, randomization, and assignment of interventions
The sachets of LNS-LQ were labeled with a unique 3-letter code that corresponded to the different formulations. Two unique codes were given to each of the 4 formulations and a further 2 codes were created for the reference group so that 10 unique codes were used in the allocation sequence list. Only the manufacturer (Nutriset) had access to the blinding code. Two allocation sequence lists, one for each study site, were computer generated using R (R Foundation for Statistical Computing). These were generated and sealed by a member of staff at the University of Copenhagen, Denmark, who was otherwise not involved in the study. Site-stratified, block randomization, with variable block sizes of 10 and 20 were used to allocate the sequential list of ID numbers to the 10 unique codes.
Upon inclusion, administrative staff allocated a unique ID from a sequentially ordered list. After completion of baseline activities, the study pharmacist allocated the intervention according to a hard-copy random allocation list. Only the pharmacist had access to the allocation list, which was checked for each participant, at each visit. Using QR codes, the pharmacist recorded the code of what was distributed in a spreadsheet, which was regularly monitored by an independent assessor in Copenhagen. Hard copies of the allocation lists were kept securely in sealed envelopes at the University of Copenhagen.
Outcome assessors and data analysts were blinded both with respect to the allocation of the intervention and to the type of ingredients contained in differently coded LNS sachets. Caregivers were blinded with respect to the type of LNS allocated, since the taste, smell, and appearance of all 4 products were indistinguishable. Caregivers were not, however, blinded with respect to receiving LNS or not. Only the Data Safety Monitoring Board, which operated independently of the study, could choose to break the blinding in order to monitor safety parameters.
Adherence
The LNS was distributed in packs of 14 sachets. To counteract the likelihood of sharing, an additional pack of the same LNS product code was distributed every 2 wk to caregivers with other children aged between 6 and 59 mo living in the same household. The additional stock provided to the household increased the likelihood that the participating child had access to the required daily quota. When collecting new sachets, the caregiver was requested to return any empty and unused sachets from the previous 2-wk supply, including those from the additional pack.
Outcomes
Primary outcomes
The primary outcomes were changes in knee-heel length (mm) and total length/height (cm) from baseline to 12 wk.
Secondary outcomes
All secondary outcomes were measured over time from baseline to 12 wk. Child development was assessed at baseline and at discharge using a locally adapted version of the Malawi Development Assessment Tool (MDAT). Anthropometric indices HAZ, WAZ, and WHZ were assessed as well as weight (g), MUAC (cm), and head circumference (mm). Body composition was assessed using bioimpedance and the triceps and subscapular skinfold thicknesses (mm). The raw data from bioimpedance were used to calculate the fat mass (FM) (kg), fat-free mass (FFM) (kg), fat mass index (kg/m2), and fat-free mass index (kg/m2). Hemoglobin concentration was assessed from blood samples collected at baseline and 12 wk.
Tertiary outcomes
Biological samples
Blood and stool samples were collected at baseline and at week 12. Blood samples will be analyzed for growth factors (IGF-I and insulin), markers of micronutrient status [i.e., iron (ferritin, soluble transferrin receptor), folate (serum folate), vitamin B-12 (cobalamin, methylmalonic acid), and vitamin A (retinol binding protein)], markers of systemic inflammation [C-reactive protein and α1-acid glycoprotein (AGP)], and markers of intestinal function (citrulline), together with other amino acids. Stool samples will be analyzed for markers of intestinal inflammation [myeloperoxidase (MPO), neopterin (NEO)] and function (α1-antitrypsin (AAT)] and the gut microbiota.
Safety, morbidity, and loss to follow-up
Data will be reported on the proportion of children who, during the intervention period, deteriorated to moderate acute malnutrition or SAM according to the WHO classifications (49). The proportion of parti-cipants who died during the study period will be reported, as well as the number of morbidity episodes including the duration and severity of the illness. Finally, the number of children who were lost to follow-up will be reported. Caregivers were called with reminders to attend upcoming or missed appointments. Loss to follow-up was defined as those who had not returned for the 12-wk follow-up visit by 14 wk post-inclusion.
Baseline participant characteristics
Additional information collected at baseline included demographics, a dietary intake assessment, and a WASH assessment taken at the initial home visit.
Measurements
Time points for each measurement are shown in Figure 2.
Anthropometrics
Knee-heel length was measured using a digital caliper with a resolution of 0.01 mm (Mitutoyo) mounted with knee and heel caps, cast in hard plastic. The distance between the knee (from the lateral condyle) and the heel (calcaneus) was measured 5 times consecutively on the left leg while the child was seated with both legs hanging over the edge of a table or the caregiver's lap. All other anthropometric measurements were repeated in triplicate. Participant length and height measurements were taken using a wooden Shorrboard (Weight and Measure), ensuring 4 points of contact with repositioning between measurements. Maternal height was measured using a fixed wall stadiometer (SECA 206). The weights of the mother and participant were measured using an electronic double-weighing scale (SECA 876). Head circumference, MUAC, and skinfold thickness were measured using a windowed, nonelastic head circumference tape (SECA 212); a nonelastic MUAC tape (UNICEF SD); and a Harpenden skinfold caliper (Baty International), respectively. Height or length, weight, MUAC, and head circumference were measured according to accepted international standards for anthropometric measurement (51). Skinfold thicknesses were measured on the left side, according to the manufacturer's instructions. For referral and inclusion, z scores were calculated using the WHO field growth charts. The WHO Anthro program will be used to calculate z scores for data analysis (52).
Bioimpedance
Bioimpedance was measured using the Bodystat 500 (50 kHz) and in accordance with the manufacturer's instructions (Bodystat Ltd.). Measurements were taken while the child was lying on his/her back, with limbs spread apart, preferably at rest and with removal of wet or soiled diapers. A measure was repeated a minimum of 2 times but up to 3 times if the child's positioning or movement rated poorly. Measurements for impedance, resistance, reactance, and phase angle were recorded. Using an equation, the raw data will be used to calculate FM and FFM.
Child development
Child development officers, trained in use of the MDAT (Manual V06, March 2018), took the participant through a series of activities adapted for the Ugandan context. The activities were related to 4 domains of development: gross motor, fine motor, language, and social development (53). The participant was graded as to whether or not he/she could complete each task successfully. The assessment continued until the child had failed to complete 6 tasks consecutively. At baseline, an interviewer-administered questionnaire was also used to gather information from the caregiver about household and family indicators for the support of child development (54).
Clinical assessment
A thorough clinical examination was carried out at baseline. It included rapid tests for HIV and malaria, a thorough medical history with questions related to signs and symptoms of wasting or hospitalization due to SAM, and assessment of vital signs (pulse, blood pressure, and respiratory rate). At follow-up visits, a short review was conducted, assessing the most recent medical history, milk intake, and where applicable, monitoring of adverse events. To maintain blinding, the pharmacist distributing LNS inquired about adherence and if the caregiver had experienced problems with the LNS.
Biological sample collection
Stool samples were collected at 2 time points and stored for later analysis of markers of gut function and microbiota. If not collected on site, a sample collection kit was given to caregivers along with specific instruction on stool sample collection at home. Collection vials contained StayRNA (A&A Biotechnology), allowing samples to be stored at room temperature for up to 5 d after collection. A maximum of 6.0 mL of venous blood was collected on site at 2 time points. A small amount was used for rapid tests: HIV status, malaria, and hemoglobin status. The remaining sample was processed and stored within hours for later analysis of selected markers. All biological samples were stored at −20°C until delivery to the main storage site in Kampala where they were stored at −80°C until they were shipped on dry ice to the University of Copenhagen, Denmark, for additional analyses.
Other measurements, baseline questionnaires, and WASH assessment
At baseline, the child's age and birth weight were recorded, wherever possible, using a birth information card. Information on sociodemographic characteristics, breastfeeding status, food frequency, and diet diversity was collected via interviewer-administered questionnaires. At the baseline home visit, GPS coordinates of the home site location were collected to facilitate later follow-up. In addition, trained staff conducted a short assessment of observed household WASH characteristics, including water source, access to basic sanitation, and the use of soap. To minimize response bias, local study staff with a good knowledge of the language and the culture were trained in asking questions to get as clear and precise answers as possible.
Participant retention, reimbursement, referral, and withdrawal
If visits were missed and phone contact was unsuccessful, attempts were made to visit the caregiver's home. To facilitate attendance, a travel reimbursement was provided at each visit to cover the cost of return transport and food while at the clinic visit. Any participants requiring hospital attention were referred for treatment. If a caregiver requested for their child to stop receiving LNS, this was permitted; however, all included participants continued to be followed up for the remainder of the 12-wk intervention period. In case of participant withdrawal, all available data up to the point of withdrawal were used in data analysis.
Data management
Participant data were collected in a paper case report form and were double entered using Epidata software (https://www.epidata.dk/) with inbuilt range checks. The secure electronic data collection platform REDCap (Open Source; Vanderbilt University) was used to monitor participant registration and visits but not for primary data collection. All source data will be kept securely on file for a minimum of 5 y after completion of the study. Adverse and serious adverse events occurring during the intervention period were recorded and reported to the sponsor and the institutional review board. Events occurring after a subject was discontinued from the study were not reported unless the investigator suspected that the event was related to the LNS-LQ intervention.
Sample size calculation
To detect a 0.35-SD or greater difference between any 2 groups, with 5% significance and 80% power, 129 children were required in each group. To allow for 10% loss to follow-up, 150 children were included in each group, based on the 4 combinations of MP and WP. If there were no interactions between the 2 experimental interventions, 2 groups of 300 children could be compared, enabling differences of 0.24 SD to be detected. In the Treatfood trial (18), the SD of knee‐heel length at baseline was 18.1 mm (18), so that a 0.24-SD difference corresponded to 4.3 mm. In secondary analysis, to assess the effect of LNS, 600 supplemented children were compared with 150 unsupplemented children, with the ability to detect a 0.27-SD difference, corresponding to 4.9 mm.
Statistical methods
Primary and secondary outcomes will be analyzed using linear mixed models that account for the correlation between repeated measurements from the same participant, whereas tertiary outcomes will be analyzed using ordinary ANCOVA models. In all of these ANCOVA models, the baseline value will be included as a covariate. Additional covariates may be included as appropriate. Results will be reported as estimated differences with corresponding 95% CIs and P values. A statistical analysis plan was prepared before unblinding of the trial and uploaded to the ISRCTN registry.
This is an effectiveness trial. Therefore, the primary statistical analysis will be carried out as intention to treat. In subsequent per-protocol statistical analysis, participants with major protocol deviations or violations are excluded.
Ethics approval and consent to participate
The study was conducted in accordance with the ethical principles set forth in the current version of the Declaration of Helsinki and all applicable local regulatory requirements. The study was approved by the School of Medicine Research Ethics Committee at Makerere University and The Ugandan National Council of Science and Technology. The study also received consultative approval from the Danish National Committee on Biomedical Research Ethics. The study was initiated only after approval was given by all aforementioned authorities. Written informed consent was obtained from all caregivers who consented to study participation of the child in their care. The rights, safety, and well-being of the children involved in the study prevailed over science and society. Before participant recruitment, the study was registered at www.isrctn.com as ISRCTN13093195.
Discussion
The findings from the MAGNUS trial will help to clarify to what extent MP and WP, given in LNS, or the LNS per se, play a role in linear catch-up growth and benefit functional outcomes such as cognition and the gut. In this, we will explore to what extent functional benefits are possible with or without effects on linear growth. Our results will also contribute to current knowledge on whether stunted children will predominantly gain lean mass when supplemented with LNS-LQ. Since our study population is aged between 12 and 59 mo, we will also be exploring the potential for catch-up growth in children beyond 2 y of age.
The gut is thought to play a role in the pathogenesis of stunting, but studies aiming to minimize environmental pathogen exposures, and so reduce the risk of EED, have not seen improvements in linear growth. It may be that, once damaged, the gut requires larger quantities of essential nutrients in order to repair and facilitate nutrient absorption. We will explore whether milk components provided in LNS-LQ can improve reparation of the gut in already stunted children and to what extent gut function and inflammation act as mediators and effect modifiers of the effect of LNS-LQ on linear growth.
The high lactose content in WP may have positive effects on the microbiota and growth. If this is demonstrated in our study, it may have implications on the future development of LNS, since WP has the potential to be used as a nutritious substitute for maltodextrin or sugar.
This is the first randomized controlled trial we are aware of that explores the effects of LNS-LQ supplementation in already stunted children. The strengths of this study are the randomized 2 × 2 factorial design, which allows us to assess both the individual and combined effects of the milk ingredients, as well as the unsupplemented reference group, allowing us to assess the main effects of LNS per se. It is also a strength that the study includes several secondary functional outcomes alongside anthropometrics and body composition, as well as tertiary mechanistic outcomes. It is a limitation that we are unable to include a longer follow-up period. A follow-up study of the cohort would be of great benefit to measure the benefits and chronic disease risks associated with the 12-wk LNS-LQ supplementation.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—HP: is a co-investigator, assisted in the study design, prepared the manuscript, and wrote the study protocol; RM: is a co-investigator and provided clinical oversight and critical revisions to the manuscript; JM: is a co-investigator and provided oversight of anthropometry and critical revisions to the manuscript; MFO: is a co-investigator and provided oversight of child development and critical revisions to the manuscript; CM: is a co-investigator and contributed to the study design and provided critical revisions to the manuscript; KFM: is a co-investigator, contributed to study design, provided oversight of knee-heel length, and provided critical revisions to the manuscript; CR: performed the sample size calculations and supervised the sections on randomization and statistics; SF and AB: are co-investigators and contributed to the study design and provided critical revisions to the manuscript; EM: is a principal investigator and contributed to the study design and provided critical revisions to the manuscript; HF: is a principal investigator and sponsor representative, and conceived of the study design and provided critical revisions to the manuscript; BG: is the co-principal investigator, led the development of the protocol, conceived of the study design, and provided critical revisions to the manuscript; and all authors: read and approved the final manuscript.
Notes
This research is funded by Arla Food for Health; the Danish Dairy Research Foundation; the University of Copenhagen, Department of Nutrition, Exercise, and Sports; the Augustinus Foundation; the Dr Sofus Carl Emil Friis Fund; and the AP Møller Fonden.
Author disclosures: KFM has received research grants from the US Dairy Export Council and the Danish Dairy Research Foundation, and reports research collaboration with Nutriset, a producer of lipid-based nutrient supplement (LNS) products and the patent owner; HF, CM, and BG have received research grants from the Arla Food for Health Centre and the Danish Dairy Research Foundation and report research collaboration with Nutriset; AB was the inventor of LNS, for which Nutriset has the patent, but abandoned claims to royalties in 2003. The other authors report no conflicts of interest. The funders Arla Food for Health and the Danish Dairy Research Foundation did not have any influence on the analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Abbreviations used: DIAAS, Digestible Indispensable Amino Acid Score; EED, environmental enteric dysfunction; FFM, fat-free mass; FM, fat mass; HAZ, height-for-age z score; IGF-I, insulin-like growth factor I; LNS, lipid-based nutrient supplement(s); LNS-LQ, large-quantity lipid-based nutrient supplement(s); LNS-SQ, small-quantity lipid-based nutrient supplement(s); MAGNUS, Milk Affecting Growth, Cognition, and the Gut in Child Stunting; MDAT, Malawi Development Assessment Tool; MP, milk protein; MPI, milk protein isolate; MUAC, midupper arm circumference; SAM, severe acute malnutrition; WASH, water, sanitation, and hygiene; WAZ, weight-for-age z score; WHZ, weight-for-height z score; WP, whey permeate.
Contributor Information
Hannah Pesu, Department of Nutrition, Exercise, and Sports, University of Copenhagen, Copenhagen, Denmark.
Rolland Mutumba, Department of Nutrition, Exercise, and Sports, University of Copenhagen, Copenhagen, Denmark; Department of Paediatrics and Child Health, School of Medicine College of Health Sciences, Makerere University, Kampala, Uganda.
Joseph Mbabazi, Department of Nutrition, Exercise, and Sports, University of Copenhagen, Copenhagen, Denmark; Department of Paediatrics and Child Health, School of Medicine College of Health Sciences, Makerere University, Kampala, Uganda.
Mette F Olsen, Department of Nutrition, Exercise, and Sports, University of Copenhagen, Copenhagen, Denmark.
Christian Mølgaard, Department of Nutrition, Exercise, and Sports, University of Copenhagen, Copenhagen, Denmark.
Kim F Michaelsen, Department of Nutrition, Exercise, and Sports, University of Copenhagen, Copenhagen, Denmark.
Christian Ritz, Department of Nutrition, Exercise, and Sports, University of Copenhagen, Copenhagen, Denmark.
Suzanne Filteau, Department of Population Health, London School of Hygiene and Tropical Medicine, London, United Kingdom.
André Briend, Department of Nutrition, Exercise, and Sports, University of Copenhagen, Copenhagen, Denmark; Tampere Centre for Child Health Research, Tampere University, Tampere, Finland.
Ezekiel Mupere, Department of Paediatrics and Child Health, School of Medicine College of Health Sciences, Makerere University, Kampala, Uganda.
Henrik Friis, Department of Nutrition, Exercise, and Sports, University of Copenhagen, Copenhagen, Denmark.
Benedikte Grenov, Email: bgr@nexs.ku.dk, Department of Nutrition, Exercise, and Sports, University of Copenhagen, Copenhagen, Denmark.
References
- 1. UNICEF; WHO; The World Bank Group . UNICEF/WHO/The World Bank Group joint child malnutrition estimates: levels and trends in child malnutrition: key findings of the 2020 edition. [Internet]. Available from: https://www.who.int/publications-detail/jme-2020-edition, (accessed 18 March 2021). [Google Scholar]
- 2. Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, Caulfield LE, Danaei G; Nutrition Impact Model Study (anthropometry cohort pooling) . Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS One. 2013;8(5):e64636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDonald CM, Olofin I, Flaxman S, Fawzi WW, Spiegelman D, Caulfield LE, Black RE, Ezzati M, Danaei G. The effect of multiple anthropometric deficits on child mortality: meta-analysis of individual data in 10 prospective studies from developing countries. Am J Clin Nutr. 2013;97(4):896–901. [DOI] [PubMed] [Google Scholar]
- 4. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell Ret al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet North Am Ed. 2013;382(9890):427–51. [DOI] [PubMed] [Google Scholar]
- 5. Leroy JL, Frongillo EA. Perspective: what does stunting really mean? A critical review of the evidence. Adv Nutr. 2019;10(2):196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Onis M, Branca F. Childhood stunting: a global perspective. Matern Child Nutr. 2016;12(Suppl 1):12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014;34(4):250–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Victora CG, Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125(3):e473–80. [DOI] [PubMed] [Google Scholar]
- 9. Khan GN, Kureishy S, Ariff S, Rizvi A, Sajid M, Garzon C, Khan AA, de Pee S, Soofi SB, Bhutta ZA. Effect of lipid-based nutrient supplement—medium quantity on reduction of stunting in children 6–23 months of age in Sindh, Pakistan: a cluster randomized controlled trial. PLoS One. 2020;15(8):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panjwani A, Heidkamp R. Complementary feeding interventions have a small but significant impact on linear and ponderal growth of children in low- and middle-income countries: a systematic review and meta-analysis. J Nutr. 2017;147:2169S–78S. [DOI] [PubMed] [Google Scholar]
- 11. Crane RJ, Jones KDJ, Berkley JA. Environmental enteric dysfunction: an overview. Food Nutr Bull. 2015;36(1 Suppl 1):S76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weisz AJ, Manary MJ, Stephenson K, Agapova S, Manary FG, Thakwalakwa C, Shulman RJ, Manary MJ. Abnormal gut integrity is associated with reduced linear growth in rural Malawian children. J Pediatr Gastroenterol Nutr. 2012;55(6):747–50. [DOI] [PubMed] [Google Scholar]
- 13. Hess SY, Abbeddou S, Jimenez EY, Somé JW, Vosti SA, Ouédraogo ZP, Guissou RM, Ouédraogo J-B, Brown KH. Small-quantity lipid-based nutrient supplements, regardless of their zinc content, increase growth and reduce the prevalence of stunting and wasting in young Burkinabe children: a cluster-randomized trial. PLoS One. 2015;10(3):e0122242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cichon B, Fabiansen C, Iuel-Brockdorf A-S, Yaméogo CW, Ritz C, Christensen VB, Filteau S, Briend A, Michaelsen KF, Friis H. Impact of food supplements on hemoglobin, iron status, and inflammation in children with moderate acute malnutrition: a 2 × 2 × 3 factorial randomized trial in Burkina Faso. Am J Clin Nutr. 2018;107(2):278–86. [DOI] [PubMed] [Google Scholar]
- 15. Kumordzie SM, Adu-Afarwuah S, Arimond M, Young RR, Adom T, Boatin R, Ocansey ME, Okronipa H, Prado EL, Oaks BMet al. Maternal and infant lipid-based nutritional supplementation increases height of Ghanaian children at 4–6 years only if the mother was not overweight before conception. J Nutr. 2019;149(5):847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart CP, Dewey KG, Lin A, Pickering AJ, Byrd KA, Jannat K, Ali S, Rao G, Dentz HN, Kiprotich Met al. Effects of lipid-based nutrient supplements and infant and young child feeding counseling with or without improved water, sanitation, and hygiene (WASH) on anemia and micronutrient status: results from 2 cluster-randomized trials in Kenya and Bangladesh. Am J Clin Nutr. 2019;109(1):148–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Style S, Tondeur M, Grijalva-Eternod C, Pringle J, Kassim I, Wilkinson C, Oman A, Dolan C, Spiegel P, Seal A. Assessment of the effectiveness of a small quantity lipid-based nutrient supplement on reducing anaemia and stunting in refugee populations in the Horn of Africa: secondary data analysis. PLoS One. 2017;12(6):e0177556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fabiansen C, Yaméogo CW, Iuel-Brockdorf A-S, Cichon B, Rytter MJH, Kurpad A, Wells JC, Ritz C, Ashorn P, Filteau Set al. Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: a randomised 2 × 2 × 3 factorial trial in Burkina Faso. PLoS Med. 2017;14(9):e1002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Binns P, Myatt M. Does treatment of short or stunted children aged 6–59 months for severe acute malnutrition using ready to use therapeutic food make them overweight?. Arch Public Health. 2018;76(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fabiansen C, Phelan KPQ, Cichon B, Yaméogo CW, Iuel-Brockdorff A-S, Kurpad A, Wells JC, Ritz C, Filteau S, Briend Aet al. Short malnourished children and fat accumulation with food supplementation. Pediatrics. 2018;142(3):e20180679. [DOI] [PubMed] [Google Scholar]
- 21. Leroy JL, Ruel M, Habicht J-P, Frongillo EA. Linear growth deficit continues to accumulate beyond the first 1000 days in low- and middle-income countries: global evidence from 51 national surveys. J Nutr. 2014;144(9):1460–6. [DOI] [PubMed] [Google Scholar]
- 22. Roberts JL, Stein AD. The impact of nutritional interventions beyond the first 2 years of life on linear growth: a systematic review and meta-analysis. Adv Nutr. 2017;8(2):323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Owino VO, Murphy-Alford AJ, Kerac M, Bahwere P, Friis H, Berkley JA, Jackson AA. Measuring growth and medium- and longer-term outcomes in malnourished children. Matern Child Nutr. 2019;15(3):e12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leroy JL, Ruel M, Habicht J-P, Frongillo EA. Using height-for-age differences (HAD) instead of height-for-age z-scores (HAZ) for the meaningful measurement of population-level catch-up in linear growth in children less than 5 years of age. BMC Pediatrics. 2015;15(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Briend A. The complex relationship between wasting and stunting. Am J Clin Nutr. 2019;110:271–2. [DOI] [PubMed] [Google Scholar]
- 26. Myatt M, Khara T, Schoenbuchner S, Pietzsch S, Dolan C, Lelijveld N, Briend A. Children who are both wasted and stunted are also underweight and have a high risk of death: a descriptive epidemiology of multiple anthropometric deficits using data from 51 countries. Arch Public Health. 2018;76(28):eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prado EL, Yakes Jimenez E, Vosti S, Stewart R, Stewart CP, Somé J, Pulakka A, Ouédraogo JB, Okronipa H, Ocansey Eet al. Path analyses of risk factors for linear growth faltering in four prospective cohorts of young children in Ghana, Malawi and Burkina Faso. BMJ Global Health. 2019;4(1):e001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schoenbuchner SM, Dolan C, Mwangome M, Hall A, Richard SA, Wells JC, Khara T, Sonko B, Prentice AM, Moore SE. The relationship between wasting and stunting: a retrospective cohort analysis of longitudinal data in Gambian children from 1976 to 2016. Am J Clin Nutr. 2019;110(2):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Beer H. Dairy products and physical stature: a systematic review and meta-analysis of controlled trials. Econ Hum Biol. 2012;10(3):299–309. [DOI] [PubMed] [Google Scholar]
- 30. Hoppe C, Mølgaard C, Michaelsen KF. Cow's milk and linear growth in industrialized and developing countries. Annu Rev Nutr. 2006;26(1):131–73. [DOI] [PubMed] [Google Scholar]
- 31. Kang K, Sotunde OF, Weiler HA. Effects of milk and milk-product consumption on growth among children and adolescents aged 6–18 years: a meta-analysis of randomized controlled trials. Adv Nutr. 2019;10(2):250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yackobovitch-Gavan M, Bhutta ZA. Malnutrition and catch-up growth during childhood and puberty. In: Nutrition and growth. Koletzko B, Shamir R, Turck D, Phillip M. Basel, Switzerland: Karger Publishers; 2017. p. 134–51. [DOI] [PubMed] [Google Scholar]
- 33. Manary M, Callaghan M, Singh L, Briend A. Protein quality and growth in malnourished children. Food Nutr Bull. 2016;37(1 Suppl):S29–36. [DOI] [PubMed] [Google Scholar]
- 34. Oakley E, Reinking J, Sandige H, Trehan I, Kennedy G, Maleta K, Manary M. A ready-to-use therapeutic food containing 10% milk is less effective than one with 25% milk in the treatment of severely malnourished children. J Nutr. 2010;140(12):2248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stobaugh HC, Ryan KN, Kennedy JA, Grise JB, Crocker AH, Thakwalakwa C, Litkowski PE, Maleta KM, Manary MJ, Trehan I. Including whey protein and whey permeate in ready-to-use supplementary food improves recovery rates in children with moderate acute malnutrition: a randomized, double-blind clinical trial. Am J Clin Nutr. 2016;103(3):926–33. [DOI] [PubMed] [Google Scholar]
- 36. Bahwere P, Balaluka B, Wells JCK, Mbiribindi CN, Sadler K, Akomo P, Dramaix-Wilmet M, Collins S. Cereals and pulse-based ready-to-use therapeutic food as an alternative to the standard milk- and peanut paste-based formulation for treating severe acute malnutrition: a noninferiority, individually randomized controlled efficacy clinical trial. Am J Clin Nutr. 2016;103(4):1145–61. [DOI] [PubMed] [Google Scholar]
- 37. Irena AH, Bahwere P, Owino VO, Diop EI, Bachmann MO, Mbwili-Muleya C, Dibari F, Sadler K, Collins S. Comparison of the effectiveness of a milk-free soy-maize-sorghum-based ready-to-use therapeutic food to standard ready-to-use therapeutic food with 25% milk in nutrition management of severely acutely malnourished Zambian children: an equivalence non-blinded cluster randomised controlled trial. Matern Child Nutr. 2015;11(Suppl 4):105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kerac M, Bunn J, Chagaluka G, Bahwere P, Tomkins A, Collins S, Seal A. Follow-up of post-discharge growth and mortality after treatment for severe acute malnutrition (FuSAM study): a prospective cohort study. PLoS One. 2014;9(6):e96030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carmela L, María José C, Mercedes G-C, Ángel G, María LC, Rosaura L. Effects of dairy product consumption on height and bonemineral content in children: a systematic review of controlled trials. Adv Nutr. 2019;10:S88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Olsen MF, Tuel-Brockdorff A-S, Yameogo CW, Cichon B, Fabiansen C. Impact of food supplements on early child development in children with moderate acute malnutrition: a randomised 2 × 2 × 3 factorial trial in Burkina Faso. PLoS Med. 2020;17:e1003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee R, Singh L, van Liefde D, Callaghan-Gillespie M, Steiner-Asiedu M, Saalia K, Edwards C, Serena A, Hershey T, Manary MJ. Milk powder added to a school meal increases cognitive test scores in Ghanaian children. J Nutr. 2018;148:1177–84. [DOI] [PubMed] [Google Scholar]
- 42. Yackobovitch-Gavan M, Phillip M, Gat-Yablonski G. How milk and its proteins affect growth, bone health, and weight. Horm Res Paediatr. 2017;88(1):63–9. [DOI] [PubMed] [Google Scholar]
- 43. Grenov B, Michaelsen KF. Growth components of cow's milk: emphasis on effects in undernourished children. Food Nutr Bull. 2018;39(2 Suppl):S45–53. [DOI] [PubMed] [Google Scholar]
- 44. Hoppe C, Mølgaard C, Dalum C, Vaag A, Michaelsen KF. Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/IGFBP-3: results from a randomized 7-day supplementation study in prepubertal boys. Eur J Clin Nutr. 2009;63(9):1076–83. [DOI] [PubMed] [Google Scholar]
- 45. Grenov B, Briend A, Sangild PT, Thymann T, Rytter MH, Hother A-L, Molgaard C, Michaelsen KF. Undernourished children and milk lactose. Food Nutr Bull. 2016;37(1):85–99. [DOI] [PubMed] [Google Scholar]
- 46. Hoppe C, Andersen GS, Jacobsen S, Mølgaard C, Friis H, Sangild PT, Michaelsen KF. The use of whey or skimmed milk powder in fortified blended foods for vulnerable groups. J Nutr. 2008;138(1):145S–61S. [DOI] [PubMed] [Google Scholar]
- 47. Mathai JK, Liu Y, Stein HH. Values for Digestible Indispensable Amino Acid Scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for Protein Digestibility-Corrected Amino Acid Scores (PDCAAS). Br J Nutr. 2017;117(4):490–9. [DOI] [PubMed] [Google Scholar]
- 48. Uganda Bureau of Statistics (UBOS) . Uganda Demographic Health Survey, 2016. Key indicators report [Internet]. Kampala (Uganda): UBOS and ICF; 2017. Available from: https://dhsprogram.com/pubs/pdf/FR333/FR333.pdf, (accessed 18 March 2021). [Google Scholar]
- 49. WHO Mulitcentre Growth Reference Study Group . WHO Child Growth Standards: methods and development. [Internet]. Geneva (Switzerland): World Health Organization; 2006. Available from: http://www.who.int/childgrowth/standards/Technical_report.pdf?ua=1, (accessed 18 March 2021). [Google Scholar]
- 50. WHO; UNICEF . WHO child growth standards and the identification of severe acute malnutrition in infants and children: a joint statement by the World Health Organization and the United Nations Children's Fund. [Internet]. 2009. Available from: http://www.ncbi.nlm.nih.gov/books/NBK200775/, (accessed 18 March 2021). [PubMed] [Google Scholar]
- 51. Gibson RS. Principles of nutritional assessment. 2nd ed.New York: Oxford University Press; 2005. [Google Scholar]
- 52. WHO . WHO Child Growth Standards. WHO Anthro. [Internet]. 2021. Available from: https://www.who.int/toolkits/child-growth-standards/software, (accessed 18 March 2021). [Google Scholar]
- 53. Gladstone M, Lancaster GA, Umar E, Nyirenda M, Kayira E, den B NR, Smyth RL. The Malawi Developmental Assessment Tool (MDAT): the creation, validation, and reliability of a tool to assess child development in rural African settings. PLoS Med. 2010;7(5):e1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kariger P, Frongillo EA, Engle P, Britto PMR, Sywulka SM, Menon P. Indicators of family care for development for use in multicountry surveys. J Health Popul Nutr. 2012;30:472–86. [DOI] [PMC free article] [PubMed] [Google Scholar]