ABSTRACT
Background
Women of reproductive age (WRA) are a high-risk population for anemia and micronutrient deficiencies. However, there are few representative population-level data from India, which could help inform evidence-based recommendations and policy.
Objective
To conduct a population-based biomarker survey of anemia and vitamin B-12 and folate status in WRA as part of a periconceptional surveillance program in southern India.
Methods
Participants were WRA (15–40 y) who were not pregnant or lactating. Whole blood (n = 979) was analyzed for hemoglobin via a Coulter counter (Coulter HMX). Plasma, serum, and RBCs were processed and stored at −80°C or less until batch analysis. Vitamin B-12 concentrations were measured via chemiluminescence; RBC and serum folate concentrations were evaluated via microbiological assay. Anemia and severe anemia were defined as hemoglobin <12.0 g/dL and <8.0 g/dL, respectively. Vitamin B-12 deficiency and insufficiency were defined as total vitamin B-12 <148 pmol/L and <221 pmol/L, respectively. Folate deficiency and insufficiency were defined as RBC folate <305 nmol/L and <748 nmol/L. A previously developed Bayesian model was used to predict neural tube defect (NTD) prevalence per 10,000 births.
Results
A total of 41.5% of WRA had anemia and 3.0% had severe anemia. A total of 48.3% of WRA had vitamin B-12 deficiency and 74.3% had vitamin B-12 insufficiency. The prevalence of RBC folate deficiency was 7.6%, and 79.3% of WRA had RBC folate <748 nmol/L, the threshold for optimal NTD prevention. Predicted NTD prevalence per 10,000 births based on RBC folate concentrations was 20.6 (95% uncertainty interval: 16.5–25.5).
Conclusions
The substantial burden of anemia, vitamin B-12 deficiency, and RBC folate insufficiency in WRA in this setting suggests an opportunity for anemia and birth defects prevention. Findings will directly inform the development of a randomized trial for anemia and birth defects prevention in southern India.
This study was registered at clinicaltrials.gov as NCT04048330.
Keywords: anemia, folate, vitamin B-12, NTDs, periconceptional, surveillance, India
Introduction
Women of reproductive age (WRA) are a high-risk population for anemia and micronutrient deficiencies (1–3). Anemia is common in WRA and is associated with increased risk of maternal and infant mortality, low birth weight, and preterm birth (4–10). Deficiencies in vitamin B-12 and/or folate result in megaloblastic anemia (11, 12); inadequate folate status periconceptionally causes neural tube defects (NTDs) and there is emerging evidence that vitamin B-12 may be an independent risk factor for NTDs (13–15).
NTDs are structural birth defects associated with the failure of neural tube closure during embryonic development. There are >260,000 new NTD cases per year globally; this estimate varies from 1 to 80 NTDs per 10,000 births, with a higher prevalence in low and middle-income countries (16–22). It is estimated that the burden of NTDs in India is among the highest globally (18, 23, 24); however, representative population-level data can establish the prevalence of NTDs and biomarkers that predict NTD risk.
The association between lower periconceptional folate status and increased risk of NTDs was first observed over 50 years ago (25, 26). Randomized trials demonstrated that periconceptional folic acid supplementation reduces the risk and recurrence of NTDs (15, 27–29). This led to dietary intake recommendations by the Institute of Medicine for WRA in the United States and folic acid fortification of flour in >80 countries (30). Fortification of staple foods with folic acid has been associated with a reduction in NTDs (31–34). In order to evaluate population-level NTD risk, the WHO recommends monitoring RBC folate concentrations in WRA (35, 36). This recommendation is supported by evidence from studies in the United States, China, and Ireland, which found that increases in RBC folate concentrations were associated with up to a 10-fold reduction in NTD risk (37–39).
Emerging evidence from laboratory and small observational studies has identified maternal vitamin B-12 deficiency as a risk factor for NTDs (13, 14) and other adverse pregnancy outcomes, including spontaneous abortion and low birth weight (13, 40, 41). Vitamin B-12 may also modify folate biomarkers that predict NTD risk at the population level (38). The burden of vitamin B-12 deficiency in India is estimated to be the highest in the world (41–44). The high prevalence of vitamin B-12 deficiency, and increasing evidence that low periconceptional vitamin B-12 status is a risk factor for NTDs, has stimulated interest in mandatory food fortification with vitamin B-12 (45). However, there are limited representative population-based data on the burden of vitamin B-12 and folate deficiencies in southern India (46). Surveillance programs can help establish critical biomarker data and inform interventions to improve maternal and child health.
The objective of this study was to conduct a biomarker survey of anemia and vitamin B-12 and folate status in WRA, as part of a periconceptional surveillance program in southern India. Findings from this biomarker survey will directly inform the development of a randomized trial of quadruple-fortified salt (i.e., iodine, iron, folic acid, vitamin B-12) for the prevention of anemia and birth defects in southern India.
Methods
Study design
This biomarker survey was conducted as part of a periconceptional surveillance program in southern India. The detailed design of this study has been previously described (47). Briefly, a census was conducted of all households within a 50-km2 catchment area of our research site, Arogyavaram Medical Centre. Data collection was completed for all households within the catchment area (n = 6552 households; rural: 3124; urban: 3428).
Study population
Participants were eligible for the biomarker survey if they were female, aged 15–40 y, and were not pregnant or lactating. In order to obtain population-level biomarker data for WRA in this setting, all rural households (n = 3124) and a simple random sample of urban households (n = 1000) were selected for screening and recruitment. Trained nurse enumerators returned to selected households to confirm household member rosters and assess household member eligibility for the biomarker survey. For households with >1 eligible WRA, an a priori algorithm was used to randomly select 1 eligible WRA as the proband. Analyses included one WRA per household, but programmatically, all eligible WRA in the household were invited to participate in the biomarker survey. Research staff were blinded to the identity of the WRA proband until after data collection was completed. Women who were currently pregnant (self-reported, based on last menstrual period) or who had severe anemia (<8.0 g/dL) were referred to a local clinic for follow-up and standard of care.
Informed consent or assent
Written informed consent (≥18 y) or assent (15 to <18 y) was obtained from all participants prior to the start of data collection, with audio-visual recording in accordance with national regulations for clinical trials in India. If a woman was not able to read, a literate witness was asked to sign the form and the woman signed the form via a thumbprint.
Ethics
The study protocol was reviewed and approved by the Institutional Review Board at Cornell University, the Institutional Ethics Committee (IEC) at Arogyavaram Medical Centre, and the IEC at St John's Research Institute. The protocol was reviewed in accordance with CDC human research protection procedures and was determined to be a nonresearch, routine surveillance activity. A nondisclosure agreement for personally identifiable information and data-sharing agreement for de-identified data were established. This study received clearance from the Indian Council of Medical Research Health Ministry Screening Committee. The protocol was registered at ClincialTrials.gov (NCT04048330). Findings from this biomarker survey will directly inform the development of a randomized efficacy trial of quadruple-fortified salt (NCT03853304) in WRA in southern India. The protocol for the randomized trial was reviewed according to the CDC human research protection procedures and was determined to be research, but CDC involvement did not constitute engagement in human subjects research.
Data collection
Data were collected at Arogyavaram Medical Centre, the central research facility for the periconceptional surveillance program. Eligibility was confirmed (i.e., 15–40 y, not currently pregnant or lactating) before initiation of data collection. Transportation was provided for participants as needed. Individuals who missed their scheduled appointments were followed up to re-schedule appointments.
All data collection was conducted by trained nurse enumerators via interviewer-administered structured questionnaires on electronic tablets (48). Data collection procedures have been described in detail previously (47). Briefly, data collection (self-reported via interview) included sociodemographic data, dietary intake (24-h recall, including specific food items containing iron, vitamin B-12, and folate), anthropometric data, overall health, reproductive and pregnancy history (including birth defects and exposures that have been linked to increased risk of birth defects), and biological specimens (i.e., blood, saliva, urine). Anthropometric measurements, including weight, height, midupper arm, waist, and hip circumferences, and triceps skinfold thickness, were measured in triplicate by trained research assistants.
Laboratory investigations
Biological specimen collection
Blood samples were collected from each participant using standardized protocols. Venous blood (12 mL) was collected in three Vacutainers [i.e., red-top, purple-top di-potassium EDTA (K2EDTA), and blue-top metal-free K2EDTA; BD Vacutainers] for each participant. After collection, blood samples were immediately stored in a portable freezer unit that was set to optimal refrigeration temperature of 4 to 6°C, until processing within 4 h.
Sample processing and storage
Red-top vacutainers were centrifuged (∼1400 × g, 10 min, room temperature) to separate serum from cells, and serum was placed into aliquots and archived at −80°C or less until batch analysis. Purple-top Vacutainers were allowed to reach room temperature, re-mixed by inversion, and 100 μL of whole blood was added to 1 mL of 1% ascorbic acid to generate whole-blood lysate for the microbiological assay for assessment of RBC folate concentrations. Purple-top Vacutainers were analyzed for complete blood count, the remaining sample was centrifuged (˜1400 × g, 10 min, room temperature), and plasma samples were stored at −80°C or less until batch analysis.
Laboratory analyses
Laboratory analyses included hemoglobin, total vitamin B-12, and RBC and serum folate concentrations. Complete blood count (including hemoglobin) was analyzed using an automated Coulter counter (Coulter HMX). Serum total vitamin B-12 concentrations were assessed via chemiluminescence (E411; Roche Diagnostics). RBC folate and serum folate concentrations were measured using the WHO-recommended microbiological assay.
Definitions of outcomes
Anemia was defined as hemoglobin <12.0 g/dL, and severe anemia was defined as hemoglobin <8.0 g/dL (49). Vitamin B-12 deficiency and insufficiency were defined as total vitamin B-12 <148 pmol/L and <221 pmol/L, respectively (41, 50). Folate deficiency was defined as RBC folate <305 nmol/L, and folate insufficiency was defined as RBC folate <748 nmol/L, the recommended calibrator-adjusted equivalent of the threshold for optimal prevention of NTDs (36, 51).
Statistical analyses
Continuous variables were defined using conventional cutoffs, where available. Continuous biomarker and household income variables were natural logarithmically transformed for all analyses. Hemoglobin was adjusted for smoking status (self-reported). For biomarker analyses, values that were outside of the assay limits of detection (LOD) were set to half the LOD (if below the LOD) or 2 times the LOD (if above the LOD). Geometric means (GMs) and 95% CIs were calculated to facilitate statistical inference. Chi-square tests for contingency tables and 1-factor ANOVA were used to evaluate differences in categorial and continuous variables, respectively, and P values <0.05 were considered significant. All rural households and a random sample of urban households were included in unweighted totals. Population weights were constructed to account for differences in the study sample compared with the overall surveillance population and were used to calculate overall weighted population characteristics. Preliminary analyses indicated there were no substantial differences between the weighted and unweighted analyses and all results presented are for the unweighted analyses. We used the RBC folate distributions and vitamin B-12 status in this population and a previously developed Bayesian model (37) to predict NTD prevalence per 10,000 births (37). Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Inc.).
Results
Characteristics of participants in this study are presented in Table 1, and a flowchart of households and participants is presented in Figure 1. The overall characteristics of the catchment area and households within the periconceptional surveillance program are described in Supplemental Tables 1 and 2. On average, there were 4.5 family members per household, and half of households reported a monthly income <5500 Indian rupees (INR; 1 US dollar ≅ 70 INR). Participating WRA (aged 15–40 y who were not pregnant or lactating) had a mean age of 28.8 y, and most women had some formal education (83.8%), although few reported finishing high school (10.9%) or college (15.7%). Women residing in rural or urban households were similar in terms of sociodemographic characteristics (e.g., age, household size, marital status, education, parity) (Table 1).
TABLE 1.
Sociodemographic characteristics of the study population1
| GM (95% CI) or n (%) | |||||
|---|---|---|---|---|---|
| Variables | n | Total (n = 980) | Rural (n = 788) | Urban (n = 192) | P 2 |
| Household | |||||
| Household size | 980 | 4.5 (4.4, 4.7) | 4.6 (4.4, 4.7) | 4.4 (4.1, 4.7) | 0.25 |
| Monthly household income,3 INR | 979 | 5452.1 (5260.9, 5650.2) | 5474.3 (5260.7, 5696.6) | 5361.8 (4946.6, 5811.8) | 0.65 |
| Type of house | 980 | ||||
| Kutcha | 41 (4.2) | 27 (3.4) | 14 (7.3) | 0.04 | |
| Semi-pucca | 3 (0.3) | 3 (0.4) | 0 (0.0) | ||
| Pucca | 936 (95.5) | 758 (96.2) | 178 (92.7) | ||
| Purchases iodized salt | 974 | 654 (67.1) | 525 (67.0) | 129 (67.5) | 0.66 |
| Woman of reproductive age | |||||
| Age, y | 980 | 28.8 (28.4, 29.3) | 28.8 (28.3, 29.3) | 29.1 (28.1, 30.2) | 0.57 |
| 15 to <18 y | 49 (5.0) | 41 (5.2) | 8 (4.2) | 0.81 | |
| 18 to <26 y | 229 (23.4) | 187 (23.7) | 42 (21.9) | ||
| 26 to <36 y | 453 (46.2) | 359 (45.6) | 94 (49.0) | ||
| 36 to 40 y | 249 (25.4) | 201 (25.5) | 48 (25.0) | ||
| Highest level of education | 975 | ||||
| No formal schooling | 158 (16.2) | 125 (15.9) | 33 (17.3) | 0.65 | |
| Grades 1–5 | 174 (17.8) | 138 (17.6) | 36 (18.8) | ||
| Grades 6–8 | 177 (18.2) | 142 (18.1) | 35 (18.3) | ||
| Grades 9–10 | 207 (21.2) | 165 (21.0) | 42 (22.0) | ||
| Grades 11–12 | 106 (10.9) | 83 (10.6) | 23 (12.0) | ||
| College or graduate degree | 153 (15.7) | 131 (16.7) | 22 (11.5) | ||
| Marital status | 975 | ||||
| Currently married | 774 (79.4) | 617 (78.7) | 157 (82.2) | 0.56 | |
| Widowed, divorced, separated | 35 (3.6) | 29 (3.7) | 6 (3.1) | ||
| Never married | 166 (17.0) | 138 (17.6) | 28 (14.7) | ||
| Gravidity | 975 | 2.0 (1.9, 2.1) | 2.0 (1.9, 2.1) | 2.0 (1.8, 2.3) | 0.72 |
| 0 | 207 (21.2) | 169 (21.6) | 38 (19.9) | 0.84 | |
| 1 | 68 (7.0) | 53 (6.8) | 15 (7.9) | ||
| 2 | 385 (39.5) | 306 (39.0) | 79 (41.4) | ||
| ≥3 | 315 (32.3) | 256 (32.7) | 59 (30.9) | ||
| Parity | 975 | 1.7 (1.6, 1.8) | 1.7 (1.6, 1.8) | 1.6 (1.5, 1.8) | 0.68 |
| Nulliparous | 231 (23.7) | 187 (23.9) | 44 (23.0) | 0.41 | |
| Primiparous | 88 (9.0) | 66 (8.4) | 22 (11.5) | ||
| Multiparous | 656 (67.3) | 531 (67.7) | 125 (65.4) | ||
| Currently has children | 746 | 737 (98.8) | 592 (99.0) | 145 (98.0) | 0.31 |
| Number of children | 2.0 (1.9, 2.1) | 2.0 (1.9, 2.1) | 1.9 (1.7, 2.2) | 0.54 | |
| 0 | 9 (1.2) | 6 (1.0) | 3 (2.0) | 0.43 | |
| 1 | 118 (15.8) | 90 (15.1) | 28 (18.9) | ||
| 2 | 514 (68.9) | 415 (69.4) | 99 (66.9) | ||
| ≥3 | 105 (14.1) | 87 (14.5) | 18 (12.2) | ||
Values are GMs (95% CI) or n (%). Total (ns) are true sample ns. GM, geometric mean; INR, Indian rupees; USD, US dollar.
Chi-square statistics and 1-factor ANOVA were used to evaluate differences in categorical and continuous variables, respectively; household income was natural logarithmically transformed prior to analyses; Poisson regressions were used for count variables (e.g., household size, gravidity, parity, and number of children)
INR 70 ≅ 1 USD.
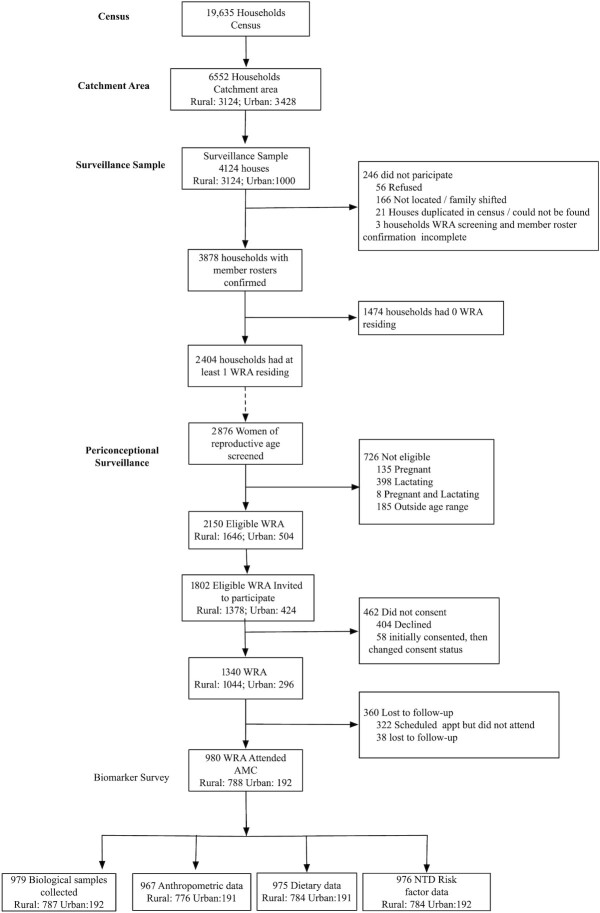
FIGURE 1.
Participant flow chart. AMC, Arogyavaram Medical Center; appt, appointment; NTD, neural tube defect; WRA, women of reproductive age.
Anthropometric and dietary characteristics of participants are presented in Table 2. Among adult women (≥18 y), 19.2% were underweight [BMI (kg/m2) <18.5], 23.4% were overweight (BMI: 25.0 to <30.0), and 9.6% had obesity (BMI ≥30.0). Women in urban areas had significantly higher BMI (P = 0.01), midupper arm circumference (P = 0.03), and waist circumference (P = 0.03). The prevalence of veganism (<1%) or vegetarianism (6.6%) was low, with 93.1% of WRA self-reporting as nonvegetarian; however, overall, the frequency of consumption of animal-source foods (ASFs; e.g., egg, poultry, meat, and fish) was low in both vegetarian and nonvegetarian individuals. WRA residing in urban areas were significantly more likely to be nonvegetarian (urban vs. rural: 99.5% vs. 91.6%; P = 0.0001) and reported more frequent consumption of ASFs compared with women in rural areas (Table 2).
TABLE 2.
Anthropometric and dietary characteristics of the study population1
| GM (95% CI) or n (%) | |||||
|---|---|---|---|---|---|
| Variables | n | Total (n = 980) | Rural (n = 788) | Urban (n = 192) | P 2 |
| Anthropometric | |||||
| Weight, kg | 969 | 53.0 (52.2, 53.7) | 52.6 (51.7, 53.4) | 54.6 (52.8, 56.4) | 0.06 |
| Height, cm | 969 | 153.2 (152.9, 153.6) | 153.4 (153.0, 153.8) | 152.7 (151.9, 153.5) | 0.14 |
| BMI, kg/m2 | 969 | 22.6 (22.2, 22.9) | 22.3 (22.0, 22.7) | 23.4 (22.7, 24.2) | 0.01 |
| BMI (kg/m2)3,4 | 920 | ||||
| <18.5 | 177 (19.2) | 153 (20.8) | 24 (13.1) | 0.02 | |
| 18.5 to <25.0 | 440 (47.8) | 354 (48.0) | 86 (47.0) | ||
| 25.0 to <30.0 | 215 (23.4) | 159 (21.6) | 56 (30.6) | ||
| ≥30.0 | 88 (9.6) | 71 (9.6) | 17 (9.3) | ||
| BMI (kg/m2)3,5 | 920 | ||||
| <18.5 | 177 (19.2) | 153 (20.8) | 24 (13.1) | 0.04 | |
| 18.5 to <23.0 | 308 (33.5) | 250 (33.9) | 58 (31.7) | ||
| 23.0 to <27.5 | 253 (27.5) | 198 (26.9) | 55 (30.1) | ||
| ≥27.5 | 182 (19.8) | 136 (18.5) | 46 (25.1) | ||
| Midupper arm circumference, cm | 969 | 26.7 (26.5, 27.0) | 26.6 (26.3, 26.9) | 27.3 (26.7, 27.9) | 0.03 |
| Waist circumference, cm | 969 | 74.6 (73.9, 75.3) | 74.2 (73.4, 75.0) | 76.3 (74.7, 78.0) | 0.03 |
| >88.9 cm3,6 | 920 | 123 (13.4) | 93 (12.6) | 30 (16.4) | 0.18 |
| Dietary preference7 | 974 | ||||
| Vegan | 3 (0.3) | 3 (0.4) | 0 (0.0) | 0.001 | |
| Vegetarian | 64 (6.6) | 63 (8.0) | 1 (0.5) | ||
| Nonvegetarian | 907 (93.1) | 717 (91.6) | 190 (99.5) | ||
| Dietary preference7 | 974 | ||||
| Vegan or vegetarian | 67 (6.9) | 66 (8.4) | 1 (0.5) | 0.0001 | |
| Nonvegetarian | 907 (93.1) | 717 (91.6) | 190 (99.5) | ||
| Frequency of animal-source foods consumption | |||||
| Dairy | 974 | ||||
| Never | 53 (5.4) | 42 (5.4) | 11 (5.8) | 0.02 | |
| Almost never (<1×/mo) | 35 (3.6) | 35 (4.5) | 0 (0.0) | ||
| Occasionally (<1×/wk) | 79 (8.1) | 63 (8.0) | 16 (8.4) | ||
| Often (∼1×/wk) | 152 (15.6) | 114 (14.6) | 38 (19.9) | ||
| Very often (>1×/wk) | 655 (67.2) | 529 (67.6) | 126 (66.0) | ||
| Eggs | 969 | ||||
| Never | 72 (7.4) | 65 (8.4) | 7 (3.7) | 0.003 | |
| Almost never (<1×/mo) | 107 (11.0) | 95 (12.2) | 12 (6.3) | ||
| Occasionally (<1×/wk) | 178 (18.4) | 134 (17.2) | 44 (23.0) | ||
| Often (∼1×/wk) | 376 (38.8) | 289 (37.1) | 87 (45.5) | ||
| Very often (>1×/wk) | 236 (24.4) | 195 (25.1) | 41 (21.5) | ||
| Poultry | 973 | ||||
| Never | 106 (10.9) | 99 (12.7) | 7 (3.7) | 0.0007 | |
| Almost never (<1×/mo) | 81 (8.3) | 67 (8.6) | 14 (7.3) | ||
| Occasionally (<1×/wk) | 119 (12.2) | 100 (12.8) | 19 (9.9) | ||
| Often (∼1×/wk) | 537 (55.2) | 409 (52.3) | 128 (67.0) | ||
| Very often (>1×/wk) | 130 (13.4) | 107 (13.7) | 23 (12.0) | ||
| Meat | 973 | ||||
| Never | 219 (22.5) | 197 (25.2) | 22 (11.5) | <0.0001 | |
| Almost never (<1×/mo) | 303 (31.1) | 240 (30.7) | 63 (33.0) | ||
| Occasionally (<1×/wk) | 213 (21.9) | 148 (18.9) | 65 (34.0) | ||
| Often (∼1×/wk) | 209 (21.5) | 169 (21.6) | 40 (20.9) | ||
| Very often (>1×/wk) | 29 (3.0) | 28 (3.6) | 1 (0.5) | ||
| Fish | 973 | ||||
| Never | 308 (31.7) | 261 (33.4) | 47 (24.6) | 0.04 | |
| Almost never (<1×/mo) | 388 (39.9) | 298 (38.1) | 90 (47.1) | ||
| Occasionally (<1×/wk) | 178 (18.3) | 139 (17.8) | 39 (20.4) | ||
| Often (∼1×/wk) | 74 (7.6) | 61 (7.8) | 13 (6.8) | ||
| Very often (>1×/wk) | 25 (2.6) | 23 (2.9) | 2 (1.0) | ||
Values GMs (95% CI) or n (%). Total (ns) are true sample ns. GM, geometric mean.
Chi-square statistics and 1-factor ANOVA were used to evaluate differences in categorical and continuous variables, respectively.
Among participants ≥18 y old (n = 931; rural: 747; urban: 184).
BMI categories as defined by the WHO (52).
BMI categories for Asian populations (53).
Defined as >35 inches, converted to >88.9 cm (54).
Vegetarian: consumed milk and/or eggs; Nonvegetarian: consumed poultry, meat, and/or fish.
The prevalences of anemia, vitamin B-12, and folate status in WRA are presented in Table 3. A total of 41.5% of women had anemia (<12.0 g/dL) and 3.0% had severe anemia (<8.0 g/dL), with mean hemoglobin concentrations of 11.9 g/dL (95% CI: 11.7, 12.0 g/dL). The prevalence of vitamin B-12 deficiency (<148 pmol/L) was 48.3%, and 74.3% of WRA were vitamin B-12 insufficient (<221 pmol/L); mean vitamin B-12 concentration was 156.0 pmol/L (95% CI: 150.3, 162.0 pmol/L). The mean serum folate concentration in this population was 16.5 nmol/L (95% CI: 16.0, 17.0 nmol/L); 3.5% of women were serum folate deficient (<7.0 nmol/L). Mean RBC folate concentrations were 540.5 nmol/L (95% CI: 526.2, 555.1 nmol/L). The prevalence of RBC folate deficiency (<305 nmol/L) was 7.6%, and 79.3% of WRA had RBC folate concentrations <748 nmol/L, the threshold for optimal NTD prevention.
TABLE 3.
Anemia and vitamin B-12 and folate status in women of reproductive age1
| GM (95% CI) or n (%) | |||||
|---|---|---|---|---|---|
| n | Total (n = 979) | Rural (n = 787) | Urban (n = 192) | P 2 | |
| Hemoglobin,3 g/dL | 979 | 11.9 (11.7, 12.0) | 11.8 (11.7, 11.9) | 12.1 (11.8, 12.4) | 0.07 |
| <12.0 g/dL | 406 (41.5) | 332 (42.2) | 74 (38.5) | 0.36 | |
| <8.0 g/dL | 29 (3.0) | 27 (3.4) | 2 (1.0) | 0.08 | |
| RBC folate,4 nmol/L | 977 | 540.5 (526.2, 555.1) | 528.5 (513.0, 544.4) | 592.3 (557.8, 628.9) | 0.0009 |
| <305 nmol/L | 74 (7.6) | 62 (7.9) | 12 (6.3) | 0.44 | |
| <748 nmol/L | 775 (79.3) | 635 (80.9) | 140 (72.9) | 0.01 | |
| Serum folate,4 nmol/L | 977 | 16.5 (16.0, 17.0) | 17.0 (16.4, 17.6) | 14.8 (13.8, 15.9) | 0.0007 |
| <7 nmol/L | 34 (3.5) | 28 (3.6) | 6 (3.1) | 0.76 | |
| Serum vitamin B-12, pmol/L | 978 | 156.0 (150.3, 162.0) | 155.0 (148.7, 161.6) | 160.2 (147.3, 174.4) | 0.49 |
| <148 pmol/L | 472 (48.3) | 386 (49.1) | 86 (44.8) | 0.28 | |
| <221 pmol/L | 727 (74.3) | 583 (74.2) | 144 (75.0) | 0.81 | |
| Vitamin B-12 and RBC folate deficient | 47 (4.8) | 39 (5.0) | 8 (4.2) | 0.64 | |
| Vitamin B-12 and RBC folate insufficient | 622 (63.7) | 510 (65.0) | 112 (58.3) | 0.09 | |
| Vitamin B-12 or RBC folate deficient | 499 (51.1) | 409 (52.1) | 90 (46.9) | 0.19 | |
| Vitamin B-12 or RBC folate insufficient | 880 (90.1) | 708 (90.2) | 172 (89.6) | 0.80 | |
| Vitamin B-12 ≥148 pmol/L and RBC folate <748 nmol/L | 355 (36.3) | 285 (36.3) | 70 (36.5) | 0.97 | |
| Vitamin B-12 ≥221 pmol/L and RBC folate <748 nmol/L | 153 (15.7) | 125 (15.9) | 28 (14.6) | 0.65 | |
| Vitamin B-12 <148 pmol/L and RBC folate ≥748 nmol/L | 52 (5.3) | 36 (4.6) | 16 (8.3) | 0.04 | |
| Vitamin B-12 <221 pmol/L and RBC folate ≥748 nmol/L | 105 (10.8) | 73 (9.3) | 32 (16.7) | 0.003 | |
Values are GMs (95% CI) or n (%). Total (ns) are true sample ns. Results outside assay limits of detection (LOD) were set to 0.50 × LOD (if below LOD) or 2 × LOD (if above LOD); results outside assay LODs: serum folate (n = 1 below LOD), vitamin B-12 (n = 4 below LOD, n = 6 above LOD). GM, geometric mean.
Chi-square statistics and 1-factor ANOVA were used to evaluate differences in categorical and continuous variables, respectively; hemoglobin, serum folate, RBC folate, and vitamin B-12 were natural logarithmically transformed prior to analyses.
Hemoglobin values from complete blood count; adjusted for smoking status (49).
Microbiological assay.
The prevalence of concurrent vitamin B-12 and folate deficiency and combinations of vitamin B-12 and folate status in WRA are summarized in Table 3. A total of 10.0% of women had adequate vitamin B-12 and RBC folate status; in contrast, 51.1% of women were vitamin B-12 or RBC folate deficient, and 90.1% of participants had either vitamin B-12 or RBC folate insufficiency. A total of 5.3% or 10.8% of women had sufficient RBC folate (≥748 nmol/L) status in combination with vitamin B-12 deficiency or vitamin B-12 insufficiency, respectively.
Anemia and vitamin B-12 and folate statuses in WRA, stratified by rural or urban residence, are also presented in Table 3. Hemoglobin and vitamin B-12 concentrations were not significantly different between rural and urban strata. RBC folate concentrations (P = 0.0009) were significantly lower among women residing in rural areas compared with those in urban areas [rural vs. urban: GM 528.5 nmol/L (95% CI: 513.0, 544.4) vs. 592.3 nmol/L (95% CI: 557.8, 628.9)]. In contrast, serum folate concentrations were higher in women residing in rural compared with urban settings (P = 0.0007). The prevalence of anemia (rural vs. urban: 42.2% vs. 38.5%), vitamin B-12 deficiency (rural vs. urban: 49.1% vs. 44.8%), vitamin B-12 insufficiency (rural vs. urban: 74.2% vs. 75.0%), and RBC folate deficiency (rural vs. urban: 7.9% vs. 6.3%) or serum folate deficiency (rural vs. urban: 3.6% vs. 3.1%) were not significantly different comparing women residing in rural and urban areas (P > 0.05). However, the prevalence of RBC folate insufficiency (<748 nmol/L) was significantly higher in women residing in rural areas compared with those in urban areas (rural vs. urban: 80.9% vs. 72.9%; P = 0.01); WRA residing in rural areas had a 1.11 times greater risk of RBC folate insufficiency (RR: 1.11; 95% CI: 1.01, 1.22; P = 0.03; data not shown) compared with those in urban areas.
Sociodemographic characteristics by vitamin B-12 status in WRA are presented in Table 4. Household size, household income, type of house, purchasing of iodized salt, WRA level of education, and WRA gravidity, parity, and number of children were similar across vitamin B-12–deficient and not vitamin B-12–deficient strata. Women who had vitamin B-12 deficiency were younger (P = 0.04) and less likely to report being married (vitamin B-12 deficient vs. not vitamin B-12 deficient: 75.8% vs. 82.9%; P = 0.002). Dietary patterns by vitamin B-12 status in WRA are presented in Table 5. Women with vitamin B-12 deficiency were significantly more likely to be vegetarian/vegan compared with women who were not vitamin B-12 deficient (vitamin B-12 deficient vs. not vitamin B-12 deficient: 9.8% vs. 4.2%; P = 0.0006). In terms of ASF intake, WRA who were vitamin B-12 deficient reported similar frequency of dairy, egg, and fish consumption compared with WRA who were not vitamin B-12 deficient (P > 0.05) but had significantly lower frequency of poultry (P = 0.006) and meat (P = 0.001) intakes. WRA who were vegetarian/vegan had 1.47 times greater risk of vitamin B-12 deficiency compared with women who were nonvegetarian (RR: 1.47; 95% CI: 1.23,1.75; P < 0.0001; data not shown).
TABLE 4.
Sociodemographic characteristics of the study population by vitamin B-12 deficiency (<148 pmol/L)1
| GM (95% CI) or n (%) | ||||
|---|---|---|---|---|
| Variables | n | Vitamin B-12 deficient (n = 472) | Not vitamin B-12 deficient (n = 506) | P 2 |
| Household | ||||
| Household size | 978 | 4.5 (4.3, 4.7) | 4.6 (4.4, 4.7) | 0.79 |
| Rural location | 978 | 386 (81.8) | 400 (79.1) | 0.28 |
| Monthly household income,3 INR | 977 | 5490.6 (5215.0, 5780.8) | 5421.3 (5158.5, 5697.5) | 0.73 |
| Type of house | 978 | |||
| Kutcha | 21 (4.4) | 20 (4.0) | 0.18 | |
| Semi-pucca | 3 (0.6) | 0 (0.0) | ||
| Pucca | 448 (94.9) | 486 (96.0) | ||
| Household purchases iodized salt | 972 | 327 (69.4) | 327 (65.3) | 0.30 |
| Woman of reproductive age | ||||
| Age, y | 978 | 28.3 (27.6, 28.9) | 29.4 (28.7, 30.0) | 0.04 |
| 15 to <18 y | 33 (7.0) | 16 (3.2) | 0.04 | |
| 18 to <26 y | 110 (23.3) | 118 (23.3) | ||
| 26 to <36 y | 218 (46.2) | 235 (46.4) | ||
| 36 to 40 y | 111 (23.5) | 137 (27.1) | ||
| Highest level of education completed | 973 | |||
| No formal schooling | 69 (14.6) | 89 (17.7) | 0.42 | |
| Grades 1–5 | 80 (17.0) | 93 (18.5) | ||
| Grades 6–8 | 84 (17.8) | 93 (18.5) | ||
| Grades 9–10 | 100 (21.2) | 107 (21.3) | ||
| Grades 11–12 | 54 (11.5) | 52 (10.4) | ||
| College or graduate degree | 84 (17.8) | 68 (13.5) | ||
| Marital status | 973 | |||
| Currently married | 357 (75.8) | 416 (82.9) | 0.002 | |
| Widowed, divorced, separated | 14 (3.0) | 21 (4.2) | ||
| Never married | 100 (21.2) | 65 (12.9) | ||
| Gravidity | 973 | 1.9 (1.8, 2.1) | 2.1 (2.0, 2.2) | 0.06 |
| 0 | 113 (24.0) | 93 (18.5) | 0.17 | |
| 1 | 29 (6.2) | 38 (7.6) | ||
| 2 | 185 (39.3) | 200 (39.8) | ||
| ≥3 | 144 (30.6) | 171 (34.1) | ||
| Parity | 973 | 1.6 (1.5, 1.7) | 1.7 (1.6, 1.9) | 0.10 |
| Nulliparous | 123 (26.1) | 106 (21.1) | 0.18 | |
| Primiparous | 42 (8.9) | 46 (9.2) | ||
| Multiparous | 306 (65.0) | 350 (69.7) | ||
| Currently has children | 746 | 347 (99.1) | 390 (98.5) | 0.41 |
| Number of children | 2.0 (1.8, 2.1) | 2.0 (1.9, 2.1) | 0.68 | |
| 0 | 3 (0.9) | 6 (1.5) | 0.54 | |
| 1 | 61 (17.4) | 57 (14.4) | ||
| 2 | 240 (68.6) | 274 (69.2) | ||
| ≥3 | 46 (13.1) | 59 (14.9) | ||
Vitamin B-12 missing for n = 1 woman of reproductive age. Results outside assay limits of detection (LOD) were set to 0.50 × LOD (if below LOD) or 2 × LOD (if above LOD); results outside assay LODs: vitamin B-12 (n = 4 below LOD, n = 6 above LOD). GM, geometric mean; INR, Indian rupees; USD, US dollar.
Chi-square statistics and 1-factor ANOVA were used to evaluate differences in categorical and continuous variables, respectively; household income was natural logarithmically transformed prior to analyses; Poisson regressions were used for count variables (e.g., household size, gravidity, parity, and number of children).
INR 70 ≅ 1 USD.
TABLE 5.
Dietary characteristics of the study population by vitamin B-12 deficiency (<148 pmol/L)1
| GM (95% CI) or n (%) | ||||
|---|---|---|---|---|
| Variables | n | Vitamin B-12 deficient (n = 472) | Not vitamin B-12 deficient (n = 506) | P 2 |
| Dietary preference3 | 972 | |||
| Vegan | 2 (0.4) | 1 (0.2) | 0.003 | |
| Vegetarian | 44 (9.4) | 20 (4.0) | ||
| Nonvegetarian | 424 (90.2) | 481 (95.8) | ||
| Dietary preference3 | 972 | |||
| Vegan or vegetarian | 46 (9.8) | 21 (4.2) | 0.0006 | |
| Nonvegetarian | 424 (90.2) | 481 (95.8) | ||
| Frequency of animal-source foods consumption | ||||
| Dairy | 972 | |||
| Never | 23 (4.9) | 30 (6.0) | 0.55 | |
| Almost never (<1×/mo) | 14 (3.0) | 21 (4.2) | ||
| Occasionally (<1×/wk) | 40 (8.5) | 38 (7.6) | ||
| Often (∼1×/wk) | 68 (14.5) | 84 (16.7) | ||
| Very often (>1×/wk) | 325 (69.1) | 329 (65.5) | ||
| Eggs | 967 | |||
| Never | 46 (9.8) | 26 (5.2) | 0.07 | |
| Almost never (<1×/mo) | 49 (10.5) | 58 (11.6) | ||
| Occasionally (<1×/wk) | 82 (17.5) | 96 (19.2) | ||
| Often (∼1×/wk) | 184 (39.3) | 190 (38.1) | ||
| Very often (>1×/wk) | 107 (22.9) | 129 (25.9) | ||
| Poultry | 971 | |||
| Never | 67 (14.3) | 38 (7.6) | 0.006 | |
| Almost never (<1×/mo) | 36 (7.7) | 45 (9.0) | ||
| Occasionally (<1×/wk) | 55 (11.7) | 64 (12.8) | ||
| Often (∼1×/wk) | 260 (55.3) | 276 (55.1) | ||
| Very often (>1×/wk) | 52 (11.1) | 78 (15.6) | ||
| Meat | 971 | |||
| Never | 128 (27.3) | 91 (18.1) | 0.001 | |
| Almost never (<1×/mo) | 153 (32.6) | 150 (29.9) | ||
| Occasionally (<1×/wk) | 91 (19.4) | 121 (24.1) | ||
| Often (∼1×/wk) | 88 (18.8) | 120 (23.9) | ||
| Very often (>1×/wk) | 9 (1.9) | 20 (4.0) | ||
| Fish | 971 | |||
| Never | 162 (34.5) | 146 (29.1) | 0.18 | |
| Almost never (<1×/mo) | 185 (39.4) | 202 (40.3) | ||
| Occasionally (<1×/wk) | 84 (17.9) | 93 (18.6) | ||
| Often (∼1×/wk) | 31(6.6) | 43 (8.6) | ||
| Very often (>1×/wk) | 8 (1.7) | 17 (3.4) | ||
Vitamin B-12 missing for n = 1 woman of reproductive age. Results outside assay limits of detection (LOD) were set to 0.50 × LOD (if below LOD) or 2 × LOD (if above LOD); results outside assay LODs: vitamin B-12 (n = 4 below LOD, n = 6 above LOD). GM, geometric mean.
Chi-square statistics were used to evaluate differences in categorical variables.
Vegetarian: consumed milk and/or eggs; Nonvegetarian: consumed poultry, meat, and/or fish.
Sociodemographic characteristics by RBC folate status in WRA are presented in Table 6. Household size, household income, and purchasing of iodized salt were similar across RBC folate–insufficient and RBC folate–sufficient strata. Women who were RBC folate insufficient were younger (P = 0.0001), more likely to live in rural households (RBC folate insufficient vs. RBC folate sufficient: 81.9% vs. 74.3%; P = 0.01), less likely to report being married (RBC folate insufficient vs. RBC folate sufficient: 77.4% vs. 87.1%; P = 0.003), and reported fewer pregnancies (P = 0.02) compared with women with RBC folate sufficiency. There were no differences in RBC folate insufficiency among self-reported dietary patterns (vegetarian vs. nonvegetarian) (Table 7).
TABLE 6.
Sociodemographic characteristics of the study population by RBC folate insufficiency (<748 nmol/L)1
| GM (95% CI) or n (%) | ||||
|---|---|---|---|---|
| Variables | n | RBC folate insufficient (n = 775) | RBC folate sufficient (n = 202) | P 2 |
| Household | ||||
| Household size | 977 | 4.6 (4.4, 4.7) | 4.5 (4.2, 4.8) | 0.53 |
| Rural location | 977 | 635 (81.9) | 150 (74.3) | 0.01 |
| Monthly household income,3 INR | 976 | 5393.1 (5180.7, 5614.1) | 5702.4 (5271.3, 6168.9) | 0.22 |
| Type of house | 977 | |||
| Kutcha | 35 (4.5) | 6 (3.0) | 0.42 | |
| Semi-pucca | 3 (0.4) | 0 (0.0) | ||
| Pucca | 737 (95.1) | 196 (97.0) | ||
| Household purchases iodized salt | 971 | 521 (67.7) | 132 (65.7) | 0.33 |
| Woman of reproductive age | ||||
| Age, y | 977 | 28.4 (27.9, 28.9) | 30.6 (29.6, 31.7) | 0.0001 |
| 15 to <18 y | 45 (5.8) | 4 (2.0) | 0.004 | |
| 18 to <26 y | 192 (24.8) | 36 (17.8) | ||
| 26 to <36 y | 356 (45.9) | 96 (47.5) | ||
| 36 to 40 y | 182 (23.5) | 66 (32.7) | ||
| Highest level of education completed | 972 | |||
| No formal schooling | 129 (16.7) | 29 (14.4) | 0.03 | |
| Grades 1–5 | 139 (18.0) | 34 (16.9) | ||
| Grades 6–8 | 127 (16.5) | 49 (24.4) | ||
| Grades 9–10 | 157 (20.4) | 50 (24.9) | ||
| Grades 11–12 | 89 (11.5) | 17 (8.5) | ||
| College or graduate degree | 130 (16.9) | 22 (10.9) | ||
| Marital status | 972 | |||
| Currently married | 597 (77.4) | 175 (87.1) | 0.003 | |
| Widowed, divorced, separated | 27 (3.5) | 8 (4.0) | ||
| Never married | 147 (19.1) | 18 (9.0) | ||
| Gravidity | 972 | 2.0 (1.9, 2.1) | 2.2 (2.0, 2.4) | 0.02 |
| 0 | 178 (23.1) | 28 (13.9) | 0.04 | |
| 1 | 51 (6.6) | 16 (8.0) | ||
| 2 | 300 (38.9) | 85 (42.3) | ||
| ≥3 | 242 (31.4) | 72 (35.8) | ||
| Parity | 972 | 1.6 (1.5, 1.7) | 1.8 (1.7, 2.0) | 0.03 |
| Nulliparous | 194 (25.2) | 35 (17.4) | 0.06 | |
| Primiparous | 70 (9.1) | 18 (9.0) | ||
| Multiparous | 507 (65.8) | 148 (73.6) | ||
| Currently has children | 745 | 571 (98.6) | 165 (99.4) | 0.42 |
| Number of children | 1.9 (1.9, 2.1) | 2.0 (1.8, 2.3) | 0.50 | |
| 0 | 8 (1.4) | 1 (0.6) | 0.36 | |
| 1 | 97 (16.8) | 21 (12.7) | ||
| 2 | 397 (68.6) | 116 (69.9) | ||
| ≥3 | 77 (13.3) | 28 (16.9) | ||
RBC folate missing for n = 2 women of reproductive age. Results outside assay limits of detection (LOD) were set to 0.50 × LOD (if below LOD) or 2 × LOD (if above LOD); results outside assay LODs: serum folate (n = 1 below LOD). GM, geometric mean; INR, Indian rupees; USD, US dollar.
Chi-square statistics and 1-factor ANOVA were used to evaluate differences in categorical and continuous variables, respectively; household income was natural logarithmically transformed prior to analyses; Poisson regressions were used for count variables (e.g., household size, gravidity, parity, and number of children).
INR 70 ≅ 1 USD.
TABLE 7.
Dietary characteristics of the study population by RBC folate insufficiency (<748 nmol/L)1
| GM (95% CI) or n (%) | ||||
|---|---|---|---|---|
| Variables | n | RBC folate insufficient (n = 775) | RBC folate sufficient (n = 202) | P 2 |
| Dietary preference3 | 971 | |||
| Vegan | 2 (0.3) | 1 (0.5) | 0.86 | |
| Vegetarian | 51 (6.6) | 13 (6.5) | ||
| Nonvegetarian | 717 (93.1) | 187 (93.0) | ||
| Dietary preference3 | 971 | |||
| Vegan or vegetarian | 53 (6.9) | 14 (7.0) | 0.97 | |
| Nonvegetarian | 717 (93.1) | 187 (93.0) | ||
| Animal-source foods consumption | ||||
| Dairy | 971 | |||
| Never | 42 (5.5) | 11 (5.5) | 0.91 | |
| Almost never (<1×/mo) | 30 (3.9) | 5 (2.5) | ||
| Occasionally (<1×/wk) | 61 (7.9) | 17 (8.5) | ||
| Often (∼1×/wk) | 121 (15.7) | 31 (15.4) | ||
| Very often (>1×/wk) | 516 (67.0) | 137 (68.2) | ||
| Eggs | 966 | |||
| Never | 57 (7.5) | 15 (7.5) | 0.75 | |
| Almost never (<1×/mo) | 81 (10.6) | 25 (12.4) | ||
| Occasionally (<1×/wk) | 142 (18.6) | 36 (17.9) | ||
| Often (∼1×/wk) | 303 (39.6) | 71 (35.3) | ||
| Very often (>1×/wk) | 182 (23.8) | 54 (26.9) | ||
| Poultry | 970 | |||
| Never | 81 (10.5) | 24 (11.9) | 0.96 | |
| Almost never (<1×/mo) | 64 (8.3) | 17 (8.5) | ||
| Occasionally (<1×/wk) | 93 (12.1) | 26 (12.9) | ||
| Often (∼1×/wk) | 426 (55.4) | 109 (54.2) | ||
| Very often (>1×/wk) | 105 (13.7) | 25 (12.4) | ||
| Meat | 970 | |||
| Never | 178 (23.1) | 41 (20.4) | 0.12 | |
| Almost never (<1×/mo) | 250 (32.5) | 52 (25.9) | ||
| Occasionally (<1×/wk) | 156 (20.3) | 56 (27.9) | ||
| Often (∼1×/wk) | 163 (21.2) | 45 (22.4) | ||
| Very often (>1×/wk) | 22 (2.9) | 7 (3.5) | ||
| Fish | 970 | |||
| Never | 240 (31.2) | 67 (33.3) | 0.29 | |
| Almost never (<1×/mo) | 314 (40.8) | 73 (36.3) | ||
| Occasionally (<1×/wk) | 137 (17.8) | 40 (19.9) | ||
| Often (∼1×/wk) | 55 (7.2) | 19 (9.5) | ||
| Very often (>1×/wk) | 23 (3.0) | 2 (1.0) | ||
RBC folate missing for n = 2 women of reproductive age. Results outside assay limits of detection (LOD) were set to 0.50 × LOD (if below LOD) or 2 × LOD (if above LOD); results outside assay LODs: serum folate (n = 1 below LOD). GM, geometric mean.
Vegetarian: consumed milk and/or eggs; Nonvegetarian: consumed poultry, meat, and/or fish.
Chi-square statistics were used to evaluate differences in categorical variables.
Sociodemographic characteristics by anemia and serum folate status in WRA are presented in the supplemental tables (Supplemental Tables 3–6). There were no differences in anemia among self-reported dietary patterns (vegetarian vs. nonvegetarian) or reported consumption of ASFs (i.e., egg, dairy, poultry, or meat products; P > 0.05), except for fish products (P = 0.03); all other sociodemographic characteristics were similar by anemia strata (Supplemental Tables 3 and 4) and by serum folate deficiency strata (Supplemental Tables 5 and 6).
Overall, the predicted NTD prevalence per 10,000 births based on RBC folate concentrations was 20.6 [95% uncertainty interval (UI): 16.5–25.5]. Predicted NTD prevalence in rural WRA was 21.3 per 10,000 births (95% UI: 16.9–26.3) and was 18.2 per 10,000 births (95% UI: 14.4–22.6) in urban WRA. The predicted NTD prevalences in rural and urban strata were not significantly different: the median difference between these 2 groups was 3.1 NTDs per 10,000 births, with a 0.95 probability of the true value being between −1.0 and 7.0.
Discussion
In this population-based biomarker survey among WRA, there was a high burden of anemia, vitamin B-12 deficiency, and RBC folate insufficiency. A total of 41.5% of WRA had anemia; 48.3% had vitamin B-12 deficiency, and 74.3% had vitamin B-12 insufficiency. Although the prevalence of RBC folate deficiency was 7.6%, 79.3% of women had RBC folate concentrations below the threshold for optimal NTD prevention. The substantial burden of anemia, vitamin B-12 deficiency, and RBC folate insufficiency in this population suggests an opportunity for anemia and birth defects prevention through interventions such as the fortification of staple foods.
Anemia, vitamin B-12 deficiency, and folate insufficiencies have documented risks to WRA, their pregnancies, and children. The WHO estimates that ∼33% of nonpregnant WRA are anemic worldwide (2016 estimates; 55), with the highest prevalence of 45.6% in the WHO–South-East Asia Regional Office region (55), including India [52.3%; National Family Health Survey (NFHS)-4, 2014–2015] (56). The prevalence of anemia (41.5%) and severe anemia (3.0%) in the current study is consistent with findings from studies in other parts of India where the reported prevalence of anemia in WRA ranged from 28% to 64% (57–60) and the prevalence of severe anemia ranged from 2.9% to 4.0% (58–60). The prevalence of anemia in the current study (41.5%) was slightly lower than the most recent NFHS-5 (NFHS-5, 2019–2020; nonpregnant WRA aged 15–49 y) district-level (Chittoor: 51.8%, NFHS-5, 2019–2020; 48.4%, NFHS-4, 2014–2015) and state-level (Andhra Pradesh: 59.0%; urban: 57.8%, rural: 59.5%; NHFS-4: 60.2%) data, although the use of capillary blood, inclusion of lactating women, and different time periods constrain direct comparability of findings (61, 62).
Vitamin B-12 deficiency in its most severe form is a cause of megaloblastic anemia and can be associated with fatigue and neurological manifestations (44). The high prevalence of vitamin B-12 deficiency (<148 pmol/L; 48.3%) and insufficiency (<221 pmol/L; 74.3%) among WRA in this study is consistent with other studies among WRA in India (31–58%) (57, 63–65): in rural Nagpur (31%) (63), rural Telangana (45.0%) (57), and vegetarian WRA in Pune (50%) (65). There are limited population-based data on vitamin B-12 status worldwide, including in WRA (41, 44, 66–68). To date, 14 countries have conducted national surveys evaluating vitamin B-12 concentrations, with reported prevalences of vitamin B-12 deficiency in WRA ranging from 3.3% to 52.4% (66, 67, 69–82). The prevalence of vitamin B-12 deficiency in the current study is higher than in WRA in surveys conducted in most other settings (including the United States, Canada, Mexico, Argentina, Colombia, Ecuador, Guatemala, Costa Rica, Belize, the United Kingdom, Germany, Jordan, Bangladesh) (66, 67, 69–82), with prevalences ranging from 3.3% in the United States (69) to 24.1% in Mexico (71), although lower than national data in Pakistan (52.4%) (74).
In this study, the prevalence of vitamin B-12 deficiency was significantly higher in vegetarian/vegan WRA compared with nonvegetarian women (P = 0.0006): women who were vegetarian or vegan had 1.47 times greater risk of vitamin B-12 deficiency compared with nonvegetarian women. Dietary intake of ASFs was associated with vitamin B-12 status: women who had vitamin B-12 deficiency had significantly lower consumption of ASFs, including poultry (P = 0.006) and other meat (P = 0.001), but not milk, eggs, or fish (P > 0.05). Findings are consistent with previous research that identified vegetarianism and low ASF intake as risk factors for vitamin B-12 deficiency (as dietary vitamin B-12 is found exclusively in ASFs) (44) and other studies in India, which reported associations between ASF intake and vitamin B-12 status, including among children (83) and pregnant women (84). In contrast, in a study in rural Nagpur with a lower prevalence of vitamin B-12 deficiency (31%) and higher prevalence of vegetarianism (28%), self-reported vegetarian diet was not significantly associated with vitamin B-12 concentrations (63). Low dietary intake of ASFs may be due to limited availability or access in the food supply, poverty, or other socioeconomic, cultural, religious, or personal factors (44). Low ASF intake is a risk factor for vitamin B-12 deficiency, in addition to other risk factors such as poor bioavailability, pernicious anemia (an autoimmune disease), gastrointestinal infections, and certain medications (e.g., metformin) (44, 85).
In the current study, we noted a high prevalence of vitamin B-12 deficiency (48.3%) in combination with a low prevalence of self-reported vegan/vegetarianism (7%). Findings highlight the low frequency of ASF intake even among nonvegetarians in this population. Although this finding may be unexpected in other settings, it is consistent with national and regional data in India. While ∼30% of women aged 15–49 y in NFHS-4 reported being vegetarian (i.e., never consuming fish, chicken, or meat), among nonvegetarians, ∼27% reported consuming fish, chicken, or meat occasionally; 37% reported consuming weekly; and only 1% reported daily consumption (86). Similarly, in a study among pregnant women in Uttar Pradesh, India (87), the prevalence of self-reported lacto-vegetarians was high (46.4%); however, among nonvegetarians, only 8.0% and 4.0% reported consuming flesh foods and eggs, respectively, in the past 24 h. This study found that dairy consumption accounted for 99.6% and 89.6% of vitamin B-12 intake for lacto-vegetarians and nonvegetarians, respectively. Although vitamin B-12 intake was significantly higher in the nonvegetarian group [median (IQR): 0.6 μg (0.2, 1.4) vs. 0.3 μg (0.1, 1.0)], the absolute intake for both groups was considerably lower than the US or Indian Recommended Dietary Allowance (77). In the current study, the low frequency of ASF intake (even among nonvegetarians) and lack of vitamin B-12 supplementation or fortification may explain the high prevalence of vitamin B-12 deficiency in this population.
While RBC folate concentrations are directly linked to NTD risk and represent an average of the last 120 d of folate intake, serum folate reflects recent intake. In the current study, the prevalence of serum folate deficiency (<7.0 nmol/L) was 3.5%. Previous studies in India have evaluated serum folate, with the prevalence of deficiency ranging from 0% to 54% (57, 63, 65). In a recent systematic review of folate status in WRA (12–49 y) globally, the prevalence of serum folate deficiency (39 surveys) ranged from <1% to 79% (46). However, serum folate was evaluated using a variety of laboratory methods and cutoffs, data included both pregnant and nonpregnant women, and did not report lactation status. To date, few national surveys have evaluated serum folate in nonpregnant WRA using the microbiological assay (MBA) (46).
Although the prevalence of RBC folate deficiency (<305 nmol/L) was 7.6% in the current study, 79.3% of WRA had RBC folate <748 nmol/L, the threshold for optimal NTD prevention. In the current study, the prevalence of RBC folate insufficiency was higher among WRA who lived in rural areas compared with those in urban settings, and among WRA who were younger, not married, and who had not previously given birth. There were no differences in the prevalence of RBC folate insufficiency by self-reported dietary patterns. Globally, few studies have evaluated RBC folate status, including nonpregnant WRA, and there are limited population-based data using the MBA. In the systematic review of folate status in WRA globally, 18 surveys assessed RBC folate, with deficiency ranging from ∼0% (<305 nmol/L, protein-binding assay, Canada) (88) to 49% (<342 nmol/L, MBA with folic acid calibrator, Kyrgyzstan) (46). In 10 of these surveys, RBC folate was evaluated using an MBA; however, surveys included pregnant and nonpregnant women and did not report lactation status, which constrains comparability of findings (46).
The RBC folate distribution in this population was used to estimate the prevalence of folate-sensitive NTDs, as has been done in previous studies of populations in the United States and Guatemala (37, 89, 90). The estimated NTD risk in this population (20.6 NTDs per 10,000 live births) was considerably higher than in the United States—as estimated with RBC folate concentrations (7.3 NTDs per 10,000 live births; 95% UI: 5.5–9.4) or through high-quality NTD surveillance (7 NTDs per 10,000 live births)—where moderate–low-dosage folic acid fortification is mandatory (89, 91). Estimates from the current study are also higher than in a Guatemalan population (14 NTDs per 10,000 live births; 95% UI: 11.1–18.6) but similar to one region of Guatemala likely unreached by fortification (Norte region: 26 NTDS per 10,000 births) (90). The rural/urban differences in RBC folate concentrations in the current study were moderate and did not result in substantial differences between estimated NTD prevalence. Findings suggest an NTD risk more than twice as high as observed in populations with mandatory folic acid fortification programs.
The current study is a population-based biomarker survey among WRA—among the largest of its kind to date—and is the first to use the folate microbiological assay in southern India. There was a high prevalence of concurrent vitamin B-12 and RBC folate insufficiency (63.7% both vitamin B-12 and RBC folate insufficiency; 90.1% vitamin B-12 or RBC folate insufficiency). Due to the interrelated metabolism of vitamin B-12 and folate and their roles in the development of anemia and NTDs (38, 51), assessment of the dual burden of vitamin B-12 and folate insufficiencies is of particular importance for WRA.
This study has several limitations. The cross-sectional design does not enable evaluation of changes in biomarkers over time or determine effects of micronutrients on anemia. This study included WRA who are not currently pregnant or lactating but was not limited to women planning to become pregnant; this constrains evaluation of micronutrient status during the preconception, pregnancy, and lactation periods. In addition, the eligibility age range and heterogeneity in WRA definition in the literature limit comparability to some studies. The response rate was ∼54%; although participants in the biomarker survey were similar to women who did not participate, they may differ on unmeasured confounders; this response rate is consistent with other recent population-based biomarker surveys (e.g., NHANES) (92). In this study, vitamin B-12 status was assessed via total vitamin B-12; inclusion of other circulating and functional (e.g., holo-transcobalamin, methylmalonic acid) biomarkers would improve assessment of vitamin B-12 status.
In summary, in this population-based biomarker survey of WRA, there was a high prevalence of anemia, vitamin B-12 deficiency, and RBC folate insufficiency. The substantial burden of anemia and micronutrient deficiencies in WRA in this setting suggests an opportunity for screening and prevention. Findings provide critical preintervention biomarker data that will directly inform the development (i.e., micronutrient concentrations for salt formulation, dose, power calculations) of a randomized efficacy trial of quadruple-fortified salt for the prevention of anemia and birth defects in southern India.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Anura Kurpad, who served as co-principal investigator at St John's Research Institute in Bangalore, India. The authors’ responsibilities were as follows—JLF: designed the research and wrote the initial draft manuscript, with feedback from the study collaborators, and had primary responsibility for final content; AF, HMG, and KSC: revised the manuscript; CBJ and AF: supervised data collection and field activities, under the guidance of the investigators; BB: conducted the laboratory analyses with expert guidance on the folate microbiological assay from SJ, MZ, and CMP; AF and KSC: conducted data analyses; and all authors: contributed to the development of the manuscript, provided feedback, and read and approved the final manuscript.
Notes
This study was supported by the Centers for Disease Control and Prevention (CDC); the Division of Nutritional Sciences, Cornell University; and the University of South Carolina's Disability Research and Dissemination Center (DRDC) through its cooperative agreement (6U19DD001218) with the CDC. AF was supported by the National Institutes of Health under award 5 T32 HD087137.
Author disclosures: The authors report no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official positions of the CDC, DRDC, Eunice Kennedy Shriver National Institute of Child Health and Human Development, or the National Institutes of Health.
Supplemental Tables 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: ASF, animal-source food; GM, geometric mean; IEC, Institutional Ethics Committee; INR, Indian rupees; K2EDTA, di-potassium EDTA; LOD, limit(s) of detection; MBA, Microbiological Assay; NFHS, National Family Health Survey; NTD, neural tube defect; UI, uncertainty interval; WRA, women of reproductive age.
Contributor Information
Julia L Finkelstein, Email: jfinkelstein@cornell.edu, Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA; St John's Research Institute, Bangalore, Karnataka, India.
Amy Fothergill, Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA.
Christina B Johnson, Arogyavaram Medical Centre, Andhra Pradesh, India.
Heather M Guetterman, Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA.
Beena Bose, St John's Research Institute, Bangalore, Karnataka, India.
Shameem Jabbar, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Mindy Zhang, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Christine M Pfeiffer, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Yan Ping Qi, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Charles E Rose, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Jennifer L Williams, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Wesley Bonam, Arogyavaram Medical Centre, Andhra Pradesh, India.
Krista S Crider, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, GA, USA.
References
- 1. World Health Organization (WHO) . The global prevalence of anaemia in 2011. WHO report. Geneva (Switzerland): World Health Organization; 2015. [Google Scholar]
- 2. World Health Organization (WHO) . Nutritional anaemias tools for effective prevention and control. Geneva (Switzerland): World Health Organization; 2017. [Google Scholar]
- 3. World Health Organization (WHO) . Global anaemia reduction efforts among women of reproductive age: impact, achievement of targets and the way forward for optimizing efforts. Geneva (Switzerland): World Health Organization; 2020. [Google Scholar]
- 4. Rahman MM, Abe SK, Rahman MS, Kanda M, Narita S, Bilano V, Ota E, Gilmour S, Shibuya K. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103(2):495–504. [DOI] [PubMed] [Google Scholar]
- 5. Geelhoed D, Agadzi F, Visser L, Ablordeppey E, Asare K, O'Rourke P, Van Leeuwen JS, Van Roosmalen J. Maternal and fetal outcome after severe anemia in pregnancy in rural Ghana. Acta Obstet Gynecol Scand. 2006;85(1):49–55. [DOI] [PubMed] [Google Scholar]
- 6. Rahman A, Khan N, Rahman M. Maternal anaemia and risk of adverse obstetric and neonatal outcomes in South Asian countries: a systematic review and meta-analysis. Public Health in Practice. 2020;1:100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anand T, Rahi M, Sharma P, Ingle GK. Issues in prevention of iron deficiency anemia in India. Nutrition. 2014;30(7-8):764–70. [DOI] [PubMed] [Google Scholar]
- 8. Lone FW, Qureshi RN, Emanuel F. Maternal anaemia and its impact on perinatal outcome. Trop Med Int Health. 2004;9(4):486–90. [DOI] [PubMed] [Google Scholar]
- 9. Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr. 2001;131(2):604S–15S.; discussion 14S–15S. [DOI] [PubMed] [Google Scholar]
- 10. Daru J, Zamora J, Fernández-Félix BM, Vogel J, Oladapo OT, Morisaki N, Tunçalp Ö, Torloni MR, Mittal S, Jayaratne Ket al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. Lancet Global Health. 2018;6(5):e548–54. [DOI] [PubMed] [Google Scholar]
- 11. Molloy AM, Kirke PN, Brody LC, Scott JM, Mills JL. Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant, and child development. Food Nutr Bull. 2008;29(2 Suppl1):S101–11.; discussion S12–15. [DOI] [PubMed] [Google Scholar]
- 12. Green R, Datta Mitra A. Megaloblastic anemias: nutritional and other causes. Med Clin North Am. 2017;101(2):297–317. [DOI] [PubMed] [Google Scholar]
- 13. Finkelstein JL, Layden AJ, Stover PJ. Vitamin B-12 and perinatal health. Adv Nutr. 2015;6(5):552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Molloy AM. Should vitamin B12 status be considered in assessing risk of neural tube defects?. Ann NY Acad Sci. 2018;1414(1):109–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De-Regil LM, Pena-Rosas JP, Fernandez-Gaxiola AC, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla Set al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet North Am Ed. 2012;380(9859):2197–223. [DOI] [PubMed] [Google Scholar]
- 17. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SYet al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet North Am Ed. 2012;380(9859):2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaganjor I, Sekkarie A, Tsang BL, Williams J, Razzaghi H, Mulinare J, Sniezek JE, Cannon MJ, Rosenthal J. Describing the prevalence of neural tube defects worldwide: a systematic literature review. PLoS One. 2016;11(4):e0151586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blencowe H, Kancherla V, Moorthie S, Darlison MW, Modell B. Estimates of global and regional prevalence of neural tube defects for 2015: a systematic analysis. Ann NY Acad Sci. 2018;1414(1):31–46. [DOI] [PubMed] [Google Scholar]
- 20. Liu J, Li Z, Ye R, Liu J, Ren A. Periconceptional folic acid supplementation and sex difference in prevention of neural tube defects and their subtypes in China: results from a large prospective cohort study. Nutr J. 2018;17(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lo A, Polsek D, Sidhu S. Estimating the burden of neural tube defects in low- and middle-income countries. J Global Health. 2014;4(1):010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atta CA, Fiest KM, Frolkis AD, Jette N, Pringsheim T, St Germaine-Smith C, Rajapakse T, Kaplan GG, Metcalfe A. Global birth prevalence of spina bifida by folic acid fortification status: a systematic review and meta-analysis. Am J Public Health. 2016;106(1):e24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allagh KP, Shamanna BR, Murthy GV, Ness AR, Doyle P, Neogi SB, Pant HB; Wellcome Trust–PHFI Folic Acid Project Team . Birth prevalence of neural tube defects and orofacial clefts in India: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0118961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhide P, Sagoo GS, Moorthie S, Burton H, Kar A. Systematic review of birth prevalence of neural tube defects in India. Birth Defects Res A Clin Mol Teratol. 2013;97(7):437–43. [DOI] [PubMed] [Google Scholar]
- 25. Hibbard ED, Smithells RW. Folic acid metabolism and human embryopathy. Lancet North Am Ed. 1965;285(7398):1254. [Google Scholar]
- 26. Smithells RW, Sheppard S, Schorah CJ. Vitamin deficiencies and neural tube defects. Arch Dis Child. 1976;51(12):944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MRC Vitamin Study Research Group . Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet North Am Ed. 1991;338(8760):131–7. [PubMed] [Google Scholar]
- 28. Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327(26):1832–5. [DOI] [PubMed] [Google Scholar]
- 29. Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, Mulinare J, Zhao P, Wong LY, Gindler Jet al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341(20):1485–90. [DOI] [PubMed] [Google Scholar]
- 30. Institute of Medicine . Dietary Reference Intakes guiding principles for nutrition labeling and fortification. Washington (DC): National Academies Press;2003. [PubMed] [Google Scholar]
- 31. Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011;3(3):370–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang H, De Steur H, Chen G, Zhang X, Pei L, Gellynck X, Zheng X. Effectiveness of folic acid fortified flour for prevention of neural tube defects in a high risk region. Nutrients. 2016;8(3):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, Frohnert B, Kirby RS. Updated estimates of neural tube defects prevented by mandatory folic acid fortification—United States, 1995–2011. MMWR Morb Mortal Wkly Rep. 2015;64(1). [PMC free article] [PubMed] [Google Scholar]
- 34. Centeno Tablante E, Pachon H, Guetterman HM, Finkelstein JL. Fortification of wheat and maize flour with folic acid for population health outcomes. Cochrane Database Syst Rev. 2019;7:CD012150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization . Guideline: optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects. Geneva (Switzerland): World Health Organization; 2015. [PubMed] [Google Scholar]
- 36. Cordero AM, Crider KS, Rogers LM, Cannon MJ, Berry RJ. Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health Organization guidelines. MMWR Morb Mortal Wkly Rep. 2015;64(15):421–3. [PMC free article] [PubMed] [Google Scholar]
- 37. Crider KS, Devine O, Hao L, Dowling NF, Li S, Molloy AM, Li Z, Zhu J, Berry RJ. Population red blood cell folate concentrations for prevention of neural tube defects: Bayesian model. BMJ. 2014;349:g4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen MY, Rose CE, Qi YP, Williams JL, Yeung LF, Berry RJ, Hao L, Cannon MJ, Crider KS. Defining the plasma folate concentration associated with the red blood cell folate concentration threshold for optimal neural tube defects prevention: a population-based, randomized trial of folic acid supplementation. Am J Clin Nutr. 2019;109(5):1452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tinker SC, Hamner HC, Qi YP, Crider KS. U.S. women of childbearing age who are at possible increased risk of a neural tube defect-affected pregnancy due to suboptimal red blood cell folate concentrations, National Health and Nutrition Examination Survey 2007 to 2012. Birth Defects Res A Clin Mol Teratol. 2015;103(6):517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rogne T, Tielemans MJ, Chong MF, Yajnik CS, Krishnaveni GV, Poston L, Jaddoe VW, Steegers EA, Joshi S, Chong YSet al. Associations of maternal vitamin B12 concentration in pregnancy with the risks of preterm birth and low birth weight: a systematic review and meta-analysis of individual participant data. Am J Epidemiol. 2017;185(3):212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Allen LH, Miller JW, de Groot L, Rosenberg IH, Smith AD, Refsum H, Raiten DJ. Biomarkers of Nutrition for Development (BOND): vitamin B-12 review. J Nutr. 2018;148(Suppl 4):1995S–2027S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. World Health Organization . Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Geneva (Switzerland): World Health Organization; 2008. [Google Scholar]
- 43. Gonmei Z, Toteja GS. Micronutrient status of Indian population. Indian J Med Res. 2018;148(5):511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Green R, Allen LH, Bjorke-Monsen AL, Brito A, Gueant JL, Miller JW, Molloy AM, Nexo E, Stabler S, Toh BHet al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3(1):17040. [DOI] [PubMed] [Google Scholar]
- 45. Allen L, Rosenberg IH, Oakley GP, Omenn GS. Considering the case for vitamin B12 fortification of flour. Food Nutr Bull. 2010;31(1_suppl1):S36–46. [DOI] [PubMed] [Google Scholar]
- 46. Rogers LM, Cordero AM, Pfeiffer CM, Hausman DB, Tsang BL, De-Regil LM, Rosenthal J, Razzaghi H, Wong EC, Weakland APet al. Global folate status in women of reproductive age: a systematic review with emphasis on methodological issues. Ann NY Acad Sci. 2018;1431(1):35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Finkelstein JL, Fothergill A, Johnson CB, Guetterman HM, Bose B, Jabbar S, Zhang M, Pfeiffer C, Qi YP, Rose CEet al. Periconceptional surveillance for prevention of anaemia and birth defects in southern India: protocol for a biomarker survey in women of reproductive age. BMJ Open. 2020;10(10):e038305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruth CJ, Huey SL, Krisher JT, Fothergill A, Gannon BM, Jones CE, Centeno-Tablante E, Hackl LS, Colt S, Finkelstein JLet al. An electronic data capture framework (ConnEDCt) for global and public health research: design and implementation. J Med Internet Res. 2020;22(8):e18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. World Health Organization . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva (Switzerland): World Health Organization; 2011. [Google Scholar]
- 50. Yetley EA, Pfeiffer CM, Phinney KW, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, Curtin LR, Durazo-Arvizu RAet al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr. 2011;94(1):313S–21S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF 3rd, Mills JL, Pfeiffer CM, Fazili Z, Zhang M, Ueland PMet al. Biomarkers of Nutrition for Development—folate review. J Nutr. 2015;145(7):1636S–80S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. World Health Organization . Obesity: preventing and managing the global epidemic. Report of a WHO consultation. WHO Technical Report. Geneva (Switzerland): World Health Organization; 2000. [PubMed] [Google Scholar]
- 53. WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet North Am Ed. 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- 54. US Department of Health and Human Services . Managing overweight and obesity in adults: systematic evidence review from the Obesity Expert Panel, 2013. Washington (DC): US Department of Health and Human Services; NIH; National Heart, Lung and Blood Institute; 2013. [Google Scholar]
- 55. World Health Organization [Internet]. Global Health Observatory data repository. [Cited 2021 Feb 24]. Available from: http://apps.who.int/gho/data/view.main.GSWCAH28v?lang=en. [Google Scholar]
- 56. International Institute for Population Sciences (IIPS) . National Family Health Survey (NFHS-4) 2015–2016. Mumbai (India): International Institute for Population Sciences; 2017. [Google Scholar]
- 57. Singh S, Geddam JJB, Reddy GB, Pallepogula DR, Pant HB, Neogi SB, John N, Kolli SR, Doyle P, Kinra Set al. Folate, vitamin B12, ferritin and haemoglobin levels among women of childbearing age from a rural district in South India. BMC Nutr. 2017;3(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chowdhury R, Taneja S, Dhabhai N, Mazumder S, Upadhyay RP, Sharma S, Tupaki-Sreepurna A, Dewan R, Mittal P, Chellani Het al. Burden of preconception morbidity in women of reproductive age from an urban setting in North India. PLoS One. 2020;15(6):e0234768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lilare RR, Sahoo DP. Prevalence of anaemia and its epidemiological correlates among women of reproductive age group in an urban slum of Mumbai. Int J Community Med Public Health. 2017;4(8):2841. [Google Scholar]
- 60. Sathya P, Gandhimathi R, Viruthasurani K, Rodriguez PM, Rajeswari PM, Subhathra N, Merlin MS. A study to assess the prevalence of anemia among women in a selected urban area in Coimbatore district. J Sci Innovative Res. 2017;6:11–5. [Google Scholar]
- 61. International Institute for Population Sciences . National Family Health Survey (NFHS-5) 2019–2020; state fact sheet Andhra Pradesh. Mumbai (India): International Institute for Population Sciences; 2020. [Google Scholar]
- 62. International Institute for Population Sciences . National Family Health Survey (NFHS-5) 2019–2020; district fact sheet Chittoor. Mumbai (India): International Institute for Population Sciences; 2020. [Google Scholar]
- 63. Menon KC, Skeaff SA, Thomson CD, Gray AR, Ferguson EL, Zodpey S, Saraf A, Das PK, Toteja GS, Pandav CS. Concurrent micronutrient deficiencies are prevalent in nonpregnant rural and tribal women from central India. Nutrition. 2011;27(4):496–502. [DOI] [PubMed] [Google Scholar]
- 64. Chakraborty S, Chopra M, Mani K, Giri AK, Banerjee P, Sahni NS, Siddhu A, Tandon N, Bharadwaj D. Prevalence of vitamin B12 deficiency in healthy Indian school-going adolescents from rural and urban localities and its relationship with various anthropometric indices: a cross-sectional study. J Hum Nutr Diet. 2018;31(4):513–22. [DOI] [PubMed] [Google Scholar]
- 65. Naik S, Mahalle N, Bhide V. Identification of vitamin B12 deficiency in vegetarian Indians. Br J Nutr. 2018;119(6):629–35. [DOI] [PubMed] [Google Scholar]
- 66. McLean E, Benoist B, Allen L. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull. 2008;29(2 Suppl 1):S38–51. [DOI] [PubMed] [Google Scholar]
- 67. Brito A, Mujica-Coopman MF, Lopez de Romana D, Cori H, Allen LH. Folate and vitamin B12 status in Latin America and the Caribbean: an update. Food Nutr Bull. 2015;36(2 Suppl):S109–18. [DOI] [PubMed] [Google Scholar]
- 68. Allen LH. Folate and vitamin B12 status in the Americas. Nutr Rev. 2004;62(6):29–33. [DOI] [PubMed] [Google Scholar]
- 69. Bailey RL, Carmel R, Green R, Pfeiffer CM, Cogswell ME, Osterloh JD, Sempos CT, Yetley EA. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am J Clin Nutr. 2011;94(2):552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. International Centre for Diarrhoeal Diseases Research Bangladesh (ICDDR B); Global Alliance for Improved Nutrition (GAIN); Institude of Public Health and Nutrition (IoPHa) . National Micronutrient Status Survey 2011–2012. Final report. Dhaka (Bangladesh): International Centre for Diarrhoeal Diseases Research; 2013. [Google Scholar]
- 71. Anaya-Loyola MA, Brito A, Villalpando S, Allen LH. Prevalence of low serum vitamin B12 in Mexican children and women: results from the first National Nutrition Survey (1999) as a basis for interventions and progress. Int J Vitam Nutr Res. 2020;90(3–4):325–32. [DOI] [PubMed] [Google Scholar]
- 72. Rosenthal J, Largaespada N, Bailey LB, Cannon M, Alverson CJ, Ortiz D, Kauwell GP, Sniezek J, Figueroa R, Daly Ret al. Folate deficiency is prevalent in women of childbearing age in Belize and is negatively affected by coexisting vitamin B-12 deficiency: Belize National Micronutrient Survey 2011. J Nutr. 2017;147(6):1183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rosenthal J, Lopez-Pazos E, Dowling NF, Pfeiffer CM, Mulinare J, Vellozzi C, Zhang M, Lavoie DJ, Molina R, Ramirez Net al. Folate and vitamin B12 deficiency among non-pregnant women of childbearing-age in Guatemala 2009–2010: prevalence and identification of vulnerable populations. Matern Child Health J. 2015;19(10):2272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Soofi S, Khan GN, Sadiq K, Ariff S, Habib A, Kureishy S, Hussain I, Umer M, Suhag Z, Rizvi Aet al. Prevalence and possible factors associated with anaemia, and vitamin B 12 and folate deficiencies in women of reproductive age in Pakistan: analysis of national-level secondary survey data. BMJ Open. 2017;7(12):e018007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. MacFarlane AJ, Greene-Finestone LS, Shi Y. Vitamin B-12 and homocysteine status in a folate-replete population: results from the Canadian Health Measures Survey. Am J Clin Nutr. 2011;94(4):1079–87. [DOI] [PubMed] [Google Scholar]
- 76. Finkelstein JL, Leal CG, Chu W, Krisher JT, Haas JD, Mehta S, Freire W. Anemia and iron, vitamin B12, and folate deficiencies in women of reproductive age in Ecuador: results from the Ecuadorian National Health and Nutrition Survey. Curr Dev Nutr. 2019;3(Suppl 1):782. [Google Scholar]
- 77. Jordan Ministry of Health; Global Alliance for Improved Nutrition; Centers for Disease Control and Prevention; United Nations Children's Fund–Jordan . National Micronutrient Survey, Jordan 2010. Amman (Jordan): Jordan Ministry of Health; 2011. [Google Scholar]
- 78. Abeyá E, Durán P, Mangialavori G, Biglieri A, Kogan L. Encuesta Nacional De Nutricion Y Salud; Documento de Resultados. Ministerio De Salud Presidencia de la Nacion. Buenos Aires, Argentina; 2007. [Google Scholar]
- 79. Herran OF, Ward JB, Villamor E. Vitamin B12 serostatus in Colombian children and adult women: results from a nationally representative survey. Public Health Nutr. 2015;18(5):836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ministerio De Salud, Instituto Costarricense de investigacion y ensenanza en nutricion y salud, Caja costarricenses de seguro social, instituto nacional de estadistica y censos, drogas ics. Encuesta Nacional de Nutrition 2008–2009. Fasciculo Micronutrientes. 2. San Jose (Costa Rica); 2012. [Google Scholar]
- 81. Ruston D, Hoare J, Henderson L, Gregory J, Bates C, Prentice A, Birch M, Swan G, Farron M. The National Diet and Nutrition Survey: adults aged 19 to 64 years. Nutritional status (anthropometry and blood analytes), blood pressure and physical activity. London: Her Majesty's Stationery Office; 2004. [Google Scholar]
- 82. Thamm M, Mensink GB, Thierfelder W. [Folic acid intake of women in childbearing age] Gesundheitswesen. 1999;61(Spec No):S207–12. [PubMed] [Google Scholar]
- 83. Christian AM, Krishnaveni GV, Kehoe SH, Veena SR, Khanum R, Marley-Zagar E, Edwards P, Margetts BM, Fall CH. Contribution of food sources to the vitamin B12 status of South Indian children from a birth cohort recruited in the city of Mysore. Public Health Nutr. 2015;18(4):596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Samuel TM, Duggan C, Thomas T, Bosch R, Rajendran R, Virtanen SM, Srinivasan K, Kurpad AV. Vitamin B12 intake and status in early pregnancy among urban South Indian women. Ann Nutr Metab. 2013;62(2):113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Allen LH. Causes of vitamin B12 and folate deficiency. Food Nutr Bull. 2008;29(2 Suppl 1):S20–34.; discussion S5–7. [DOI] [PubMed] [Google Scholar]
- 86. Indian Institute for Population Sciences . National Family Health Survey (NFHS-4) 2015–16: India. Mumbai (India): Indian Institute for Population Sciences; 2017. [Google Scholar]
- 87. Bellows AL, Kachwaha S, Ghosh S, Kappos K, Escobar-Alegria J, Menon P, Nguyen PH. Nutrient adequacy is low among both self-declared lacto-vegetarian and non-vegetarian pregnant women in Uttar Pradesh. Nutrients. 2020;12(7):2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Colapinto CK, O'Connor D, Tremblay MS. Folate status of the population in the Canadian Health Measures Survey. Can Med Assoc J. 2011;183(2):E100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Crider KS, Qi YP, Devine O, Tinker SC, Berry RJ. Modeling the impact of folic acid fortification and supplementation on red blood cell folate concentrations and predicted neural tube defect risk in the United States: have we reached optimal prevention?. Am J Clin Nutr. 2018;107(6):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rosenthal J, Reeve ME, Ramirez N, Crider KS, Sniezek J, Vellozzi C, Devine O, Lopez-Pazos E. Red blood cell folate insufficiency among nonpregnant women of childbearing age in Guatemala 2009 to 2010: prevalence and predicted neural tube defects risk. Birth Defects Res A Clin Mol Teratol. 2016;106(7):587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, Lupo PJ, Riehle-Colarusso T, Cho SJ, Aggarwal Det al. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019;111(18):1420–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. National Center for Health Statistics [Internet]. NHANES Response Rates and Population Totals. [Cited 2021 Feb 24]. Available from: https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.