Introduction
Pacemaker allergy is a rare phenomenon that can present with a spectrum of mild local inflammation to severe systemic manifestations. Many treatment modalities for the allergy have been described in the literature, with a majority resulting in removal or substitution of the offending allergen. We describe a case of severe erythroderma that occurred shortly after pacemaker implantation. Definitive management was removal of transvenous leads and replacement with an epicardial system, which resulted in complete resolution of the patient’s systemic inflammatory reaction.
Case report
An 80-year-old man with a past medical history of pulmonary embolism, severe eczema, and intermittent high-grade heart block status post pacemaker placement initially presented to his electrophysiologist in a virtual visit with a chief complaint of worsening rash. Three months prior to presentation he had his permanent pacemaker placed, and the rash developed shortly after placement. He described the rash, which initially started out on his gluteal area, as “small pimples,” but then progressed over weeks to a red, flat eruption that covered the majority of his body. The lesions were pruritic and initially not painful, but over time started feeling like “a bad sunburn.” He denied any oral lesions or pain with defecation or urination. He did admit to dry eyes, but otherwise no eye pain or change in vision. He denied use of any new soaps or detergents. A complete review of systems was otherwise negative.
He was seen by a dermatologist and there was initial concern for allergy to the apixaban he was prescribed approximately 1 month before the rash developed. He was switched to warfarin without any improvement. A punch biopsy was then performed on 1 of the lesions and demonstrated mild spongiosis with superficial dermal lymphohistiocytic inflammation containing eosinophils. Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and fibrinogen. The findings favored drug eruption. A 21-day course of prednisone starting at 40 mg was tried without any improvement. He was then given dupilumab 600 mg 1 week prior to presentation, with no significant improvement. His history of severe eczema and previous skin testing demonstrating allergies to formaldehyde and ethylenediamine dihydrochloride prompted suspicion for allergy to his pacemaker.
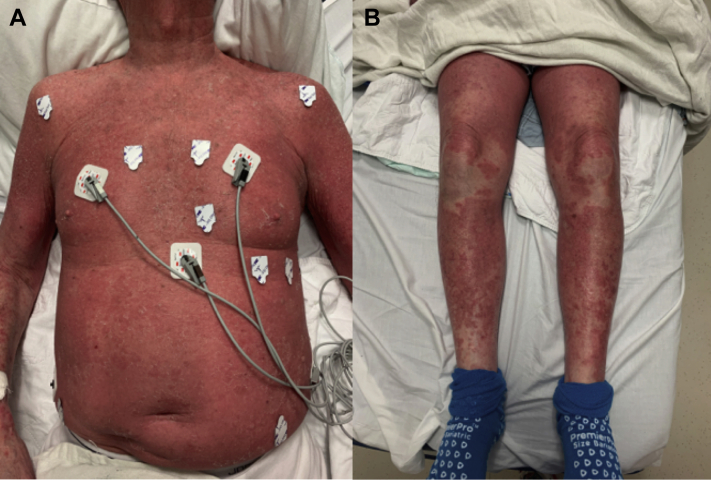
He was referred to the emergency department, where vital signs were blood pressure of 150/68, temperature of 95.7°F, pulse of 99 beats per minute, and saturating 98% on room air. His physical exam was notable for a diffuse, erythematous, nonraised rash covering approximately 90% of his body (Figure 1). There were no oral mucosa lesions. Pacemaker site was normal without any bruising or tenderness. The remainder of the physical exam was unremarkable. Labs were significant for elevated C-reactive protein of 1.01 mg/dL and normal white blood cell count with elevated absolute eosinophils (1940/μL).
Figure 1.
Dermatologic findings on presentation. A: Erythroderma of torso. B: Erythroderma of lower extremities.
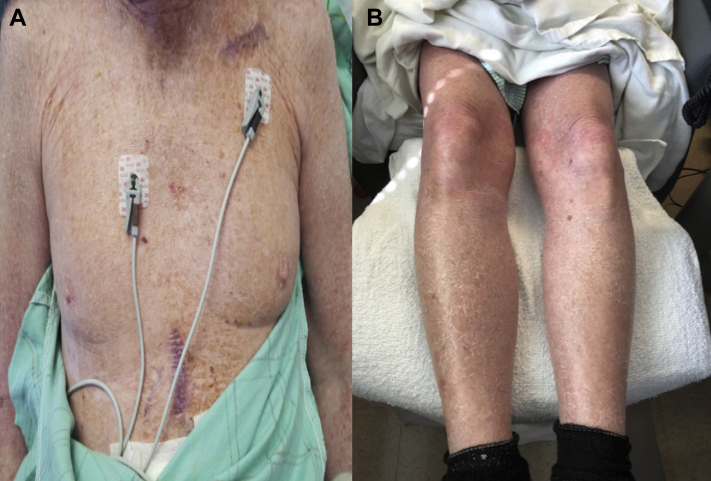
He was seen by the electrophysiology service while inpatient regarding the concern for his pacemaker being the cause of his rash. Ethylenediamine dihydrochloride is a component of polyurethane used in the manufacturing of pacemakers. After discussion with the pacemaker manufacturer it was determined that neither ethylenediamine dihydrochloride nor any of the allergens noted from the patient’s patch testing were in direct contact with the patient. The other potential etiology for the allergy then could be the titanium or silicone in the pacemaker, which is not typically included in empiric patch testing. Given the systemic nature of his reaction, with no local irritation around the generator site, it was felt the reaction was likely an allergy to a component of the leads. After consultation with dermatology and discussion with the patient, a decision was made to remove the pacemaker. Given his adequate chronotropic response during exercise tolerance testing and minimal pacing burden (right atrium 0.3%, right ventricle 0.1%), a single-chamber pacemaker would be adequate. The patient underwent extraction of his pacemaker system and implantation of a single-lead epicardial system via minithoracotomy. The components of this lead included titanium, Mp35n nickel alloy, and silicone. Within 2 days of the exchange, there was marked improvement in his rash (Figure 2) and the patient was visibly less symptomatic. He was discharged home the following day with moisturizing cream for residual dry skin. Three months after this hospitalization the patient no longer has any rash and his pacing burden remains minimal.
Figure 2.
Dermatologic findings after extraction. A: Torso with complete resolution of erythroderma. Presence of subxiphoid scar from epicardial pacemaker insertion. B: Resolution of erythoderma of lower extremities with residual xerosis.
Discussion
We present a case of severe erythroderma with a unique etiology. Erythroderma, also known as exfoliative dermatitis, is a rare condition with an estimated annual incidence of 1–2 per 100,000 patients.1 Literally translating to “red skin,” erythroderma is an intense, generalized erythema that is classically defined to involve ≥90% of the body.2 The majority of patients affected are of older age with a male predominance.1 There are multiple etiologies described, with the most common being evolution of pre-existing dermatoses such as psoriasis or eczema, with the second-leading cause being hypersensitivity reactions to medications.3 Of the pre-existing dermatoses, contact dermatitis accounts for approximately 3%.4 Diagnosis is clinical, as histopathology is typically nonspecific, with findings of acanthosis, parakeratosis, hyperkeratosis, and chronic perivascular inflammatory infiltrate, which may or may not involve eosinophils.5 Our patient likely experienced a contact hypersensitivity reaction to a component of his pacemaker, which then evolved into a more systemic manifestation of erythroderma.
Permanent pacemaker insertion is a common cardiovascular intervention. As of 2009, approximately 200,000 pacemakers were implanted in the United States, with numbers expected to increase in coming years.6 There are several indications for pacemaker insertion and the recommendations have been consolidated jointly by American Heart Association, American College of Cardiology, and Heart Rhythm Society.7 The pacemaker can be in the form of a transvenous, leadless, or epicardial system, but all forms can be divided into 2 essential components: a pulse generator and 1 or more electrodes/leads. The composition of the pulse generator includes the motherboard and battery, which is typically a lithium/iodine cell. The leads are composed of a metal alloy and coated with an insulator such as silicone or polyurethane. The final product is then most commonly encased in titanium or titanium alloy. The manufacturer of this patient’s pacemaker disclosed that the components were made of titanium, Mp35n nickel alloy, polyurethane, silicone rubber, and stainless steel (Table 1).8 In correlation with our patient’s known allergies, one of the concerns was the ethylenediamine dihydrochloride being a component of the polyurethane; however, we were reassured the polyurethane used was within the pulse generator and had no direct contact with the patient. Furthermore, his patch testing showed no allergy to nickel; therefore the possible offending agents could have been titanium or silicone. Since silicone allergy is rare, there is also consideration that the device lost its integrity and internal components were exposed to the patient. Though a leadless pacemaker was considered, given the uncertain nature of the patient’s allergy we determined that the safest course of action would be to implant an epicardial system to remove any potential allergens from the patient’s bloodstream.
Table 1.
Explanted and implanted device information and components
| Explanted Devices | Implanted Devices | |
|---|---|---|
| Pacemaker | W1DR01 PACEMAKER CARDIAC 7.4MM 50.8X46.6MM AZURE XT DR MRI SURESCAN | W1SR01 PACEMAKER CARDIAC 7.4MM 50.8X42.6MM AZURE XT SR MRI SURESCAN |
| Lead | 5076-58 LEAD PACING 58CM 6.2FR 2MM 10MM SPC SMSTRG ATR VNTR 5076-52 LEAD PACING 52CM 6.2FR 2MM 10MM SPC SMSTRG ATR VNTR |
511211 LEAD PACING 35CM BIPOLAR ACTFX MYOPORE SIL MYOCRD |
| Components | Pacemaker: Titanium, polyurethane, silicone rubber Leads: Silicone, MP35N, platinum |
Pacemaker: Titanium, polyurethane, silicone rubber Lead: Silicone, MP35N, platinum, titanium, stainless steel |
There are numerous cases of allergies to pacemaker components cited in the literature. Among these cases, multiple allergens have been identified as culprits, including cobalt, titanium, polyurethane, and silicone.9 The majority of the literature describes contact dermatitis and there are very few cases of severe systemic reactions.10 Identification of the specific agent can be made with patch testing, though the reliability of this test for certain metallic agents may be unreliable, especially in the case of titanium.10 Active skin reaction makes patch testing impossible. In any case, especially if severe, it is ideal to remove or substitute the offending component. There have been reports that describe successful resolution of symptoms with use of a gold-coated pacemaker in substitution of titanium.11 Other methods described include wrapping the generator or leads in a polytetrafluoroethylene sheet.12 To the best of our knowledge, our alternative of switching to an epicardial system while maintaining the same components is the only one described in the literature.
Given the patient’s known history of atopic dermatitis—in addition to many known allergens—the question arises if future patients like this should be patch tested to the components prior to pacemaker insertion. Reed and colleagues13 retrospectively analyzed patients undergoing metallic device implantation both prior to implantation and after the surgery. They noted that positive preoperative patch testing to metallic components influenced the selection of devices in all cases. In contrast, Carlsson and Moller14 described 18 patients who underwent device implantation despite having known metallic allergies from preprocedure patch testing. After a 6.3-year follow-up, there were no reported dermatologic complications. These, however, were metallic orthopedic devices; thus it is difficult to extrapolate these findings to endovascular implants such as a pacemaker. There are currently no guidelines on patch testing or other forms of allergen testing prior to pacemaker implantation. This would be difficult to accomplish, as pacemaker placement is often an acute issue. Patch testing would have to be done inpatient, results can take up to a week, and depending on the severity of conduction disease the patient would have to have a temporary wire placed. This exposes the patient to risk of infection and increased healthcare costs through increased length of stay. With a low incidence of approximately 571 per 1 million, patch testing may be an impractical solution.15
Conclusion
Pacemaker allergy is a rare entity that can range from mild contact dermatitis to severe systemic manifestations including erythroderma. Multiple components of the pacemaker have been described as allergens in the literature, and treatment typically involves removal of the offending agent or substitution with a hypoallergenic alternative. Our treatment strategy of utilizing the same components but switching to an epicardial system proved to be effective, with almost immediate results. Currently there are no official recommendations on allergen testing prior to implantation.
Key Teaching Points.
-
•
Cutaneous manifestations resulting from allergies to pacemaker components can be systemic and severe.
-
•
Numerous components of pacemakers are known allergens.
-
•
A newly described management option includes the replacement of intravenous pacemaker leads with an epicardial system.
-
•
There are no guidelines for patch testing prior to pacemaker placement.
Footnotes
Funding: There was no funding for this submission. Disclosures/Conflict of Interest: None of the authors have any disclosures or conflicts of interest.
References
- 1.Hasan T., Jansén C.T. Erythroderma: a follow-up of fifty cases. J Am Acad Dermatol. 1983;8:836–840. doi: 10.1016/s0190-9622(83)80013-9. 6. [DOI] [PubMed] [Google Scholar]
- 2.Okoduwa C., Lambert W.C., Schwartz R.A. Erythroderma: review of a potentially life-threatening dermatosis. Indian J Dermatol. 2009;54:1–6. doi: 10.4103/0019-5154.48976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botella-Estrada R., Sanmartín O., Oliver V., Febrer I., Aliaga A. Erythroderma: a clinicopathological study of 56 cases. Arch Dermatol. 1994;130:1503–1507. doi: 10.1001/archderm.130.12.1503. [DOI] [PubMed] [Google Scholar]
- 4.Harper-Kirksey K. Life-Threatening Rashes. Springer; Cham: 2018. Erythroderma; pp. 265–277. [Google Scholar]
- 5.Karakayli G., Beckham G., Orengo I., Rosen T. Exfoliative dermatitis. Am Fam Physician. 1999;59:625–630. [PubMed] [Google Scholar]
- 6.Greenspon A.J., Patel J.D., Lau E. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012;60:1540–1545. doi: 10.1016/j.jacc.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Kusumoto F.M., Schoenfeld M.H., Barrett C. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:932–987. doi: 10.1016/j.jacc.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Medtronic. Manual Library. Available at http://manuals.medtronic.com/manuals/main/en_US/manual/index.
- 9.Peters M.S., Schroeter A.L., van Hale H.M., Broadbent J.C. Pacemaker contact sensitivity. Contact Dermatitis. 1984;11:214–218. doi: 10.1111/j.1600-0536.1984.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 10.Honari G., Ellis S.G., Wilkoff B.L., Aronica M.A., Svensson L.G., Taylor J.S. Hypersensitivity reactions associated with endovascular devices. Contact Dermatitis. 2008;59:7–22. doi: 10.1111/j.1600-0536.2008.01351.x. [DOI] [PubMed] [Google Scholar]
- 11.Syburra T., Schurr U., Rahn M., Graves K., Genoni M. Gold-coated pacemaker implantation after allergic reactions to pacemaker compounds. Europace. 2010;12:749–750. doi: 10.1093/europace/eup411. [DOI] [PubMed] [Google Scholar]
- 12.Ishii K., Kodani E., Miyamoto S. Pacemaker contact dermatitis: the effective use of a polytetrafluoroethylene sheet. Pacing Clin Electrophysiol. 2006;29:1299–1302. doi: 10.1111/j.1540-8159.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- 13.Reed K.B., Davis M.D., Nakamura K., Hanson L., Richardson D.M. Retrospective evaluation of patch testing before or after metal device implantation. Arch Dermatol. 2008;144:999–1007. doi: 10.1001/archderm.144.8.999. [DOI] [PubMed] [Google Scholar]
- 14.Carlsson A., Möller H. Implantation of orthopaedic devices in patients with metal allergy. Acta Derm Venereol. 1989;69:62–66. [PubMed] [Google Scholar]
- 15.Shittu M., Shah P., Elkhalili W. A rare case of recurrent pacemaker allergic reaction. Heart Views. 2015;16:59–61. doi: 10.4103/1995-705X.159222. [DOI] [PMC free article] [PubMed] [Google Scholar]