Abstract
Post-anoxic leukoencephalopathy is a rare event that causes global demyelination secondary to anoxic injury. Given the nature and extent of the damage, cognitive and functional deficits are typically chronic even after standard therapies. Here, we describe a novel treatment approach that used high definition transcranial direct-current stimulation (HD-tDCS) with a 62-year-old male who was 5 years post-anoxic leukoencephalopathy secondary to an accidental drug overdose. HD-tDCS was administered over the left lateral prefrontal cortex across 29 daily sessions at 2 mA (20 minutes/session) in order to address dysexecutive behaviors. Results demonstrated improved delayed memory and trends for improved visuospatial and semantic fluency performance as well as improved insight and daily functioning, all of which returned to baseline by the end of a 10 week no-contact follow up period. Resting state fMRI connectivity results mirrored these changes by showing increased dorsal attention and cingulo-opercular but reduced ventral attention network connectivity after session 29, all of which returned to baseline at follow-up. These findings suggest HD-tDCS may benefit functioning even following serious and pervasive anoxic injury. Findings also suggest the need for continued HD-tDCS for maintenance purposes, though future work is needed to identify optimal dose-response information.
Keywords: leukoencephalopathy, HD-tDCS, neurostimulation, fMRI, prefrontal cortex
Introduction
Neuropathology of Post-Anoxic Leukoencephalopathy
Anoxic brain injury results from either lower circulating arterial oxygen or reduced cerebral perfusion. Anoxia initiates numerous complex changes in neuronal functioning, and the amount and length of oxygen deprivation greatly influences the extent of brain injury (Caine & Watson, 1998). In the event of reduced oxygen to the brain, dilation of blood vessels temporarily maintains blood flow and allows tissue to continue receiving adequate oxygen. However, in prolonged anoxic conditions membrane depolarization, influx of uncontrolled calcium, and activation of phospholopases and proteases lead to cell swelling and eventual cell death (Victor & Ropper, 2001; Michaels, 2004). While injury occurs on a whole-brain level, specific areas of the brain such as watershed territories and those with high metabolic demands (e.g., hippocampus, basal ganglia, cerebellum, thalamus) are more vulnerable to the effects of anoxic injury (Anderson & Arciniegas, 2010; Caine & Watson, 1998).
Post-anoxic leukoencephalopathy (PAL) can occur in more severe cases of anoxia and is a rare condition in which patients show initial recovery, sometimes returning to their baseline status, only to later show clinical decline consistent with hypoxic/anoxic injury days or weeks later (Shprecher & Mehta, 2010; Thacker et al., 1994). PAL causes damage to the brain via global demyelination, though typically sparing the cerebellum and brainstem (Shprecher & Mehta, 2010). While PAL etiology is unknown (Shprecher & Mehta, 2010; Zamora et al., 2015), it has been reported in those who experienced benzodiazepine and opiate overdose (Peter et al., 2004; Salazar & Dubow, 2012), carbon monoxide poisoning, complications from surgical anesthesia, and cardiac arrest (Shprecher & Mehta, 2010).
PAL Clinical Presentation
Given the widespread injury that occurs with PAL, it is unsurprising that cognitive deficits have been reported in virtually all domains including attention, language, executive function, and learning and memory (Anderson, & Arciniegas, 2010; Shprecher & Mehta, 2010; Victor & Ropper, 2001). Associated personality and behavioral changes can be wide-ranging but often include emotional lability, impulsivity, and irritability (Anderson, & Arciniegas, 2010). In more severe cases, PAL can also lead to frontal release signs and symptoms typically seen in psychosis (Shprecher & Mehta, 2010).
Recovery of cognitive, motor, and behavioral factors after PAL typically occur within the first year, with limited recovery in more severe cases (Smania et al., 2011; Victor & Ropper, 2001). While the length of coma and the patient’s functional ability upon admission may serve as good predictors of prognosis following “standard” anoxia (Heinz & Rollnik, 2015), comparatively little is known about recovery from PAL. However, most patients who experience PAL are left with chronic deficits even after standard therapeutic options such as speech, occupational, and physical therapy have been exhausted (Anderson & Arciniegas, 2010; Thacker, Asthana, & Sakari, 1994). It is within this context that we considered a form of non-invasive brain stimulation, transcranial direct current stimulation (tDCS), for a patient who experienced chronic deficits due to PAL (see History below).
tDCS
tDCS uses a low intensity electrical current, delivered via scalp electrodes, to modulate the excitability of brain tissue under and between the electrodes (Bikson et al., 2004). “Traditional” tDCS passes the electricity between two relatively large pads (e.g., 25–35cm2) – an anode that introduces the current and a cathode that collects it. The current study used High Definition (HD-)tDCS, which provides more focal stimulation delivery by surrounding a small center electrode with multiple small electrodes of the opposite polarity. This center electrode uses the full current strength (e.g., 2mA) while the ring electrodes each use about ¼ of the current (e.g., ~0.5mA if 4 electrodes). The neurophysiological effects of HD-tDCS appear to exceed those of the pad-based approach (Kuo et al., 2013; Pelletier & Cicchetti, 2015).
The specific mechanisms through which tDCS exerts effects are still being uncovered but the basic premise is that the subthreshold stimulation modulates the cell membrane thereby altering the probability of neuronal transmission (Stagg & Nitsche, 2011). Effects depend on the polarity of the current (anode vs. cathode), magnitude and duration of current (Samani et al., 2019), and type and orientation of the cell (Rahman et al., 2013; Pelletier & Cicchetti, 2015). Other work suggests tDCS-induced changes along synaptic pathways (Rahman et al., 2013), which may correspond with evidence of altered neurotransmitter levels (Clark et al., 2011; Das et al, 2016; Kim et al., 2014). Evidence of neurophysiologic change outside of the motor system has been provided by altered resting state functional connectivity (Krishnamurthy et al., 2016; Polania et al., 2011; Polania et al., 2012) and task-based (Hampstead, Brown, Hartley, 2014) functional magnetic resonance imaging (fMRI). Given that PAL markedly affects white matter, it is relevant to note that homogenous isotopic white matter conductivity is typically modeled as lower then grey matter, while with anisotropic white matter can be modeled with a directional conductivity higher than grey matter (Suh et al, 2012). Notwithstanding open questions about tissue conductivity values, and specifically how to model anisotropy based on imaging ( Shahid et al., 2014), it is expected that white matter will differently impact overall brain current flow patterns. tDCS has been shown to impact axon terminals (Bikson et al., 2004), but it remains to be understood if and how tDCS would impact axons of passage (Chakraborty et al., 2018; Rahman et al., 2017). Such questions are beyond the scope of the current case study but broach important questions for the larger field.
tDCS has been used in a variety of populations for cognitive enhancement purposes, though we are not aware of any prior use in those with PAL or other anoxic injuries (aside from ischemic stroke). However, literature from related conditions appears promising. For example, a study of 9 patients with leukoaraiosis (ischemic white matter lesions) found that a single session of tDCS (2 mA for 15 minutes with the anode over the primary motor cortex and cathode over inion) in combination with gait and balance training improved both skills compared to balance training alone (Kaski et al., 2013). Likewise, studies in patients who suffered a stroke have demonstrated improved motor (Lindenberg et al., 2010) and language abilities regardless of whether tDCS was applied alone (Boggio et al., 2007) or in combination with therapy (Lindenberg et al., 2010). Specifically, past work with traditional stroke populations which investigated the use of tDCS over the left lateral prefrontal cortex has demonstrated overall improved language fluency (Polanowska et al., 2013; Richardson, et al., 2015; Wang et al., 2019), verbal learning (Yun, Chun, & Kim, 2015), and working memory (Jo et al., 2009). However, some studies have found a lack of cognitive improvement with tDCS in those suffering from traditional stroke (Shaker et al., 2018). Meta-analyses on both motor and language functioning and learning in stroke patients are generally positive (O’Brien et al., 2018; Otal et al., 2015), but some suggest small or non-statistically significant differences between active tDCS protocols and sham (Tedesco Triccas et al., 2016) or poor quality of evidence for tDCS (Elsner et al., 2016a). Conversely, there is also work that suggests no cognitive benefit after multiple tDCS sessions (Elsner et al., 2016a; Valiengo et al., 2017), though studies do not always require the presence of cognitive deficits at the time of enrollment; a limitation that renders change highly unlikely. Thus, a great deal more work is needed in this area to determine optimal dosing and targeting parameters. Such exploration is entirely feasible since tDCS is widely regarded as well tolerated and safe, including when multiple sessions are provided (Bikson et al., 2016; Brunoni et al., 2011; Paneri et al., 2016; Reckow et al., 2018).
As described below, our patient with PAL demonstrated significant cognitive and functional deficits even after exhausting other treatment options. Therefore, we investigated the use of HD-tDCS over the left lateral prefrontal cortex (PFC) for an extended treatment period of 29 sessions at 2mA for 20 minutes conducted over 6 consecutive weeks. No concurrent treatment was provided during this time. Effects were evaluated using a multi-method approach that included neuropsychological testing, informant report, and resting-state fMRI.
Case Study History
The current case (PT1) was a 62-year-old, married, right-handed, White male who, at the age of 57, sustained PAL following an accidental opioid drug overdose. He was found unresponsive the morning after taking the medication and was twice resuscitated before being transported to the hospital. PT1 was intubated in the ER. After two weeks in a medically induced coma he appeared to be recovering well for approximately four days. Consistent with the onset of PAL, he suddenly decompensated while still hospitalized on day four and remained on inpatient and rehabilitation units for the next four months. Neuroimaging (MRI) during this time noted extensive, symmetric, and diffuse periventricular and deep white matter hyperintensities as well as significant volume loss.
PT1 presented for neuropsychological evaluation at an outpatient clinic about five years after the anoxic event, at which point he required 24-hour supervision despite completing extensive courses of occupational, physical, and speech therapy. Per informant (wife) report, anterograde memory (from the time of the event) was significantly impaired while retrograde memory was relatively intact. PT1 was independent in activities of daily living (ADLs) but was generally dependent for most instrumental ADLs. He helped with some household chores as long as frequent prompts about when and how to perform the activities were provided. In this regard, he demonstrated problems with procedural knowledge (e.g., recalling and correctly sequencing the steps associated with making coffee). Speech was reportedly limited the first two years after the event but gradually improved and was characterized primarily by language issues such as short, halting phrases and a general sense of pressured production. A self-report questionnaire completed by his wife indicated that PT1 demonstrated significant executive dysfunction compared to before his anoxic event including reduced inhibitory control (e.g. frequent swearing, inappropriate social comments) that appeared to interact with the other cognitive changes and resulted in social isolation for PT1 and, to some extent, his wife. There was no history of learning or attentional disorders and he was reportedly an above average student. He earned a bachelor’s degree and subsequent legal degree and was a successful attorney for over 20 years at the time of the event. His previous medical history included non-Hodgkin’s Lymphoma (treated with chemotherapy 13 years prior to the neuropsychological evaluation). He had a history of alcohol dependence but had been sober for about 20 years at the time of the evaluation. Medications included Celexa, Ritalin (5mg), aspirin (81mg), fish oil, and a daily multivitamin. These medications had been stable for several months prior to participation and his spouse reported consistent, daily use for all medications throughout the entire study protocol. There were no reported medication changes during the study period.
Neuropsychological testing revealed marked and pervasive cognitive impairment. PT1 had intact simple attentional abilities, but at least mildly impaired sustained attention, working memory, and processing speed. Visuoperception was variably impaired, but visuoconstruction was intact. Speech was hypophonic and pressured with intact articulation. He was noticeably disinhibited as he frequently interrupted test instructions and initiated tasks prior to instructions being completed. Similar to his wife’s report, he tended to provide an initial “burst” of responding during testing and would then state “that’s it,” though prompting from the examiner typically elicited additional responding. His verbal fluency was mild (phonemic) to moderately (semantic) impaired. Confrontation naming and repetition were intact. Anterograde learning and memory were moderately to severely impaired regardless of modality (i.e., verbal vs. visuospatial). PT1 had mild difficulty identifying solution strategies when confronted with a novel problem solving task, and his overall performance was severely impaired on the measure, largely due to severe perseveration. His ability to shift between two concurrent lines of thought was severely impaired. A formal measure of inhibitory control was mildly impaired.
MRI findings at the time of testing revealed interval worsening as reflected by global atrophy as well as hyperintensities throughout the supratentorial white matter. Such structural changes are consonant with previous findings of progressive damage in the years following PAL (Shprecher & Mehta, 2010).
Methods
Procedures
Overview (Table 1):
Table 1.
Outline of Procedures
| Pre-Studyy (~6 months before treatment) | Baseline Session 1 (T-4 weeks) | Baseline Session 2 (T-2 weeks) | HD-tDCS Sessions (29 separate days) | Post-Treatment Session | Follow-Up Session (10-weeks post-treatment) |
|---|---|---|---|---|---|
| Initial clinical evaluation | fMRI | fMRI | HD-tDCS (2mA for 20 minutes) | fMRI | fMRI |
| Neuropsych testing RBANS1 BADS2 COWAT3 Trails A & B4 |
Questionnaires FrsBe6 ADLQ7 |
Safety Questionnaire | Neuropsych testing RBANS BADS COWAT Trails A & B |
Neuropsych testing RBANS BADS COWAT Trails A & B |
|
| Questionnaires GDS5 |
Questionnaires GDS FrsBe ADLQ |
Questionnaires GDS FrsBe ADLQ |
Repeatable Battery for Assessment of Neuropsychological Status (RBANS) (Randolph, 1998)
Behavioral Assessment of Dysexecutive Syndrome (BADS) (Wilson, Alderman, Burgess, Emslie, & Evans, 1996)
Controlled Oral Word Association Test (COWAT) (Benton, Hamsher, & Sivan, 1994)
Trail Making A & B (TMT) (Reitan, 1958)
Geriatric Depression Scale, (GDS) (Yesavage et al. 1983)
Frontal Systems Behavior Scale (FrsBe) (Locasio, Growdon, & Corkin, 1995)
Activities of Daily Living Questionnaire (ADLQ) (Grace, Stout, & Malloy, 1999).
Given the severity and functional impact of PT1’s objective and subjective deficits in executive functioning (e.g. mental flexibility, planning, inhibition), language (e.g., fluency, pressured speech), and learning and memory, we targeted the left lateral prefrontal cortex (PFC) using HD-tDCS. We evaluated potential neurophysiologic effects using resting state fMRI, neuropsychological testing, and self-/informant-report questionnaires at two time points before (baseline 1 = 4 weeks before; baseline 2 = 1 week before) and two time points after treatment (post-treatment = 1 week after; follow-up session = 10 weeks after). Tolerability and safety were assessed after each daily HD-tDCS session using a standard questionnaire (Brunoni et al., 2011). No randomization or blinding was used given the nature of the study. All procedures were approved by the institutional review board and PT1 and his wife provided written consent.
Neuropsychological Testing, Self-Report, and Informant Report
We performed additional neuropsychological testing at the time of study enrollment since there was ~6 month delay since the previously described clinical evaluation (Table 1). Baseline results (Table 2) were consistent with the previous clinical evaluation and document the persistence and severity of the cognitive deficits.
Table 2.
RCI Scores
| Baseline to Post-testing | Post-testing to Follow-up | Baseline to Follow-up | |
|---|---|---|---|
| RBANS | |||
| Immediate Memory | 0 | 1.46 | 1.46 |
| Visuospatial | 1.30 | −2.00 | −0.71 |
| Attention | −0.81 | 1.14 | 0.34 |
| Language | 1.95 | −2.43 | −0.49 |
| Delayed Memory | 2.43 | −1.46 | 0.97 |
fMRI
Anatomical and resting state images were collected through a GE MR750 3T magnetic resonance system (GE, Milwaukee, WI) with a 32-channel phased array head coil. Sequence parameters for anatomical acquisition were: Field of view (FOV) = 256, Matrix = 256 × 256, 156 slices per volume, 1 × 1 × 1 mm voxel size, TR = 12 ms, TE = 5 ms, TI = 500 ms, flip angle = 15°. For functional resting state data, PT1 was instructed to focus his gaze on a fixation cross (i.e., eyes open) on the screen and not to fall asleep. Resting state data were acquired through a multiband echo planar imaging (EPI) sequence with parameters: FOV = 240, Matrix = 74 × 74, 45 slices per volume, 3.24 × 3.24 × 3 mm voxel size, TR = 900 ms, TE = 30 ms, flip angle = 70°. We acquired 512 volumes but dropped the first 6 to allow for signal normalization, resulting in a total of 7’35.4” of resting state data.
Both anatomical and functional images were preprocessed and analyzed through SPM8 (SPM8; Wellcome Trust Centre for Neuroimaging). Anatomical images were spatially normalized to a standard MNI template using the voxel-based morphometry toolbox (VBM8 http://dbm.neuro.uni-jena.de/vbm) and DARTEL high-dimensional warping and resampled to a 3×3×3 mm voxel size. Slice timing correction was applied for functional volumes. Functional volumes were then realigned to the first volume to correct for head motion and further co-registered with the high-resolution sagittal images. Estimated deformation fields from normalization were applied to normalize functional images to MNI space and smoothed with a 5-mm FWHM Gaussian kernel. Time course data for each voxel was band-pass filtered (0.01 to 0.10 Hz band) to capture the low-frequency spontaneous BOLD oscillations. Translational and rotational motion data were added as motion regressors as well with their first derivative and quadratic terms to regress out motion artifacts from all voxels. White matter and CSF signals were also regressed out with the PCA CompCor method (Behzadai et al., 2007). Global signal regression was not used.
Resting state functional connectivity was examined using the 264 node parcellation atlas defined by Power et al. (2013). We included 8 additional nodes in the hippocampus and amygdala (bilaterally) using the Automated Anatomical Labeling (AAL) atlas as reference (2 nodes in left and right amygdala; 6 nodes in hippocampus – 1 in the each of the left and right head, body, and tail). We created 5 mm spheres at these 272 nodes and extracted time course data over all volumes and Pearson product–moment correlation coefficients were calculated between the average BOLD time course in the 272 node regions. Correlation coefficients were then transformed to z scores using a Fisher r to z transformation. Graph theory metrics, including nodal strength, were calculated through the ‘igraph’ package in R. We accepted Power’s (2013) network designation for each node and extracted graph metrics for use during subsequent data analysis. We selected the six cognitive networks most relevant to this case, which included the dorsal attention, ventral attention, salience, cingulo-opercular, frontoparietal, default mode networks.
HD-tDCS Methods
PT1 underwent 29 HD-tDCS sessions, administered once a day, on consecutive weekdays, over a six-week period (with an exception of his final week which was once a day for four days). Each stimulation session consisted of 30 second “ramp up” and “ramp down” periods with 19 minutes of intervening stimulation at 2mA that was performed using a Soterix Medical Inc. tES unit with attached 4×1 unit. The 4×1 unit passively splits the current among the 4 ring electrodes. The center HD electrode was placed at F5 (anode) while the surrounding cathodes were placed at F9, Fp1, F1, and C5 of the 10–20 International Positioning System. We selected F5 as the cite for the anode since this montage targeted both ventro- and dorso-lateral prefrontal cortices, which was deemed appropriate given his diffuse difficulty with multiple aspects of executive functioning (especially cognitive control). Each set of five electrodes were used for 10 sessions and the location of each electrode was rotated to where any given electrode was used as the center anode twice and ring cathode 8 times. The resulting finite element model (created with the ROAST platform; Huang et al., 2019) indicates this montage effectively targeted the left lateral PFC (see Figure 1). Before each session, PT1 completed a questionnaire that included medication use, hours of sleep, alcohol and recreational drug use (there were none reported), all of which were constant throughout the 29 sessions. Once these electrode locations were measured, elastic head netting (Surgilast®) was placed on his head and HD electrode holders were fitted through the mesh at the appropriate locations. Holders were filled with approximately 10ml of conductive gel. Impedance was checked at each electrode site using the “quality unit” measurement provided by the Soterix Medical Inc. 4×1 unit prior to the start of stimulation each day. Values above 1.0 were rare but were checked to ensure there were no confounding factors (e.g., hair, air bubbles). A ten-minute saturation period was given between electrode placement and start of stimulation to further reduce impedance, after which stimulation began. PT1 sat quietly in a room with the examiner during tDCS sessions. PT1 also completed a side effects questionnaire (Brunoni et al., 2011) that was administered before and immediately after stimulation (as recommended by Reckow et al., 2018).
Figure 1.
Finite element model of electric current flow on PT1 brain based on ROAST. Center anode at F5, ring cathodes at F9, Fp1, F1, C5.
Results
Tolerability of HD-tDCS
HD-tDCS was well tolerated as PT1 reported only mild tingling (79% of sessions), mild itching (72%), and mild burning sensation (39%). Other symptoms (e.g. scalp pain, neck pain) occurred during two or fewer sessions. On the morning of the 21st session (a Monday morning), red spots were noted across his forehead, including at the location of the FP1 electrode. These spots were determined as unlikely associated with HD-tDCS since they were not present at the end of the previous session and were also evident on the right side of his forehead (where no stimulation was performed). His wife speculated that these spots were from wearing an old (unwashed) hat for several days in a row. In an abundance of caution, the FP1 electrode was moved approximately 1 cm anteriorly for 5 sessions to avoid the irritated skin. No further redness was noted. No unexpected or severe adverse effects occurred. Thus, tolerability and safety were high across these 29 sessions.
Changes in Neuropsychological Performance
As noted above, PT1 demonstrated persistent deficits across baseline evaluations. However, reliable change indices (calculated by subtracting the baseline from post test score and then dividing by the standard error of the known test-retest difference; a value > 1.96 was considered significant) demonstrated improvement on the RBANS Delayed Memory Index (RCI = 2.47) and Visuospatial Index (RCI = 1.98) (see Table 2). Likewise, semantic fluency performance improved by over 1.5 SD during post-treatment. Performances returned to baseline levels at follow-up (see Table 3). Thus, there is an inverse “V”-shaped curve with improvements demonstrated immediately after the 29 HD-tDCS sessions but regression to baseline after 10 weeks of no treatment.
Table 3.
Neuropsychological Testing, Self-Report, and Informant-Report Questionnaire Results at Baseline, Post-testing, and Follow-up Sessions.
| Baseline | Post-testing | Follow-up | |
|---|---|---|---|
| ATTENTION | |||
| Trailmaking Test Part A | 0.67 | 0.67 | −2.5 |
| Trailmaking Test Part B | DC | DC | DC |
| RBANS | |||
| Digit Span | −1.05 | −1.05 | −0.57 |
| Coding | −1.66 | −2.42 | −1.78 |
| Attention Index | −1.4 | −1.75 | −1.25 |
| VISUOSPATIAL SKILLS | |||
| RBANS | |||
| Figure Copy | −5.94 | −4.18 | −4.76 |
| Line Orientation | 0.14 | 0.83 | −0.55 |
| Visuospatial Index | −1.75 | −1.25 | −2.33 |
| LANGUAGE | |||
| COWAT | −1.5 | −1.85 | −1.5 |
| RBANS | |||
| Naming | 0.6 | 0.6 | 0.6 |
| Fluency | −2.17 | −0.22 | −3.04 |
| Language Index | −1.3 | −0.25 | −1.5 |
| LEARNING AND MEMORY | |||
| RBANS | |||
| List Learning | −1.78 | −1.78 | −1.11 |
| Story Learning | −3.26 | −3.83 | −2.97 |
| Immediate Memory Index | −2.75 | −2.75 | −2.1 |
| List Recall | −2.27 | −1.36 | −2.27 |
| List Recognition | −6.17 | −4.5 | −2.83 |
| Story Recall | −3.95 | −3 | −4.42 |
| Figure Recall | −2.9 | −1.65 | −2.65 |
| Delayed Memory Index | <−3.00 | −2.67 | <−3.00 |
| EXECUTIVE FUNCTION | |||
| BADS | |||
| Rule Shift* | 3 | 4 | 4 |
| Act Plan* | 3 | 4 | 2 |
| Key Search* | 1 | 1 | 1 |
| Temp Judgment* | 0 | 1 | 0 |
| Zoo Map* | 0 | 0 | 0 |
| Total | <−3.00 | −2.67 | <−3.00 |
| SELF-REPORT/ INFORMANT REPORT | |||
| GDS | 0 | 0 | 1 |
| FrSBe* | |||
| Self-before | 98 | 56 | 106 |
| Self-after | 101 | 134 | 137 |
| Wife-before | 54 | 51 | 53 |
| Wife-after | 153 | 121 | 119 |
| Caregiver-after | 120 | 113 | 101 |
| ADLQ*(all raw scores) | |||
| Self- Self care | 0 | 11.11 | 5.56 |
| Self- House care | 33.33 | 20 | 38.89 |
| Self- Employ./Recreation | 33.33 | 0 | 33.33 |
| Self- Shopping/Money | 44.44 | 33.33 | 33.33 |
| Self- Travel | 33.33 | 11.11 | 33.33 |
| Self- Communication | 20 | 13.33 | 20 |
| Wife- Self care | 33.33 | 16.67 | 16.67 |
| Wife- House care | 83.33 | 61.11 | 55.56 |
| Wife- Employ./Recreation | 66.67 | 44.44 | 58.33 |
| Wife- Shopping/Money | 66.67 | 55.56 | 55.56 |
| Wife- Travel | 91.67 | 83.33 | 55.56 |
| Wife- Communication | 53.33 | 33.34 | 26.67 |
| Caregiver- Self care | 11.11 | 16.67 | 11.11 |
| Caregiver- House care | 77.78 | 77.78 | 72.22 |
| Caregiver- Employ./Recreation | 55.56 | 44.44 | 66.67 |
| Caregiver- Shopping/Money | 55.56 | 55.56 | 55.56 |
| Caregiver- Travel | 75 | 75 | 91.67 |
| Caregiver- Communication | 41.67 | 33.33 | 6.67 |
Note: Z-Scores unless otherwise stated
= Raw Scores; DC = Discontinued; FrSBe and ADLQ listed as Baseline session were given during Baseline session 2.
Self- and Informant-Report
Self-report measures suggested enhanced insight into deficits at both post-treatment sessions relative to baseline. Specifically, PT1’s baseline responding on the FrSBe was characterized by nominal endorsement of executive dysfunction either before or after the event (i.e., claiming no effect of PAL). In contrast, his post-treatment reports indicate nominal dysfunction prior to PAL but high levels of dysfunction following the injury. In fact, his post-treatment report of impairment was more comparable to that of his wife’s and caregiver’s baseline ratings. Informants reported improved executive functioning post-treatment. Similarly, he reported significant levels of impairment on the Activities of Daily Living Questionnaire (ADLQ) pre-treatment whereas he reported lower levels, at post-testing, consistent with the relative improvement both his wife and caregiver reported on this measure at post-treatment.
Although he remained dependent overall, qualitative reports from his wife and caregiver described him as showing greater empathy and social appropriateness with less impulsivity and pressured speech. Per his wife’s report, PT1 began independently planning and completing household chores, was better able to follow commands, and showed improved memory (e.g., remembering events, requests, conversations) within the first two weeks of HD-tDCS treatment. By the end of HD-tDCS treatment sessions, he successfully began texting (with complete sentences) and using a computer tablet independently – neither of which he had been able to do despite months of practice with his wife and caregiver. Both informants felt these improvements generally persisted at follow-up (see Table 3).
Changes in resting-state fMRI
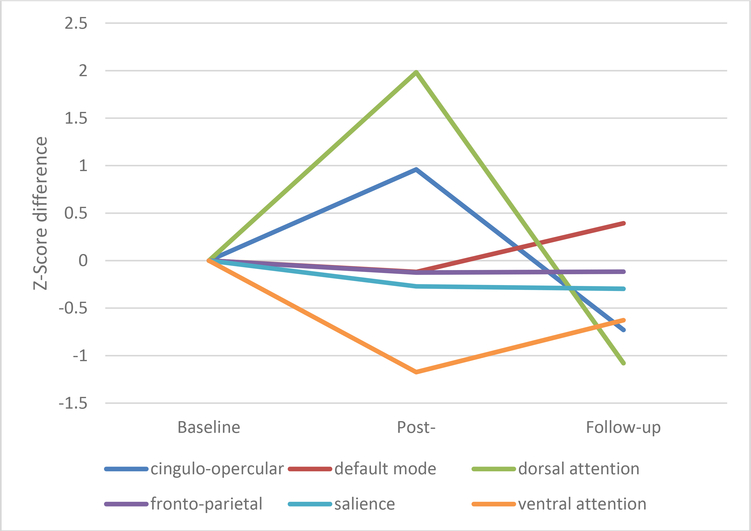
Given the case-study nature of this report, we focused on changes in the six major networks (i.e., dorsal attention, ventral attention, salience, cingulo-opercular, frontoparietal, default mode) using a raw threshold correlation value of 0.1. We examined connectivity strength (i.e., r-value of each connection for each node within a network) at baseline (using the mean values of baseline 1 and baseline 2) relative to post-treatment and the follow-up scans (see Figure 2 and Table 4). An inverse “V” emerged where network strength increased post training but returned to (or below) baseline at the 10-week follow-up in both the dorsal attention and cingulo-opercular networks. The ventral attention network showed the opposite pattern with reduced network strength at post-training and a return to baseline at follow-up. Nominal changes were evident in the other networks.
Figure 2.
Changes in resting-state fMRI
Table 4.
Means and Standard Errors for resting-state fMRI
| Network | Baseline Mean (SE) |
Post-Testing Mean (SE) |
Follow-up Mean (SE) |
|---|---|---|---|
| Cingulo-Opercular | 15.19(0.73) | 17.80(0.85) | 13.21(0.82) |
| Fronto-Parietal | 23.57(0.75) | 23.10(1.02) | 23.13(0.97) |
| Default Mode | 20.91(0.65) | 20.31(0.61) | 22.86(0.96) |
| Salience | 17.24(1.18) | 15.89(1.50) | 15.76(1.81) |
| Dorsal Attention | 21.85(0.69) | 26.37(1.58) | 19.39(1.02) |
| Ventral Attention | 21.89(0.52) | 20.05(1.23) | 20.91(1.26) |
Note: Baseline raw scores are averaged between baseline session 1 and 2.
Discussion
The current study is the first of our knowledge to investigate the use of HD-tDCS in a case of PAL with significant cognitive and daily life dysfunction. PT1 demonstrated improvement from baseline to post-testing on delayed memory performance, visuospatial skills, and verbal fluency. These improvements coincide with increased dorsal attention and cingulo-opercular network connectivity from baseline to post-testing, whereas ventral attention network connectivity decreased. PT1’s insight improved during his treatment sessions, and his wife and caregiver reported improved daily functioning, including better empathy, social appropriateness, and speech production. However, while self and informant report noted sustained improvement, cognitive test performance did not sustain at the 10-week follow-up.
Given the multi-year persistence of baseline deficits, pattern of improvement, and general regression to (or below) baseline, this case appears to add to the growing literature that HD-tDCS can have positive benefits. The sustained period of treatment is presumably central to these gains and PT1’s improvement is grossly similar to past work in large vessel stroke (Boggio et al., 2007; Lindenberg et al., 2010; Polanowska et al., 2013). The overall pattern of cognitive and neurophysiologic changes are all biologically plausible given the left lateral PFC focus, as this region is a convergence zone for aspects of attention, executive control, language, and learning/memory (Siddiqui et al., 2008). We cannot say with any certainty that 2 mA for 20 minutes or that 29 sessions are optimal dosage parameters, however, there is a general paucity of treatment parameters at this time and prior work has suggested the need for a more thorough consideration of individual differences (Esmaeilpour et al., 2017). In that respect, our individual computational model suggested a maximum electric field of about 0.5 V/m (with most current in the 0.3–0.4 range), but it is unclear whether this is optimal or whether higher (or lower) electric field would be more effective (see Esmaeilpour et al., 2017).
Relatedly, resting state fMRI findings demonstrated increased network strength at post-testing, specifically in dorsal attention and cingulo-opercular networks. The left lateral prefrontal cortex is involved in both of these networks (Dosenbach et al., 2008; Vossel et al., 2014), again supporting the biological plausibility of HD-tDCS effects. Further, as both of these networks are related to arousal, attention (specifically object oriented attention), and processing speed (Corbetta et al., 2008; Sadaghiani & D’Esposito, 2015), their increase in strength may help explain the observed cognitive improvement. The lack of findings in other networks (e.g. salience, fronto-parietal) and initial reduction in the ventral attention network also support the specificity of the effects, especially given past findings that the ventral attention system can be suppressed when the dorsal network is engaged (Corbetta et al., 2008).
The fact that nearly all changes regressed to baseline by the 10-week follow-up further supports the effects of HD-tDCS. Past work has been mixed in terms of the persistence of post-tDCS change (Elsner et al., 2016b; Pisegna et al., 2016; Elsner et al., 2019; Polanowska et al., 2013) so it appears that sustained HD-tDCS or booster sessions are needed to maintain effects. We note such effects are not unique to HD-tDCS as many medications for chronic conditions require ongoing use to be effective, and we advocate that HD-tDCS and other forms of neuromodulation be considered in a similar manner.
As mentioned, our 29 day session protocol is consistent with other multi-session studies (Boggio et al., 2007; Yang et al., 2012), and past work suggests that more sessions appear to provide additional benefit with no additional side effects (Nikolin et al., 2018). There is little evidence that small breaks in protocol, such as over weekends in PT1’s protocol, is detrimental to overall cognitive or behavioral effects. In fact, one study found greater working memory improvement between sessions that were separated by a two-day weekend than those separated by 24 hours (Au et al., 2016). Further, breaks spanning 24 hours to two weeks showed no change in outcomes or effect sizes in a previous meta-analysis (Dedoncker et al., 2016).
Limitations of the current study include those associated with a single case study; however, the rarity of PAL and associated mortality rates render large-scale studies unlikely. Likewise, far more work is needed to establish dose-response data across populations (e.g., Thair et al., 2017), which is the focus of our (BMH) ongoing triple blind RCT (NCT03875326). While we focused on the effects of HD-tDCS in isolation, it is entirely possible that combined non-pharmacologic (e.g., cognitive training + HD-tDCS) or pharmacologic (e.g., medication + HD-tDCS) approaches may be particularly effective, as suggested by the concept of functional targeting (Bikson et al., 2013) and prior evidence of pharmacologic enhancement (Batsikadze, et al., 2013; Kuo et al., 2016). Further, use of reliable alternative forms may be helpful in future studies in order to minimize stimulus specific practice effects. With these caveats in mind, our current findings provide some initial evidence supporting the benefits of HD-tDCS in a population lacking therapeutic options.
Acknowledgements
This work was supported by the NIH/NIA via R01AG058724 (to BMH), the Michigan Alzheimer’s Disease Research Center (MADRC) (P30AG053760), and an MADRC pilot grant (to BMH). The authors would like to thank Sean Ma, Ph.D., Oliver Calhoun, B.A., and Alina Lesnovskaya, B.A., for their assistance with this case.
Footnotes
Conflict of Interest Statement
The Authors declare that there is no conflict of interest.
References
- Anderson CA, & Arciniegas DB (2010). Cognitive sequelae of hypoxic-ischemic brain injury: a review. NeuroRehabilitation, 26, 47–63. 10.3233/NRE-2010-0535. [DOI] [PubMed] [Google Scholar]
- Au J, Katz B, Buschkuehl M, Bunarjo K, Senger T, Zabel C, Jaeggi SM, & Jonides J (2016). Enhancing working memory training with transcranial direct current stimulation. Journal of Cognitive Neuroscience, 28(9), 1419–1432. 10.1162/jocn_1_00979 [DOI] [PubMed] [Google Scholar]
- Batsikadze G, Paulus W, Kuo M, & Nitsche MA (2013). Effect of serotonin on paired associative stimulation-induced plasticity in the human motor cortex. Neuropsychopharmacology, 38, 2260–2267. 10.1038/npp.2013.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, & Sivan AB (1994). Multilingual Aphasia Examination: Manual of Instructions. Iowa City, IA: AJA Associates. [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A Component Based Noise Correction Method (CompCor) for BOLD and Perfusion Based fMRI. NeuroImage, 37, 90–101. 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, Mourdoukoutas AP, Kronberg G, Truong D, Boggio P, Brunoni AR, Charvet L, Fregni F, Fritsch B, Gillick B, Hamilton RH, Hampstead BM, Jankord R, Kirton A, … Woods AJ (2016). Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimulation, 9, 641–661. 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, & Jeffreys JG (2004). Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. Journal of Physiology, 557, 175–190. 10.1113/jphysiol.2003.055772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, & Rahman A (2013). Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Frontiers in Human Neuroscience, 7, 5. 10.3389/fnhum.2013.00688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, & Fregni F (2007). Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restorative Neurology and Neuroscience, 25, 123–129. [PubMed] [Google Scholar]
- Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, & Fregni F (2011). A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. International Journal of Neuropsychopharmacology, 14(8), 1133–1145. 10.1017/S1461145710001690 [DOI] [PubMed] [Google Scholar]
- Caine D, & Watson JDG (1998). Neuropsychological and neuropathological sequelae of cerebral anoxia: A critical review. Journal of the International Neuropsychological Society, 6, 86–99. 10.1017/S1355617700611116 [DOI] [PubMed] [Google Scholar]
- Chakraborty D, Truong DQ, Bikson M, & Kaphzan H (2018). Neuromodulation of axon terminals. Cerebral Cortex, 28(8), 2786–2794. 10.1093/cercor/bhx158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Coffman BA, Trumbo MC, & Gasparovic C (2011). Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: a (1)H magnetic resonance spectroscopy study. Neuroscience Letters, 500, 67–71. 10.1016/j.neulet.2011.05.244 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, & Shulman GL (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron, 58(3), 306–324. 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Holland P, Frens MA, & Donchin O (2016). Impact of transcranial direct current stimulation (tDCS) on neuronal functions. Frontiers in Neuroscience, 10: 550. 10.3389/fnins.2016.00550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedoncker J, Brunoni AR, Baeken C, & Vanderhasselt A (2016). The effect of the interval-between-sessions on prefrontal transcranial direct current stimulation (tDCS) on cognitive outcomes: a systematic review and meta-analysis. Journal of Neural Transmission, 123(10), 1159–1172. 10.1007/s00702-016-1558-x [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, & Petersen SE (2008). A dual-networks architecture of top-down control. Trends in Cognitive Science, 12, 99–105. 10.1016/j.tics.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner B, Kugler J, Pohl M, & Mehrholz J (2016a). Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database of Systematic Reviews, 3. 10.1002/14651858.CD009645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner B, Kufler J, Pohl M, & Merholz J (2016b). Transcranial direct current stimulation for improving spasticity after stroke: a systematic review with meta-analysis. Journal of Rehabilitation Medicine, 48(7), 565–570. 10.2340/16501977-2097 [DOI] [PubMed] [Google Scholar]
- Elsner B, Kugler J, Pohl M, & Mehrholz J (2019). Transcranial direct current stimulation (tDCS) for improving aphasia in adults with aphasia after stroke. Cochrane Database of Systematic Reviews, 5. 10.1002/14651858.CD009760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeilpour Z, Marangolo P, Hampstead BM, Bestmann S, Galletta E, Knotkova H, & Bikson M (2017). Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimulation, 11, 310–321. 10.1016/j.brs.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace J, Stout J, & Malloy P (1999). Assessing frontal behavior syndromes with the Frontal Lobe Personality Scale. Assessment, 6(3), 269–284. 10.1177/107319119900600307 [DOI] [PubMed] [Google Scholar]
- Hampstead BM, Brown GS, & Hartley JF (2014). Transcranial direct current stimulation modulates activation and effective connectivity during spatial navigation. Brain Stimulation 7, 314–324. 10.1016/j.brs.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Heinz UE, & Rollnik JD (2015). Outcome and prognosis of hypoxic brain damage patients undergoing neurological early rehabilitation. BMC Research Notes, 8, 243. 10.1186/s13104-015-1175-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Datta A, Bikson M, & Parra LC, (2019). Realistic volumetric-Approach to Simulate Transcranial Electric Stimulation -- ROAST -- a fully automated open-source pipeline. Journal of Neural Engineering, 16(5). 10.1088/1741-2552/ab208d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo JM, Kim Y, Ko M, Ohn SH, Joen B, & Lee KH (2009). Enhancing the working memory of stroke patients using tDCS. American Journal of Physical Medicine & Rehabilitation, 88(5), 404–409. 10.1097/PHM.0b013e3181a0e4cb [DOI] [PubMed] [Google Scholar]
- Kaski D, Dominguez RO, Allum JH, & Bronstein AM (2013). Improving gait and balance in patients with leukoaraiosis using transcranial direct current stimulation and physical training: an exploratory study. Neurorehabilitation and Neural Repair, 27, 864–871. 10.1177/1545968313496328 [DOI] [PubMed] [Google Scholar]
- Kim S, Stephenson MC, Morris PG, & Jackson SR (2014). tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: A 7 T magnetic resonance spectroscopy study. Neuroimage, 99, 237–243. 10.1016/j.neuroimage.2014.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy V, Kaundinya G, Brown GS, & Hampstead BM (2015). Resting-state fMRI reveals enhanced functional connectivity in spatial navigation networks after transcranial direct current stimulation. Neuroscience Letters, 604, 80–85. 10.1016/j.neulet.2015.07.042 [DOI] [PubMed] [Google Scholar]
- Kuo H, Bikson M, Datta A, Minhas P, Paulus W, Kuo M, & Nitsche MA (2013). Comparing cortical plasticity induced by conventional and high-definition 4×1 ring tDCS: a neurophysiological study. Brain Stimulation, 6, 644–648. 10.1016/j.brs.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Kuo H, Paulus W, Batsikadze G, Jamil A, Kuo M, & Nitsche MA (2016). Chronic enhancement of serotonin facilitates excitatory transcranial direct current stimulation-induced neuroplasticity. Neuropsychopharmacology, 41, 1223–1230. 10.1038/npp.2015.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. (2010). Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology, 75, 2176–2184. 10.1212/WNL.0b013e318202013a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio JJ, Growdon JH, & Corkin S (1995). Cognitive test performance in detecting, staging, and tracking Alzheimer’s disease. Archives of Neurology, 52(11), 1087–1099. 10.1001/archneur.1995.00540350081020 [DOI] [PubMed] [Google Scholar]
- Michels C (2004). Physiological and Pathological Responses to Hypoxia. American Journal of Pathology, 164, 1875–1882. 10.1016/S0002-9440(10)63747-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolin S, Huggins C, Martin S, Alonzo A, & Loo CK (2017). Safety of repeated sessions of transcranial direct current stimulation: a systematic review. Brain Stimulation, 11(2), 278–288. 10.1016/j.brs.2017.10.020 [DOI] [PubMed] [Google Scholar]
- O’Brien AT, Bertolucci F, Torrealba-Acosta G, Huerta R, Fregni F, & Thibaut A (2018). Non-invasive brain stimulation for fine motor improvement after stroke: a meta-analysis. European Journal of Neurology, 25(8), 1017–1026. 10.1111/ene.13643 [DOI] [PubMed] [Google Scholar]
- Otal B, Olma MC, Floel A, & Wellwood I (2015). Inhibitory non-invasive brain stimulation to homologous language regions as an adjunct to speech and language therapy in post-stroke aphasia: a meta-analysis. Frontiers of Human Neuroscience, 9. 10.3389/fnhum.2015.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneri B, Khadka N, Patel V, Thomas C, Tyler W, Parra LC, & Bokson M (2016). The tolerability of transcranial electrical stimulation used across extended periods in a naturalistic context by healthy individuals. Brain Stimulation, 9, 740–754. 10.1016/j.brs.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier SJ, & Cicchetti F (2015). Cellular and Molecular Mechanisms of Action of Transcranial Direct Current Stimulation: Evidence from In Vitro and In Vivo Models. International Journal of Neuropsychopharmacology, 18, 1–13. 10.1093/ijnp/pyu047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter L, Nighoghossian N, Jouvet A, Derex L, Hermier M, Philippeau F, Honnorat J, & Trouillas P (2004). Delayed post-anoxic leukoencephalopathy. Revue Neurologique, 160, 1085–1088. 10.1016/s0035-3787(04)71148-0 [DOI] [PubMed] [Google Scholar]
- Pisegna JM, Kaneoka A, Pearson WG, Kumar S, & Langmore SE (2016). Effects of non-invasive brain stimulation on post-stroke dysphagia: A systematic review and meta-analysis of randomized controlled trials. Clinical Neurophysiology, 127(1), 956–968. 10.1016/j.clinph.2015.04.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polania R, Paulus W, Antal A, & Nitsche MA (2011). Introducing graph theory to track for neuroplastic alterations in the resting human brain: A transcranial direct current stimulation study. Neuroimage, 54, 2287–2296. 10.1016/j.neuroimage.2010.09.085 [DOI] [PubMed] [Google Scholar]
- Polania R, Paulus W, & Nitsche MA (2012). Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Human Brain Mapping, 33, 2499–2508. 10.1002/hbm.21380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanowska KE, Lesniak MM, Seniow JB, Czepiel W, & Czlonkowska A (2013). Anodal transcranial direct current stimulation in early rehabilitation of patients with post-stroke non-fluent aphasia: a randomized, double-blind, sham-controlled pilot study. Restorative Neurology and Neuroscience, 31, 761–71. 10.3233/RNN-130333 [DOI] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, & Petersen SE (2013). Evidence for hubs in human functional brain networks. Neuron, 79(4), 798–813. 10.1016/j.neuron.2013.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, & Bikson M (2013). Cellular effects of acute direct current stimulation: Somatic and synaptic terminal effects. Journal of Physiology, 591, 2563–2578. 10.1113/jphysiol.2012.247171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C (1998). Repeatable battery for the assessment of neuropsychological status manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Reckow J, Rahman-Filipiak A, Garcia S, Schlaefflin S, Calhoun O, DaSilva AF, Bikson M, & Hampstead BM: Tolerability and blinding of 4×1 High-Definition transcranial direct current stimulation (HD-tDCS) at two and three milliamps. Brain Stimulation, 11, 991–997, 2018. 10.1016/j.brs.2018.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Lafon B, Parra LC, & Bikson M (2017). Direct current stimulation boosts synaptic gain and cooperativity in vitro. The Journal of Physiology, 595(11), 3535–3547. 10.1113/jp273005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM (1958). The validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills, 8, 271–276. 10.2466/pms.1958.8.3.271 [DOI] [Google Scholar]
- Richardson J, Datta A, Dmochowski J, Parra LC, & Fridriksson J (2015). Feasibility of using high-definition transcranial direct current stimulation (HD-tDCS) to enhance treatment outcomes in persons with aphasia. Neurorehabilitation, 36, 115–126. 10.3233/NRE-141199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, & D’Esposito M (2015). Functional Characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cerebral Cortex, 25(9), 2763–2773. 10.1093/cercor/bhu072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar R, & Dubow J (2012). Delayed posthypoxic leukoencephalopathy following a morphine overdose. Journal of Clinical Neuroscience, 19, 1060–1062. 10.1016/j.jocn.2012.01.001 [DOI] [PubMed] [Google Scholar]
- Samani MM, Agboada D, Jamil A, Kuo M, & Nitsche MA (2019). Titrating the neuroplastic effects of cathodal transcranial direct current stimulation (tDCS) over the primary motor cortex. Cortex, 119, 350–361. 10.1016/j.cortex.2019.04.016 [DOI] [PubMed] [Google Scholar]
- Shahid SS, Bikson M, Salman H, Wen P, & Ahfock T (2014). The value and cost of complexity in predictive modelling: role of tissue anisotropic conductivity and fibre tracts in neuromodulation. Journal of Neural Engineering, 11(3). https://doi/org/10.1088/1741-2560/11/3/036002 [DOI] [PubMed] [Google Scholar]
- Shaker HA, Sawan SAE, Fahmy EM, Ismail RS, & Elrahman SAEA (2018). Effect of transcranial direct current stimulation on cognitive function in stroke patients. The Egyptian Journal of Neurology, Psychiatry, and Neurosurgery, 54(32). 10.1186/s41983-018-0037-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprecher D, & Mehta L (2010). The syndrome of delayed post-hypoxic leukoencephalopathy. NeuroRehabilitation, 26, 65–72. [PMC free article] [PubMed] [Google Scholar]
- Siddiqui SV, Chatterjee U, Kumar D, Siddiqui A, & Goyal N (2008). Neuropsychology of prefrontal cortex. Indian Journal of Psychiatry 50(3), 202–208. 10.4103/0019-5545.43634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smania N, Avesani R, Roncari L, Ianes P, Giradi P, Varalta V, Gambini MG, Fiaschi A, & Gandolfini M (2011). Factors predicting functional and cognitive recovery following severe traumatic, anoxic, and cerebrovascular brain damage. The Journal of Head Trauma Rehabilitation, 28(2), 131–140. 10.1097/HTR.0b013e31823c0127 [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA (2011). Physiological basis of transcranial direct current stimulation. The Neuroscientist, 17, 37–53. 10.1177/1073858410386614 [DOI] [PubMed] [Google Scholar]
- Suh HS, Lee WH, & Kim T (2012). Influence of enisotropic conductivity in the skull and white matter on transcranial direct current stimulation via an anatomically realistic finite head model. Physics in Medicine & Biology, 57(21), 6961–6981. 10.1088/0031-9155/57/21/6961 [DOI] [PubMed] [Google Scholar]
- Thacker AK, Asthana AB, Sakari NBS (1995). Delayed post-anoxic encephalopathy. Postgraduate Medical Journal, 71, 373–374. 10.1136/pgmj.71.836.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thair H, Holloway AL, Newport R, & Smith AD (2017). Transcranial direct current stimulation (tDCS): A beginner’s guide for design and implementation. Frontiers in Neuroscience, 11, 1–13. 10.3389/fnins.2017.00641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco Triccas L, Burridge JH, Hughes AM, Pickering RM, Desikan M, Rothwell JC, & Verheyden G (2015). Multiple sessions of transcranial direct current stimulation and upper extremity rehabilitation in stroke: a review and meta-analysis. Clinical Neurophysiology, 127(1), 946–955. 10.1016/j.clinph.2015.04.067 [DOI] [PubMed] [Google Scholar]
- Valiengo LCL, Goulart AC, de Oliveira JF, Bensenor IM, Lotufo PA, & Brunoni AR (2017). Transcranial direct current stimulation for the treatment of post-stroke depression: Results from a randomized, sham-controlled, double-blinded trial. Journal of Neurology, Neurosurgery and Psychiatry, 88(20), 170–175. 10.1136/jnnp-2016-314075 [DOI] [PubMed] [Google Scholar]
- Victor M, & Ropper AH (2001). Principles of Neurology (7th edition). New York, NY: McGraw-Hill Professional. [Google Scholar]
- Vossel S, Geng JJ, & Fink GR (2014). Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist, 20(2), 150–159. 10.1177/107385841349426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wu D, Cheng Y, Song W, Yuan Y, Zhang X, Zhang D, Zhang T, Wang Z, Tang J, & Yin L (2019). Effects of transcranial direct current stimulation on apraxia of speech and cortical activation in patients with stroke: a randomized sham-controlled study. American Journal of Speech-Language Pathology, 28(4), 1625–1637. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Alderman N, Burgess PW, Emslie H, & Evans JJ (1996). Behavioural assessment of the dysexecutive syndrome: test manual. Edmunds, England: Thames Valley Test Company. [Google Scholar]
- Yang EJ, Baek S, Shin J, Lim JY, Jang HJ, Kim YK, & Paik NJ (2012). Effects of transcranial direct current stimulation (tDCS) on post-stroke dysphagia. Restorative Neurology and Neuroscience, 30(4), 303–311. 10.3233/RNN-2012-110213 [DOI] [PubMed] [Google Scholar]
- Yesavage A, Brink TL, Rose TL, Lum O, Huang V, Adey M, & Leirer WO (1983). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17, 37–49. 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- Yun GJ, Chun MH, & Kim BR (2015). The effects of transcranial direct-current stimulation on cognition in stroke patients. Journal of Stroke, 17(3), 354–358. 10.5853/jos.2015.17.3.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora CA, Nauen D, Hynecek R, Ilica A, Izbudak I, Sair HI, Gujar SK, & Pillai JJ (2015). Delayed posthypoxic leukoencephalopathy: a case series and review of the literature. Brain and Behavior, 5(8), e00364. 10.1002/brb3.364 [DOI] [PMC free article] [PubMed] [Google Scholar]