Abstract
BACKGROUND:
Human cytomegalovirus (HCMV) infection is associated with renal allograft dysfunction and loss, particularly in combination with acute rejection. Emerging literature suggests that non-HLA antibodies may contribute to antibody-mediated rejection, but pathogen-induced antibodies have not been investigated in this context. This study examines the presence of CMV-induced antibodies in murine CMV (MCMV) infected renal allografts during acute rejection.
METHODS:
Intragraft IgG and complement C3 immunostaining were compared among allogeneic MCMV D−/R−, D+/R−, and D+/R+ renal transplants. Intragraft antibody deposition was examined in B cell deficient (μMT) recipients treated with MCMV immune sera. Antibody binding and complement dependent cytotoxicity (CDC) of D−/R− and D+/R+ sera against infected renal tubular epithelial cells (TECs) were measured in-vitro. IgG immunostaining was performed in D+/R+ allografts and native kidneys, and in D+/R− allografts treated with ganciclovir to inhibit viral replication.
RESULTS:
D+/R− and D+/R+ transplants had more abundant IgG and C3 deposition compared to D−/R− recipients. Greater IgG deposition was associated with more severe allograft injury in μMT recipients treated with MCMV immune sera compared to nonimmune sera. D+/R+ sera induced greater CDC of infected TECs compared to D−/R− sera. Native kidneys had lower IgG deposition compared to allografts, despite similar organ viral loads. Ganciclovir-treated allografts had reduced IgG deposition compared to untreated allografts.
CONCLUSIONS:
In this murine model, complement-fixing antibodies can deposit into MCMV-infected renal allografts, are associated with allograft damage, and can induce CDC of MCMV-infected renal TECs. The allogeneic response and viral replication may also contribute to intragraft antibody deposition.
INTRODUCTION
Cytomegalovirus (CMV) infection is associated with adverse effects in renal transplantation.1 The “direct effects” of CMV are caused by viral reactivation, replication (DNAemia), and CMV end-organ disease. The “indirect effects” of CMV include associations with acute rejection and late graft loss; virus-induced systemic immunomodulation conferring increased susceptibility to bacterial, fungal, and other viral infections; post-transplant vasculopathy; new onset diabetes after transplantation; and other transplant co-morbidities.1–5 Human CMV (HCMV) positive serostatus is associated with inferior graft outcome, with the highest graft loss observed among HCMV seropositive patients with acute rejection (AR) episodes.6–9 Patients who develop HCMV DNAemia are also more likely to experience graft dysfunction and loss compared to those without DNAemia, suggesting that viral reactivation or replication may contribute to graft dysregulation.10–14 HCMV antigens can be identified in acutely rejecting allografts, as well as those explanted due to graft failure.15–17 Antiviral treatment with ganciclovir or valganciclovir to prevent HCMV disease (prophylaxis or pre-emptive therapy) is associated with improved graft survival and reduced interstitial fibrosis and tubular atrophy, suggesting that inhibition of viral replication may be beneficial for graft outcome.18–21 However, the exact mechanisms by which HCMV might contribute to renal allograft dysfunction remain incompletely understood.
Animal models for CMV pathogenesis have been utilized to demonstrate essential immunologic and host-pathogen interactions. In rodent renal transplant models, rat CMV (RCMV) or murine CMV (MCMV) infection are associated with increased inflammation and accelerated graft injury compared to uninfected allografts.22–26 In rat kidney transplants, RCMV infection interferes with tolerance induced by anti-CD4 monoclonal antibodies, and increases both antiviral and alloreactive T cell responses resulting in chronic allograft damage.27 In the murine model, MCMV reactivation from latency in the donor kidney is induced after allogeneic transplantation via cytokines such as tumor necrosis factor-α and by ischemia-reperfusion injury.28–31 MCMV infection of the donor allograft exacerbates intragraft infiltration of CD8+ T cells, macrophages, neutrophils, and natural killer (NK) cells.26,32 Ganciclovir administration ameliorates MCMV-associated allograft injury, supporting a role for viral reactivation in the pathogenesis of allograft damage.26
Acute rejection can be precipitated by T cell mediated rejection (TCMR) and/or antibody mediated rejection (AMR).33–37 AMR is known to be mediated by donor specific antibodies (DSA) directed against HLA antigens. However, circulating DSAs are sometimes not found during episodes of AMR, raising the possibility that non-HLA antibodies might contribute to AMR.38–40 Recent literature suggests that patients who have antibodies directed against self-proteins, such as angiotensin-II type-1 receptor, MHC class I-related chain A, and endothelin type A receptor, may also have adverse kidney transplant outcomes.38,41–48 The role of pathogen-induced antibodies in AMR has not been explored. Although pathogen-induced antibodies serve important functions in host protection from infectious diseases, deposition of these antibodies could conceivably occur in the pathologic setting of allograft rejection, particularly for viruses infecting the transplant organ such as HCMV. Anti-HCMV antibody titers vary among kidney transplant recipients and correlate with CMV DNAemia, indicating that antiviral antibody quantities differ among individuals and increase after viral reactivation, even in the immunocompromised host.49 The aim of the present study was to examine whether pathogen-induced antibodies can be detected in acutely rejecting allografts, using murine CMV (MCMV) as a model pathogen that infects donor kidneys.
MATERIALS AND METHODS
Animals and viruses
BALB/cJ (“BALB/c”), C57BL/6J (“B6”), and C57BL/6-Igh-6tm1Cgn (“μMT”) mice were purchased from Jackson Laboratory (Bar Harbor, ME). The μMT mice have a targeted gene deletion in the μ-chain resulting in deficiency of mature B cells and absence of detectable IgG in serum.50 Mice were maintained in animal facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care under the Animal Resources Program of the University of Alabama at Birmingham (UAB, Birmingham AL) or the Animal Resources Core of The Abigail Wexner Research Institute at Nationwide Children’s Hospital (AWRI, Columbus OH) under specific pathogen-free conditions. Murine experimental protocols were approved by the Institutional Animal Care and Use Committees at UAB and AWRI.
MCMV strain Smith deleted for open reading frame (ORF) m157 (“MCMVΔ157”), which infects BALB/c and B6 mice similarly, and MCMV-GFP viruses were provided by Ulrich Koszinowski (Maz von Pettenkofer-Institute, Ludwig Maximilians-University, Munich Germany).51,52 Virus stocks were propagated using murine embryonic fibroblasts (MEFs) as described, frozen in aliquots at −80°C and discarded after single use.26
Murine kidney transplantation
Donor and recipient mice were infected by intraperitoneal (IP) injection with 104 plaque-forming units (PFU) MCMVΔ157 at least 12 weeks prior to renal transplantation (D+ or R+ transplants).26,32,53 Kidney transplantation was performed using vessel and bladder cuffs according to published techniques, with one or both native kidneys retained to maintain renal function post-transplant.26,54 BALB/c (allogeneic) or C57BL/6 (syngeneic) kidneys were transplanted into either B6 or μMT recipients. For some experiments, recipients were treated with cyclosporine (Novartis Pharmaceuticals, Cambridge MA) at 10 mg/kg/day subcutaneously (SC), once daily until terminal sacrifice. Each experimental transplant cohort consisted of 3–6 mice per group.
Tissues stored from a previously published ganciclovir-treated cohort were also analyzed for this study.26 This group was treated with cyclosporine and with ganciclovir (GCV, Genentech, Inc., South San Francisco CA) at 15 mg/kg/day SC once daily for 14 days, and sacrificed at either day 14 or day 21 (n=4/group).
Immunofluorescence analysis of kidney sections
Recipients were sacrificed, perfused to organ pallor with ice-cold saline, and organs were processed for formalin fixation and paraffin embedding (FFPE). Kidney sections were deparaffinized, blocked with goat serum, and incubated with either AlexaFluor-488 conjugated goat anti-mouse IgG antibodies (Invitrogen, Carlsbad, CA) or FITC-conjugated goat anti-mouse complement C3 antibodies (MP Biomedicals LLC, Solon, OH), counterstained with DAPI and mounted using ProLong anti-fade reagent (Invitrogen, Carlsbad, CA). For each experiment, images were collected under identical exposure times and gain using an Olympus BX51 fluorescence microscope and Olympus image processing software. AF488- or FITC-positive immunofluorescence signals per high power field (HPF) were quantified using ImageJ (NIH, https://imagej.net). For large, confluent areas of AF488- or FITC-positive staining, positive signals were quantitated by counting the number of DAPI-positive nuclei within or immediately adjacent to each AF488 or FITC-positive area. For each slide, 10 HPF were quantified (5 cortex, 5 medulla) and the average number of positive signals per HPF calculated for each sample. Kidneys from non-transplant B6 mice were procured and processed in identical fashion as staining controls.
Passive transfer of MCMV antisera in μMT transplants
To generate anti-MCMV antisera, B6 mice were infected with 106 PFU of MCMVΔ157 IP on day 1, boosted with 105 PFU MCMVΔ157 at days 14 and 21, sacrificed at day 24, and MCMV immune sera collected, pooled and analyzed by ELISA (“MCMV+ sera”). Sera from uninfected B6 mice were also collected and pooled (“MCMV− sera”). Recipient μMT mice were either untreated (no sera) or injected at day +1 post-transplant with 200 μl of MCMV− or MCMV+ sera IP and sacrificed at post-transplant day 3 or 14.
ELISA for anti-MCMV antibodies
96 well EIA/RIA plates (Costar, Corning NY) were coated with MCMV-infected MEF cell lysate, blocked with goat serum, and incubated with 2-fold serial dilutions of B6 MCMV− sera, MCMV+ sera, or transplant sera (D−/R−, D+/R−, D+/R+). Plates were developed using HRP-conjugated goat anti-mouse IgG antibodies and TMB substrate solution (Pierce Biotechnology, Rockford IL). The optical density was measured with an ELX808 Ultra Microplate Reader (BioTek Instruments, Winooski, VT) at 450 nm wavelength.
Western blotting of kidney lysates
Portions of kidneys were snap-frozen at −80°C on the day of sacrifice. Tissues were homogenized in RIPA lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 2mM EDTA, 1% NP-40, 0.1% SDS) containing protease inhibitor cocktail (Pierce Biotechnology). For each sample, 20 μg protein were separated by 10% SDS-PAGE, and western blot performed using HRP-conjugated goat anti-mouse IgG (H+L chain) and West Pico reagent (Pierce Biotechnology). Membranes were stripped and re-probed using a rabbit anti-actin antibody (Sigma-Aldrich, St. Louis MO), HRP-conjugated goat anti-rabbit-IgG secondary antibody, and West Pico reagent. Band intensities were quantitated by densitometry using ImageJ.
Histopathology
FFPE transplant and native kidney sections were stained with hematoxylin and eosin (H&E). Histopathology was scored in blinded fashion by veterinary pathologists (T.R.S. or R.C.), using a published scale of 8 pathologic criteria (range 0–3, maximum score 24) reflecting criteria used for clinical allograft rejection.32,53,55 Concordance of scoring between the pathologists was confirmed using pathologic samples previously utilized in other studies.32,53
Renal epithelial cell isolation
Murine primary renal tubular epithelial cells (TECs) were isolated using selective media as described, with minor modifications.56 In brief, B6 or BALB/c kidneys were minced in Dulbecco’s Modification of Eagle’s Medium (Mediatech Inc., Manassas VA) with Ham’s F12 (Cellgro, Herndon VA) at 1:1 ratio, dissociated using collagenase IV at 1mg/ml (Worthington Biochemical Corp., Lakewood NJ), homogenized by needle disruption and propagated on plates coated with bovine collagen I at 1 μg/ml (Advanced BioMatrix, Carlsbad CA) using complete epithelial cell media (Cell Biologics, Chicago IL).
Antibody binding assay
B6 TECs, BALB/c TECs or BALB/c 3T3 cells (American Type Culture Collection, Manassas VA) were infected with MCMV-GFP virus at a multiplicity of infection (MOI) of 1 for each cell type. At 72 hours post infection, cells were detached and incubated with either MCMV− or MCMV+ B6 sera, or with transplant sera (D−/R− or D+/R+) at 1:20 dilution for 1 hour on ice. MCMV uninfected cells were also incubated with sera. Cells were washed and stained for viability (Ghost Dye UV450, TONBO Biosciences, San Diego CA), EpCAM-PE (CD326, Clone G8.8, Biolegend, San Diego CA), and anti-mouse IgG1-APC (Clone A85–1, BD Biosciences, San Jose CA). Samples were analyzed using a LSRFortessa (BD Biosciences) and FlowJo v10 software (TreeStar Inc, Ashland OR). Experiments were performed 3 times independently.
Complement dependent cytotoxicity assay
BALB/c TECs or 3T3 cells were infected with MCMVΔ157 at MOI of 1 for 72 hours, then incubated with B6 MCMV− sera, MCMV+ sera, or transplant sera at 1:20 dilution, with or without rabbit complement at 1:10 dilution (Cedarlane, Burlington Ontario, Canada). Control wells were treated with no sera/no complement, or with complement alone. After incubation at 37°C for 3 hours, media was replaced with 50 μl Fluorobrite DMEM (Gibco Inc., Gaithersburg MD) containing 7-AAD at 1:50 dilution (BD Biosciences, San Jose CA) for 15 minutes. Images were acquired and quantitated using the EVOS FL Auto 2 cell imaging system and software (Thermo Fisher Scientific, Waltham MA). Experiments were performed 3 times independently.
MCMV quantitative DNA PCR
MCMV viral loads were quantified from D+/R+ transplant and native kidneys as previously described.26,57 Briefly, total DNA from snap-frozen tissue was isolated using the Qiagen QiaAMP DNA Kit (Qiagen, Germantown MD), quantitative DNA PCR performed in triplicate for each sample using an ABI StepOnePlus Real-Time PCR cycler (Applied Biosystems, Waltham MA), and copy number calculated by comparison to plasmid-derived standards. Viral loads were depicted as copies per gram tissue.
CD45+ cellular infiltrates by flow cytometry
At day 14 post-transplant, D+/R+ allografts and native kidneys were mechanically disrupted and digested with 0.05 μg/ml collagenase A (Roche, Indianapolis IN) for 30 min at 37°C. After blocking with anti-mouse CD16/CD32 (clone 93, eBiosciences, San Diego CA), cells were stained with propidium iodide and CD45-FITC (clone 30-F11, eBiosciences) and analyzed by flow cytometry using a dual laser FACSCalibur (BD Biosciences) and FlowJo software.
Statistical Analysis
Groups were compared using Student’s t-test or one-way analysis of variance with Tukey’s multiple comparison test. Continuous variables were compared using linear regression. Statistically significant differences were accepted at a p-value of <0.05. Results were depicted as mean±standard deviation (SD). All statistical testing was performed using Prism 7.0 (GraphPad, San Diego CA).
RESULTS
IgG and complement component C3 deposit into MCMV infected allografts.
We have previously shown that MCMV infection accelerates renal allograft injury compared to uninfected grafts, and is associated with T cell and NK cell infiltrates consistent with T cell mediated rejection.26 Antibody mediated rejection (AMR) was not previously assessed in this model. To identify AMR, IgG deposition was examined by immunofluorescence staining of D−/R− and D+/R− allografts without immunosuppression at day 7 post-transplant. FFPE sections were stained with goat anti-mouse IgG-AF488 (Fig. 1A), and positive staining quantitated using Image J (Fig. 1B). IgG staining was detected in both D−/R− and D+/R− allografts and was more abundant in D+/R− grafts (157±28 vs. 36±10 spots/HPF, p=0.0426). D+/R− grafts had greater IgG immunostaining in the medulla (Fig. 1A, lower panel) compared to the cortex (Fig. 1A, upper panel). Non-transplant B6 kidney controls showed no IgG staining (Fig. 1A, left panel; Fig. 1B).
FIGURE 1.
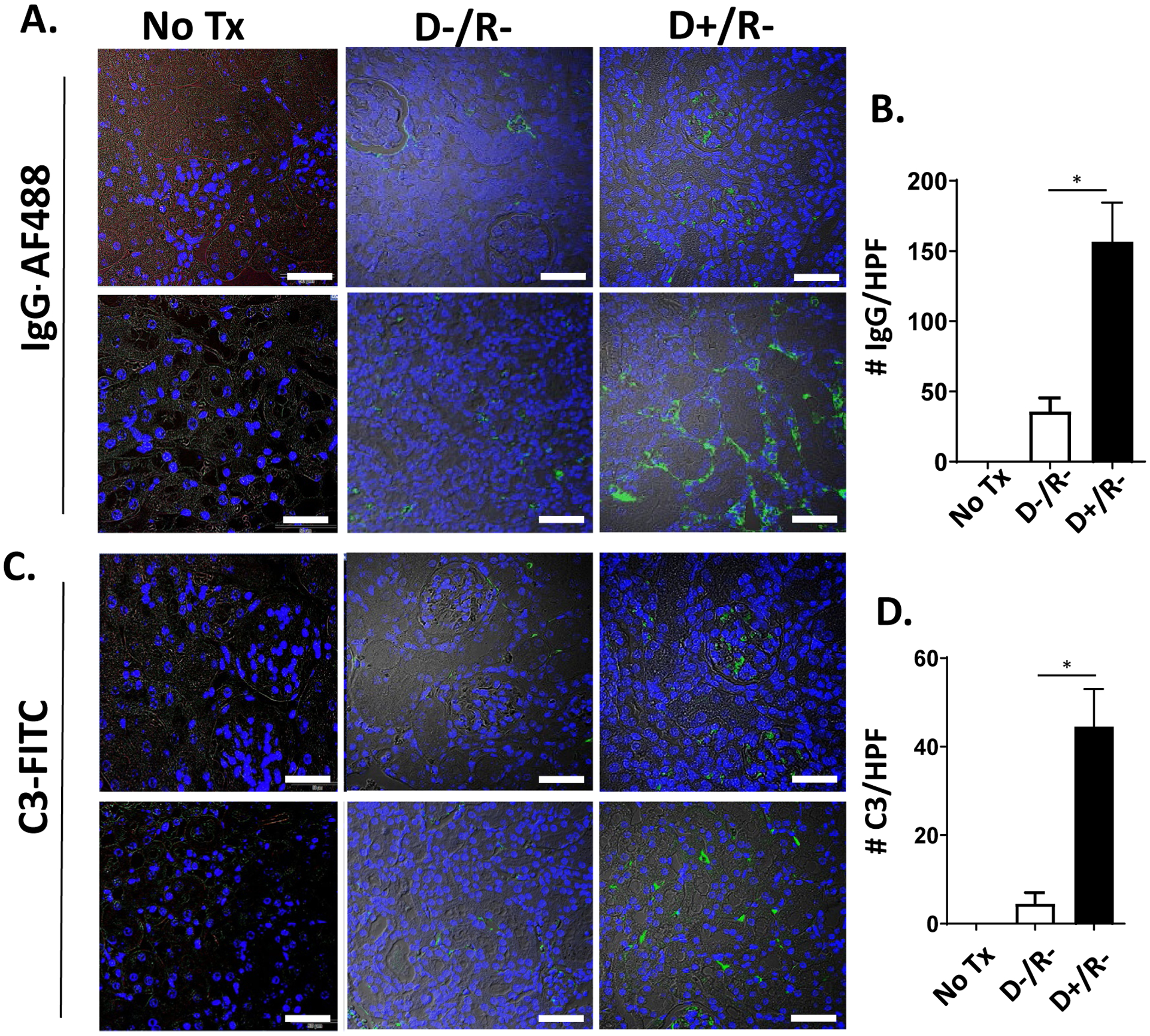
IgG and C3 staining of CMV infected and uninfected renal allografts.
(A, C) Allografts of MCMV D−/R− and D+/R− transplants, without immunosuppression, were fixed at post-transplant day 7 and stained with IgG-AF488 (A) or C3-FITC (C). Kidneys from non-transplant C57BL/6 mice (“No Tx”) served as staining controls (left panels). Representative images of cortex (upper panels) and medulla (lower panels) are shown, with brightfield overlay to orient tissue morphology (60x; white bar=50 μm). (B, D) Fluorescence immunostaining for IgG (B) or C3 (D) was quantitated for 10 high power fields (HPF) per kidney using Image J software. Average positive staining was calculated for each graft and compared among the groups (n=3/group).
* p<0.05.
The detection of the complement split product C4d is a diagnostic criterion for the histopathologic assignment of AMR in clinical transplant biopsies.55,58 However, attempts to quantify C4 deposition in the murine allografts by western blotting and immunofluorescent staining were not successful. Therefore, immunofluorescent staining for the complement split product C3 was performed (Fig. 1C), which showed no staining in control (non-transplant) B6 kidneys, minimal staining in D−/R− grafts, and greater staining in the D+/R− grafts (5±3 spots/HPF vs. 45±12 spots/HPF, p=0.0457)(Fig. 1D), with more abundant staining in the medulla (lower panel) compared to the cortex (upper panel). Together, results of immunostaining for IgG and C3 indicate that this model of acute rejection includes findings consistent with AMR in the MCMV infected allografts.
Passively transferred antibodies from MCMV immune sera deposit into MCMV infected allografts of μMT mice
Although IgG deposition was evident in D+/R− transplants, immunofluorescent staining could not distinguish whether these were alloantibodies or antiviral antibodies. To investigate this question, B cell deficient (μMT) recipients were used to prevent alloantibody generation after transplant.50 Pooled sera from MCMV uninfected (MCMV− Ig) or MCMV infected (MCMV+ Ig) B6 mice were compared by ELISA against MCMV-infected MEF cell lysates and confirmed the presence of anti-MCMV antibodies in pooled B6 MCMV+ Ig (Fig. 2A). Binding characteristics of these sera to syngeneic or allogeneic target cells ex-vivo were shown to be similar in later experiments, indicating no significant differences in auto- or allo-antibody quantities between these pooled sera. D+/R− transplants using μMT recipients without immunosuppression were treated with either no sera (“no Ig”), sera from uninfected B6 mice without MCMV antibodies (MCMV− Ig), or sera from MCMV infected B6 mice (MCMV+ Ig) at post-transplant day 1 and sacrificed at either post-transplant day 3 or day 14.
FIGURE 2.
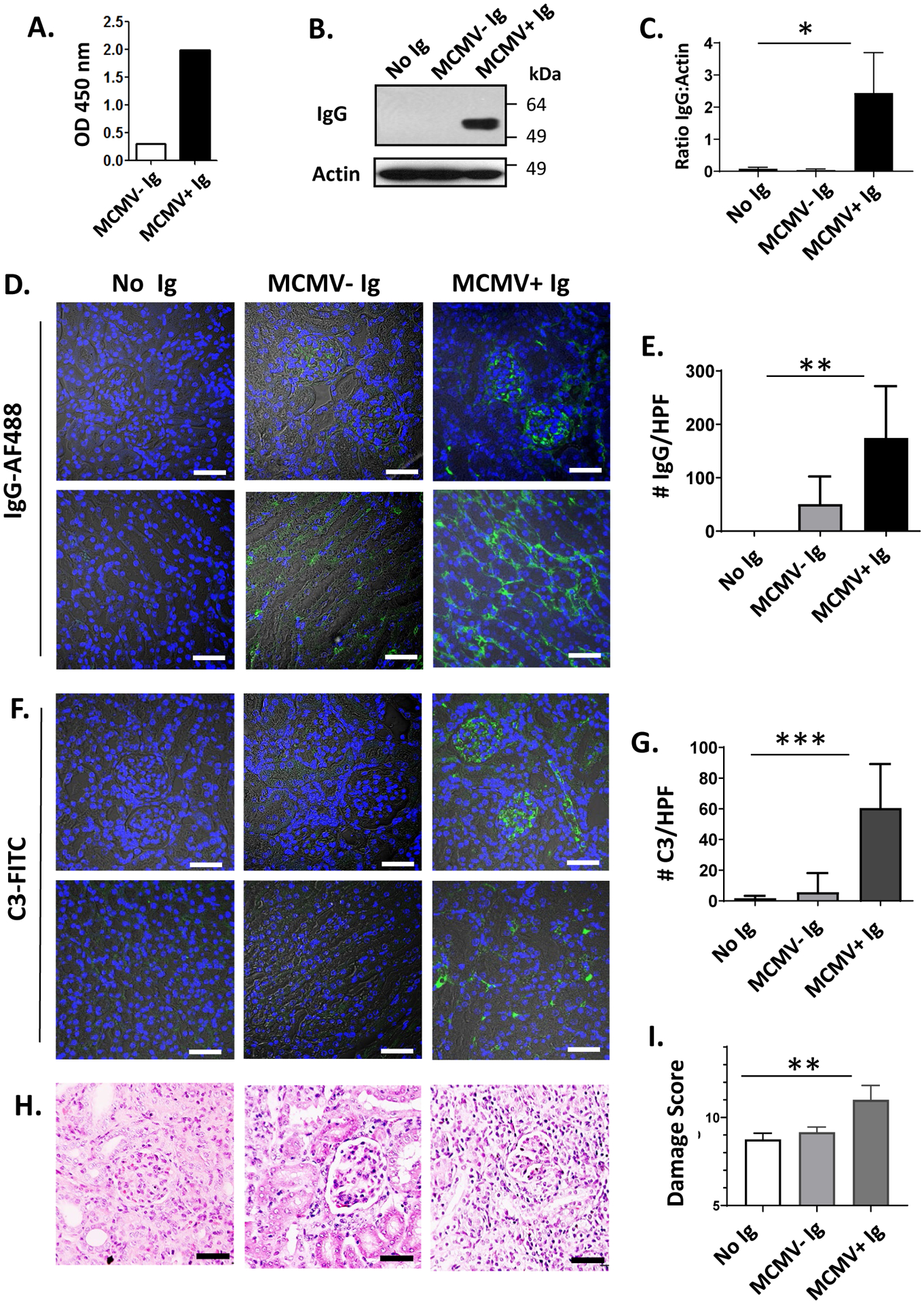
Adoptive transfer of MCMV immune or nonimmune sera into D+/R− μMT transplants.
(A) Sera from uninfected (MCMV− Ig) or MCMV infected (MCMV+ Ig) C57BL/6 mice were pooled and anti-MCMV antibody titers measured by ELISA at 450 nm optical density (O.D.). (B-I). D+/R− transplants were performed using μMT recipients, which were treated post-transplant with no Ig, MCMV− Ig, or MCMV+ Ig (n=3/experimental group). (B, C) Western blots of allograft lysates at post-transplant day 3 were probed using anti-mouse IgG and anti-actin antibodies (B), and the IgG:actin ratio quantitated (C) using Image J. (D-G) Allografts from μMT recipients at post-transplant day 3 were stained for IgG-AF488 (D) or C3-FITC (F). Representative images of cortex (upper panels) and medulla (lower panels) are shown, with brightfield overlay to orient tissue morphology (60x; white bar=50 μm). Immunostaining for IgG (E) or C3 (G) was quantitated using Image J as in Figure 1, and compared between groups receiving no Ig, MCMV− Ig, or MCMV+ Ig. (H-I) Allografts at day 14 post-transplant were stained using hematoxylin and eosin (H) and damage scores quantitated (I).
* p<0.05; ** p<0.01; *** p<0.001.
At day 3, allograft lysates were analyzed by western blot for IgG deposition (Fig. 2B) and showed no intragraft IgG in μMT recipients receiving no Ig or MCMV− Ig, whereas MCMV+ Ig-treated μMT recipients had significantly greater intragraft IgG deposition by densitometry (Fig. 2C, p=0.0107). Intragraft IgG-AF488 immunostaining was absent in μMT recipients receiving no sera (Fig. 2D, left column), sparsely detected in allografts treated with MCMV− Ig (Fig. 2D, middle column), and detected in allografts from MCMV+ Ig treated μMT recipients (Fig. 2D, right column) at significantly higher levels (Fig. 2E, p=0.0016). C3-FITC immunostaining showed similar results (Fig. 2F), with MCMV+ Ig treated allografts showing greater staining compared to the other groups (Fig. 2G, p=0.0005). Allograft histopathology scores at day 14 post-transplant (Fig. 2H–I) showed similar damage scores for allografts treated with no Ig or MCMV− Ig (8.75±0.25 vs. 9.17±0.17, p=n.s.), but more severe damage in MCMV+ Ig treated grafts (11.00±0.41, p=0.0081). Together, these findings indicate that the deposition of antibodies derived from B6 MCMV+ Ig is sufficient to result in complement component C3 fixation in MCMV infected allografts and is associated with more severe histopathologic allograft injury.
IgG deposition in immunosuppressed mice.
To test whether immunosuppression influences the intragraft deposition of MCMV-induced antibodies, day 14 allografts from cyclosporine-treated D−/R− and D+/R− transplants were stained for IgG (Fig. 3A) and showed less abundant IgG deposition compared to non-immunosuppressed mice at day 7 (Fig. 1), indicating that humoral responses were impaired by cyclosporine. Next, IgG immunostaining was examined in a cohort of immunosuppressed MCMV D+/R+ transplants (Fig. 3A), so that recipients had pre-transplant anti-MCMV immunity, and showed significantly greater IgG deposition compared to D−/R− and D+/R− transplants (Fig. 3B, p=0.0002). Similar to transplants without immunosuppression, immunostaining was more abundant in the medulla (lower panels) compared to the cortex (upper panels). Staining controls of non-transplant B6 kidneys showed no IgG immunostaining (Fig. 3A, left panel). Sera from immunosuppressed D−/R−, D+/R−, and D+/R+ recipients were analyzed by ELISA for anti-MCMV antibodies (Fig. 3C), with pooled B6 MCMV− Ig and MCMV+ Ig included as negative and positive controls. Anti-MCMV antibodies were less abundant in D+/R− mice compared to both D+/R+ and MCMV+ Ig (p<0.0001). A positive correlation was observed between the ELISA O.D. of D+/R− or D+/R+ sera and the quantity of intragraft IgG immunostaining for D+/R− and D+/R+ transplants (R2=0.82) (Fig. 3D), indicating that the intensity of antibody deposition in allografts correlated with quantities of anti-MCMV antibodies in recipient blood.
FIGURE 3.
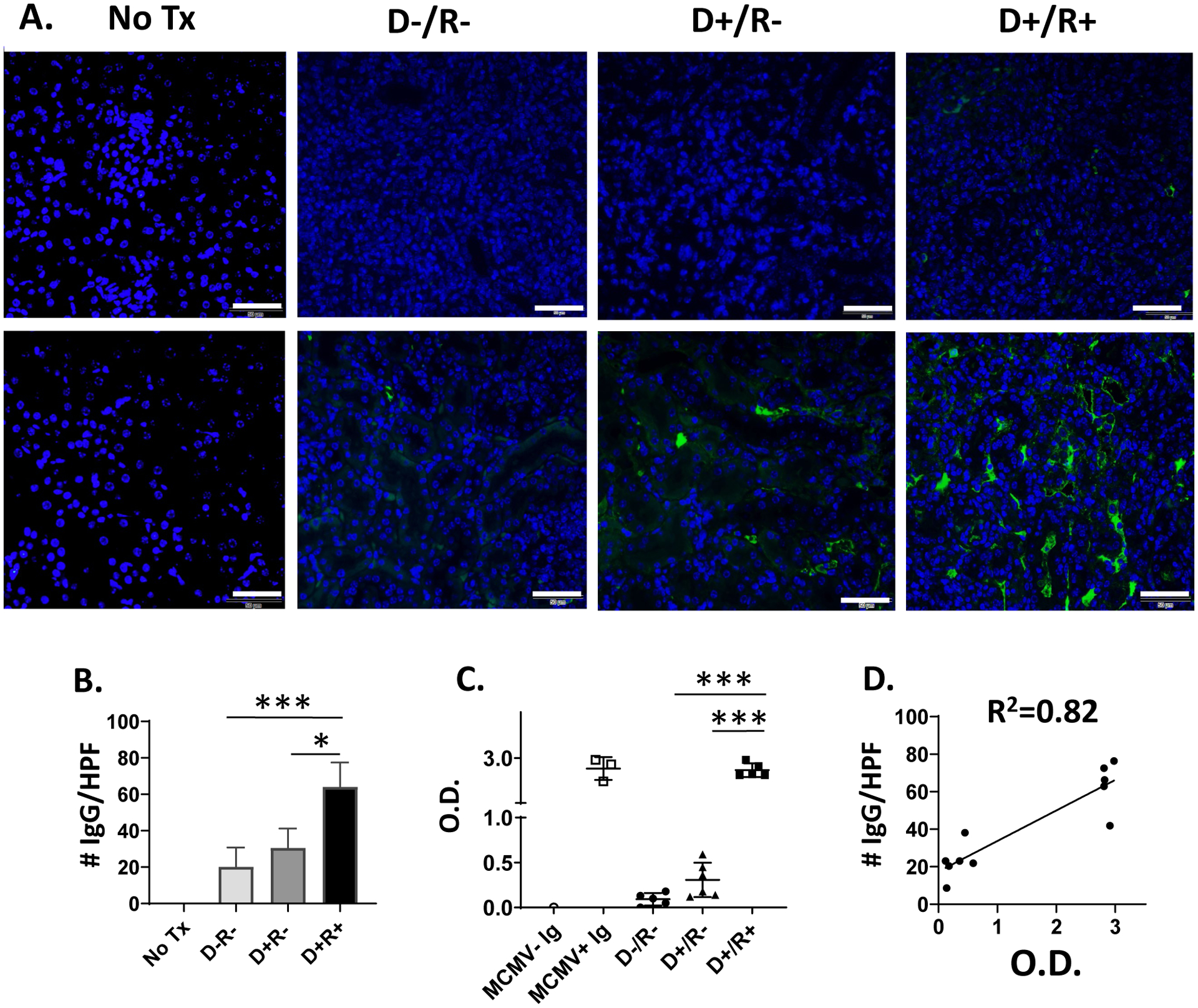
IgG deposition in allografts with immunosuppression.
(A) Allografts of cyclosporine-immunosuppressed MCMV D−/R−, D+/R− and D+/R+ recipients were stained with IgG-AF488 at day 14 post-transplant (n=5–6/group). Kidneys from non-transplant C57BL/6 mice (“No Tx”) served as staining controls (left panels, n=3). Representative images of cortex (upper panel) and medulla (lower panel) are shown (40x; white bar=50 μm). (B) Fluorescence immunostaining was quantitated for 10 HPF per kidney using Image J and compared between groups. (C) MCMV− Ig, MCMV+ Ig, or sera from immunosuppressed D−/R−, D+/R− or D+/R+ transplant recipients were analyzed by ELISA for anti-MCMV antibodies at OD450 (O.D). (D) Serologic anti-MCMV antibody levels (O.D.) were correlated with allograft IgG immunostaining for D+/R− and D+/R+ transplant recipients.
* p<0.05; *** p<0.001.
Virus-induced antibodies bind to infected renal tubular epithelial cells in-vitro
CMV can infect renal tubular epithelial cells.59–62 Because IgG deposition was observed by immunostaining in the medulla, IgG binding to MCMV infected renal tubular epithelial cells (TECs) was examined in-vitro (Fig. 4A). B6 or BALB/c TECs were either uninfected (“MCMV− TECs”) or infected with MCMV-GFP (“MCMV+ TECs”), then incubated with either MCMV− sera or MCMV+ sera from non-transplant B6 mice, or with pooled sera from cyclosporine-immunosuppressed D−/R− or D+/R+ transplant recipients. MCMV+ TECs were identified by flow cytometry via GFP+ expression among EpCAM+ cells, and IgG binding identified by staining with anti-mouse IgG-APC (Figs. 4A, S1).
FIGURE 4.
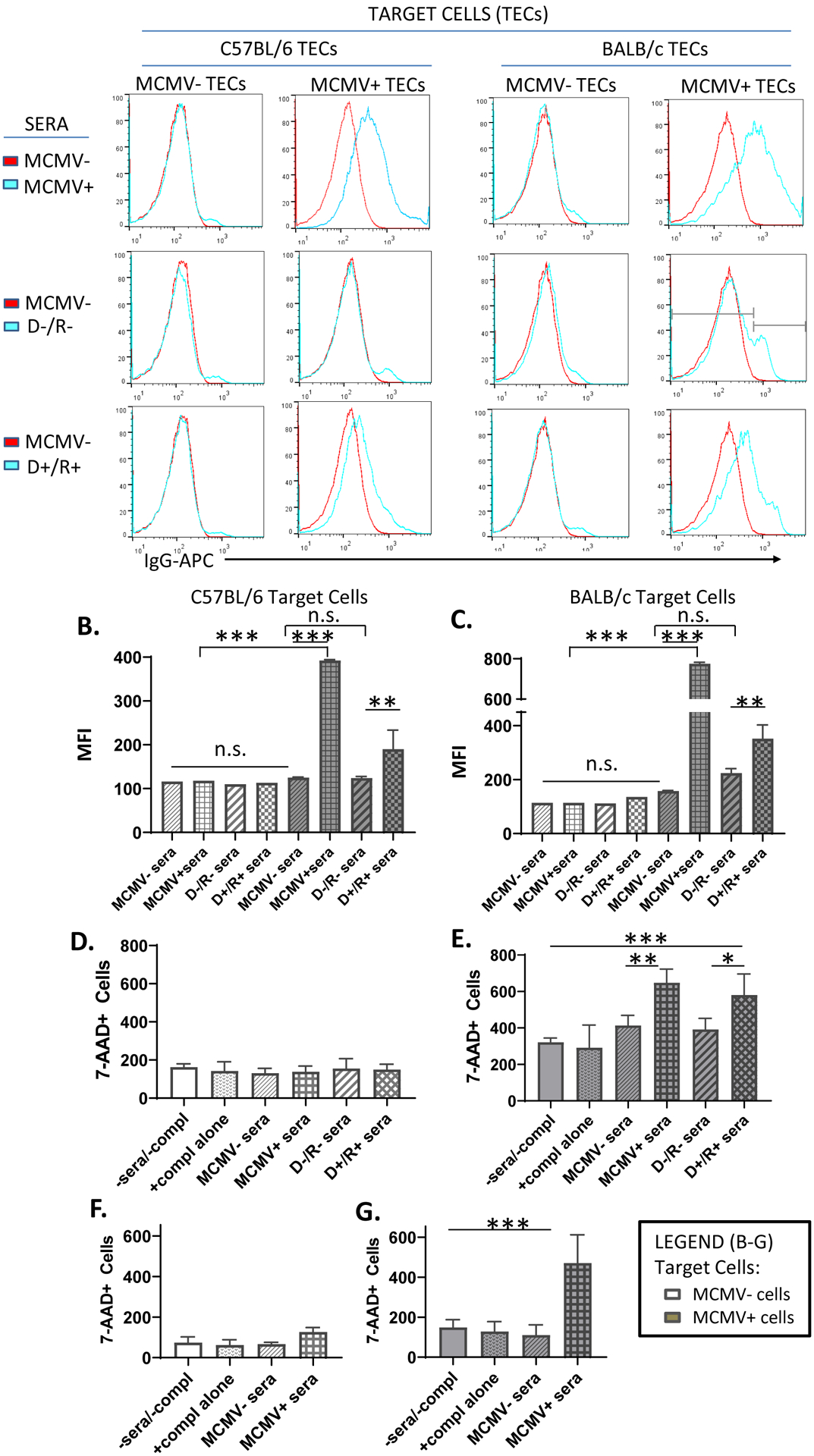
Antibody binding and complement dependent cytotoxicity of MCMV-infected target cells.
(A) Uninfected or MCMV-GFP infected B6 or BALB/c renal tubular epithelial cells (TECs) were incubated either with sera from MCMV nonimmune B6 mice (MCMV−, red lines), or from MCMV immune (MCMV+) B6 mice, D−/R− or D+/R+ recipients (blue lines). IgG binding to target cells was identified by staining with anti-mouse IgG-APC antibodies and detection by flow cytometry. Histogram subgating is shown with gray bars. (B, C) Mean fluorescence intensity (MFI) of IgG binding was compared among groups for both uninfected (white bars) and MCMV-infected (gray bars) B6 (B) or BALB/c (C) TEC target cells.
(D-E) A complement dependent cytotoxicity (CDC) assay using uninfected (D) or MCMV-GFP infected (E) BALB/c 3T3 target cells was performed by incubation of target cells with MCMV−/+ sera or transplant sera as in (A), in the presence of rabbit complement for 3 hours. Cytotoxicity was quantitated using 7-AAD staining. Cells incubated without sera or complement (-sera/-compl) or with complement alone (+compl alone) were included as controls. (F-G). CDC assay was repeated using MCMV−/+ sera and BALB/c TEC target cells, as described for (D-E).
(A-G) All experiments were performed 3 times and representative experiments are shown.
* p<0.05; ** p<0.01; *** p<0.001.
In Figure 4A (top row), B6 MCMV− sera (red line) and MCMV+ sera (blue line) bound similarly to MCMV uninfected B6 and BALB/c TECs (“MCMV-TECs”), indicating that antibodies recognizing non-viral TEC antigens (auto- and allo-antibodies) were present at similar quantities in non-transplant B6 MCMV+/− sera. MCMV− sera (red) bound at a similar mean fluorescence intensity (MFI) to both MCMV uninfected and MCMV infected B6 and BALB/c TECs (Fig. 4A, top row; Fig. 4B), indicating that MCMV infection of TECs did not upregulate antigens that significantly altered binding by antibodies found in MCMV− sera. In contrast, MCMV+ sera bound to MCMV infected B6 and BALB/c TECs at a significantly higher MFI than the MCMV-sera (Fig. 4A, top row; Fig. 4B, gray bars, p≤0.0001). Since MCMV− and MCMV+ sera bound similarly to uninfected B6 and BALB/c TECs, this difference was unlikely to be due to increased auto- or allo-antibodies in MCMV+ sera. As these pooled sera were used for adoptive transfer to μMT recipients (Figure 2), it is likely that the difference in antibody deposition observed in μMT recipients treated with MCMV immune sera was not due to auto- or allo-antibodies, which were similarly present in MCMV+/− sera.
Sera from immunosuppressed D−/R− transplants bound to MCMV uninfected B6 and BALB/c TECs at similar MFI as MCMV− sera (Fig. 4A, middle row; Fig. 4B, white bars). Binding of D−/R− sera to MCMV infected B6 and BALB/c TECs resembled that of MCMV− sera, except that D−/R− sera showed a second binding peak with an MFI significantly higher than MCMV− sera (p<0.01). D+/R+ sera had significantly greater binding to MCMV infected B6 and BALB/c TECs compared to D−/R− sera (Fig. 4A, bottom row; Fig. 4B, gray bars, p≤0.01). D+/R+ sera bound to uninfected B6 and BALB/c TECs similarly to non-transplant MCMV− sera (Fig. 4B, white bars), indicating no significant difference in auto- and allo-reactive antibody quantities. However, D+/R+ binding to MCMV infected BALB/c TECs did show a small peak at ≥103 fluorescence intensity, similar to D−/R− sera (Fig. 4A, right column). Taken together, these results indicate that MCMV+ and D+/R+ sera contain virus-induced antibodies, not present in MCMV− or D−/R− sera, that bind to antigens expressed by MCMV infected but not uninfected TECs.
Antibodies in MCMV+ and D+/R+ sera induce complement dependent cytotoxicity
Next, complement dependent cytotoxicity (CDC) assays were performed using BALB 3T3 cells, with or without MCMV infection, by incubation with MCMV− or MCMV+ B6 sera in the presence or absence of rabbit complement, and cytotoxicity was quantified by 7-AAD staining. Control cells were incubated without sera or complement (-sera/-compl), or with complement alone (+compl alone). B6 MCMV− and + sera without complement did not induce cytotoxicity above control wells (data not shown). In the presence of complement (Fig. 4D–E), MCMV− sera did not induce cytotoxicity above that observed in control wells, whereas MCMV+ sera induced cytotoxicity of MCMV+ cells (Fig. 4E) but not MCMV− cells (Fig. 4D). Similarly, D−/R− sera did not induce cytotoxicity, whereas D+/R+ sera induced cytotoxicity only in MCMV+ cells (Fig. 4D–E). The CDC assay was repeated using BALB/c TECs with similar results for MCMV+ and MCMV− sera (Fig. 4F–G), but insufficient D−/R− and D+/R+ sera was available to perform the assay using BALB/c TECs. However, in aggregate, these results indicate that MCMV+ sera and D+/R+ sera can induce CDC of MCMV-infected 3T3 cells or TECs in-vitro.
Native kidneys of D+/R+ transplant recipients lack IgG deposition.
The renal transplants in this model are implanted heterotopically, retaining one or both native kidneys. For R+ recipients, the native kidneys harbor MCMV prior to transplant. To examine whether MCMV antibodies deposit into the native kidneys of D+/R+ transplant recipients, IgG staining of R+ native kidneys was compared to the D+ allografts (Fig. 5A–B) and showed minimal IgG deposition (Fig. 5C, p<0.0001). Histopathology (Fig. 5D–F) showed significantly greater damage scores for allografts compared to native kidneys (12.00±0.71 vs. 0.40±0.40, p<0.0001). Similarly, CD45+ cell infiltrates were significantly greater in allografts compared to native kidneys (Fig. 5G, p=0.0002). In contrast, allografts and native kidneys had similar viral loads by quantitative DNA PCR (Fig. 5H). Taken together, these results indicate that R+ native kidneys lack significant antibody deposition and cellular infiltrates despite MCMV viral loads comparable to those found in the contralateral allografts, suggesting that the presence of MCMV in the native kidney is not sufficient to elicit intrarenal IgG deposition.
FIGURE 5.
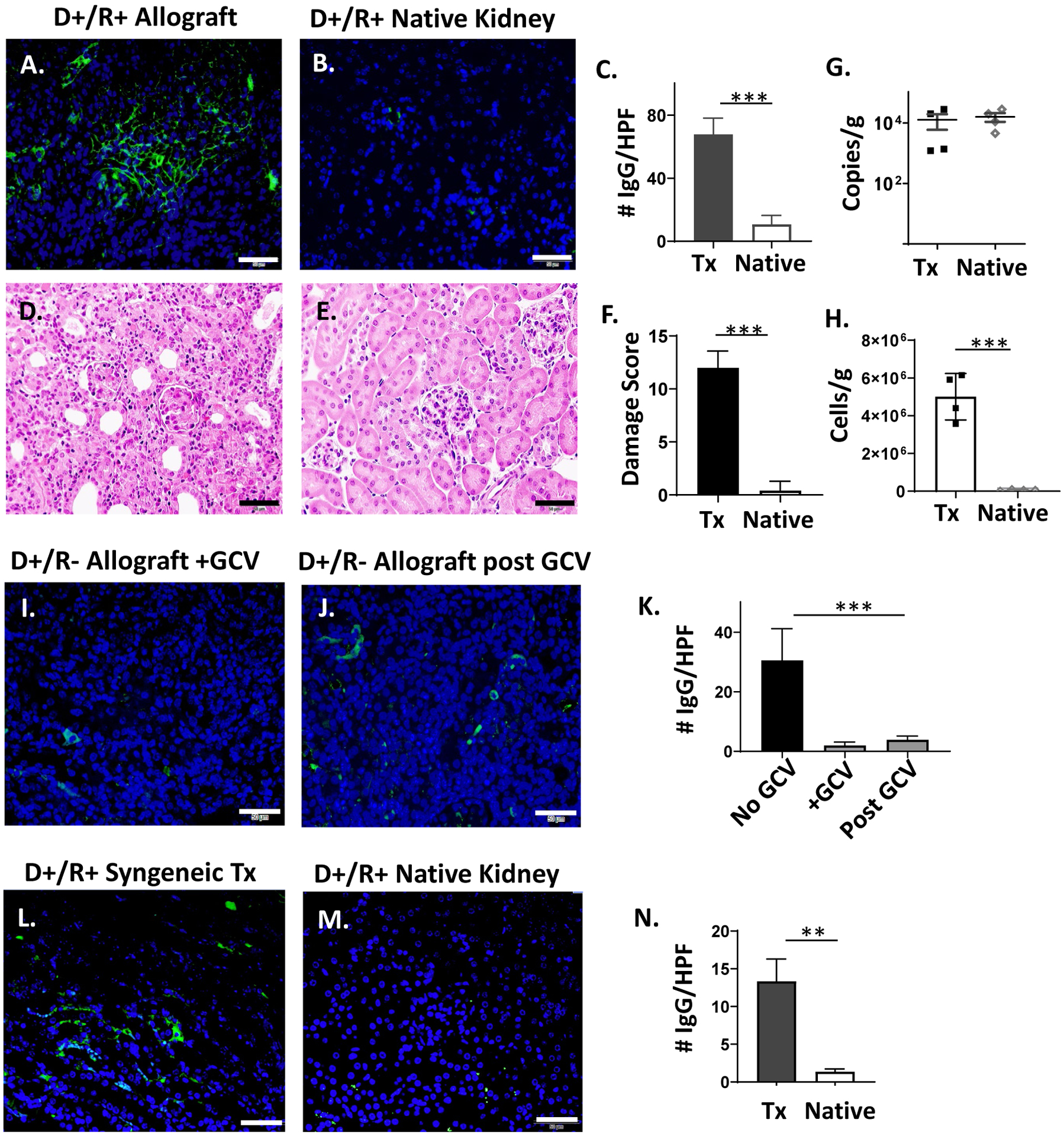
IgG immunostaining of D+/R+ native kidneys and D+/R− allografts treated with ganciclovir.
(A-B) Allografts and native kidneys of D+/R+ recipients were stained with IgG-AF488 as in Fig. 1. (C) IgG immunostaining was quantitated for 10 HPF and compared between transplant (Tx) and native kidneys (n=4/group). (D-F) Hematoxylin and eosin staining of allografts (D) and native kidneys (E) were scored for organ injury, and damage scores (F) compared between Tx and native kidneys. (G) MCMV viral loads in Tx and native kidneys were compared by quantitative DNA PCR. (H) CD45+ cell infiltrates in Tx and native kidneys were quantified by flow cytometry. (I-K) D+/R− recipients were treated with ganciclovir (GCV) for 14 days (I), or for 14 days followed by 7 days without antiviral treatment (J). Allografts were stained for IgG-AF488 (I-J) and quantitated (K) using Image J in comparison with IgG staining of day 14 D+/R− allografts without ganciclovir treatment (Fig. 3). (L-N) D+/R+ syngeneic grafts and native kidneys of immunosuppressed recipients were stained for IgG and quantitated using Image J (n=3).
(A-J) Representative images are shown (40x, bar=50 μm).
** p<0.01; *** p<0.001.
Ganciclovir reduces intragraft IgG deposition.
Ganciclovir (GCV) treatment of D+/R− transplant recipients inhibits viral replication and reduces intragraft leukocyte infiltrates.26 To determine whether GCV treatment affects intragraft IgG deposition, allografts from D+/R− recipients treated with GCV for 14 days were stained for IgG (Fig. 5I), as well as grafts from mice sacrificed 7 days after discontinuing GCV (Fig. 5J). Allografts from GCV-treated mice showed lower IgG staining for both conditions, compared to untreated mice (Fig. 5K, p=0.0002). This result suggests that pharmacologic inhibition of viral antigen expression may reduce IgG deposition within MCMV-infected allografts either via inhibition of primary antiviral antibody generation or by reduced binding of antiviral antibodies in the absence of viral antigen expression.
IgG deposits into syngeneic D+/R+ transplants.
Transplant ischemia-reperfusion injury (IRI) can induce MCMV reactivation in syngeneic D+ grafts of immunosuppressed mice.31 To induce MCMV reactivation while eliminating the contribution of alloantibodies in our model, syngeneic D+/R+ transplants were performed using B6 donors and recipients with cyclosporine immunosuppression. IgG immunostaining in the syngeneic grafts was significantly greater than that observed in the native kidneys (p=0.0023). This result indicates that antiviral antibodies, in the absence of alloantibodies, can deposit into D+ kidneys of immunosuppressed R+ recipients after IRI.
DISCUSSION
In this study, a murine renal transplant model was utilized to determine whether pathogen-induced antibodies could deposit within allografts during acute rejection, using murine cytomegalovirus as a model pathogen. MCMV naturally infects the kidney and reactivates after allogeneic transplantation.28,29,63 In this model, IgG and C3 deposited into MCMV infected allografts during acute rejection, consistent with AMR, and was recapitulated by passive transfer of MCMV immune sera in μMT mice. In-vitro, MCMV+ and D+/R+ sera could induce CDC of MCMV-infected renal TECs. These data suggest that MCMV-induced antibodies might deposit into infected allografts during acute rejection and may be capable of triggering CDC. However, MCMV-induced antibodies did not deposit into MCMV-infected native kidneys, suggesting that intrarenal MCMV infection alone is not sufficient to induce antibody deposition in the kidney. Together, these data suggest the possibility that pathogen-induced antibodies might be capable of contributing to acute AMR.
HCMV positive serostatus and infection (DNAemia) are risk factors for late allograft dysfunction and loss via “indirect effects”. Although CMV-induced antibodies protect the host against CMV disease, their potential role in allograft injury has not been previously examined. Our prior work in this transplant model showed that other cell types that are important for control of MCMV infection, such as NK cells and CD8+ T cells, can also induce allograft injury during acute rejection.26,32 The current study extends the evidence supporting a model that immune mechanisms necessary for protection of the host against systemic infection might, conversely, contribute to injury of the CMV-infected allograft. As anti-CMV antibody titers vary in kidney transplant recipients depending on their history of viral reactivation49, future work could examine the possibility that these varying antibody titers might perhaps contribute to varying degrees of allograft dysfunction among HCMV-infected patients.
In the current study, MCMV-induced antibodies were shown to be capable of fixing complement and lysing MCMV-infected TECs in-vitro, suggesting that virus-induced antibodies may participate in CDC during AMR. Further studies to investigate CDC in vivo could define the functional relevance of this pathway within rejecting allografts. In addition, pathogen-induced IgM antibodies were not analyzed in this work and deserve further study to define their role in complement fixation and CDC during AMR. Our prior work in this model also showed that NK cells are recruited to MCMV-infected allografts and manifest both cytolytic and cytotoxic phenotypes, raising the untested possibility that virus-induced antibodies might also contribute to NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC). Further studies in animal models and clinical populations could examine whether CDC or ADCC triggered by pathogen-induced antibodies might constitute possible mechanisms contributing to the “indirect effects” of CMV in renal transplantation.
In this animal model, MCMV-induced antibodies were identified in allografts but not native kidneys of D+/R+ transplants, despite the presence of similar quantities of CMV DNA in both organs. A possible explanation for this observation may be that CMV reactivation in allografts during acute rejection results in expression of viral antigens recognized by virus-induced antibodies. Consistent with this interpretation, inhibition of viral replication via ganciclovir treatment was associated with a lack of intragraft antibody deposition, although this result could also have been due to inhibition of primary antiviral antibody generation in R- recipients by suppressing viral antigen expression. These observations align with clinical studies showing that HCMV antigens can be identified in acutely rejecting allografts, and that antiviral drugs used to prevent viral reactivation are associated with improved graft longevity among HCMV-infected renal transplant patients.17,19,20 Other groups have also shown that reactivation of latent CMV from murine renal allografts can be induced by inflammatory cytokines such as TNF-α that are expressed after allogeneic transplantation, as well as by ischemia-reperfusion injury (IRI) after syngeneic transplantation.29,31 Zhang et al. also showed that immunosuppression alone failed to induce reactivation from latency in native kidneys of non-transplant mice,31 supporting the possibility that viral antigens might not be expressed in the native kidney of the immunosuppressed transplant recipient, and consistent with our observations in the R+ native kidneys. However, a limitation of the current study is that we were not able to characterize MCMV antigen expression in allografts and native kidneys. Unfortunately, attempts to quantify MCMV antigen expression in this renal transplant model using commercially available mouse anti-MCMV antibodies (Center for Proteomics, Rijeka Croatia) were unsuccessful due to high background staining (data not shown).
Another limitation to interpretation of this study is the possibility that the antibodies observed in the MCMV-infected allografts were directed against nonviral antigens. In this work, the MCMV immune and nonimmune sera used for adoptive transfer in the μMT mice showed similar quantities of auto- and allo-reactive antibodies (measured by ex vivo binding to syngeneic (B6) and allogeneic (BALB/c) TECs), which would be predicted to deposit similarly into allografts. Since intragraft IgG staining was more intense after adoptive transfer of MCMV immune sera compared to nonimmune sera, these data indirectly support the likelihood that virus-directed antibodies, rather than auto- or allo-antibodies, comprised the observed differential staining. However, an alternative explanation might be that MCMV-infected cells within rejecting allografts could upregulate expression of antigens that bind yet-uncharacterized non-viral antibodies that might be present only in MCMV immune sera. In-vitro, MCMV infection does alter numerous transcriptional pathways that may affect host cellular protein expression by infected cells.64 MCMV infection also induces expression of interferon-regulated genes and other cell stress-response genes.65 It is therefore also possible that intragraft MCMV infected cells might express stress-induced molecules that promote non-viral antibody binding and complement activation; however, this pathway would be predicted to induce deposition of non-viral antibodies similarly by MCMV nonimmune and immune sera in μMT mice. Finally, in a syngeneic model with intragraft MCMV reactivation induced by ischemia-reperfusion injury, IgG deposition was observed in the absence of alloantigens, supporting that the intragraft IgGs are antiviral antibodies and not alloantibodies.
In summary, in this murine model, complement-fixing antibodies localized to MCMV-infected acutely rejecting allografts. In-vitro, MCMV-induced antibodies could induce CDC of infected renal tubular epithelial cells. The relevance of these findings may merit further exploration in the animal model and clinical populations to elucidate the “indirect effects” of CMV in renal transplantation.
Supplementary Material
FUNDING:
Funding for this work was provided by NIH R01AI101138 (M.S.); NIH NIDDK P30 DK079337 (L.G., B.C.); NIH NIDDK P30 DK064400 (UAB Digestive Diseases Research Development Center Core); the Children’s Center for Research and Innovation of The Alabama Children’s Hospital Foundation (M.S.), and the Abigail Wexner Research Institute at Nationwide Children’s Hospital (M.S.).
ABBREVIATIONS:
- AMR
antibody mediated rejection
- AR
acute rejection
- CDC
complement dependent cytotoxicity
- CSA
cyclosporine A
- DSA
donor specific antibodies
- FFPE
formalin fixed and paraffin embedded
- GCV
ganciclovir
- HCMV
human cytomegalovirus
- HLA
human leukocyte antigen
- HPF
high power field
- IgG
immunoglobulin G
- IP
intraperitoneal
- MCMV
murine cytomegalovirus
- MEFs
murine embryonic fibroblasts
- MOI
multiplicity of infection
- O.D.
optical density
- PFU
plaque forming units
- RCMV
rat cytomegalovirus
- SD
standard deviation
- TCMR
T cell mediated rejection
- TECs
tubular epithelial cells
Footnotes
DISCLOSURE: The authors declare no conflicts of interest.
REFERENCES
- 1.Freeman RB Jr. The ‘Indirect’ effects of cytomegalovirus infection. American Journal of Transplantation. 2009;9(11):2453–2458. [DOI] [PubMed] [Google Scholar]
- 2.Tong CYW, Bakran A, Peiris JSM, et al. The association of viral infection and chronic allograft nephropathy with graft dysfunction after renal transplantation. Transplantation. 2002;74:576–578. [DOI] [PubMed] [Google Scholar]
- 3.Streblow DN, Orloff SL, Nelson JA. Acceleration of allograft failure by cytomegalovirus. Curr Opin Immunol. 2007;19(5):577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legendre C, Pascual M. Improving outcomes for solid organ transplant recipients at risk from cytomegalovirus infection: late onset disease and indirect consequences. Clinical Infectious Diseases. 2008;46(5):732–740. [DOI] [PubMed] [Google Scholar]
- 5.Einollahi B, Motalebi M, Salesi M, et al. The impact of cytomegalovirus infection on new-onset diabetes mellitus after kidney transplantation: a review on current findings. Journal of nephropathology. 2014;3(4):139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin RH, Tolkoff-Rubin NE, Oliver D, et al. Multicenter seroepidemiologic study of the impact of cytomegalovirus infection on renal transplantation. Transplantation. 1985;40(3):243–249. [DOI] [PubMed] [Google Scholar]
- 7.Schnitzler MA, Woodward RS, Brennan DC, et al. The effects of cytomegalovirus serology on graft and recipient survival in cadaveric renal transplantation: implications for organ allocation. American Journal of Kidney Diseases. 1997;29(3):428–434. [DOI] [PubMed] [Google Scholar]
- 8.Schnitzler MA, Woodward RS, Brennan DC, et al. Impact of cytomegalovirus serology on graft survival in living related kidney transplantation: implications for donor selection. Surgery. 1997;121(5):563–568. [DOI] [PubMed] [Google Scholar]
- 9.Humar A, Gillingham KJ, Payne WD, et al. Association between cytomegalovirus disease and chronic rejection in kidney transplant recipients. Transplantation. 1999;68(12):1879–1883. [DOI] [PubMed] [Google Scholar]
- 10.Browne BJ, Young JA, Dunn TB, et al. The impact of cytomegalovirus infection >/=1 year after primary renal transplantation. Clin Transplant. 2010;24(4):572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erdbruegger U, Scheffner I, Mengel M, et al. Impact of CMV infection on acute rejection and long-term renal allograft function: a systematic analysis in patients with protocol biopsies and indicated biopsies. Nephrol Dial Transplant. 2012;27(1):435–443. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Oliva MO, Flores J, Madero R, et al. Cytomegalovirus infection after kidney transplantation and long-term graft loss. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia. 2017;37(5):515–525. [DOI] [PubMed] [Google Scholar]
- 13.Selvey LA, Lim WH, Boan P, et al. Cytomegalovirus viraemia and mortality in renal transplant recipients in the era of antiviral prophylaxis. Lessons from the western Australian experience. BMC infectious diseases. 2017;17(1):501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blazquez-Navarro A, Dang-Heine C, Wittenbrink N, et al. BKV, CMV, and EBV interactions and their effect on graft function one year post-renal transplantation: results from a large multi-centre study. EBioMedicine. 2018;34:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markovic-Lipkovski J, Muller CA, Engler-Blum G, et al. Human cytomegalovirus in rejected kidney grafts; detection by polymerase chain reaction. Nephrol Dial Transplant. 1992;7(8):865–870. [PubMed] [Google Scholar]
- 16.Gerstenkorn C, Robertson H, Mohamed MA, et al. Detection of cytomegalovirus (CMV) antigens in kidney biopsies and transplant nephrectomies as a marker for renal graft dysfunction. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2000;38(11):1201–1203. [DOI] [PubMed] [Google Scholar]
- 17.Holma K, Tornroth T, Gronhagen-Riska C, et al. Expression of the cytomegalovirus genome in kidney allografts during active and latent infection. Transpl Int. 2000;13 Suppl 1:S363–365. [DOI] [PubMed] [Google Scholar]
- 18.Opelz G, Dohler B, Ruhenstroth A. Cytomegalovirus prophylaxis and graft outcome in solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(6):928–936. [DOI] [PubMed] [Google Scholar]
- 19.Reischig T, Hribova P, Jindra P, et al. Long-term outcomes of pre-emptive valganciclovir compared with valacyclovir prophylaxis for prevention of cytomegalovirus in renal transplantation. Journal of the American Society of Nephrology. 2012;23(9):1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reischig T, Kacer M, Hruba P, et al. Less renal allograft fibrosis with valganciclovir prophylaxis for cytomegalovirus compared to high-dose valacyclovir: a parallel group, open-label, randomized controlled trial. BMC infectious diseases. 2018;18(1):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witzke O, Nitschke M, Bartels M, et al. Valganciclovir Prophylaxis Versus Preemptive Therapy in Cytomegalovirus-Positive Renal Allograft Recipients: Long-term Results After 7 Years of a Randomized Clinical Trial. Transplantation. 2018;102(5):876–882. [DOI] [PubMed] [Google Scholar]
- 22.Koskinen PK, Yilmaz S, Kallio E, et al. Rat cytomegalovirus infection and chronic kidney allograft rejection. Transplant International. 1996;9(S1):S3–4. [DOI] [PubMed] [Google Scholar]
- 23.Lautenschlager I, Soots A, Krogerus L, et al. CMV increases inflammation and accelerates chronic rejection in rat kidney allografts. Transplantation Proceedings. 1997;29(1–2):802–803. [DOI] [PubMed] [Google Scholar]
- 24.van Dam JG, Li F, Yin M, et al. Effects of cytomegalovirus infection and prolonged cold ischemia on chronic rejection of rat renal allografts. Transplant International. 2000;13(1):54–63. [DOI] [PubMed] [Google Scholar]
- 25.Inkinen K, Holma K, Soots A, et al. Expression of TGF-beta and PDGF-AA antigens and corresponding mRNAs in cytomegalovirus-infected rat kidney allografts. Transplant Proc. 2003;35(2):804–805. [DOI] [PubMed] [Google Scholar]
- 26.Shimamura M, Saunders U, Rha B, et al. Ganciclovir transiently attenuates murine cytomegalovirus-associated renal allograft inflammation. Transplantation. 2011;92(7):759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pascher A, Proesch S, Pratschke J, et al. Rat Cytomegalovirus Infection Interferes with Anti-CD4 mAb-(RIB 5/2) Mediated Tolerance and Induces Chronic Allograft Damage. American Journal of Transplantation. 2006;6(9):2035–2045. [DOI] [PubMed] [Google Scholar]
- 28.Klotman ME, Starnes D, Hamilton JD. The source of murine cytomegalovirus in mice receiving kidney allografts. J Infect Dis. 1985;152(6):1192–1196. [DOI] [PubMed] [Google Scholar]
- 29.Hummel M, Zhang Z, Yan S, et al. Allogeneic transplantation induces expression of cytomegalovirus immediate-early genes in vivo: a model for reactivation from latency. J Virol. 2001;75(10):4814–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hummel M, Kurian SM, Lin S, et al. Intragraft TNF receptor signaling contributes to activation of innate and adaptive immunity in a renal allograft model. Transplantation. 2009;87(2):178–188. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Qiu L, Yan S, et al. A clinically relevant murine model unmasks a “two-hit” mechanism for reactivation and dissemination of cytomegalovirus after kidney transplant. Am J Transplant. 2019;19(9):2421–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimamura M, Seleme MC, Guo L, et al. Ganciclovir prophylaxis improves late murine cytomegalovirus-induced renal allograft damage. Transplantation. 2013;95(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh N, Pirsch J, Samaniego M. Antibody-mediated rejection: treatment alternatives and outcomes. Transplantation Reviews. 2009;23(1):34–46. [DOI] [PubMed] [Google Scholar]
- 34.Pouliquen E, Koenig A, Chen CC, et al. Recent advances in renal transplantation: antibody-mediated rejection takes center stage. F1000prime reports. 2015;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randhawa P T-cell-mediated rejection of the kidney in the era of donor-specific antibodies: diagnostic challenges and clinical significance. Curr Opin Organ Transplant. 2015;20(3):325–332. [DOI] [PubMed] [Google Scholar]
- 36.Halloran PF, Venner JM, Madill-Thomsen KS, et al. Review: The transcripts associated with organ allograft rejection. Am J Transplant. 2018;18(4):785–795. [DOI] [PubMed] [Google Scholar]
- 37.Chong AS, Rothstein DM, Safa K, et al. Outstanding questions in transplantation: B cells, alloantibodies, and humoral rejection. Am J Transplant. 2019;19(8):2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delville M, Lamarthee B, Pagie S, et al. Early acute microvascular kidney transplant rejection in the absence of anti-hla antibodies is associated with preformed igg antibodies against diverse glomerular endothelial cell antigens. J Am Soc Nephrol. 2019;30(4):692–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parajuli S, Redfield RR, Garg N, et al. Clinical significance of microvascular inflammation in the absence of anti-hla dsa in kidney transplantation. Transplantation. 2019;103(7):1468–1476. [DOI] [PubMed] [Google Scholar]
- 40.Senev A, Otten HG, Kamburova EG, et al. Antibodies against ARHGDIB and ARHGDIB gene expression associate with kidney allograft outcome. Transplantation. epub ahead of print, 2019. doi: 10.1097/TP.0000000000003005 [DOI] [PubMed] [Google Scholar]
- 41.Costa C, Touscoz GA, Bergallo M, et al. Non-organ-specific and anti-endothelial antibodies in relation to CMV infection and acute rejection in renal transplant recipients. Clin Transplant. 2010;24(4):488–492. [DOI] [PubMed] [Google Scholar]
- 42.Philogene MC, Jackson AM. Non-HLA antibodies in transplantation: when do they matter? Curr Opin Organ Transplant. 2016;21(4):427–432. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q, Reed EF. The importance of non-HLA antibodies in transplantation. Nature reviews. Nephrology 2016;12(8):484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cardinal H, Dieude M, Hebert MJ. The emerging importance of non-hla autoantibodies in kidney transplant complications. J Am Soc Nephrol. 2017;28(2):400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamburova EG, Gruijters ML, Kardol-Hoefnagel T, et al. Antibodies against ARHGDIB are associated with long-term kidney graft loss. Am J Transplant. 2019;19(12):3335–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lefaucheur C, Viglietti D, Bouatou Y, et al. Non-HLA agonistic anti-angiotensin II type 1 receptor antibodies induce a distinctive phenotype of antibody-mediated rejection in kidney transplant recipients. Kidney Int. 2019;96(1):189–201. [DOI] [PubMed] [Google Scholar]
- 47.Philogene MC, Johnson T, Vaught AJ, et al. Antibodies against angiotensin ii type 1 and endothelin a receptors: relevance and pathogenicity. Hum Immunol. 2019;80(8):561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farouk S, Zhang Z, Menon MC. Non-HLA donor-recipient mismatches in kidney transplantation-A stone left unturned. Am J Transplant. 2020;20(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iglesias-Escudero M, Moro-Garcia MA, Marcos-Fernandez R, et al. Levels of anti-CMV antibodies are modulated by the frequency and intensity of virus reactivations in kidney transplant patients. PLoS ONE. 2018;13(4):e0194789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitamura D, Roes J, Kuhn R, et al. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350(6317):423–426. [DOI] [PubMed] [Google Scholar]
- 51.Brune W, Menard C, Heesemann J, et al. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science. 2001;291(5502):303–305. [DOI] [PubMed] [Google Scholar]
- 52.Bubic I, Wagner M, Krmpotic A, et al. Gain of virulence caused by loss of a gene in murine cytomegalovirus. Journal of Virology. 2004;78(14):7536–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li M, Boddeda SR, Chen B, et al. NK cell and Th17 responses are differentially induced in murine cytomegalovirus infected renal allografts and vary according to recipient virus dose and strain. Am J Transplant. 2018;18(11):2647–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z, Schlachta C, Duff J, et al. Improved techniques for kidney transplantation in mice. Microsurgery. 1995;16(2):103–109. [DOI] [PubMed] [Google Scholar]
- 55.Roufosse C, Simmonds N, Clahsen-van Groningen M, et al. A 2018 reference guide to the banff classification of renal allograft pathology. Transplantation. 2018;102(11):1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y, Zhao H, Zhang Y, et al. Isolation and epithelial co-culture of mouse renal peritubular endothelial cells. BMC Cell Biol. 2014;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bantug GR, Cekinovic D, Bradford R, et al. CD8+ T lymphocytes control murine cytomegalovirus replication in the central nervous system of newborn animals. J Immunol. 2008;181(3):2111–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haas M, Loupy A, Lefaucheur C, et al. The banff 2017 kidney meeting report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18(2):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heieren MH, Kim YK, Balfour HH Jr. Human cytomegalovirus infection of kidney glomerular visceral epithelial and tubular epithelial cells in culture. Transplantation. 1988;46(3):426–432. [DOI] [PubMed] [Google Scholar]
- 60.Ustinov JA, Loginov RJ, Mattila PM, et al. Cytomegalovirus infection of human kidney cells in vitro. Kidney Int. 1991;40(5):954–960. [DOI] [PubMed] [Google Scholar]
- 61.Hendrix RM, Wagenaar M, Slobbe RL, et al. Widespread presence of cytomegalovirus DNA in tissues of healthy trauma victims. Journal of Clinical Pathology. 1997;50(1):59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimamura M, Murphy-Ullrich JE, Britt WJ. Human cytomegalovirus induces TGF-beta1 activation in renal tubular epithelial cells after epithelial-to-mesenchymal transition. PLoS Pathog. 2010;6(11):e1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pollock JL, Virgin HWt. Latency, without persistence, of murine cytomegalovirus in the spleen and kidney. J Virol. 1995;69(3):1762–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Juranic Lisnic V, Babic Cac M, Lisnic B, et al. Dual analysis of the murine cytomegalovirus and host cell transcriptomes reveal new aspects of the virus-host cell interface. PLoS Pathog. 2013;9(9):e1003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marcinowski L, Lidschreiber M, Windhager L, et al. Real-time transcriptional profiling of cellular and viral gene expression during lytic cytomegalovirus infection. PLoS Pathog. 2012;8(9):e1002908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


