Introduction
Interatrial conduction block (IACB) has been well described and is mostly seen in patients at risk of atrial fibrosis from advanced age, cardiac structural abnormalities, prior cardiac surgery, and extensive atrial ablation.1 In advanced forms, IACB is associated with increased burden of tachyarrhythmia, interatrial dyssynchrony with shortened left atrioventricular intervals as well as poor left atrial contractility, and functional atrioventricular (AV) block owing to delayed or absent conduction of sinoatrial nodal impulses to the AV node.2, 3, 4 Even with a dual-chamber pacemaker, conduction block of the atrial paced impulse owing to the lead tip being positioned proximal to the region of block can result in unnecessary ventricular pacing. This can potentially cause pacemaker syndrome if ventricular-paced beats are able to conduct retrograde via the AV node to the atrial tissue on the distal aspect of the region of block.5
Dual-site atrial pacing has been performed temporarily for the prevention of atrial fibrillation in post–coronary artery bypass graft patients as well as permanently in those at high risk for recurrence of atrial fibrillation.6,7 We report a rare case where dual-site atrial pacemaker “upgrade” was performed in a patient with IACB and a pre-existent traditional dual-chamber pacemaker, leading to resolution of unnecessary ventricular pacing and systemic AV dyssynchrony.
Key Teaching Points.
-
•
Cardiac surgery and catheter ablation can increase the risk of interatrial conduction block, which can be associated with functional atrioventricular (AV) block.
-
•
Dual-chamber pacing with the right atrial lead positioned in the atrial compartment proximal to the site of block can lead to unnecessary ventricular pacing and retrograde ventriculoatrial conduction into the distal atrial compartment, with associated AV dyssynchrony that can be biatrial or predominantly left-sided, based on the site of conduction block.
-
•
Dual-site atrial pacing can overcome interatrial conduction block and achieve AV synchrony as well as reduce unnecessary ventricular pacing.
Case report
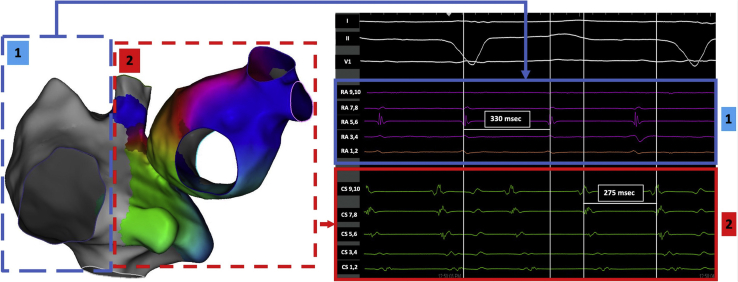
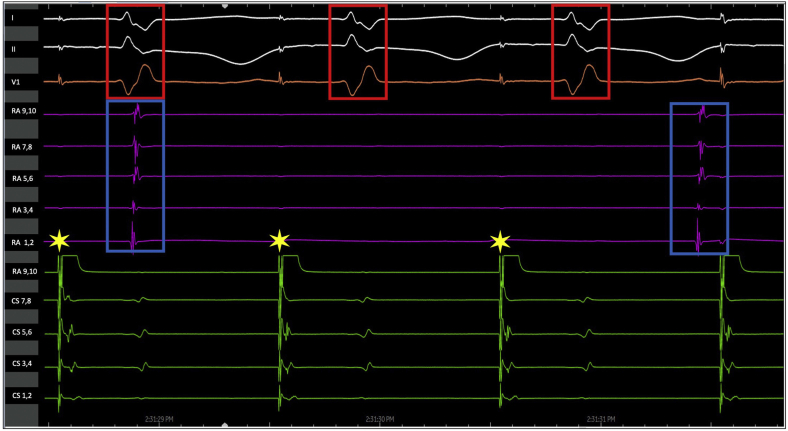
A 75-year-old man with a history of mechanical mitral valve replacement, and with multiple subsequent catheter ablations for atrial fibrillation and flutter, presented in February 2020 with recurrent atrial flutter while on sotalol therapy. He also had a history of reported sick sinus syndrome and “AV block,” for which he had received a dual-chamber pacemaker (Boston Scientific, Boston, MA). Table 1 displays the year and details of his prior procedures. After discussion of treatment options, it was decided to proceed with repeat catheter ablation. During electrophysiology study, he was found to be in an atypical atrial flutter with a tachycardia cycle length of ∼275 ms in the coronary sinus with proximal-to-distal activation (Figure 1, flutter 2). Interestingly, most of the lateral right atrium appeared to be dissociated from the circuit at a much slower tachycardia cycle length (Figure 1, flutter 1). Mapping the right atrial septum and the left atrium revealed a mitral isthmus–dependent mechanism for the faster atrial flutter and ablation was performed along the anterior mitral isthmus area where there was previous scar, which resulted in slowing and termination of the flutter. The lateral right atrial flutter terminated during mapping and could not be reinduced. When dual-chamber pacing resumed after termination of atrial flutter, the dissociated aspect of the right atrium (where the atrial pacing lead was present) resulted in no ventricular conduction owing to IACB (“exit block”), and hence ventricular pacing was needed. Following ventricular pacing, there was retrograde conduction up the septum and left atrium, resulting in systemic (left-sided) AV dyssynchrony (Figure 2). With proximal coronary sinus (CS) pacing, there was conduction to the ventricle via the AV node; however, there was no conduction into the dissociated aspect of the right atrium (Supplemental Figure S1; “entrance block”).
Table 1.
Procedures performed prior to final ablation
| Year | Procedure |
|---|---|
| 2008 | Mechanical mitral valve replacement |
| 2015 | Atrial fibrillation ablation† |
| 2016 | Dual-chamber pacemaker insertion |
| 2019 (January) | Atrial tachycardia ablation‡ |
| 2019 (March) | Complex right and left atrial flutter ablation§ |
Presumably underwent pulmonary vein isolation and cavotricuspid isthmus ablation at outside hospital.
Ablation performed at the site of earliest activation on the right atrial septum.
Anterior mitral isthmus line, left atrial posterior wall isolation, completion of pulmonary vein isolation, right atrial ablation near atriotomy, and confirmation of cavotricuspid isthmus block.
Figure 1.
Electroanatomic map (left panel) and intracardiac electrograms (right panel) demonstrating 2 different atrial flutters: (1) flutter involving lateral right atrium (blue dashed box) with tachycardia cycle length of 330 ms (blue box; could not be mapped owing to termination/lack of reinduction); (2) flutter involving right atrial septum and left atrium (red dashed box) with tachycardia cycle length of 275 ms (red box).
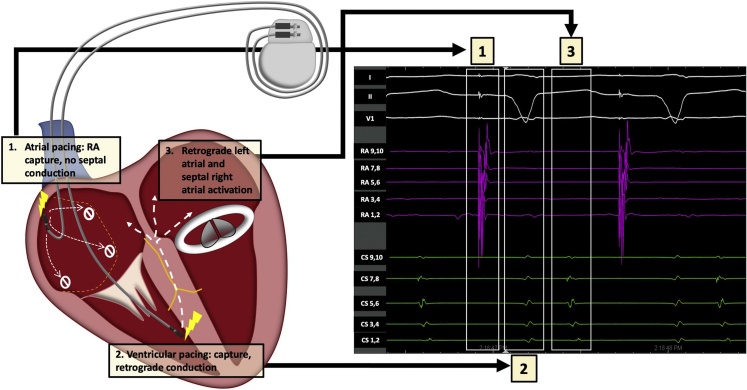
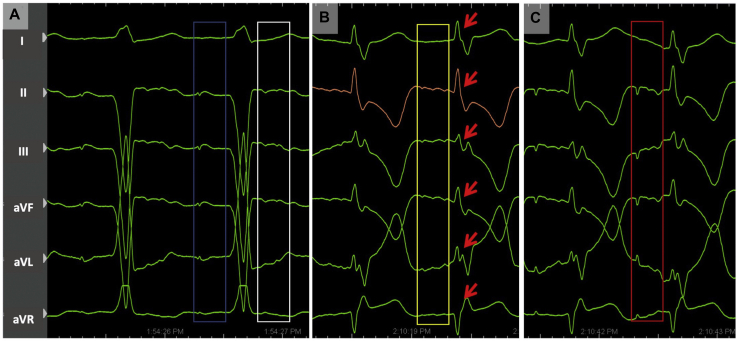
Figure 2.
Representative schematic (left panel) and intracardiac electrograms (right panel) illustrating electrical perturbations owing to interatrial conduction block, with hemodynamic consequences: (1) Atrial pacing leading to local capture but with no conduction to septum and AV nodal area; (2) Ventricular pacing resulting in ventricular capture and leading to retrograde conduction that activates the right atrial septum and left atrium (3). RA = right atrium.
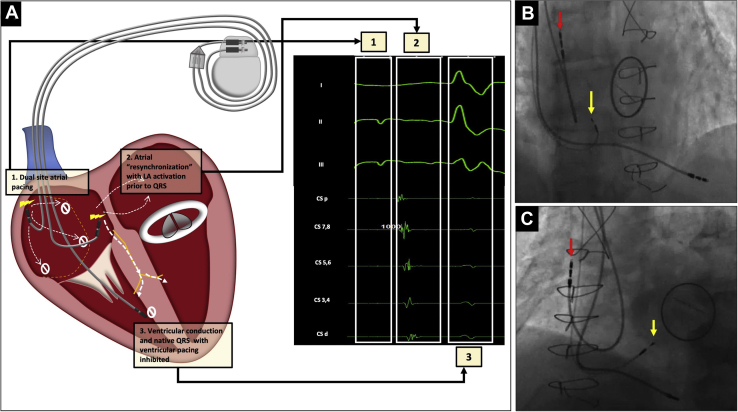
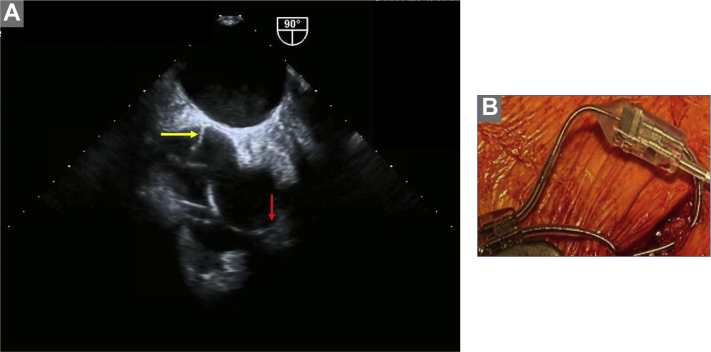
In follow-up, although the patient felt better when he was not in atrial flutter, he continued to feel fatigued and had intermittent pedal edema requiring diuretics. The complex physiology owing to IACB with unnecessary ventricular pacing and systemic AV dyssynchrony was discussed with the patient. Options of care were then discussed, including atrial resynchronization therapy; and after shared decision making, we proceeded with the upgrade to dual-site atrial pacing. A concomitant electrophysiology study with CS recordings was also planned to aid in lead placement and document resolution of the left-sided AV dyssynchrony. Right femoral venous access was obtained and a decapolar CS catheter was placed (Bard, Lowell, MA). After ipsilateral (left-sided) axillary venous access was obtained, a 7F C315 sheath (Medtronic Inc, Minneapolis, MN) was placed, through which a model 3830 lead (SelectSecure®; Medtronic Inc, Minneapolis, MN) was advanced to the right atrial septal area. The entire septal area was evaluated for areas of adequate sensing and capture. Capture was assessed by change in the P-wave morphology compared to high right atrial pacing as well as conducted ventricular beats (Supplemental Figure S2). The superior and the mid septal areas did not have any good areas of capture; however, an acceptable pacing threshold was obtained in the lower septal area where the lead was actively fixated, and the septal position was confirmed via fluoroscopy (Figure 3, yellow arrows) and transesophageal echocardiogram (Supplemental Figure S3, panel A, yellow arrow). The right atrial septal lead capture threshold was 1 V at 0.4 ms; the pre-existing right atrial lead demonstrated a stable threshold (1.20 V at 0.4 ms). With the help of a Y-connector (2XBiS Y-connector; Oscor, Palm Harbor, FL), both atrial leads were connected to the atrial port of the new dual-chamber pacemaker (Supplemental Figure S3, panel B). The right ventricular lead was then connected to the pacemaker, followed by pocket closure and removal of the CS catheter. With biatrial pacing, there was evidence of P-wave fusion between high right atrial and atrial septal pacing (Supplemental Figure S2, Panel C) with resolution of the left-sided AV dyssynchrony and restoration of AV conduction (Figure 3). The device was programmed in the Managed Ventricular PacingTM mode (Medtronic, Inc, Minneapolis, MN), to minimize ventricular pacing. On 2-month follow-up, the patient was doing very well, with increased energy levels and lack of pedal edema. Device interrogation showed adequate estimated device longevity, minimal ventricular pacing (<1%), and a combined atrial lead impedance of 266 ohms.
Figure 3.
A: Representative schematic (left) with intracardiac electrocardiograms (right) after dual-site atrial pacing: (1) dual-site pacing; (2) atrial resynchronization with synchronous right and left atrial activation and left atrial capture documented in coronary sinus (CS) / left atrium (LA) electrograms; (3) intact native ventricular conduction without ventricular pacing. B,C: Right anterior oblique and left anterior oblique fluoroscopic view (right) demonstrating high right atrial (red arrow) and low atrial septal (yellow arrow) pacemaker leads.
Discussion
We report an unusual case where IACB owing to prior cardiac surgery and catheter ablations resulted in unnecessary ventricular pacing and left-sided AV dyssynchrony, which we were able to demonstrate with intracardiac recordings. To the best of our knowledge, this is the first documented case of dual-site atrial pacing that led to a reduction in ventricular pacing and resolution of systemic AV dyssynchrony in a patient with IACB.
IACB occurs when circumferential conduction block occurs in the atrium, usually owing to some combination of extensive idiopathic fibrosis, structural heart disease, prior cardiac surgery, and ablation procedures.1 While IACB can lead to either significant conduction delay or complete conduction block to left-sided atrial activation, it may also lead to functional AV block owing to the AV node and His-Purkinje system being electrically connected to atrial tissue on the distal side of the zone of conduction block. Placement of a traditional dual-chamber pacemaker in this situation could lead to incomplete atrial activation from the atrial paced impulse, followed by ventricular pacing with retrograde conduction via the AV node to the atrial tissue beyond the point of block, resulting in AV dyssynchrony.5 In our case, most of the lateral right atrium was dissociated from the septum and left atrium, and ventricular pacing resulted in predominantly left-sided AV dyssynchrony (owing to retrograde conduction), as demonstrated with intracardiac electrocardiograms.
Dual-site atrial pacing has previously been reported for patients at risk for atrial fibrillation. It has been implemented via temporary pacing wires in the immediate post–cardiac surgical period to decrease postoperative atrial fibrillation burden and length of stay.6 Permanent dual-site atrial pacing was also studied in a randomized, multicenter crossover trial of 118 patients, where pacing the high right atrium and septum outside the CS increased the time to recurrence of atrial fibrillation.7 Given advances in therapies for atrial fibrillation, including catheter ablation, and the preference to avoid additional leads, permanent dual-site atrial pacing is not routinely performed. However, in documented cases of IACB leading to electrical and hemodynamic perturbation, dual-site atrial pacing should be considered.
Single-site left atrial pacing has been performed in patients with IACB with short left atrioventricular intervals and heart failure with preserved ejection fraction, where it has shown to improve symptoms without the need for dual-site pacing.8 In our case, single-site atrial septal or left atrial pacing could have resolved both unnecessary ventricular pacing and left-sided AV dyssynchrony. However, given that a significant part of the right atrium, including the right atrial appendage, was dissociated from the interatrial septum (with documentation of entrance block), right-sided AV dyssynchrony would result from atrial septal pacing alone, and hence we performed dual-site atrial pacing. There are several techniques to perform left atrial pacing instead of septal pacing, including active or passive fixation within the main body of the CS, prolapse of a canted left ventricular lead, or wedging a lead into the vein of Marshall.9, 10, 11, 12, 13 These techniques are complex and can be associated with high thresholds and dislodgements (up to 42% with passive leads).10 We found that pacing the interatrial septum in this case was easily facilitated by use of a dedicated sheath that directs the lead to the septum. We were able to achieve right- and left-sided atrioventricular synchrony with a significant reduction in unnecessary ventricular pacing.
Currently, dual-site atrial pacing with ventricular pacing can only be performed with the help of a bifurcated Y-connector. Assessing atrial capture with dual-site pacing can be challenging, and it may be aided by observing P-wave morphology on a 12-lead electrocardiogram during threshold testing, along with watching for appearance of intrinsic electrograms when 1 or both leads lose capture, assuming 1 or both compartments have intrinsic rhythms in the absence of pacing. At the time of implant, we assessed the capture threshold of the right atrial septal lead by confirming the presence of CS atrial electrograms and conducted native QRS complexes that were only present during atrial septal capture. We assessed capture of the high right atrial lead by watching the surface P-wave morphology, which showed fusion when both leads were capturing atrial tissue. The lead impedance is lower with dual-site pacing using a Y-connecter owing to the principle of total resistance in the setting of parallel circuits; in some instances, this could lead to premature battery depletion, which is a trade-off for the benefit of dual-site pacing. In our case, the estimated battery longevity did not appear to be significantly different from expected with the lead impedance values seen during Y-connected dual-site atrial pacing.
Conclusion
In conclusion, we report a unique case of IACB in a patient with a dual-chamber pacemaker, which led to unnecessary ventricular pacing and systemic AV dyssynchrony that was overcome with dual-site atrial pacing to achieve interatrial, atrioventricular, and ventricular synchrony. Dual-site atrial pacing should be considered in patients where IACB can result in significant hemodynamic perturbances with single-site pacing alone.
Footnotes
Conflict of interest statement: The authors have no conflicts of interest pertinent to this manuscript to disclose.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2021.01.004.
Appendix. Supplementary data
Supplemental Figure S1.
Coronary sinus pacing (yellow stars) results in conduction to the ventricle (red boxes) but no conduction into the dissociated aspect of the right atrium where isolated potentials (blue boxes) are seen confirming ‘entrance block’.
Supplemental Figure S2.
A- High right atrial pacing showing local atrial capture (blue box) followed by ventricular pacing and retrograde additional p-waves within T-waves (white box); B-Atrial septal pacing showing atrial capture (yellow box) followed by conducted QRS complexes (red arrows) with T-wave memory; C- Dual site atrial pacing showing subtle fusion between high right atrial and atrial septal pacing (red box) followed by conducted QRS complexes.
Supplemental Figure S3.
A- Transesophageal echocardiography (left) demonstrating dual-site atrial pacemaker with high right atrial (red arrow) and low atrial septal (yellow arrow) leads; B- Picture of Y-connector connecting bi-atrial pacemaker leads.
References
- 1.Johner N., Namdar M., Shah D.C. Intra- and interatrial conduction abnormalities: hemodynamic and arrhythmic significance. J Interv Card Electrophysiol. 2018;52:293–302. doi: 10.1007/s10840-018-0413-4. [DOI] [PubMed] [Google Scholar]
- 2.de Luna A.B., Massó-van Roessel A., Robledo L.A.E. The diagnosis and clinical implications of interatrial block. Eur Cardiol. 2015;10:54–59. doi: 10.15420/ecr.2015.10.01.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal S.B., Spodick D.H. Electromechanical dysfunction of the left atrium associated with interatrial block. Am Heart J. 2001;142:823–827. doi: 10.1067/mhj.2001.118110. [DOI] [PubMed] [Google Scholar]
- 4.Eicher J.C., Laurent G., Mathé A. Atrial dyssynchrony syndrome: an overlooked phenomenon and a potential cause of ‘diastolic’heart failure. Eur J Heart Fail. 2012;14:248–258. doi: 10.1093/eurjhf/hfr169. [DOI] [PubMed] [Google Scholar]
- 5.Schüller H., Brant J., Camm A. The pacemaker syndrome: old and new causes. Clin Cardiol. 1991;14:336–340. doi: 10.1002/clc.4960140410. [DOI] [PubMed] [Google Scholar]
- 6.Burgess D.C., Kilborn M.J., Keech A.C. Interventions for prevention of post-operative atrial fibrillation and its complications after cardiac surgery: a meta-analysis. Eur Heart J. 2006;27:2846–2857. doi: 10.1093/eurheartj/ehl272. [DOI] [PubMed] [Google Scholar]
- 7.Saksena S., Prakash A., Ziegler P. Improved suppression of recurrent atrial fibrillation with dual-site right atrial pacing and antiarrhythmic drug therapy. J Am Coll Cardiol. 2002;40:1140–1150. doi: 10.1016/s0735-1097(02)02068-5. [DOI] [PubMed] [Google Scholar]
- 8.Laurent G., Eicher J.C., Mathe A. Permanent left atrial pacing therapy may improve symptoms in heart failure patients with preserved ejection fraction and atrial dyssynchrony: a pilot study prior to a national clinical research programme. Eur J Heart Fail. 2013;15:85–93. doi: 10.1093/eurjhf/hfs150. [DOI] [PubMed] [Google Scholar]
- 9.Corbisiero R., Kazemian P., Kos C. Chronic performance of standard pacing leads placed in the coronary sinus: an answer to chronic pacing for diastolic heart failure? J Card Fail. 2019;25:S153. [Google Scholar]
- 10.Levy T., Walker S., Rex S. A comparison between passive and active fixation leads in the coronary sinus for biatrial pacing: initial experience. Europace. 2000;2:228–232. doi: 10.1053/eupc.2000.0105. [DOI] [PubMed] [Google Scholar]
- 11.Worley S.J., Gohn D.C. Prolapsed double-canted bipolar left ventricular lead for pacing the left atrium via the coronary sinus: experience in 11 patients. Europace. 2012;14:445–448. doi: 10.1093/europace/eur331. [DOI] [PubMed] [Google Scholar]
- 12.Verma S. Permanent left atrial pacing via the vein of Marshall. J Cardiovasc Electrophysiol. 2007;18:785. doi: 10.1111/j.1540-8167.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 13.Mirza I., James S., Holt P. Permanent left atrial pacing: a 2-year follow-up of coronary sinus leads. Pacing Clin Electrophysiol. 2004;27:314–317. doi: 10.1111/j.1540-8159.2004.00434.x. [DOI] [PubMed] [Google Scholar]