Abstract
The speed of mRNA translation depends in part on the amino acid to be incorporated into the nascent chain. Peptide bond formation is especially slow with proline and two adjacent prolines can even cause ribosome stalling. While previous studies focused on how the amino acid context of a Pro-Pro motif determines the stalling strength, we extend this question to the mRNA level. Bioinformatics analysis of the Escherichia coli genome revealed significantly differing codon usage between single and consecutive prolines. We therefore developed a luminescence reporter to detect ribosome pausing in living cells, enabling us to dissect the roles of codon choice and tRNA selection as well as to explain the genome scale observations. Specifically, we found a strong selective pressure against CCC/U-C, a sequon causing ribosomal frameshifting even under wild-type conditions. On the other hand, translation efficiency as positive evolutionary driving force led to an overrepresentation of CCG. This codon is not only translated the fastest, but the corresponding prolyl-tRNA reaches almost saturating levels. By contrast, CCA, for which the cognate prolyl-tRNA amounts are limiting, is used to regulate pausing strength. Thus, codon selection both in discrete positions but especially in proline codon pairs can tune protein copy numbers.
Subject terms: Ribosome, Evolutionary genetics, tRNAs, Bacterial genetics
Ralph Krafczyk et al. demonstrate that both proline codon usage and prolyl-tRNA abundance affect mRNA translation rates of polyproline-motifs in bacteria. These results provide additional insight into sequence-specific translation regulation.
Introduction
Proline has a set of characteristics that is not found in other proteinogenic amino acids. It is the only n-alkyl amino acid and thus has unique chemical properties. Its pyrrolidine ring makes proline conformationally rigid and thus it can shape protein structure: depending on its configuration—cis or trans—the binding axis rotation of amide bonds changes with major consequences for folding1. Peptide stretches enriched in prolines can even form a distinct type of secondary structure, the so called polyproline helix2. However, all these unique features come at a price. Not only is peptide bond formation with proline the slowest compared to all other proteinogenic amino acid3–5, but ribosomes can even be arrested when translating stretches of proline residues6–8. However, consecutive prolines occur frequently in eukaryotic and prokaryotic proteomes9,10. For example, in Escherichia coli every third protein contains at least one polyproline motif (PP-motif, at least diproline)11 and in Streptomyces species there is more than one PP-motif per protein on average12. The explanation for this apparent oddity is the existence of a ubiquitous elongation factor (termed EF-P in bacteria and a/eIF5A in archaea/eukaryotes) that alleviates ribosome stalling13–16. Nevertheless, EF-P cannot fully compensate for the translational burden caused by PP-motifs11. Intriguingly, bacteria can even benefit from ribosomal pausing by using it to regulate translation rates14. PP-motifs are enriched in inter-domain linker regions, which might promote correct folding, upstream of transmembrane regions, where they could facilitate correct insertion, and close to the protein N-terminus11. Here, similar to rare codons17, PP-motifs might be instrumental in generating a translational ramp and helping to avoid ribosome collisions18.
It is well accepted that the amino acids bracketing PP-motifs influence the pausing strength19–22, thus representing a specific regulatory mechanism of translation. The role of proline codon choice, however, has not yet been investigated, although the incorporation speed of proline into the nascent chain differs significantly depending on which of the four codons (CCA/C/G/U) (Fig. 1a) and three tRNAs (ProK/ProL/ProM) are used (Fig. 1b)5. Here, we have comprehensively investigated how the interplay of codon choice and tRNA abundance affect the translation of PP-motifs.
Fig. 1. Diversity of proline codons and their corresponding tRNAs.
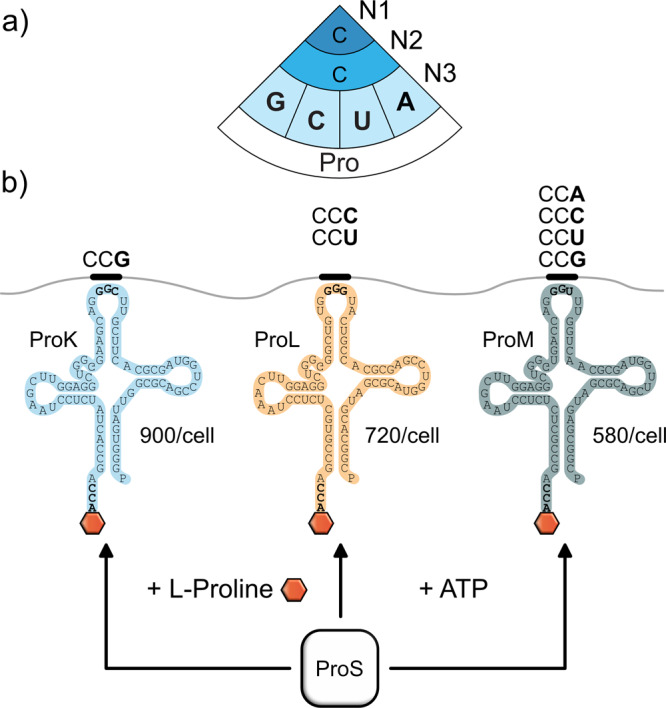
a The genetic code contains four codons for proline: CCG, CCC, CCU, and CCA. b The three tRNAs ProK, ProL, and ProM recognize distinct sets of proline codons and exhibit different levels of abundance within the cell34. All three prolyl-tRNAs are charged by the prolyl-tRNA synthetase ProS.
Results
Distribution of proline codon pairs suggests their regulatory role in translation
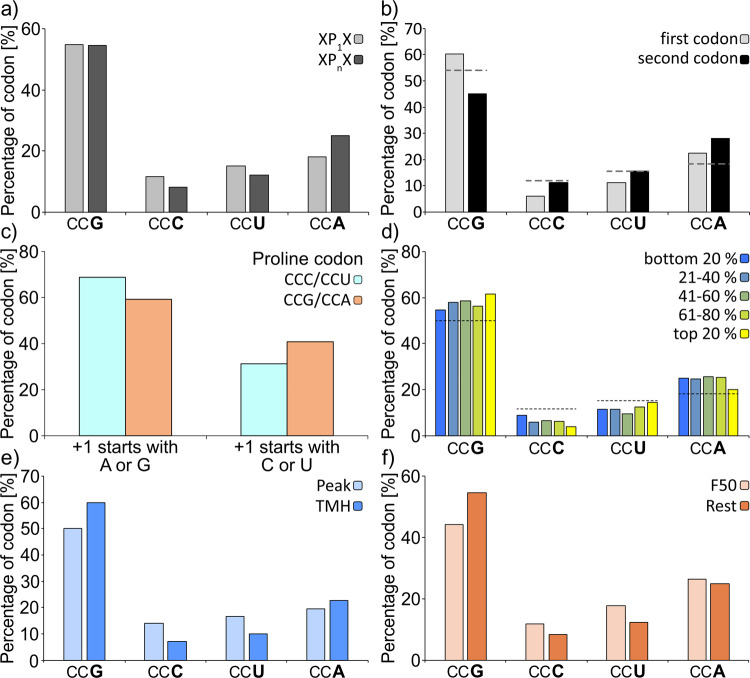
Our study started with a bioinformatics analysis, in which we investigated whether codon usage differs between single prolines and proline pairs in the proteome of E. coli MG1655 (Figs. 2 and 3). We observed a depletion of CCC (8.1 vs. 11.6%) and CCU (12.3 vs. 15.3%) in codon pairs as compared to single prolines (Fig. 2a). Both of these codons delay diproline synthesis more (tdip[CCC]) = ~116.3 ms; tdip[CCU]) = ~71.4 ms) than the other two codons (tdip[CCA]) = ~66.7 ms; tdip[CCG]) = ~62.5 ms)5. Selection against slowly translating proline codon pairs is not restricted to E. coli: Out of 15 bacterial genomes with a broad range of GC-content values CCC and CCU are disfavored in 13 and 11 genomes, respectively (Fig. S1 and Supplementary data file S1). We next asked whether this bias might be related to codon order. Reportedly, an mRNA sequence of CCC/U-C/UCN promotes +1 ribosomal frameshifting, which is in principle counteracted by methylation of the corresponding isoacceptor tRNAs ProL and ProM at the 3′ side of the anticodon (m1G37)23,24. However, this modification cannot fully prevent ribosome slipping, as we could demonstrate with a bioreporter in vivo (Fig. S2). Accordingly, it would be plausible that the selective pressure on proline codon pairs is most pronounced for the first codon. Indeed, our analysis unveiled strong avoidance of both CCC and CCU at the first positions, while their occurrence at the second position matches their genome-wide usage (Fig. 2b). Further, the observed bias is not restricted to proline codon pairs but also to single prolines, as long as the downstream codon starts either with “C” or “U” (Fig. 2c).
Fig. 2. Bioinformatic analysis of proline codon bias in E. coli.
a Codon usage of either single (XP1X) or consecutive (XPnX) prolines (with X being any amino acid except proline and n > 1). p value = 1.7e−30, chi-squared test. b Codon usage of the first and second proline in PP-motifs. Only PP-motifs with two consecutive proline residues were included in this analysis. The dashed lines indicate the codon usage for single prolines. p value < 2.2e−16, chi-squared test. c Codon usage for amino acids in the +1-position downstream CCC/CCU (cyan) or CCG/CCA (orange) encoded single prolines. p value < 2.2e−16, chi-squared test. d Correlation between proline codon usage in PP-motifs and translation efficiency from least efficiently translated proteins (dark blue) to most efficiently translated proteins (yellow). The dashed lines indicate the codon usage for single prolines. e Difference between proline codon usage of PP-motifs in the peak region (light blue, amino acids 49–59 from the TMH start where PP-motifs are enriched to facilitate the efficient insertion of TMH into the membrane) and TMHs (blue; transmembrane helices in which PP-motifs are depleted for proper folding of transmembrane segments11. p value = 0.13, chi-squared test. f Proline codon usage in PP-motifs in the first 50 codons (light orange) compared with the rest of proteins (orange). p value = 2.14e−7, chi-squared test.
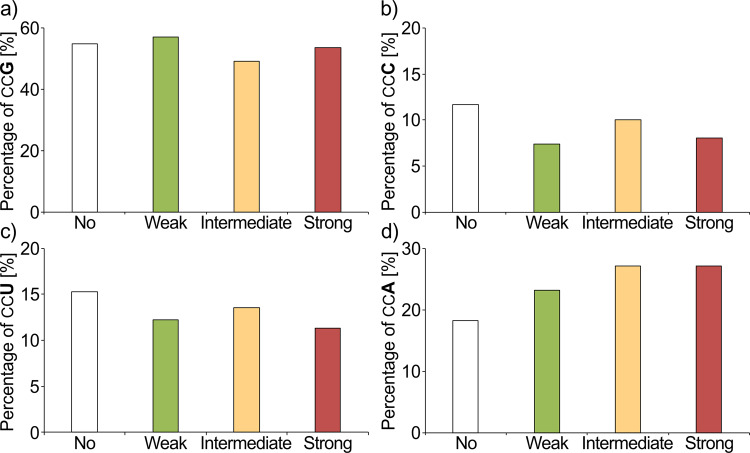
Fig. 3. Codon usage in PP-motifs of different pausing strength.
Pausing strength of PP-motifs depends on the upstream amino acid context11,20,22 resulting in weak, intermediate, and strong pausers. The pausing strength resulting from amino acid context is indicated by colored bars (no pausing—white; weak pausing—green; intermediate pausing—yellow; strong pausing—red). Codon usage in differently strong pausing motifs is shown for CCG (a), CCC (b), CCU (c), and CCA (d) codons. The difference is significant according to chi-squared test, p value = 4.2e−3.
Interestingly, the negative selection of CCC/U in proline codon pairs is not compensated by overrepresentation of CCG, being the most optimal codon in terms of diproline synthesis rates5. Instead, an enrichment of CCA (18.2 vs. 25%) was found. Ranking proteins with proline pairs according to their translation efficiency (Fig. 2d) revealed a preference for CCG in the top 20%. These findings imply a potential regulatory role of the relative CCA accumulation in PP-motifs, e.g., to slow down translation for proper membrane insertion or at the protein start to generate a translational ramp as a late stage of translation initiation thereby reducing ribosomal traffic jams17. In fact, non-CCG proline codons are enriched in these regions, further supporting the idea (Fig. 2e, f).
PP-motifs can be classified into “weak”, “intermediate”, and “strong” pausing motifs according to their interference with translation11,20,22. These differences result from the preceding amino acid. We were therefore interested whether specific proline codon biases exist within these subgroups of PP-motifs. Thus, we dissected PP-motifs accordingly (Fig. 3a–d and Supplementary data file S2). The most pronounced difference to single prolines was again the CCA usage (Fig. 3d). This codon represents 23.2% of all proline codons associated with weak pausing compared to 27.2% and 27.1% for intermediate and strong pausing, respectively. This difference is significant according to a two-sided Z test (p value = 7.0e−3). Thus, the differences between CCA and CCG in terms of pausing strength might be an additional mechanism to tune the translation efficiency.
An in vivo reporter system to quantify translational pausing
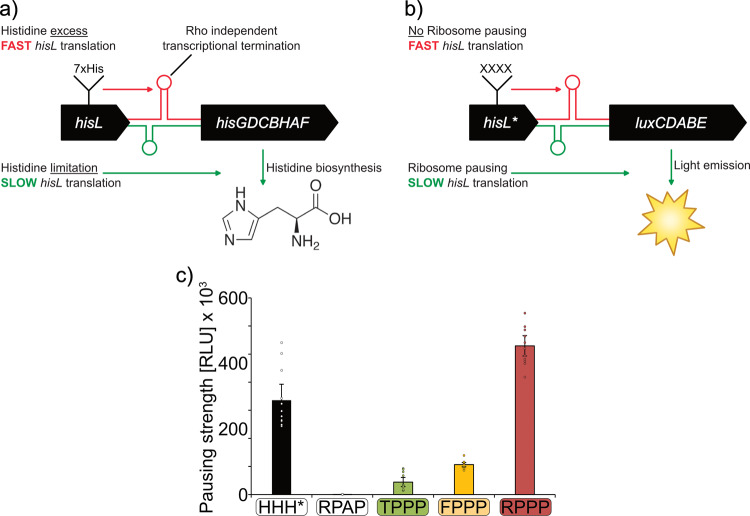
In order to measure codon effects on translational efficiency, we established a reporter system that is capable of determining translational pausing strength within living cells. The system hijacks the attenuation mechanism of the histidine biosynthesis operon hisGDCBHAF (Fig. 4a)25. Here, translational speed of the preceding His-leader peptide (HisL) controls expression of the downstream structural genes26. Naturally this peptide contains seven consecutive histidines. When charged histidyl-tRNA is present in excess, ribosomes translate HisL non-stop, which in turn results in the formation of an mRNA attenuator stem loop that prevents transcription of hisGDCBHAF. When histidine concentrations are limiting, HisL translation is decelerated due to a lack of charged histidyl-tRNAs and an alternative mRNA stem loop is formed, which in turn permits transcription of the histidine biosynthesis genes. We fused the 5′ untranslated region (5′ UTR) of hisGDCBHAF as well as the preceding hisL with the luxCDABE operon of Photorhabdus luminescens27 and integrated the resulting construct via single homologous recombination into the E. coli chromosome (Fig. 4b)28,29. Monitoring of light emission over 16 h of growth showed a maximal output of only around 500 RLU, demonstrating that almost no pausing takes place under standard growth conditions in complex medium (LB). This was expected as LB contains about 1 mM of histidine30, which means an excess of about 100-fold31.
Fig. 4. The His-pausing system for in vivo measurement of pausing strength.
a Architecture of the histidine biosynthesis operon in E. coli. In its native state, the histidine biosynthesis gene cluster (hisGDCBHAF) is regulated by the His-leader peptide (hisL). This peptide contains seven consecutive histidines. At high histidine/histidyl-tRNA levels, translation efficiently proceeds through the His-leader peptide, resulting in the formation of an attenuator stem loop (red) that prevents transcription of the downstream genes. At low histidine and histidyl-tRNA levels translation is slowed down allowing for transcription and translation of the structural genes and synthesis of histidine (green). b Architecture of the His-pausing operon. An engineered His-leader peptide (hisL*) precedes the structural genes of the lux operon (luxCDABE). Here, His1 through His4 are exchanged by artificial sequence motifs (XXXX). In case of non-consecutive proline motifs (e.g., RPAP) there is no pausing, resulting in the formation of an attenuator stem loop (red) that prevents transcription of the downstream genes and low light emission. In the presence of motifs that contain consecutive prolines (e.g., RPPP) translation is slowed down allowing for transcription and translation of the structural genes and thus increased light emission (green). c Maximal luminescence emission at PP-motifs with increasing pausing strength. HisL*_Lux operons carrying a stop codon at the position corresponding to His4 (HHH*), non-consecutive (RPAP) or consecutive prolines of varying known pausing strength at the hisL* position (Weak: TPPP; green. Intermediate: FPPP; yellow. Strong: RPPP; red) were chromosomally integrated in E. coli BW25113 and tested for maximal luminescence emission. Threonine, phenylalanine, and arginine were encoded by ACC, TTT, and CGC, respectively. CCG was used as proline codon in all constructs. n = 12, Error bars indicate 95% confidence intervals.
To assess the potential of our reporter to measure ribosome pausing we generated HisL variants encompassing PP-motifs of varying strength (Fig. 4c). Specifically, we substituted His1 through His4 by TPPP, FPPP, or RPPP being representatives of weak, intermediate, and strong pausers, respectively22. As a positive control, we placed a stop codon in the position corresponding to His4. As a negative control, we chose RPAP, which does not reduce translational speed14. As codon for alanine, we selected GCG being highly similar to the proline codon CCG. This choice was made to minimize putative effects of mRNA structural alterations.
To delineate codon effects from those caused by the peptide sequence all prolines were encoded only by CCG. The maximal light output of the corresponding E. coli strains RPAPCCG, , , and increased from 390 RLU to 44,000 RLU to 106,000 and 530,000 RLU, respectively (Fig. 4c). The results obtained here perfectly match published datasets based on completely different experimental principles14,19,20,22,32. Accordingly, the outcome of our assay is a result of ribosome pausing that is determined by sequence identity but not mRNA structure. Notably, the positive control HHH* reached a maximum RLU of 336,000, which was in the same range as the reporter activity of the RPPP construct. We can therefore conclude that ribosome pausing induced by strong stallers is comparable to a stop caused by a termination signal.
Of particular interest is, that the measurements were conducted in an E. coli wild-type strain where stalling at consecutive prolines is alleviated by EF-P14. Thus, we have a tool in hand to determine pausing strength in vivo. Using the system, we unambiguously demonstrate that the burden associated with PP-motifs is an inherent translational feature and explains the strong selective pressure causing the proteome shaping11.
Codon choice modulates pausing strength at consecutive proline motifs
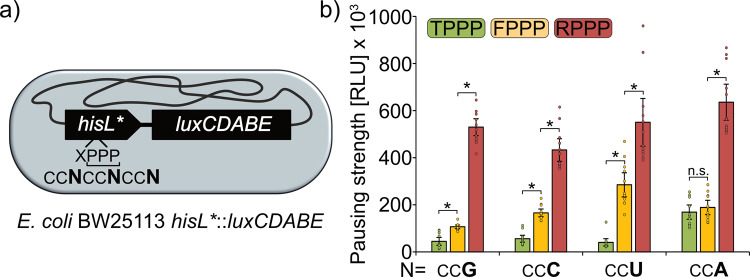
To investigate whether the statistical tendencies of codon usage in PP-motifs can be attributed to physiological differences we conducted a systematic in vivo analysis. To this end we constructed a series of 4 × 4 HisL*_Lux reporter strains (Fig. 5a and Supplementary data file S3). Utilizing the strong pauser RPPP, CCG, CCA, and CCU were indistinguishable from each other, each producing a maximal light output of over 525,000 RLUs (Fig. 5). Only with CCC codons we found around 1.2-fold reduced maximal light emission. When testing a motif with intermediate strength (FPPP) a different pattern was obtained. In this case, CCU stretches produced significantly more light than the other codons, whereas emission using CCG was significantly decreased. CCC and CCA ranged in the middle of both. Interestingly, the most pronounced effect of codon choice on pausing strength occurred with the weak pauser TPPP. The luminescence with was significantly elevated by at least threefold compared to the strains encoding , , or . Notably, such an increase is equivalent to a step in the pausing strength from weak to intermediate pausing (Figs. 5b and S3A). This result is also in perfect agreement with our genome scale analysis (Fig. 3a–d) and explains the strong selection against CCA in weak pausers. On the other hand, the bias in favor of CCA in intermediate and strong pausers might be attributed to a regulatory role that requires a further slowdown of translation. To exclude that the observed effects derive from mRNA structure alterations, we conducted another analysis utilizing a second reporter series XPPP with X being N (AAC) for weak, L (CTG) for intermediate and W (TGG) for strong22 (Fig. S3B, C). Expectedly, the activities are congruent with the T/F/RPPP derived data including the CCA effect in the weak pausing context. Taken together, these results demonstrate that codon choice in PP-motifs is capable of influencing ribosome pausing.
Fig. 5. Codon-dependent pausing strength at weak, intermediate, and strong PP-motifs.
a Genomic organization of the HisL*_Lux reporter. Synthetic His-Leader peptides (HisL*) preceding the lux genes (luxCDABE) were genomically integrated at the his-locus. In hisL*, His1 one was replaced by a variable amino acid (X) to modulate pausing strength16. His2 through His4 were replaced by proline. In this regard several reporter strains (Supplementary data file S3) were generated with hisL* varying in the proline codon usage and are denoted as where the underlined X designates the preceding amino acid and the bold N designates the wobble base used for encoding the proline residues. b HisL*_Lux carrying PP-motifs of varying pausing strength (weak—TPPP: green; intermediate—FPPP: yellow; strong—RPPP: red) with different proline codon usage were chromosomally integrated in E. coli BW25113 and tested for maximal luminescence emission. n = 12, Error bars indicate 95% confidence intervals. Data for CCG codons are duplicated from Fig. 5 for better overview. Statistically significant differences according to unpaired two-sided t-tests (p value < 0.05) are indicated by asterisks.
tRNA abundance influences pausing strength at all proline codons
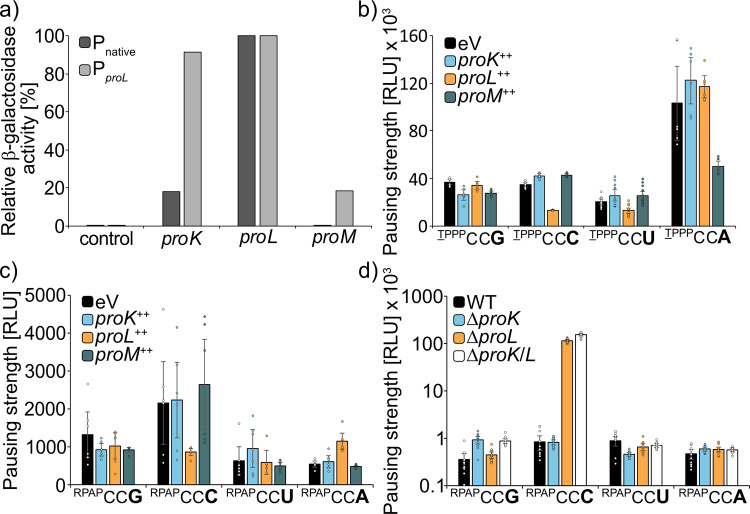
The variations in proline codon bias of PP-motifs of varying strength, particularly the one of CCA, raised the question whether tRNA abundance might contribute to pausing strength. In E. coli, three tRNAs—ProK, ProL, and ProM—are responsible for decoding of proline codons (Fig. 1). ProM represents a general tRNA that is capable of recognizing them all33, while ProL and ProK are more specialized and decode CCC/U and CCG, respectively5. These differences have a quantitative effect on the reading probabilities of the individual codons. Taking the copy numbers of ProK (900/cell), ProL (720/cell), and ProM (580/cell) into account, CCG has the highest number of the corresponding tRNAs (900 + 580 = 1480/cell)34 and thus matches very well to the general codon usage in the E. coli genome, where more than 50% of all prolines are encoded by CCG (Fig. 2). CCA is the other extreme, being recognized solely by ProM and accordingly only 580 tRNA copies per cell are available for translation.
To assess an effect of prolyl-tRNA copy numbers on pausing strength, we unbalanced the native ratios in favor of either ProK, ProL, and ProM (ProX++) by ectopically expressing them from PproL. Beforehand, the 5′ upstream sequences of proK (5′proK), proL (5′proL), and proM (5′proM) were tested on promoter activity, by generating an artificial operon with lacZ (Fig. 6a). As expected, no β-galactosidase activity could be measured when utilizing 5′proM, as proM is part of the argX polycistronic operon (argX_hisR_leuT_proM)35,36. From the remaining two regions—5′proK and 5′proL—the latter gave a higher reporter signal and was therefore chosen as constitutive promoter for all three prolyl-tRNAs.
Fig. 6. Influence of prolyl-tRNA copy number on the codon-dependent pausing strength at PP-motifs.
a Approximation of E. coli BW25113 cells carrying the weak HisL*_Lux operon (TPPP) with different proline codon usage were transformed with pBBR1 MCS4-lacZ plasmids encoding ProK, ProL, or ProM under the control of their corresponding native promoters. n = 4. b E. coli BW25113 cells carrying the weak HisL*_Lux operon (TPPP) were transformed with pBBR1 MCS4-lacZ plasmids encoding for ProK, ProL, or ProM under control of PproL and tested for bioluminescence emission. n = 6. c E. coli BW25113 cells carrying the “non-PP” HisL*_Lux operon (RPAP) were transformed with pBBR1-MCS4-lacZ plasmids encoding for ProK, ProL, or ProM under control of PproL and tested for bioluminescence emission. n = 6. d The “non-PP” HisL*Lux operon (RPAP) was genomically integrated in E. coli BW25113 deletion strains lacking either proK (ΔproK), proL (ΔproL), or both (ΔproK/L) and cells were tested for bioluminescence emission. n = 12, Error bars indicate 95% confidence intervals.
The effect of tRNA copy number increase was first assessed in the four reporter strains which harbor a HisL-TPPP variant each encoded by a series of one of the four distinct proline codons (, , , ) (Fig. 6b). The CCG-specific ProK had a positive but only mild influence on pausing strength, solely when translating . One plausible explanation is that the native copy number of 900/cell is already close to saturating levels and accordingly overexpression does not substantially add to pausing strength reduction. With ProL we observed significantly reduced pauses when testing and , being again in line with the tRNA codon specificity. Interestingly, an increase in copy number of the general tRNA ProM had no major impact on reporter activity of the strains, indicative of a selection in favor of the more specialized tRNAs (ProK and ProL). Conversely, we saw a significant pausing strength reduction (>2-fold) for the , which can only be decoded by ProM.
Second, to separate PP-motif specific effects from those also occurring only with single prolines, a reference reporter set encoding RPAP-HisL variants was included into our study (Fig. 6c). Here, the previously observed minor alleviating effect at CCG codons on translational pausing upon ProK overexpression was lost. On the contrary, an increase in the copy number of ProL still significantly reduced pausing strength at CCC codons, yet no reduction of reporter activity for RPAPCCU was observed. CCC codons are reportedly translated the slowest5, which is in line with a general increase in luminescence compared to all HisL variants encoded by other proline codons. However, this does not explain the stimulatory effect on translational speed when overexpressing ProL: In their in vitro study on dipeptide synthesis with proline Pavlov et al. always employed bulk tRNA when measuring incorporation speed5. Accordingly, tRNA abundance effects were neglected. Our findings now indicate that the observed differences in dipeptide synthesis time might be partially due to ProL limitation. This idea is supported by the fact that the in vitro experiments in Pavlov et al. revealed CCU after CCC as the slowest codon to be decoded5.
The tRNA abundance effect that differs most between consecutive and single prolines is for CCA (Fig. 6b, c). While translational pausing is alleviated by a factor of around three upon overexpression of ProM, we hardly found any changes when analyzing the RPAPCCA reporter. Thus, our findings provide a rationale for the CCA codon bias in PP-proteins.
Third, we performed the converse experiment by deleting the two non-essential tRNA genes proK and proL33, both individually—ΔproK, ΔproL—and in combination—ΔproK/L. These strains (Supplementary data file S3) were investigated on growth and cell morphology (Fig. S4) as well as on the effect they have on pausing strength (Fig. 6d). When analyzing the effects of proK and proL deletions on luminescence we saw the expected increase at RPAPCCG, when proK is missing. The most striking results were obtained upon proL deletion. The light output at CCC significantly increased almost by a factor of 100, whereas translation of the RPAPCCU reporter remained unaffected. This led us to conclude that the general tRNA ProM is a good decoder of CCU codons, as it can compensate for the lack of ProK. By contrast, the strong increase in pausing strength with CCC in ΔproL strains explains the necessity for a more specialized tRNA, which can outperform the ProM decoding capabilities at this specific codon. We therefore speculate that nature has evolved ProL predominantly to read CCC codons in order to compensate for its reduced translational speed. Additional reading of the “U” in the wobble position was acquired later, as a consequence of a mild advantage (Fig. 6b). This idea is also congruent with the identity of the ProL anticodon, which is GGG.
Taken together, we could show that tRNA abundance is a major driving force for the efficient translation of single and consecutive prolines.
Proline codon choice finetunes protein copy number of the pH sensor CadC
Based on our results, we hypothesized that codon choice within PP-motifs can be used as a regulatory means to tune the pausing strength according to stoichiometric requirements. In this regard, counterselection of certain codons would occur in order to prevent modulation of the pausing strength predetermined by the amino acid context. To test this hypothesis, we investigated codon choice in the PP-motif of the transcriptional activator CadC.
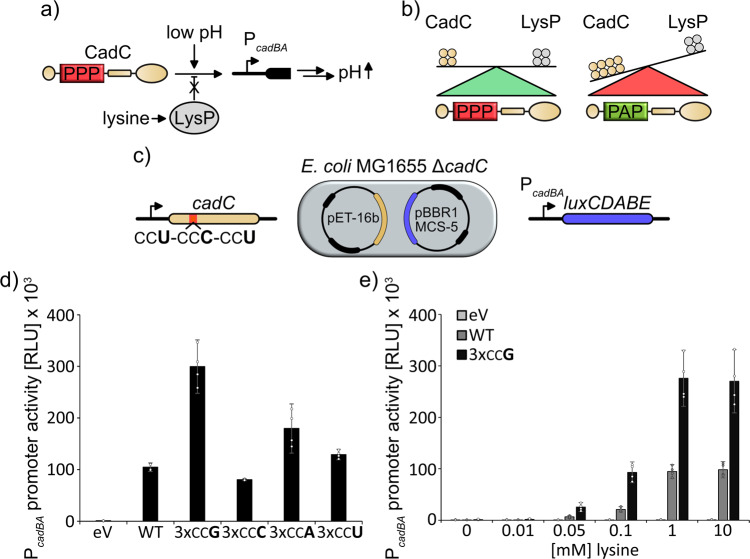
CadC is a membrane-bound transcriptional regulator and part of the E. coli acid stress response37–39. The two external stimuli, mild acidic pH (<6.5) and lysine are needed to activate expression of the cadBA operon. While acidic conditions are sensed by CadC directly, lysine is recognized by a coregulator—the permease LysP. LysP directly interacts with CadC and a specific equilibrium between both proteins is crucial for an adequate transcriptional response (Fig. 7a)14. This equilibrium is strictly dependent on a triproline motif (aa120-122) within CadC37 that is decoded from CCUP120-CCCP121-CCUP12214,32 and preceded by a serine (TCG).
Fig. 7. Codon choice modulates protein expression and ensures physiological protein stoichiometry of the Cad system.
a The Cad system. CadC is a pH sensor that induces expression of its target genes at low pH by binding to the cadBA promoter (PcadBA). Expression of the corresponding gene products ultimately leads to an increase in pH. The lysine dependency of the acid stress response depends on stoichiometric expression of CadC and the co-sensor LysP. b The equilibrium of the protein copy numbers of CadC and LysP is ensured by a triproline motif within the CadC primary structure. Absence of the triproline results in deregulation of the acid stress response due to increased CadC copy number. c Reporter system used to test the cadC translation efficiency. E. coli MG1655 ΔcadC cells were transformed with pET-16B vectors encoding for wild type or proline codon-exchanged variants of CadC. Cells were cotransformed with pBBR1MCS-5 vectors carrying the lux genes under control of the PcadBA promoter. PcadBA promoter activity was assessed by measuring luminescence emission and used as a proxy for CadC copy number14. d PcadBA promoter activity under inducing conditions (pH = 5.8; 10 mM lysine) upon expression of wild-type CadC or proline codon-exchanged CadC variants where all proline codons in the pausing motif have been substituted by the same codon. n = 4. e PcadBA promoter activity at increasing external lysine concentrations. PcadBA induction when cadC contains the natural codon composition is shown in dark gray. PcadBA induction when cadC contains only CCG codons at the relevant PP-motif is shown in black. n = 4, Error bars indicate 95% confidence intervals.
As expression of cadC from pET-16b leads to physiological protein levels and an adequate pH-stress response in E. coli MG1655 cells14, we generated plasmid-encoded CadC variants in which we unified the codons within the triproline motif (Fig. 7b). These were tested with a lux reporter controlled by PcadBA (Fig. 7c)40. Upon monitoring the maximal light output during 16 h of growth in minimal medium under CadC inducing conditions (pH 5.8 and supplemented with 10 mM lysine) we detected a threefold increase in PcadBA activity with CCG stretches in the CadC open reading frame while 3 × CCA, 3 × CCC, and 3 × CCU resulted in only subtle changes in light emission compared to the wild-type protein (Fig. 7d). As previously shown these changes in promoter activity reflect fluctuating copy numbers of the regulator14. Of note, only the changes between the CadC variants with 3 × CCA and 3 × CCG can be directly compared in terms of translation efficiency as a consequence of differences in tRNA abundance and codon anticodon pairing5. For the variants with 3 × CCC and 3 × CCU, the additional effect of ribosome slipping also causes decreased protein output.
To test for physiological repercussions of the elevated protein production with CadCCCG, we performed the same experiment again, but tested different lysine concentrations (Fig. 7e). In this setup both the wild type and the CadCCCG variant reached the highest induction level at 1 mM lysine but the latter showed a threefold increased maximal light output. More importantly, CadCCCG turned on cadBA transcription already at 100 µM lysine. This concentration, however, is insufficient for pH neutralization. Thus, codon choice within the CadC triproline motif is crucial to maintain an optimal ratio between CadC and LysP in order to achieve an adequate stress response.
Discussion
The theory of codon bias postulates the correlation between preferred codons and abundances of their iso-accepting tRNAs41, thereby increasing translation efficiency42 and accuracy43. Although the “tRNA abundance” theory also applies to proline codons (Fig. S1)34, the strong correlation with incorporation velocities seems to be more important5. This explains, for example, why CCC is a rather neglected codon in E. coli as it interacts least efficiently with the tRNAPro–EF-Tu–GTP ternary complex5. Moreover, a pair of CCC/U codons promotes ribosomal frameshifting (Fig. S2)24.
Generally, proline pairs are difficult to translate as they cause ribosome stalling7. Their frequent occurrence in nature points to a selective advantage that outweighs the concomitant translational burden16 and has even favored the emergence of a specialized elongation factor EF-P to aid in translation14. This advantage is due to the unique properties of polyprolines affecting protein structure2 and function10. Although there is an evolutionary trend to reduce the translational load, we have previously identified specific regions where pausing by PP-motifs is favored to limit translation rates and to facilitate proper membrane insertion and correct folding11.
Our previous work focused on the PP-motifs and their amino acid context. We have now extended our study to the transcript level, which led to several new insights into the relation between codon pairs and tRNA abundance.
First, we found that the codon bias in consecutive prolines differs significantly from that in single prolines, which helps to avoid slippery sequences (CCC/U-CCN) and to boost translation efficiency. Only in the regions where increased pausing time might be beneficial, such as the vicinity of the translational start and downstream of transmembrane helices, more slowly translating codons are favored (Fig. 2). Moreover, we have demonstrated the physiological importance of codon choice on one prominent example—the pH sensor CadC. Here, the silent mutation of prolines of CCUP120-CCCP121-CCUP122 into 3 × CCG led to a deregulation of the acid stress response as a result of an increased protein copy number. Thus, there is a concerted adjustment of both codon usage within PP-motifs and their amino acid context, in turn allowing for a precise adjustment of protein copy numbers. We note, that additional factors such as mRNA structure or stability might also contribute to this effect.
Second, we have uncovered the specific effects associated with isoacceptor tRNAPro. Overall, we found that both overexpression and deletion of each individual prolyl-tRNA gene—proK, proL, or proM—affected translation at their cognate codons, regardless of their amino acid context. The most pronounced effect was observed with ProL and on CCC, whereas the benefit for the other target codon CCU was comparatively small. One reason for this might be the ProL anticodon—GGG, which could lead to different affinities between both of the recognized codons. Generally, dipeptide synthesis is slowest with CCC and CCU, which can also explain their scarceness in the genome. This rare usage was also one reason for having included proL into the pRARE plasmid in order to augment the yield and fidelity of heterologously produced proteins44. Our data now show that even under natural conditions ProL is limiting and thus increasing its copy number might have a positive effect on endogenously produced proteins (Fig. 6b, c). Especially, decoding of CCC benefits from ProL overproduction (Fig. 6) and thus heterologous expression of genes from GC-rich organisms such as Streptomyces species might lead to an increased yield. In this regard, it is notable that, e.g., S. venezuelae encodes a second copy of proL, presumably to circumvent this limitation (CCG: 52%, CCC: 43%, CCU: 3% CCA: 2%). Moreover, tRNA abundance explains also the selective pressure against CCA in weak PP-motifs. One might therefore speculate that recruitment of ProM to the ribosome is the rate limiting step in the weak context. Interestingly, increased copy number of ProM did not result in a decrease of ribosome pausing at any other codon than CCA. For CCC the reason might be in the poor interaction between the cmo5U34 modified base and the 3′ cytosine of the CCC codon. Besides that, even under control of PproL, proM was less efficiently transcribed than the other tRNAs, indicating that the relative titers compared to the more specialized ProK and ProL for translation of CCG/C/U were not as strongly affected. The preference for CCG for which the cognate tRNA levels are close to saturation is consistent with this idea (Fig. 6). Further, CCG is enriched in PP-motifs at the expense of CCA in the top 20% of proteins in terms of translation efficiency (Fig. 2). In general, CCG seems to be the “best” proline codon in bacteria, when it comes to translation efficiency of codon pairs. This also explains why especially this codon is avoided in the CadC proline codon triplet, as here an extremely low copy number is crucial for a regulated acid stress response14.
Thus, codon choice in proline codon pairs represents an elegant strategy to control translation efficiency and finetune protein copy numbers in bacteria.
Material and methods
Plasmid and strain construction
All strains, plasmids, and oligonucleotides used in this study are listed and described in Supplementary data files S3–S5, respectively. All kits and enzymes were used according to manufacturer’s instructions. Plasmid DNA was isolated using the Hi Yield® Plasmid Mini Kit from Süd Laborbedarf. DNA fragments were purified from agarose gels using the Hi Yield® Gel/PCR DNA fragment extraction kit from Süd Laborbedarf. All restriction enzymes, DNA modifying enzymes, and the Q5® high fidelity DNA polymerase for PCR amplification were purchased from New England BioLabs.
The pNPTS-138-R6KT_hisL_luxCDABE vector was generated by amplification of hisGDCBHAF operon leader peptide hisL from E. coli BW25113 genomic DNA and ligation into pNPTS-138-R6KT_PBAD_luxCDABE after restriction with SphI and NcoI. All variants of hisL (hisL*, Supplementary data file S4) were generated by overlap extension PCR with mutagenized primers (Supplementary data file S5) from pNPTS-138-R6KT_hisL_luxCDABE and subsequent cut/ligation into pNPTS-138-R6KT_PBAD_luxCDABE as described above. HisL*_lux reporter strains (Supplementary data file S3) were generated by single homologous recombination as described previously29. Briefly, E. coli WM3064 cells were transformed with pNPTS-138-R6KT vectors28 carrying the lux operon preceded by either native or synthetic His-leader peptides (Supplementary data file S4). The vectors were transferred into the target E. coli BW25113 or Δefp cells by conjugation. Transformants were selected from LB agar plates supplemented with kanamycin sulfate. PCR (Pf: HisL_chk_fw Pr: LuxC_chk_rev, Supplementary data file S5) and subsequent sequencing of the amplicon were used to verify incorporation of the correct hisL*.
tRNA deletion strains (Supplementary data file S3) were generated according to the “Quick and Easy E. coli Gene Deletion Kit by Red®/ET® Recombination” protocol (Gene Bridges). In short, primers containing 50 base-pair overhangs corresponding to the tRNA loci (Supplementary data file S5) were used to amplify linear FRT-side-flanked resistance cassettes from either FRT-PGK-gb2-neo-FRT or FRT-PGK-gb2-cat-FRT (Supplementary data file S4) using PCR. E. coli BW25113 cells transformed with pRED/ET were transferred from a thick overnight culture into a fresh culture in LB by 1:100 dilution, which was grown at 37 °C for about 2 h, until an optical density at 600 nm (OD600) of 0.3 was reached. Cells were then harvested and washed in 10% glycerol three times. The cells were subsequently transformed with the linear fragment by electroporation. Successful integration was confirmed by selective growth on LB plates containing either kanamycin sulfate or chloramphenicol and by PCR. Loss of the temperature sensitive pRED/ET plasmid was confirmed by selective growth on LB plates containing carbenicillin sodium salt or no antibiotic. To remove the chromosomally integrated resistance cassettes, the corresponding strains were transformed with the 707-FLPe plasmid (Supplementary data file S4) and transformants were subsequently inoculated in LB and grew at 30 °C for 2 h before shifting the temperature to 37 °C for overnight incubation. On the next day, cells were streaked out on LB plates and incubated overnight at 37 °C. Successful removal of the resistance cassettes and the temperature sensitive 707-FLPe plasmid was confirmed by selecting cells on plates with and without antibiotic and subsequent sequencing of the corresponding loci after colony PCR.
Plasmids for expression of E. coli tRNAs under control of their native promoters were generated by amplification of the corresponding genes and putative regulatory regions from E. coli BW25113 genomic DNA using specific primers (Supplementary data file S5) and subsequent cut/ligation into the pBBR1-MCS4-lacZ vector29 (Supplementary data file S4). Plasmids for expression of E. coli tRNAs under control of the proL promoter were generated using primers with a 70 BP overhang corresponding to PproL (Supplementary data file S5) and subsequent cut/ligation into pBBR1-MCS4-lacZ.
Plasmids for quantification of ribosome slipping were generated by overlap extension PCR using primers with the sequence ATTAACCATGGGGNNNTAGGACTAAAAAAATTTCATTC (Supplementary data file S5) and pBAD_HisA-luxCDABE27 (Supplementary data file S4) as template. The first underlined sequence of the primer designates the initial open reading frame coding for a short peptide that stops at the TAG codon (italic). NNN designates the mutagenized region coding for the slipping sequence. The single base G (bold) allows the +1 frameshift into the luxCDABE open reading frame which is represented by the second underlined sequence.
Growth conditions
E. coli cells were routinely grown in Miller modified Lysogeny Broth (LB)45,46 at 37 °C aerobically under agitation, if not indicated otherwise 1.5% (w/v) agar were used to solidify media when required. Antibiotics were added at the following concentrations: 100 µg/ml carbenicillin sodium salt, 50 µg/ml kanamycin sulfate, 20 µg/ml gentamycin sulfate. Plasmids carrying PBAD47 were induced with L-arabinose at a final concentration of 0.2% (w/v).
Measurement of pausing strength in vivo
Pausing strength at PP-motifs was determined by measuring light output of the lux operon under the control of a synthetic His-leader peptide (HisL*) (Figs. 4c–6). Cells carrying the reporter were inoculated in 96-well plates (Sarstedt TC-Plate 96-Well, Standard d, F) with each well containing 200 µl of LB supplemented with kanamycin sulfate and incubated in an Eppendorf Thermomixer comfort at 37 °C and 550 rpm for at least 16 h. When expressing E. coli tRNAs from MCS4 plasmids, carbenicillin sodium salt was also added to the medium. On the next morning, Corning® 96-well flat clear bottom black polystyrene TC-treated microplates containing 200 µl of LB—supplemented with either kanamycin sulfate alone or in combination with carbenicillin sodium salt—were inoculated with 2 µl of overnight culture. The plates were directly transferred to a Tecan Spark® plate reader. Absorption at 600 nm (Number of flashes: 10; Settle time: 50 ms) and luminescence emission (Attenuation: none; Settle time: 50 ms; Integration time: 200 ms) were determined in between 10-min cycles of agitation (orbital, 180 rpm, amplitude: 3 mm) for around 16 h.
β-Galactosidase activity assay
E. coli HisL* reporter strains (Supplementary data file S3) containing plasmids for expression of E. coli tRNA (Supplementary data file S4) were inoculated in 1.5 ml LB containing kanamycin sulfate and carbenicillin sodium salt and cultivated overnight in an Eppendorf Thermomixer comfort at 37 °C under microaerobic conditions and agitation at 650 rpm. On the next day, the optical density (OD600) was determined in 1 ml volumes containing 0.5 ml overnight culture and 0.5 ml of fresh LB medium. In total, 0.5 ml of overnight culture were transferred to a new 2 ml Eppendorf reaction tube. Cells were harvested by centrifugation and subsequently resuspended in 1 ml Buffer Z (0.06 M Na2HPO4, 0.04 M NaH2PO4, 0.01 M KCl, 0.001 M MgSO4). In total, 0.1 ml Chloroform and 0.05 ml 0.1 % SDS were added and the suspension was mixed by vortexing. Samples were preincubated at 30 °C for 5 min. The reaction was started by adding 0.2 ml of ortho-Nitrophenyl-β-galactoside solution (4 mg/ml in Buffer Z) and stopped by adding 0.5 ml 1 M Na2CO3 when yellow color formation was observed or after 5 min of incubation. The time between starting and stopping the reaction was noted in seconds. The samples were centrifuged at 20,000 × g for 10 min and 1 ml of the reaction solution was transferred to a cuvette. Absorbance at 420 nm was determined and Miller units (MU) were calculated as MU = 1000 × Abs420 × t−1 × V−1 × Abs600−1,48.
Measurement of cadBA promoter activity in vivo
Activity of the cadBA promoter upon exchange of proline codons within the cadC gene (Fig. 7) was assessed using a luminescence reporter as described before40. E. coli MG1655 ΔcadC cells were cotransformed with the reporter plasmid pBBR1-MCS5-PcadBA-lux (Supplementary data file S4) and a pET16B vector for ectopic expression of either the wild-type cadC (pET16B-cadC) or a copy with silent mutations in the proline codon triplet CCUP120-CCCP121-CCUP122 leading to pET16B-cadC_3xCCG, pET16B-cadC_3xCCC, pET16B-cadC_3xCCU, and pET16B-cadC_3xCCA (Supplementary data file S4). As control the reporter plasmid was cotransformed with pET16B. Transformants were incubated in 200 µl of minimal medium developed by Epstein and Kim49 pH 7.6 supplemented with gentamycin sulfate, carbenicillin sodium salt, and 0.2% glucose (w/v) in 96-well plates in a Eppendorf Thermomixer comfort at 37 °C and agitation of 550 rpm overnight. On the next day, 2 µl of overnight culture were transferred to 200 µl of fresh medium supplemented with gentamycin sulfate and carbenicillin sodium salt in a Corning® 96-well flat clear bottom black polystyrene TC-treated microplate. Here, KE pH 5.8, 0.2% (w/v) glucose with varying concentrations of lysine was used. Bioluminescence emission (Attenuation: none; Settle time: 50 ms; Integration time: 200 ms) and growth (Wavelength: 600 nm; Number of flashes: 10; Settle time: 50 ms) were monitored in a Tecan Spark® in 10-min intervals during agitation (orbital, 180 rpm, amplitude: 3 mm) for around 16 h.
Quantification of +1 translational frameshifting in vivo
E. coli BW25113 cells were transformed with plasmids containing pBAD-HisA-luxCDABE plasmids (Supplementary data file S4) in which the luxC gene was cloned out of frame as described above. In total, 200 µl LB containing the carbenicillin sodium salt were inoculated with 2 µl of an overnight culture of the desired transformants. To induce expression of the slipping vector arabinose was added to a final concentration of 0.2% (w/v). The measurement was performed in a Tecan Spark® reader in Corning® 96-well flat clear bottom black polystyrene TC-treated microplates. Bioluminescence emission (Attenuation: none; Settle time: 50 ms; Integration time: 200 ms) and growth (Wavelength: 600 nm; Number of flashes: 10; Settle time: 50 ms) were monitored in a Tecan Spark® in 10-min intervals during agitation (orbital, 180 rpm, amplitude: 3 mm) for around 16 h.
Bioinformatic analyses
cDNA and protein sequences from E. coli
The cDNA and protein sequences of 4352 E. coli K-12 MG1655 genes were downloaded from the OMA database50. The cDNA and protein sequences of genes from the other 15 bacteria were downloaded from the Ensembl Bacteria database (Supplementary data file S1)51.
Identification of PP-motifs in protein sequences
PP-motifs in protein sequences were identified using the fuzzpro program from the EMBOSS package52. The PP-motifs were defined as in11, i.e., XX-nP-X where n ≥ 2 and X could be any non-proline amino acid.
Protein abundance and translation efficiency
We obtained the protein abundance and translation efficiency values for E. coli genes as described previously11: protein abundance data covering 2163 E. coli genes was from53,54; transcription levels of 2710 E. coli genes under standard growth conditions were downloaded from the ASAP database55. For each of the 1743 genes present in both datasets, we calculated the translation efficiency as the ratio between its protein abundance and transcription level.
Transmembrane segments of the E. coli proteins
Sequence positions of 5672 transmembrane segments within 912 α-helical transmembrane proteins were downloaded from the Uniprot database56. Data for the E. coli K-12 strain (taxonomy ID 83333) were used instead of E. coli K-12 MG1655 (taxonomy ID 511145), since the reviewed data of the latter are unavailable in the Uniprot database56.
Statistics and reproducibility
Sample size: sample size in biochemical experiments was chosen to be at least n = 4. This sample size was calculated from Lehr’s formula where the effect size was at least twice the standard deviation of experiments using wild-type cells. Biological replicates were defined as single colonies derived from culture plates. No data were excluded from the analysis. Replication: initial experiments using the His-Leader system (Fig. 4c) were conducted as technical replicates both in a Tecan Spark and a Tecan F500 reader showing qualitatively comparable results. Experiments on codon choice variation (Fig. 5b) were conducted both in 200 and 150 µl showing quantitatively comparable results.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Dr. Kerstin Lassak and Dr. Natalia Tschowri for fruitful discussions and constructive criticism. We thank Urte Tomasiunaite for her help during the revision of this manuscript. F.Q. is supported by the National Natural Science Foundation of China (Project No. 32000462) and Scientific Research Funds of Huaqiao University. J.L. & K.J. gratefully acknowledge financial support from the DFG Research Training Group GRK2062 (Molecular Principles of Synthetic Biology). Moreover, J.L. is grateful for DFG grant LA 3658/1-1 and D.F. for DFG grant FR 1411/17-1
Author contributions
The HisL*-Lux reporter concept was developed by J.L. R.K., A.S., J.M., and J.L. constructed HisL*-lux reporter strains and plasmids. Corresponding in vivo measurements were performed by R.K., A.S. and J.M. tRNA gene deletions were made by R.K. and J.M. Reporters for quantification of +1 frameshifting were constructed and corresponding assay performed by R.K. and J.M. R.K. constructed cadC mutations and recorded their effect on pH-regulation. All bioinformatic analyses were performed by F.Q. and D.F. The study was designed by F.Q., R.K., D.F. and J.L. with contributions of K.J. The manuscript was written by F.Q., R.K., and J.L. with contributions of D.F. and K.J.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information. Source data underlying graphs presented in the main figures are available in Supplementary data file S6. No datasets were generated during this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ralph Krafczyk, Fei Qi.
Contributor Information
Dmitrij Frishman, Email: d.frishman@wzw.tum.de.
Jürgen Lassak, Email: juergen.lassak@lmu.de.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-02115-z.
References
- 1.Wedemeyer WJ, Welker E, Scheraga HA. Proline cis-trans isomerization and protein folding. Biochemistry. 2002;41:14637–14644. doi: 10.1021/bi020574b. [DOI] [PubMed] [Google Scholar]
- 2.Adzhubei AA, Sternberg MJ, Makarov AA. Polyproline-II helix in proteins: structure and function. J. Mol. Biol. 2013;425:2100–2132. doi: 10.1016/j.jmb.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Muto H, Ito K. Peptidyi-prolyl-tRNA at the ribosomal P-site reacts poorly with puromycin. Biochem. Bioph. Res. Co. 2008;366:1043–1047. doi: 10.1016/j.bbrc.2007.12.072. [DOI] [PubMed] [Google Scholar]
- 4.Wohlgemuth I, Brenner S, Beringer M, Rodnina MV. Modulation of the rate of peptidyl transfer on the ribosome by the nature of substrates. J. Biol. Chem. 2008;283:32229–32235. doi: 10.1074/jbc.M805316200. [DOI] [PubMed] [Google Scholar]
- 5.Pavlov MY, et al. Slow peptide bond formation by proline and other N-alkylamino acids in translation. Proc. Natl Acad. Sci. USA. 2009;106:50–54. doi: 10.1073/pnas.0809211106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayes CS. Proline residues at the C terminus of nascent chains induce SsrA tagging during translation termination. J. Biol. Chem. 2002;277:33825–33832. doi: 10.1074/jbc.M205405200. [DOI] [PubMed] [Google Scholar]
- 7.Tanner DR, Cariello DA, Woolstenhulme CJ, Broadbent MA, Buskirk AR. Genetic identification of nascent peptides that induce ribosome stalling. J. Biol. Chem. 2009;284:34809–34818. doi: 10.1074/jbc.M109.039040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huter P, et al. Structural basis for polyproline-mediated ribosome stalling and rescue by the translation elongation factor EF-P. Mol. Cell. 2017;68:515–527 e516. doi: 10.1016/j.molcel.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Morgan AA, Rubenstein E. Proline: the distribution, frequency, positioning, and common functional roles of proline and polyproline sequences in the human proteome. PLoS ONE. 2013;8:e53785. doi: 10.1371/journal.pone.0053785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starosta AL, et al. A conserved proline triplet in Val-tRNA synthetase and the origin of elongation factor P. Cell Rep. 2014;9:476–483. doi: 10.1016/j.celrep.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi F, Motz M, Jung K, Lassak J, Frishman D. Evolutionary analysis of polyproline motifs in Escherichia coli reveals their regulatory role in translation. PLoS Comput. Biol. 2018;14:e1005987. doi: 10.1371/journal.pcbi.1005987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinheiro B, et al. Structure and function of an elongation factor P subfamily in actinobacteria. Cell Rep. 2020;30:4332–433. doi: 10.1016/j.celrep.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Doerfel LK, et al. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science. 2013;339:85–88. doi: 10.1126/science.1229017. [DOI] [PubMed] [Google Scholar]
- 14.Ude S, et al. Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science. 2013;339:82–85. doi: 10.1126/science.1228985. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez E, et al. eIF5A promotes translation of polyproline motifs. Mol. Cell. 2013;51:1–11. doi: 10.1016/j.molcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassak J, Wilson DN, Jung K. Stall no more at polyproline stretches with the translation elongation factors EF-P and IF-5A. Mol. Microbiol. 2016;99:219–235. doi: 10.1111/mmi.13233. [DOI] [PubMed] [Google Scholar]
- 17.Tuller T, et al. An evolutionarily conserved mechanism for controlling the efficiency of protein translation. Cell. 2010;141:344–354. doi: 10.1016/j.cell.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Hersch SJ, Elgamal S, Katz A, Ibba M, Navarre WW. Translation initiation rate determines the impact of ribosome stalling on bacterial protein synthesis. J. Biol. Chem. 2014;289:28160–28171. doi: 10.1074/jbc.M114.593277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hersch SJ, et al. Divergent protein motifs direct elongation factor P-mediated translational regulation in Salmonella enterica and Escherichia coli. mBio. 2013;4:e00180–00113. doi: 10.1128/mBio.00180-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peil L, et al. Distinct X/PP/X sequence motifs induce ribosome stalling, which is rescued by the translation elongation factor EF-P. Proc. Natl Acad. Sci. USA. 2013;110:15265–15270. doi: 10.1073/pnas.1310642110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elgamal S, et al. EF-P dependent pauses integrate proximal and distal signals during translation. PLoS Genet. 2014;10:e1004553. doi: 10.1371/journal.pgen.1004553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starosta AL, et al. Translational stalling at polyproline stretches is modulated by the sequence context upstream of the stall site. Nucleic Acids Res. 2014;42:10711–10719. doi: 10.1093/nar/gku768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yourno J, Tanemura S. Restoration of in-phase translation by an unlinked suppressor of a frameshift mutation in Salmonella typhimurium. Nature. 1970;225:422–426. doi: 10.1038/225422a0. [DOI] [PubMed] [Google Scholar]
- 24.Gamper HB, Masuda I, Frenkel-Morgenstern M, Hou YM. Maintenance of protein synthesis reading frame by EF-P and m(1)G37-tRNA. Nat. Commun. 2015;6:7226. doi: 10.1038/ncomms8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chevance FF, Le Guyon S, Hughes KT. The effects of codon context on in vivo translation speed. PLoS Genet. 2014;10:e1004392. doi: 10.1371/journal.pgen.1004392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston HM, Barnes WM, Chumley FG, Bossi L, Roth JR. Model for regulation of the histidine operon of Salmonella. Proc. Natl Acad. Sci. USA. 1980;77:508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volkwein W, et al. Switching the Post-translational modification of translation elongation factor EF-P. Front. Microbiol. 2019;10:1148. doi: 10.3389/fmicb.2019.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lassak J, Henche AL, Binnenkade L, Thormann KM. ArcS, the cognate sensor kinase in an atypical Arc system of Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2010;76:3263–3274. doi: 10.1128/AEM.00512-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried L, Lassak J, Jung K. A comprehensive toolbox for the rapid construction of lacZ fusion reporters. J. Microbiol. Methods. 2012;91:537–543. doi: 10.1016/j.mimet.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Sezonov G, Joseleau-Petit D, D’Ari R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007;189:8746–8749. doi: 10.1128/JB.01368-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertels F, Merker H, Kost C. Design and characterization of auxotrophy-based amino acid biosensors. PLoS ONE. 2012;7:e41349. doi: 10.1371/journal.pone.0041349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woolstenhulme CJ, Guydosh NR, Green R, Buskirk AR. High-precision analysis of translational pausing by ribosome profiling in bacteria lacking EFP. Cell Rep. 2015;11:13–21. doi: 10.1016/j.celrep.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Näsvall SJ, Chen P, Bjork GR. The modified wobble nucleoside uridine-5-oxyacetic acid in tRNAPro(cmo5UGG) promotes reading of all four proline codons in vivo. RNA. 2004;10:1662–1673. doi: 10.1261/rna.7106404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong H, Nilsson L, Kurland CG. Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J. Mol. Biol. 1996;260:649–663. doi: 10.1006/jmbi.1996.0428. [DOI] [PubMed] [Google Scholar]
- 35.Kröger C, et al. An infection-relevant transcriptomic compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe. 2013;14:683–695. doi: 10.1016/j.chom.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Mohanty BK, Petree JR, Kushner SR. Endonucleolytic cleavages by RNase E generate the mature 3’ termini of the three proline tRNAs in Escherichia coli. Nucleic Acids Res. 2016;44:6350–6362. doi: 10.1093/nar/gkw517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchner S, Schlundt A, Lassak J, Sattler M, Jung K. Structural and functional analysis of the signal-transducing linker in the pH-responsive one-component system CadC of Escherichia coli. J. Mol. Biol. 2015;427:2548–2561. doi: 10.1016/j.jmb.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Schlundt A, et al. Structure-function analysis of the DNA-binding domain of a transmembrane transcriptional activator. Sci. Rep. 2017;7:1051. doi: 10.1038/s41598-017-01031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung, K., Fabiani, F., Hoyer, E. & Lassak, J. Bacterial transmembrane signalling systems and their engineering for biosensing. Open Biol. 8, 180023 (2018). [DOI] [PMC free article] [PubMed]
- 40.Brameyer S, et al. DNA-binding directs the localization of a membrane-integrated receptor of the ToxR family. Commun. Biol. 2019;2:4. doi: 10.1038/s42003-018-0248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol. Biol. Evol. 1985;2:13–34. doi: 10.1093/oxfordjournals.molbev.a040335. [DOI] [PubMed] [Google Scholar]
- 42.Ikemura T. Correlation between the abundance of Escherichia coli transfer-RNAs and the occurrence of the respective codons in its protein genes: A proposal for a synonymous codon choice that Is optimal for the Escherichia coli translational system. J. Mol. Biol. 1981;151:389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- 43.Akashi H. Synonymous codon usage in Drosophila melanogaster: natural selection and translational accuracy. Genetics. 1994;136:927–935. doi: 10.1093/genetics/136.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novy R, Drott D, Yaeger K, Mierendorf R. Overcoming the codon bias of E. coli for enhanced protein expression. Innovations. 2001;12:1–3. [Google Scholar]
- 45.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951;62:293–300. doi: 10.1128/JB.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller JH. Experiments in molecular genetics. Cold Spring Harb. Lab. Press. 1972;221–222:263–274. [Google Scholar]
- 47.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995;177:4121–4130. doi: 10.1128/JB.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tetsch L, Koller C, Haneburger I, Jung K. The membrane-integrated transcriptional activator CadC of Escherichia coli senses lysine indirectly via the interaction with the lysine permease LysP. Mol. Microbiol. 2008;67:570–583. doi: 10.1111/j.1365-2958.2007.06070.x. [DOI] [PubMed] [Google Scholar]
- 49.Epstein W, Kim BS. Potassium transport loci in Escherichia coli K-12. J. Bacteriol. 1971;108:639–644. doi: 10.1128/JB.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altenhoff AM, et al. The OMA orthology database in 2018: retrieving evolutionary relationships among all domains of life through richer web and programmatic interfaces. Nucleic Acids Res. 2018;46:D477–D485. doi: 10.1093/nar/gkx1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howe KL, et al. Ensembl Genomes 2020-enabling non-vertebrate genomic research. Nucleic Acids Res. 2020;48:D689–D695. doi: 10.1093/nar/gkz890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rice P, Longden I, Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 2000;16:276–277. doi: 10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 53.Wisniewski JR, Rakus D. Multi-enzyme digestion FASP and the ‘Total Protein Approach’-based absolute quantification of the Escherichia coli proteome. J. Proteom. 2014;109:322–331. doi: 10.1016/j.jprot.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Wisniewski JR, Rakus D. Quantitative analysis of the Escherichia coli proteome. Data Brief. 2014;1:7–11. doi: 10.1016/j.dib.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glasner JD, et al. ASAP, a systematic annotation package for community analysis of genomes. Nucleic Acids Res. 2003;31:147–151. doi: 10.1093/nar/gkg125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–d515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information. Source data underlying graphs presented in the main figures are available in Supplementary data file S6. No datasets were generated during this study.