Abstract
Cutaneous basal cell carcinoma (cBCC) is an economic burden to health services, due to its great morbidity and increasing incidence in old people. Infiltrative cBCCs and cBCCs with micronodular pattern are considered as more aggressive. The role of p53 expression and TERTp mutation on cBCC behavior remains to be clarified. We aimed to assess TERTp mutations and p53 expression in relation to the cBCC histological subtype in a cohort of patients referred to an ENT Department of a tertiary Hospital of Northern Portugal. We performed a retrospective clinicopathological and histological review of the head and neck cBCCs followed-up at the otorhinolaryngology department of Trás-os-Montes e Alto Douro hospital (January 2007–June 2018). We assessed TERTp mutations in 142 cBCCs and p53 protein expression, through immunohistochemistry, in 157 cBCCs. We detected TERTp mutations in 43.7% of cBCCs and p53 overexpression in 60.5% of cBCCs. We spotted association of p53 overexpression and TERTp mutation with necrosis. In the infitrative-growth pattern cBCCs, there was no significant association with the clinical and histological features evaluated, except for necrosis. In the indolent-growth cBCCs, we identified a significant association of TERTp mutation status with female sex, necrosis, multiple cBCCs, and p53 positive expression. Our results suggest that TERTp mutation may be useful to identify more aggressive features in the indolent-growth pattern cBCCs (nodular and superficial subtypes). Further studies with larger cohorts are warranted to clarify the relevance of TERTp mutation in cBCCs.
Subject terms: Cancer, Cancer genetics, Skin cancer
Introduction
Cutaneous basal cell carcinoma (cBCC) is the most common cancer diagnosed in human populations, representing 70–80% of skin cancers1,2. Despite its very low mortality rate, it is an economic burden to health services, due to its great morbidity and increasing incidence in old people1,3. The main carcinogenic agent is ultraviolet light (UV), which is reflected by the cBCCs higher frequency in sun-exposed sites2.
cBCC is characterized by great morphological variability, both clinically and histologically4. cBCCs can be classified in three main histopathological subtypes: nodular, superficial, and infiltrative5, but cBCC with mixed histology is a common pattern1. cBCCs can present indolent or aggressive behavior6. Superficial and nodular cBCCs subtypes have a more indolent behavior whereas infiltrative subtypes and BCCs with micronodular features are usually more aggressive6,7. cBCCs exhibiting infiltrative growth display a higher propensity for recurrence than well-circumscribed cBCCs so the histological subtyping is crucial for evaluating the risk of relapse4.
Several genes were associated with cBCC, including the key components of the Hedgehog pathway, PTCH1 and SMO, the TP53 tumor suppressor, and members of the RAS proto-oncogene family3. The PTCH gene is the most commonly mutated, involved in 90% of cBCCs4. Inactivation of the TP53 gene is also a frequent event associated with cBCC pathogenesis3. p53 expression and its expression in actinic keratosis suggests that p53 plays a role in the early steps of carcinogenesis in skin cancers8. Moreover, it has been suggested that p53 expression seems to be associated with cBCC aggressiveness9–12, and some differences in p53 mutation frequency, types of mutation, and hot spots were seen between aggressive and nonaggressive BCCs13. However, these differences do not constitute clear-cut diagnostic or prognostic indicators of tumor aggressiveness, so tumor aggressiveness may be attributable to other genetic events that occur during tumor progression13.
Recent reports described non-coding mutations in the TERT promoter gene (TERTp) as a frequent event in cBCCs14–16. Mutations in the promoter region of TERT lead to increased TERT expression and de novo telomerase activity in cancer cells, which allow cells to acquire the ability to overcome senescence and to become immortal17. Telomerase up-regulation is a key, rate-limiting step in tumorigenesis as evidenced by the highly recurrent mutations in the TERT promoter18. Inactivating mutations in the p53 pathway and activating mutations in the TERT promoter may be critical in enabling tumor progression18. TERTp mutations have been associated with poor prognosis in several cancers including melanoma and squamous cell carcinoma15,19,20 but, so far, this has not been observed in BCC. Additionally, there are no reports describing an association of the genetic profile with specific histopathological cBCCs subtypes, namely the more aggressive infiltrative subtype.
Taking this into account, we aimed to assess p53 expression and TERTp mutation status in cBCC, and associate them with histological subtype and clinicopathological features, in a cohort of cBCC patients.
Material and methods
Cases studied
Selection criteria for inclusion in this retrospective series were all cases of histologically confirmed cutaneous BCCs (cBCCs) located in head and neck, managed at the ENT Department of the Centro Hospitalar de Trás-os-Montes e Alto Douro (CHTMAD), from January 2007 to June 2018. Exclusion criteria included age < 18 year-old, genetic diseases or syndromes predisposing to cBCC, genetic disorders associated with other neoplasms, diffuse dermatosis, and biopsies from recurrence or persistence of a previously diagnosed cBCC. Applying these criteria, 151 patients were included and signed an informed consent after proper information about the goal and scope of the study. Data from these patients were gathered in a personal interview questionnaire, using medical records (SClinico database and Clinical Process), and pathological records from the Pathology Department at CHTMAD. The Ethical Committee and the Administrative Council of CHTMAD approved the study in 16/10/2012. All the methods were performed in accordance with relevant guidelines and regulations.
Patient melanin content in the forehead and inner arm was measured with a DermaSpectrometer (Cortex Technology, Hadsund, Denmark) as described by Shriver and Parra21. Sun-exposure was assessed according to the patient’s profession and lifestyle—high exposure was considered for patients who had professions with many hours of sun exposure such as farmers and construction workers.
Patients with previous incomplete or inappropriate histological records, missing information, or histological samples in inappropriate conditions were excluded (n = 55), so we ended up with 96 patients. Of these 96 patients, 36 had more than one BCC. The final series comprised 160 cBCCs from 96 patients.
cBCC histopathological features were reviewed by a pathologist (MSF), under the supervision of a senior pathologist/dermatopathologist (JML), to assess detailed histological data for each tumor. The morphological classification of cBCCs was performed according to Rippey22 and included the following growth patterns: nodular, infiltrative (including morpheic), superficial or mixed (including a combination of at least two of the aforementioned patterns). The cBCCs were further classified as cBCCs with an infiltrative-type growth pattern, considered as more aggressive, which included BCCs with infiltrative, morphoeform or micronodular component, and BCCs with an indolent-type growth pattern, considered as less aggressive, which included the nodular and superficial subtypes6,7.
p53 immunohistochemistry
p53 protein expression in cBCCs was evaluated through immunohistochemistry. Briefly, deparaffinized and rehydrated sections were subjected to a 45’ steamer treatment in 10 mM sodium citrate buffer, pH 6.0, for antigen retrieval. Ultravision hydrogen peroxide block (Ref TA-060-H202Q, Thermo Scientific) and protein block (Ref TA-125-PBQ, Thermo Scientific) were used to block endogenous peroxidase, 10’ each. Subsequently, we used the monoclonal antibody anti-p53 (NCL-L-p53, Leica), diluted 1:550 (Ref TA-125-ADQ, Thermo Scientific), for 60’ at room temperature. The monoclonal antibody (clone DO-7) recognizes both wild type and mutant forms of human p53 protein under denaturing and non-denaturing conditions. After this step, sections were processed for the detection of positive immunohistochemical reaction with HRP polymer quantum (Ref TL-060-QPH, Thermo Scientific) for 10’, and further reaction with 3% diaminobenzidine chromogen (DAB, Ref K3468, Dako), for 3’. Finally, sections were counterstained with Mayer’s hematoxylin, cleared and mounted.
For the p53 staining evaluation, the slides were digitalized using the scanner D-sight FLUO (A-MENARINI diagnostics, UK) and its automatized analysis program with a previously developed p53 algorithm. Approximately 2000 cells were counted in each cBCC.
The scoring of tumors was done as follows: staining < 10% of tumor cells were considered as negative, and staining ≥ 10% was considered as positive23,24.
Molecular analysis: TERTp mutations
TERTp mutation analysis was performed as previously described15,25. Briefly, cBCCs areas were manually dissected from 10-µm whole sections of paraffin-embedded material. DNA extraction was performed using with GRiSP kit (GRiSP, Portugal) according to the manufacturer instructions. After the last centrifugation, DNA was quantified with the Nanodrop (ND-1000, Thermo Fisher Scientific, Lithuania). Mutation analysis was performed with Sanger sequencing. Briefly, genomic DNA (25–100 ng) was amplified by polymerase chain reaction (PCR) with the kit from Bioline (MyTaq HS Mix 2X, USA), using the following set of primers: forward TERTF, CAGCGCTGCCTGAAACTC; and reverse TERTR, GTCCTGCCCCTTCACCTT. The following cycling conditions were used: 35 s at 94 °C; 40 s at 62 °C and 45 s at 72 °C for 40 cycles. Products were enzymatically purified with Exonuclease I and Shrimp Alkaline Phosphatase and sequenced in an ABI Prism 3130 xl Automatic sequencer (Perkin-Elmer, Foster City, CA) using the BigDye v3.1 Sequencing Kit (Applied Biosystems, Washington). Cases with mutations were confirmed by an independent PCR amplification. Additionally, randomly selected cases were confirmed using real-time PCR amplification curve analysis25. Positive and negative mutation control samples were included in each run to ensure the assay’s validity.
Statistical analysis
Statistical analysis was performed using the statistical package for social sciences software (IBM SPSS Statistics 23). Proportions were compared using the X2 test or Fisher’s exact test when appropriate (Yates correction in case of multiple entries); the significance of differences between means was assessed with Student’s unpaired t-test. A p value < 0.05 was considered statistically significant with a 95% confidence interval.
Ethics approval
The Ethical Committee and the Administrative Council of CHTMAD approved the study in 16/10/2012.
Consent to participate
Patients signed an informed consent after proper information about the goal and scope of the study.
Results
p53 immunostaining
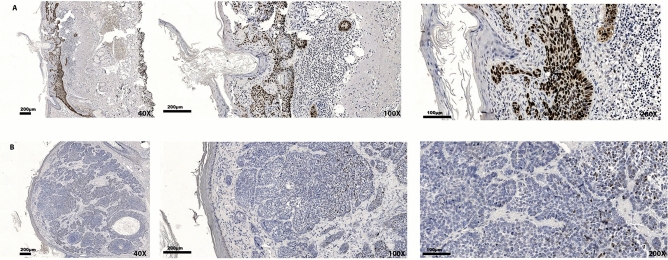
p53 positive staining (≥ 10% of the stained tumor cells) was observed in the nucleus of 95 out of 157 cBCCs (60.5%; in three cases the evaluation was not possible due to unspecific staining) (Fig. 1). None of the cases displayed either absence of expression or cytoplasm expression of p53 in the tumor cells. No significant differences were observed concerning age at diagnosis, sex, skin melanin content in inner arm, solar exposure, and presence of multiple cBCCs, between p53 positive and p53 negative cBCCs (Table 1).
Figure 1.
Representative examples of p53 immunoexpression in cBCCs: (A) positive (90.6%) expression; (B) negative (2.3%) expression.
Table 1.
Clinicopathological features and p53 expression in cBCCs.
| Clinicopathological features | Total cBCC | p53 negative (< 10%) | p53 positive (≥ 10%) | p value |
|---|---|---|---|---|
| Age at diagnosis (mean ± SD) (n = 157) | 73.0 ± 11.2 | 72.9 ± 12.0 | 73.1 ± 10.7 | 0.905 |
| Sex [n (%)](n = 157) | ||||
| Male | 82 (52.2) | 35 (56.5) | 47 (49.5) | 0.467 |
| Female | 75 (48.8) | 27 (43.5) | 48 (50.5) | |
| Skin melanin content (inner arm) (mean ± SD) (n = 124) | 28.3 ± 3.3 | 28.2 ± 2.8 | 28.3 ± 3.5 | 0.773 |
| Sun exposure (mean ± SD) (n = 134) | ||||
| Low | 34 (25.4) | 13 (25.5) | 21 (25.3) | 0.981 |
| High | 100 (74.6) | 38 (74.5) | 62 (74.7) | |
| Location [n (%)] (n = 157) | ||||
| Nose | 39 (24.9) | 14 (22.6) | 25 (26.3) | 0.997 |
| Face | 47 (29.9) | 19 (30.6) | 28 (29.5) | |
| Eyelid | 31 (19.8) | 14 (22.6) | 17 (17.9) | |
| Preauricular / auricular region | 27 (17.2) | 9 (14.5) | 18 (18.9) | |
| Scalp | 1 (0.6) | 1 (1.6) | 0 (0) | |
| Lip | 6 (3.8) | 3 (4.8) | 3 (3.2) | |
| Tumor dimension (mean ± SD) (n = 154) | 13.2 ± 9.8 | 13.3 ± 8.1 | 13.2 ± 10.8 | 0.987 |
| Focality [n (%)] (n = 157) | ||||
| Single | 61 (38.9) | 24 (38.7) | 37 (38.9) | 0.976 |
| Multiple | 96 (61.1) | 38 (61.3) | 58 (61.1) | |
Concerning cBCCs histopathological features (tumor thickness, tumor dimension, location, histological subtype, invasion level, ulceration, associated actinic keratosis, necrosis, tumor pigmentation, elastosis, and growth pattern), no significant differences were found according to p53 positive expression except for necrosis, which was a more frequent finding in the p53 positive cBCCs (25.5%) comparing with the negative ones (11.7%) (p = 0.036) (Table 2).
Table 2.
cBCCs histopathological features and p53 expression.
| Histopathological features | Total cBCC | p53 negative (< 10%) | p53 positive (≥ 10%) | p value |
|---|---|---|---|---|
| Tumor thickness (mean ± SD) (n = 151) | 2.4 ± 1.7 | 2.4 ± 1.8 | 2.3 ± 1.5 | 0.713 |
| Histological subtype [n (%)] (n = 157) | ||||
| Nodular | 56 (35.7) | 24 (38.7) | 32 (33.7) | 0.444 |
| Infiltrative | 51 (32.5) | 20 (32.3) | 31 (32.6) | |
| Mixed | 44 (28.0) | 16 (25.8) | 28 (29.5) | |
| Superficial | 6 (3.8) | 2 (3.2) | 4 (4.2) | |
| Invasion level [n (%)] (n = 152) | ||||
| Papillary dermis | 8 (5.2) | 2 (1.3) | 6 (6.5) | 0.547 |
| Reticular dermis | 84 (55.3) | 30 (50.0) | 54 (58.7) | |
| Subcutaneous | 58 (38.2) | 26 (43.3) | 32 (34.8) | |
| Intramuscular | 2 (1.3) | 2 (3.3) | 0 (0) | |
| Tumor features [n (%)] | ||||
| Ulceration (n = 153) | 95 (62.1) | 34 (58.6) | 61 (64.2) | 0.489 |
| Actinic keratosis (n = 152) | 14 (9.2) | 4 (6.8) | 8 (8.6) | 0.767 |
| Necrosis (n = 154) | 31 (20.1) | 7 (11.7) | 24 (25.5) | 0.036 |
| Pigmentation (n = 154) | 14 (9.1) | 8 (13.3) | 6 (6.4) | 0.143 |
| Elastosis (n = 153) | 14 (9.2) | 6 (10.0) | 8 (8.6) | 0.77 |
| Lymphocytic infiltrate [n (%)] (n = 156) | ||||
| Absent/Rare | 82 (52.6) | 34 (55.7) | 48 (50.5) | 0.525 |
| Moderate/Intense | 74 (47.4) | 27 (44.3) | 47 (49.5) | |
| Lymphovascular invasion [n (%)](n = 150) | ||||
| Not identified | 145 (96.7) | 56 (94.9) | 89 (97.8) | 0.807 |
| Present | 5 (3.3) | 3 (5.1) | 2 (2.2) | |
| Perineural invasion [n (%)] (n = 152) | ||||
| Not identified | 142 (94.0) | 56 (93.3) | 86 (94.5) | 0.967 |
| Present | 9 (6.0) | 4 (6.7) | 5 (5.5) | |
| Growth pattern [n (%)] (n = 157) | ||||
| Indolent-type | 56 (35.7) | 22 (35.5) | 34 (35.8) | 0.969 |
| Aggressive-type | 101 (64.3) | 40 (64.5) | 61 (64.2) | |
Bold - significant difference (p-value < 0.05).
Additionally, we repeated the same analysis separately for cBCC with infiltrative-type growth pattern vs indolent-type growth pattern, as this classification reflects BCC aggressiveness. In cBCCs with indolent-type growth pattern, we observed a significant association of p53 expression with TERTp mutation, which will be described in the next section. We also observed an association of necrosis with p53 expression in this subgroup, which had been already observed when analyzing all the cBCCs together. A higher frequency of necrosis was observed in cBCCs with positive p53 expression (11/34, 32.4%) comparing with p53 negative cBCCs (1/22, 4.5%) (p = 0.018). No significant associations were found in the infiltrative-type growth pattern group.
TERTp mutations
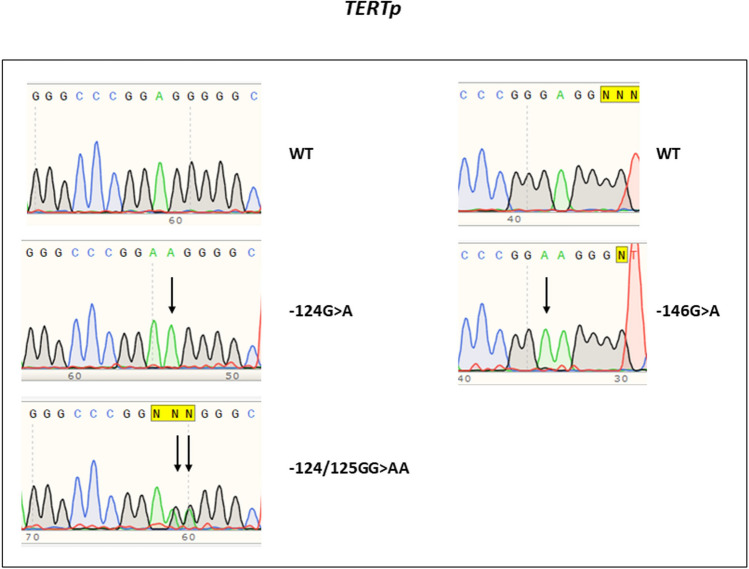
We attained information on TERTp mutation status in 142 cBCCs of the 160 cBCCs evaluated (88.8%); in 18 cases, we could not obtain enough DNA or DNA quality to ensure a proper amplification. In these 142 cases we did not achieve p53 evaluation in threes cases, meaning we had 139 cases with evaluation of both, TERTp mutations and p53 expression. In the142 cBCCs, 62 were TERTp mutated (43.7%). The most frequent mutation was the − 146:G > A mutation, which occurred in 48 of 62 cases (77.4%). The− 124:G > A occurred in 13 out of 62 cases (21.0%) (Fig. 2). In one cBCC, we observed the tandem mutation − 124/125:GG > AA (Fig. 2), and in another cBCC the − 124:G > A and − 146:G > A simultaneous mutations were observed (1.6%).
Figure 2.
Electrophoregram with representative examples of the TERTp mutations observed.
No significant differences were observed concerning age at diagnosis, sex, skin melanin content in the forearm, solar exposure, and presence of multiple cBCCs, between TERTp mutated and TERTp wild type (wt) cBCCs (Table 3).
Table 3.
Clinicopathological features in cBCCs and TERTp mutation status.
| Clinicopathological features | Total cBCCs (n = 142) | TERTwt (n = 80) | TERTmut (n = 62) | p value |
|---|---|---|---|---|
| Age at diagnosis (mean ± SD) (n = 142) | 72.4 ± 11.2 | 72.4 ± 11.1 | 72.7 ± 11.3 | 0.887 |
| Sex [n (%)] (n = 142) | ||||
| Male | 78 (54.9) | 46 (57.5) | 32 (51.6) | 0.484 |
| Female | 64 (45.1) | 34 (42.5) | 30 (48.4) | |
| Skin melanin content (inner arm) (mean ± SD) (n = 114) | 28.3 ± 3.3 | 28.3 ± 3.7 | 28.2 ± 2.8 | 0.924 |
| Sun exposure (mean ± SD) (n = 121) | ||||
| Low | 30 (24.8) | 17 (25.4) | 13 (24.1) | 0.869 |
| High | 91 (75.2) | 50 (74.6) | 41 (75.9) | |
| Location [n (%)] (n = 142) | ||||
| Nose | 31 (21.8) | 17 (21.3) | 14 (22.6) | 0.876 |
| Face | 43 (30.3) | 23 (28.7) | 20 (32.3) | |
| Eyelid | 30 (21.1) | 21 (26.3) | 9 (14.5) | |
| Preauricular/Auricular region | 26 (18.3) | 14 (17.5) | 12 (19.4) | |
| Scalp | 1 (0.7) | 0 (0) | 1 (1.6) | |
| Lip | 5 (3.5) | 3 (3.8) | 2 (3.2) | |
| Other | 6 (4.2) | 2 (2.5) | 4 (6.5) | |
| Tumor dimension (mean ± SD) (n = 142) | 13.6 ± 10.1 | 14.0 ± 11.7 | 13.1 ± 7.7 | 0.612 |
| Other | ||||
| Focality [n (%)] (n = 142) | ||||
| Single | 56 (39.4) | 35 (43.8) | 21 (33.9) | 0.232 |
| Multiple | 86 (60.6) | 45 (56.2) | 41 (66.1) | |
Concerning cBCCs histopathological features (tumor thickness, tumor dimension, location, histological subtype, invasion level, ulceration, associated actinic keratosis, skin pigmentation, elastosis, and growth pattern) no significant differences were found according to the TERTp mutation status (Table 4), except for a significant association with necrosis, which was more frequent in TERTp mutated cBCCs (33.9%), compared with the TERTp wt cBCCs (10.3%) (p = 0.001). No association was found between p53 expression and TERTp mutation status in this cohort of cBCCs.
Table 4.
cBCCs histopathological features and TERTp mutation status.
| Histopathological features | Total cBCCs (n = 142) | TERTwt (n = 80) | TERTmut (n = 62) | p value |
|---|---|---|---|---|
| Tumor thickness (mean ± SD) (n = 136) | 2.44 ± 1.72 | 2.21 ± 1.57 | 2.73 ± 1.87 | 0.081 |
| Histological subtype (n = 142) | ||||
| Nodular | 52 (36.6) | 25 (31.3) | 27 (43.5) | 0.095 |
| Infiltrative | 45 (31.7) | 30 (37.5) | 15 (24.2) | |
| Mixed | 39 (27.5) | 19 (23.8) | 20 (32.3) | |
| Superficial | 6 (4.2) | 6 (7.5) | 0 (0) | |
| Invasion level | ||||
| Papillary dermis | 7 (5.1) | 7 (9.2) | 0 (0) | 0.178 |
| Reticular dermis | 76 (55.1) | 42 (55.3) | 34 (54.8) | |
| Subcutaneous | 53 (38.4) | 26 (34.2) | 27 (43.5) | |
| Intramuscular | 2 (1.4) | 1 (1.3) | 1 (1.6) | |
| Tumor features | ||||
| Ulceration (n = 139) | 50 (36.0) | 27 (34.6) | 23 (37.7) | 0.706 |
| Actinic keratosis (n = 139) | 11 (7.9) | 7 (9.0) | 4 (6.6) | 0.755 |
| Necrosis (n = 140) | 29 (20.7) | 8 (10.3) | 21 (33.9) | 0.001 |
| Pigmentation (n = 140) | 13 (9.3) | 9 (11.5) | 4 (6.5) | 0.303 |
| Elastosis (n = 139) | 13 (9.4) | 9 (11.7) | 4 (6.5) | 0.292 |
| Lymphocytic infiltrate (n = 142) | ||||
| Absent/Rare | 76 (53.5) | 39 (48.8) | 37 (59.7) | 0.195 |
| Moderate/Intense | 66 (46.5) | 41 (51.2) | 25 (40.3) | |
| Lymphovascular invasion (n = 136) | ||||
| Not identified | 132 (97.1) | 71 (94.7) | 61 (100) | 0.187 |
| Present | 4 (2.9) | 4 (5.3) | 0 (0) | |
| Perineural invasion (n = 137) | ||||
| Not identified | 128 (93.4) | 70 (92.1) | 58 (95.1) | 0.725 |
| Present | 9 (6.6) | 6 (7.9) | 3 (4.9) | |
| Growth pattern (n = 142) | ||||
| Indolent-type | 51 (35.9) | 32 (40.0) | 19 (30.6) | 0.249 |
| Infiltrative-type | 91 (64.1) | 48 (60.0) | 43 (69.4) | |
Bold - significant difference (p-value < 0.05).
The significant association with necrosis, observed when we considered the whole cBCCs analyzed, persisted when the groups were split according to the growth pattern. In cBCCs with infiltrative-type growth pattern, necrosis was present in 12 out of 43 TERTp mutated cBCCs (27.9%), compared with 5 out of 46 TERTp wt cBCCs (10.9%) (p = 0.041). No other significant associations were found in the infiltrative-type growth pattern group. In cBCCs with indolent-type growth pattern, significant associations were observed for TERTp mutation status and female sex, multiple cBCC, and p53 positive expression (Table 5).
Table 5.
Features significantly associated with TERTp mutation status in indolent-type growth pattern cBCCs.
| Features | Total (n = 51) | wt (n = 32) | TERTp (n = 19) | p value |
|---|---|---|---|---|
| Sex [n (%)] | ||||
| Male | 23 (45.1) | 18 (56.3) | 5 (26.3) | 0.038 |
| Female | 28 (54.9) | 14 (43.7) | 14 (73.7) | |
| Focality [n (%)] | ||||
| Single | 20 (39.2) | 16 (50.0) | 4 (21.1) | 0.041 |
| Multiple | 31 (60.8) | 16 (50.0) | 15 (78.9) | |
| p53 | ||||
| p53 negative (< 10%) | 16 (38.0) | 3 (15.8) | 13 (46.4) | 0.020 |
| p53 positive (≥ 10%) | 31 (62.0) | 16 (84.2) | 15 (53.6) | |
| Necrosis | ||||
| Absent | 38 (74.5) | 29 (90.6) | 10 (52.6) | 0.005 |
| Present | 23 (23.5) | 3 (9.4) | 9 (47.4) | |
Bold - significant difference (p-value < 0.05).
Finally, we compared p53 positive and TERTp mutated cases, with p53 negative and TERTp wild type cases (data not shown). The only significant difference found was the frequency of necrosis. cBCCs p53 positive and TERTp mutated had more frequently necrosis (17/ 37, 45.9%) when compared with cBCCs p53 negative and TERTp wild type (4/31, 12.9%) (p = 0.003).
Discussion
In the present study, TERTp mutations and p53 expression were evaluated in cBCCs from patients referred to an ENT Hospital Department. We observed that 32% of the cBCCs were of the infiltrative subtype, which is considered as more aggressive due to its higher risk of subclinical tumor extension26. In most series, lower frequencies of infiltrative cBCC were reported, ranging from 4.2 to 8.7%27–32. Leibovitch et al. reported a higher frequency, 28.3%, which they ascribed to a referral bias for Micrographic Mohs Surgery (MMS)33. The second most frequent subtype detected in the present study was the mixed cBCC subtype. Again, this may be related to a more aggressive pattern, since mixed type cBCCs have been reported as more frequently composed of aggressive subtypes34.
The cBCC location distribution of our series, with the face and nose as the main locations, is in line with what was been reported in other studies30,33,35. A relevant proportion of the cBCCs invaded the deep skin layers (reticular dermis and subcutaneous layer) when compared with other studies35–37. Indeed, Bandeira et al. did not report invasion deeper than the reticular dermis36, but Betti et al. reported subcutaneous fat invasion in infiltrative cBCCs, including the micronodular subtype37. Our results can be related to the presence of a high proportion of the infiltrative subtype since infiltrative cBCCs usually invade deeper than nodular cBCCs36–38.
Multiple cBCCs were more frequent in our series (38.9%) than the 16% reported by Scrivener et al.29 in the largest BCCs series described to date. In previous studies from our group, we found a frequency ranging from 30.0 to 38.1%15,32. Multiple cBCCs are a sign of aggressiveness as they increase the risk of relapse2.
In our study, most cBCCs overexpressed the p53 protein (60.5%), a frequency higher than the 45% found by Stamatelli et al.23 but similar to the values observed by Haghighi et al.24; both authors used the ≥ 10% cut-off to consider p53 positive expression. No associations were found between p53 overexpression and the clinical and histological features of the cBCCs, except for necrosis. Necrosis was significantly more frequent in cBCCs with p53 overexpression. Information about necrosis as a prognosis factor in BCC is scarce. Welsch et al.38 observed this feature in 61% of their cBCC series and reported that cBCCs without necrosis had a significantly deeper invasion than those with necrosis. Recent studies revealed a role for p53 in regulating necrotic cell death by activating independent signaling pathways that include induction of mitochondrial outer and inner membrane permeability, and altered mitochondrial dynamics39.
It has been reported that p53 overexpression is significantly higher in aggressive cBCCs compared with the non-aggressive ones10–12. Our data do not corroborate this finding since cBCCs with indolent-type or infiltrative–type growth pattern displayed an equivalent frequency of p53 overexpression. Furthermore, other authors reported that overexpression of p53 does not always reflect the degree of malignancy in cutaneous neoplasms40.
Regarding TERTp mutations, 42% of the cBCCs were mutated, a lower frequency than the previously reported (51–78%)14–16. The most frequent mutation was the − 146:G > A, which occurred in 79% of the cases. In a previous study from our group, a similar frequency of the − 146:G > A mutation was detected in a cohort of cBCCs occurring after irradiation in childhood for tinea capitis (75%), but in the sporadic context (non-irradiated control group) only 25% of the mutated cBCCs harbored this mutation15. Other studies, such as the one from Griewank et al.16 reported a 55.6% frequency of the − 146:G > A mutation. Skin cancers seem to be the only cancers where the − 146:G > A mutation is more common than − 124:G > A mutation20. One cBCC harbored concurrently the − 124:G > A and the − 146:G > A mutations, and other harbored the tandem mutation at position − 124/− 125, rare events previously reported by our group15.
Griewank et al. found no statistically significant associations of TERTp mutation with cBCC clinical and histopathologic features16, nor did our group in previous studies15. Noteworthy, in the present study, we observed an association of TERTp mutation with necrosis, which also occurred for p53 overexpression.
TERTp mutation has not been reported as a prognostic factor in cBCCs14–16, at variance with other skin cancers, such as squamous cell carcinoma41 and melanoma18,33 in which TERTp mutations were associated with an ominous outcome. Contrarily, in the bladder cancer model, the − 146:G > A was an independent predictor of nonrecurrence after BCG therapy in the BCG-NMI tumors42.
We have compared p53 positive and TERTp mutated cases, with p53 negative and TERTp wild type cases (data not shown), observing that cases with concomitantly p53 positive and TERTp mutated did not present more aggressive features, as it could be anticipated. Again, the only significant association found was with necrosis.
Then, we decided to evaluate the cBCCs according to the type of growth pattern (indolent vs infiltrative), and we found that in the infiltrative-type cBCCs, considered to be the most aggressive, the only feature associated with the TERTp mutation was necrosis. In the less aggressive indolent-type growth pattern cBCCs, besides necrosis, there was a significant association of TERTp mutation with female sex, multiple cBCC, and p53 positive expression. Contrarily to what happened when considering all cBCCs together, where no association between p53 positivity and TERTp mutation was observed, this association was observed in the indolent-type growth pattern cBCCs.
Few studies have evaluated the concomitant presence of p53 and TERTp mutations, none in BCC. In solid fibrous tumours, Machado et al.43 reported that tumours with TP53 and TERTp mutations were almost always classified as high risk, and the patients developed metastases and/or died of the disease. Morevover, Akaike et al. reported that TP53 mutations, which result in its overexpression, in combination with TERT promoter mutations seem to play an important role in the dedifferentiation process in these tumours44. In thyroid cancer, the concomitant presence of TERTp and TP53 mutations may be useful for the identification of more aggressive tumours45,46. In the hepatocellular carcinoma model, Shulze et al. found that TERTp mutations were early event in tumour progression whereas TP53 alterations appeared at more advanced stages in aggressive tumors47. In urogenital cancer, Wu et al. have observed a significant co-occurrence of mutations between the TERT promoter and the tumor protein 51/retinoblastoma1 (TP53/RB1) signaling pathway, indicating that they may cooperatively contribute to the genesis and progression of bladder cancer48. Overall, these data show that the concomitant presence of TERTp and p53 mutations is these tumours is associated with a more aggressive pattern. Although this kind of associations were not described in cBCC, p53 positive expression, and presence of multiple cBCCs, are features independently related to cBCCs aggressiveness10,11,22,49.
This study has limitations which do not allow establishing TERTp as a new prognostic marker for cBCCs. First, we would need to have a validation cohort, which was not possible in the present study. Second, additional experiments would be needed to establish the association between p53 over expression and TERTp mutation.
In conclusion, it seems that TERTp mutation may potentially be useful to discriminate more aggressive indolent-type growth pattern cBCCs, but further studies with larger cohorts are warranted to clarify the relevance of TERTp mutations in cBCCs.
Date availability
Full date will be made available upon request to the corresponding author.
Author contributions
A.C., A.M., J.M.L., P.S. conceived the study, A.C., M.J.V. and P.B.; wrote the main manuscript text, A.C. and P.B. did the statistical analysis and prepared the tables, A.C. and M.J.V prepared Fig. 1, C.D., M.J.V. and M.P. did the genetic analysis, L.P., M.J.V. and S.M. did the immunocytochemistry, A.C., F.V., J.M.L. and M.S.F. did the histological review of the cases. All authors reviewed the manuscript.
Funding
This study was supported by FCT, the Portuguese Foundation for Science and Technology through a Ph.D. Grant to SM (SFRH/BD/137802/2018). This work was financed by FEDER—Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020—Operacional Programme for Competitiveness and Internationalization (POCI), Portugal 2020, and by Portuguese funds through FCT-Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Inovação in the framework of the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274). Additional funding by the European Regional Development Fund (ERDF) through the Operational Programme for Competitiveness and Internationalization—COMPETE2020, and Portuguese national funds via FCT, under project POCI-01-0145-FEDER-016390: CANCEL STEM and from the FCT, under the project POCI-01-0145-FEDER-031438: The other faces of telomerase: Looking beyond tumour immortalization (PDTC/MED-ONC/31438/2017).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: António Castanheira, Maria João Vieira and Mafalda Pinto.
References
- 1.Correia de Sá TR, Silva R, Lopes JM. Basal cell carcinoma of the skin (part 1): epidemiology, pathology and genetic syndromes. Future Oncol. 2015;11(22):3011–3021. doi: 10.2217/fon.15.246. [DOI] [PubMed] [Google Scholar]
- 2.Peris K, et al. Diagnosis and treatment of basal cell carcinoma: European consensus–based interdisciplinary guidelines. Eur. J. Cancer. 2019;118:10–34. doi: 10.1016/j.ejca.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Pellegrini C, et al. Understanding the molecular genetics of basal cell carcinoma. Int. J. Mol. Sci. 2017;18(11):2485. doi: 10.3390/ijms18112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang BM, et al. S2k guidelines for cutaneous basal cell carcinoma-part 1: epidemiology, genetics and diagnosis. JDDG. 2019;17(1):94–103. doi: 10.1111/ddg.13733. [DOI] [PubMed] [Google Scholar]
- 5.Verkouteren J, et al. Epidemiology of basal cell carcinoma: scholarly review. Br. J. Dermatol. 2017;177(2):359–372. doi: 10.1111/bjd.15321. [DOI] [PubMed] [Google Scholar]
- 6.Pyne J, Sapkota D, Wong JC. Aggressive basal cell carcinoma: dermatoscopy vascular features as clues to the diagnosis. Br. J. Dermatol. 2012;2(3):0203a02. doi: 10.5826/dpc.0203a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crowson AN. Basal cell carcinoma: biology, morphology and clinical implications. Mod. Pathol. 2006;19(2):S127–S147. doi: 10.1038/modpathol.3800512. [DOI] [PubMed] [Google Scholar]
- 8.Yalçin UK, Seçkin S. The expression of p53 and COX-2 in basal cell carcinoma, squamous cell carcinoma and actinic keratosis cases. Turk Patoloji Derg. 2012;28(2):119–127. doi: 10.5146/tjpath.2012.01110. [DOI] [PubMed] [Google Scholar]
- 9.Enache AO, et al. Immunoexpression of p53 and COX-2 in basal cell carcinoma. Rom. J. Morphol. Embryol. 2018;59(4):1115–1120. [PubMed] [Google Scholar]
- 10.Ansarin H, Daliri M, Soltani-Arabshahi R. Expression of p53 in aggressive and non-aggressive histologic variants of basal cell carcinoma. Eur. J. Dermatol. 2006;16(5):543–547. [PubMed] [Google Scholar]
- 11.Auepemkiate S, Boonyaphiphat P, Thongsuksai P. p53 expression related to the aggressive infiltrative histopathological feature of basal cell carcinoma. Histopathology. 2002;40(6):568–573. doi: 10.1046/j.1365-2559.2002.01393.x. [DOI] [PubMed] [Google Scholar]
- 12.Koseoglu RD, et al. Expressions of p53, cyclinD1 and histopathological features in basal cell carcinomas. J. Cutan. Pathol. 2009;36(9):958–965. doi: 10.1111/j.1600-0560.2009.01204.x. [DOI] [PubMed] [Google Scholar]
- 13.Bolshakov S, et al. p53 mutations in human aggressive and nonaggressive basal and squamous cell carcinomas. Clin. Cancer Res. 2003;9(1):228–234. [PubMed] [Google Scholar]
- 14.Scott GA, Laughlin TS, Rothberg PG. Mutations of the TERT promoter are common in basal cell carcinoma and squamous cell carcinoma. Mod. Pathol. 2014;27(4):516. doi: 10.1038/modpathol.2013.167. [DOI] [PubMed] [Google Scholar]
- 15.Populo H, et al. TERT promoter mutations in skin cancer: the effects of sun exposure and X-irradiation. J. Invest. Dermatol. 2014;134(8):2251–2257. doi: 10.1038/jid.2014.163. [DOI] [PubMed] [Google Scholar]
- 16.Griewank KG, et al. TERT promoter mutations are frequent in cutaneous basal cell carcinoma and squamous cell carcinoma. PLoS ONE. 2013;8(11):e80354. doi: 10.1371/journal.pone.0080354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lombardo D, et al. Frequency of somatic mutations in TERT promoter, TP53 and CTNNB1 genes in patients with hepatocellular carcinoma from Southern Italy. Oncol. Lett. 2020;19(3):2368–2374. doi: 10.3892/ol.2020.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roake CM, Artandi SE. Control of cellular aging, tissue function, and cancer by p53 downstream of telomeres. Cold Spring Harb. Perspect. Med. 2017;7(5):1–16. doi: 10.1101/cshperspect.a026088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campos MA, et al. TERT promoter mutations are associated with poor prognosis in cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2019;80(3):660–669. doi: 10.1016/j.jaad.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Hafezi F, Perez Bercoff D. The solo play of TERT promoter mutations. Cells. 2020;9(3):749. doi: 10.3390/cells9030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shriver MD, Parra EJ. Comparison of narrow-band reflectance spectroscopy and tristimulus colorimetry for measurements of skin and hair color in persons of different biological ancestry. Am J Phys Anthropol Off Publ Am Assoc Phys Anthropol. 2000;112(1):17–27. doi: 10.1002/(SICI)1096-8644(200005)112:1<17::AID-AJPA3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Rippey J. Why classify basal cell carcinomas? Histopathology. 1998;32(5):393–398. doi: 10.1046/j.1365-2559.1998.00431.x. [DOI] [PubMed] [Google Scholar]
- 23.Stamatelli A, et al. B-Raf mutations, microsatellite instability and p53 protein expression in sporadic basal cell carcinomas. Pathol Oncol Res. 2011;17(3):633–637. doi: 10.1007/s12253-011-9363-1. [DOI] [PubMed] [Google Scholar]
- 24.Haghighi RGF. Immunohistochemistry assessment of p53 protein in basal cell carcinoma. Iran. J. Allergy Asthma Immunol. 2005;4(4):167–171. [PubMed] [Google Scholar]
- 25.Batista R, et al. Validation of a novel, sensitive and specific urine-based test for recurrence surveillance of patients with non-muscle invasive bladder cancer in a comprehensive multicenter study. Front. Genet. 2019;10:1237. doi: 10.3389/fgene.2019.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newlands C, et al. Non-melanoma skin cancer: United Kingdom national multidisciplinary guidelines. J. Laryngol. Otol. 2016;130(S2):S125–S132. doi: 10.1017/S0022215116000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartos V, Kullova M. Basal cell carcinoma of the skin with mixed histomorphology: a comparative study. Ceskoslov. Patol. 2016;52(4):222–226. [PubMed] [Google Scholar]
- 28.Mantese SAO, et al. Carcinoma basocelular-Análise de 300 casos observados em Uberlândia-MG Basal cell Carcinoma-Analysis of 300 cases observed in Uberlândia-MG, Brazxil. An. Bras. Dermatol. 2006;18(2):136–142. doi: 10.1590/S0365-05962006000200004. [DOI] [Google Scholar]
- 29.Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br. J. Dermatol. 2002;147(1):41–47. doi: 10.1046/j.1365-2133.2002.04804.x. [DOI] [PubMed] [Google Scholar]
- 30.Demirseren DD, et al. Basal cell carcinoma of the head and neck region: a retrospective analysis of completely excised 331 cases. J. Skin Cancer. 2014;2014:1–6. doi: 10.1155/2014/858636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sexton M, Jones DB, Maloney ME. Histologic pattern analysis of basal cell carcinoma: study of a series of 1039 consecutive neoplasms. J. Am. Acad. Dermatol. 1990;23(6):1118–1126. doi: 10.1016/0190-9622(90)70344-H. [DOI] [PubMed] [Google Scholar]
- 32.Boaventura P, et al. Mitochondrial D310 D-loop instability and histological subtypes in radiation-induced cutaneous basal cell carcinomas. J. Dermatol. Sci. 2014;73(1):31–39. doi: 10.1016/j.jdermsci.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Leibovitch I, et al. Basal cell carcinoma treated with Mohs surgery in Australia II. Outcome at 5-year follow-up. J. Am. Acad. Dermatol. 2005;53(3):452–457. doi: 10.1016/j.jaad.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 34.Ghanadan A, et al. Characteristics of mixed type basal cell carcinoma in comparison to other BCC subtypes. Indian J. Dermatol. 2014;59(1):56–59. doi: 10.4103/0019-5154.123496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourlidou E, et al. Risk factors for local recurrence of basal cell carcinoma and cutaneous squamous cell carcinoma of the middle third of the face: a 15-year retrospective analysis based on a single centre. Eur. J. Dermatol. 2019;29(5):490–499. doi: 10.1684/ejd.2019.3643. [DOI] [PubMed] [Google Scholar]
- 36.Bandeira AM, et al. Basal cell carcinomas: anatomopathological and clinical study of 704 tumors. An. Bras. Dermatol. 2003;78(1):23–34. doi: 10.1590/S0365-05962003000100003. [DOI] [Google Scholar]
- 37.Betti R, et al. Micronodular basal cell carcinoma: a distinct subtype? Relationship with nodular and infiltrative basal cell carcinomas. J. Dermatol. 2010;37(7):611–616. doi: 10.1111/j.1346-8138.2009.00772.x. [DOI] [PubMed] [Google Scholar]
- 38.Welsch MJ, et al. Basal cell carcinoma characteristics as predictors of depth of invasion. J. Am. Acad. Dermatol. 2012;67(1):47–53. doi: 10.1016/j.jaad.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 39.Ying Y, Padanilam BJ. Regulation of necrotic cell death: p53, PARP1 and cyclophilin D-overlapping pathways of regulated necrosis? Cell. Mol. Life Sci. 2016;73(11):2309–2324. doi: 10.1007/s00018-016-2202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onodera H, Nakamura S, Sugai T. Cell proliferation and p53 protein expressions in cutaneous epithelial neoplasms. Am. J. Dermatopathol. 1996;18(6):580–588. doi: 10.1097/00000372-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Campos MAC, et al. Trends of cutaneous squamous cell carcinoma in the hospital of Gaia (2004–2013) J. Portug. Soc. Dermatol. Venereol. 2018;76(3):279–286. doi: 10.29021/spdv.76.3.919. [DOI] [Google Scholar]
- 42.Batista R, et al. TERT promoter mutation as a potential predictive biomarker in BCG-treated bladder cancer patients. Int. J. Mol. Sci. 2020;21(3):947. doi: 10.3390/ijms21030947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machado I, et al. Solitary fibrous tumor: a case series identifying pathological adverse factors-implications for risk stratification and classification. Virchows Arch. 2020;476(4):597–607. doi: 10.1007/s00428-019-02660-3. [DOI] [PubMed] [Google Scholar]
- 44.Akaike K, et al. Distinct clinicopathological features of NAB2-STAT6 fusion gene variants in solitary fibrous tumor with emphasis on the acquisition of highly malignant potential. Hum. Pathol. 2015;46(3):347–356. doi: 10.1016/j.humpath.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Marotta V, et al. Application of molecular biology of differentiated thyroid cancer for clinical prognostication. Endocr. Relat. Cancer. 2016;23(11):R499–R515. doi: 10.1530/ERC-16-0372. [DOI] [PubMed] [Google Scholar]
- 46.Romei C, Elisei R. A narrative review of genetic alterations in primary thyroid epithelial cancer. Int. J. Mol. Sci. 2021;22(4):1726–1741. doi: 10.3390/ijms22041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulze K, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015;47(5):505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu S, et al. Telomerase reverse transcriptase gene promoter mutations help discern the origin of urogenital tumors: a genomic and molecular study. Eur. Urol. 2014;65(2):274–277. doi: 10.1016/j.eururo.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 49.MercuŢ R, et al. Expression of p53, D2–40 and α-smooth muscle actin in different histological subtypes of facial basal cell carcinoma. Rom. J. Morphol. Embryol. 2014;55(2):263–272. [PubMed] [Google Scholar]