Abstract
Development of practical deuteration reactions is highly valuable for organic synthesis, analytic chemistry and pharmaceutic chemistry. Deuterodehalogenation of organic chlorides tends to be an attractive strategy but remains a challenging task. We here develop a photocatalytic system consisting of an aryl-amine photocatalyst and a disulfide co-catalyst in the presence of sodium formate as an electron and hydrogen donor. Accordingly, many aryl chlorides, alkyl chlorides, and other halides are converted to deuterated products at room temperature in air (>90 examples, up to 99% D-incorporation). The mechanistic studies reveal that the aryl amine serves as reducing photoredox catalyst to initiate cleavage of the C-Cl bond, at the same time as energy transfer catalyst to induce homolysis of the disulfide for consequent deuterium transfer process. This economic and environmentally-friendly method can be used for site-selective D-labeling of a number of bioactive molecules and direct H/D exchange of some drug molecules.
Subject terms: Photocatalysis, Synthetic chemistry methodology
Deuterodehalogenation of organic chlorides is a useful strategy to install deuterium atoms at specific positions, however, it has several drawbacks. In this study, the authors report an organophotocatalytic system consisting of an aryl-amine-based photocatalyst and a common disulfide co-catalyst, for efficient deuteration of a wide range of aryl chlorides, alkyl chlorides and other halides, at room temperature in air.
Introduction
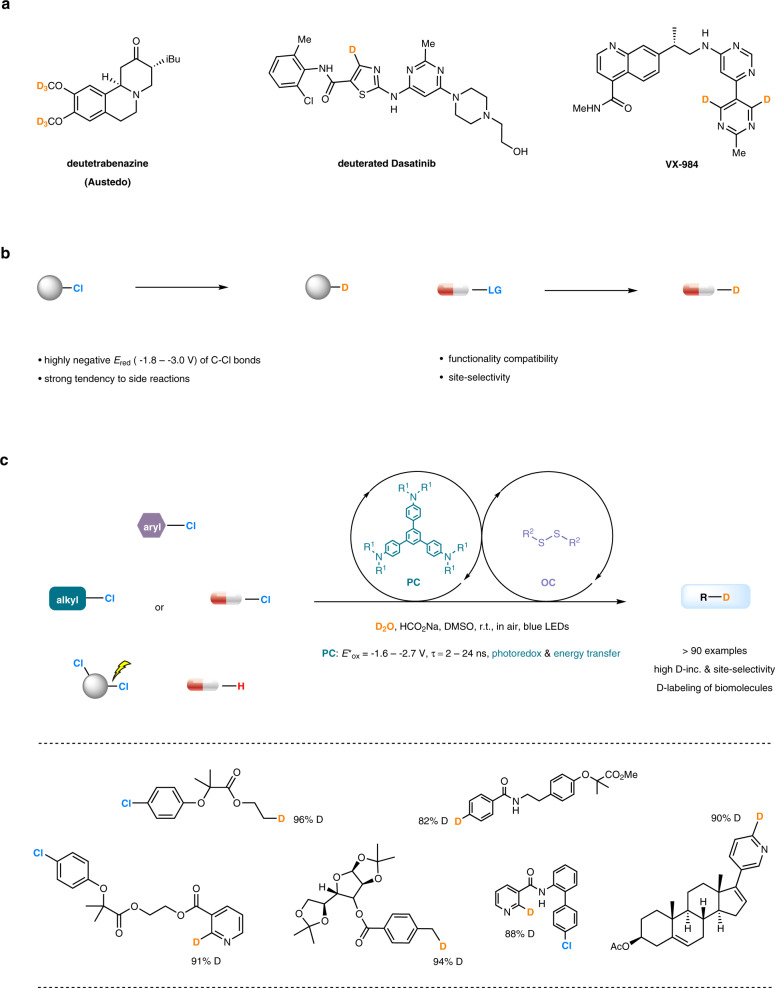
Deuterium-containing molecules are widely used in investigation of reaction mechanisms1–7, organic synthesis8–12, analytic chemistry13–15, and pharmaceutic chemistry16–19. In particular, D-labeling techniques have been established as an important tool in drug development because incorporation of deuterium atoms into bioactive compounds can dramatically affect their metabolic and pharmacokinetic properties20,21. In 2017, Austedo (deutetrabenazine) was approved by FDA as the first deuterated drug used for the treatment of chorea, a dyskinesia associated with Huntington’s disease22. Our knowledge of the distribution, metabolism and excretion properties of a number of drugs such as Tolperisone, Rofecoxib and Dasatinib has been remarkably enhanced by deuteration (Fig. 1a)23,24. Development of practical and bio-friendly deuteration protocols, in particular those being applicable to structurally complex pharmaceutical compounds, is of great interests and in high demand25–37.
Fig. 1. Overview of this work.
a Deuterated drug molecules. b Challenges in deuterodehalogenation of organic chlorides and its application to drug labeling. c This work: organophotocatalytic selective deuterodehalogenation of aryl or alkyl chlorides. LG, leaving group; PC, photocatalyst; OC, organocatalyst; r.t., room temperature; D-inc., deuterium incorporation; DMSO, dimethyl sulfoxide.
Deuterodehalogenation of organohalides is a straightforward strategy with which to incorporate deuterium atoms at specific positions. However, the available methods often rely on transition-metal catalysis and suffer from harsh reaction conditions, narrow substrate scope, and the use of toxic and expensive deuterium sources38. Achieving a high level of deuterium incorporation is also a challenging task39. These factors apparently limit their application in pharmaceutic and life science. Recently, several subtle strategies have been developed to avoid use of transition metal catalysts40–44. For example, Renaud et al. reported thiol-catalyzed deuterative deiodination of alkyl iodides with D2O as a source of deuterium atoms with a stoichiometric amount of Et3B as the reductant40. Liu et al. developed potassium methoxide/disilane-mediated deuterodehalogenation of (hetero)aryl bromides or iodides in CD3CN41. Loh et al. developed the photocatalytic deuterodehalogenation of aryl halides using porous CdSe nanosheets as the catalyst and D2O as the deuteration reagent42. Organic chlorides tend to be less expensive, more abundant and readily available than other halides. There are however very limited methods for deuterodehalogenation of aryl chlorides45,46, and to date, deuterodehalogenation of unactivated alkyl chlorides has not been reported. Several significant challenges are associated with the strategy, including (i) the highly negative reduction potentials of unactivated C-Cl bonds (for example, Ered [(CH3CH2CH2Cl)/(CH3CH2CH2Cl)·‒] = −2.8 V vs SCE)47–52, (ii) the strong possibility of side reactions such as elimination in transition metal catalysis53, (iii) the lack of an effective approach to deuterium transfer which can ensure a high incorporation of deuterium29. Moreover, precise differentiation of C–Cl bonds in polychlorinated compounds that have little or no difference in polarity or other features is highly useful in multi-step organic syntheses of deuterated biomolecules, but remains an unsolved problem (Fig. 1b).
Our group has long had an interest in the design of economic and environmentally-friendly photochemical synthesis54–58. We questioned whether implementing reducing organophotoredox catalysis could initiate the cleavage of unactivated aryl/alkyl C–Cl bonds, and well-matched organocatalysis could bridge the energy gap between the O–D bonds in D2O (BDE = 118 kcal mol−1) and C–D bonds in deuterated hydrocarbons (BDE = 88‒110 kcal mol−1)29. This would allow us establish an effective channel for deuterium transfer, suppressing side reactions, and developing a general and convenient D-labeling method.
Herein, we report photocatalytic deuterodehalogenation for both aryl and alkyl chlorides by synergistic aryl-amine-based organophotoredox and disulfide organocatalysis in the presence of sodium formate as a mild electron and hydrogen sacrificial agent. This reaction provides a practical access to structurally diverse deuterated products including many bioactive molecules and drugs, and exhibits excellent site-selectivity in deuterodehalogenation of polychlorinated compounds which would be beneficial for the assembly of libraries of deuterated compounds (Fig. 1c).
Results
Reaction development
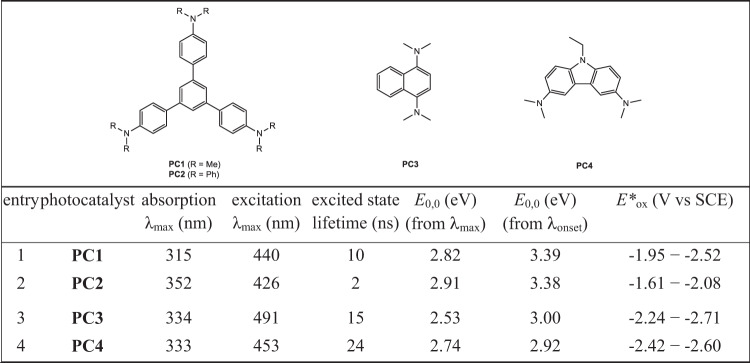
Aryl amines have been established as useful photoredox catalysts in some light-induced reactions59,60. We questioned whether adjusting structures of conjugated aryl amines would enable us find effective visible-light photocatalysts for deuterodehalogenation of organic chlorides. For example, 1,3,5-tris(4′-(N,N-dimethylamino)phenyl)benzene (PC1, Fig. 2) has been intensively used as chemo-sensors61. To investigate their applications in synthetic chemistry, photophysical and electrochemical properties of PC1 and PC2–PC4 were measured and summarized in Fig. 2, including the maximum absorption, excitation, the energies of the first singlet excited state (E0,0), and the oxidation potentials at excited state (E*ox). These compounds exhibited obvious visible-light absorption (see more details in Supplementary Fig. 15), long excited-state lifetimes (2–24 ns), and strongly reducing ability (E*ox = −1.61 to −2.71 V vs SCE), which allowed them serve as catalyst candidates being capable of cleaving unactivated C(sp2)-Cl and C(sp3)-Cl bonds (Ered = −1.8 to −3.0 V), and initiating radical process under visible light conditions.
Fig. 2. Photophysical and electrochemical properties of arylamine-based photocatalysts PC1 − PC4.
All the data are measured in DMSO (0.10 or 1.0 mM) solution at room temperature. PC, photocatalyst; DMSO, dimethyl sulfoxide.
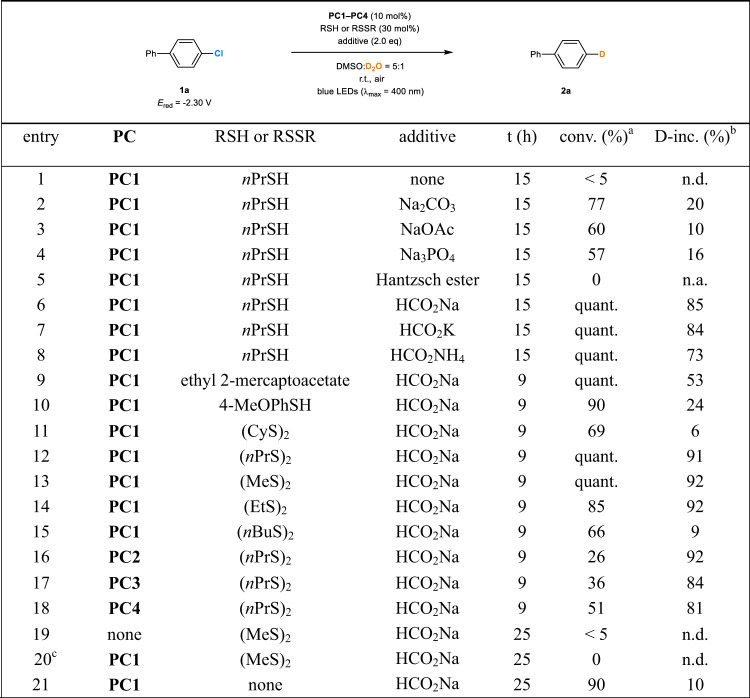
We embarked on an examination of photocatalytic deuterodehalogenation reactions by using PC1 (5 mol%) as a photocatalyst, propane-1-thiol (30 mol%) as the co-catalyst for deuterium atom transfer25,62, 4-chloro-1,1′-biphenyl (1a, Ered = −2.30 V vs SCE in DMSO) as the model substrate, and D2O as the deuterium source. Upon irradiation with a 50 W blue LEDs lamp (λmax = 400 nm) at room temperature, the reaction of 1a with excess D2O in DMSO produced only a trace amount of 2a (Fig. 3, entry 1). The reaction could be improved to some extent by the addition of inorganic bases, which provided low to moderate conversion and deuterium incorporation (entries 2–4). It was thought that introduction of certain reductive hydrogen donor could tune the reactivity of the catalytic cycles and improve the efficiency of the reaction. The Hantzsch ester was found to be inappropriate for this transformation (entry 5), but formate salts accelerated the reaction significantly and led to an obvious increase in the deuterium incorporation (entries 6–8). Sodium formate was identified as the best additive, delivering in 15 h the product (2a) in quantitative yield and with 85% deuterium incorporation (D-inc.) (entry 6). The result of using sodium formate to promote the transfer of electrons and hydrogen atoms is fully consistent with the observation in the intermolecular addition of aryl and heteroaryl radicals to enecarbamate substrates reported recently by Jui et al.63. Other sulfur-containing cocatalysts such as ethyl 2-mercaptoacetate (entry 9), 4-methoxybenzenethiol (entry 10), dicyclohexyl disulfide (entry 11), dipropyl disulfide (entry 12), dimethyl disulfide (entry 13), and diethyl disulfide were examined. The D-incorporation was significantly improved (92% D-inc.) by replacing the thiol with dimethyl disulfide (entry 13). The higher reaction efficiency might be attributed to the fact that in contrast to the thiols, organic disulfides can be easily cleaved into free thiyl radicals useful in the deuterium transfer without introduction of additional protons64. Photocatalysts PC2–PC4 could be used in the reaction, while gave reduced yields or/and deuterium incorporation (entries 16–18). Control experiments revealed that the photocatalyst and the light irradiation were essential for the reaction to proceed (entries 19, 20), and the sulfur-based cocatalyst was critical for achieving a high level of deuterium incorporation (entry 21).
Fig. 3. Initial experiments for photocatalytic deuterodehalogenation of aryl chlorides.
Reaction conditions: 1a (0.10 mmol), D2O (0.10 mL), PC1−PC4 (0.0050 mmol), thiol or disulfide (0.030 mmol), additive (0.20 mmol), DMSO (0.50 mL), a 50 W blue LEDs lamp (λmax = 400 nm), room temperature (~ 30 °C under irradiation), in air. aDegree of conversion determined by 1H-NMR. bD-inc. determined by 1H-NMR. cIn the dark. PC, photocatalyst; eq, equivalent; r.t., room temperature; conv., conversion; D-inc., deuterium incorporation; t, reaction time; quant., quantitative conversion; n.d., not determined; n.a., not applicable; DMSO, dimethyl sulfoxide.
Reaction scope of aryl chlorides and other halides
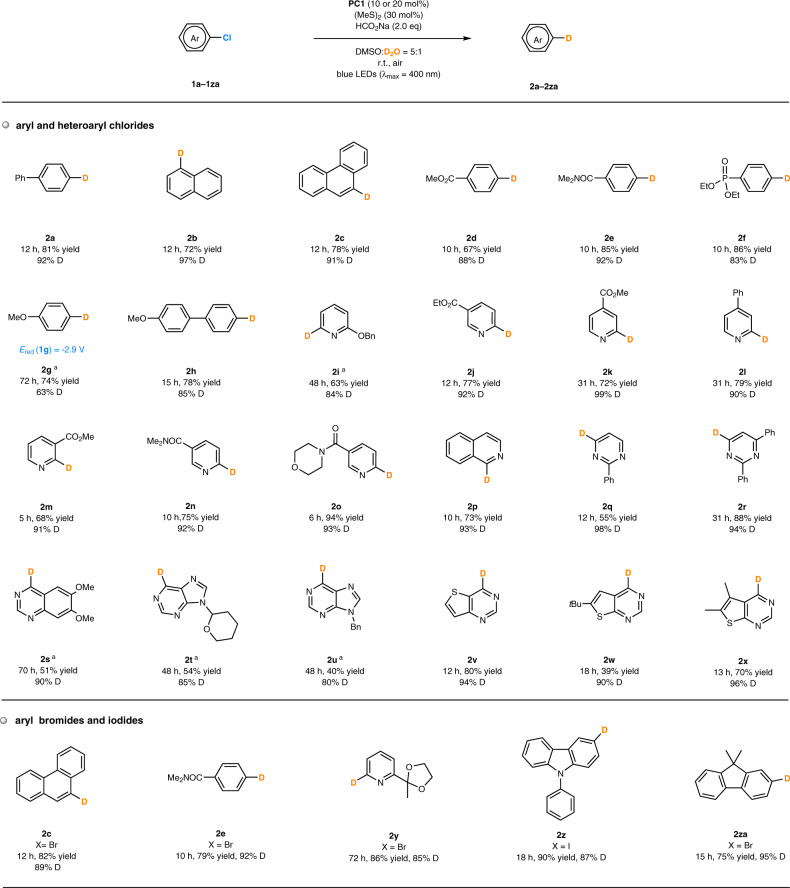
With the optimal conditions in hand, the substrate scope of the photocatalytic deuterodehalogenation of aryl chlorides was investigated (Fig. 4). Products bearing a phenyl substituent (2a), a fused ring (2b, 2c) or an electron-withdrawing group (2d–2f) were formed within 10–12 h from the chlorobenzene derivatives (67–86% yield, 83–97% D-inc.). 1-Chloro-4-methoxybenzene failed to provide the desired product (2g) when using PC1 as the photocatalyst, perhaps because the electron-rich chlorobenzene contains the C(sp2)-Cl bond with higher reduction potential (Ered = −2.90 V vs SCE in DMSO). This transformation could be improved by replacing PC1 by PC4 of stronger reducing ability (E*ox = −2.42 to −2.60 V), delivering 2g in 74% yield and with 63% D-inc. Aromatic heterocycles are common in organic compounds including natural products and bioactive molecules, and a number of pharmaceutically important heterocyclic chlorides were examined and found to be well tolerated under the standard conditions. For example, deuterated heterocycles including pyridines with an electron-donating substituent (product 2i) or an electron-withdrawing substituent (products 2j–2o), isoquinoline (product 2p), pyrimidines (products 2q, 2r), quinazoline (product 2s), purines (products 2t, 2u), thieno[3,2-d]pyrimidine (product 2v) and thieno[2,3-d]pyrimidines (products 2w, 2x) were all obtained in reasonable yields (39–94%) and with excellent deuterium incorporation (80–99%). These results also revealed the excellent functionality compatibility of the reaction, which could be useful in its further application to structurally more complex pharmaceutical molecules and drugs35. Moreover, this method was applicable to other aryl halides. For example, the D-labeled products (2c, 2e, 2y–2za) could be obtained by the reaction of the corresponding aryl bromides or iodides under the standard conditions, providing similar deuterium incorporation while increasing the reaction rates.
Fig. 4. Scope of photocatalytic deuterodehalogenation of aryl chlorides and other halides.
aPC4 (20 mol%), (nPrS)2 (30 mol%), in argon. PC, photocatalyst; r.t., room temperature; eq, equivalent; DMSO, dimethyl sulfoxide.
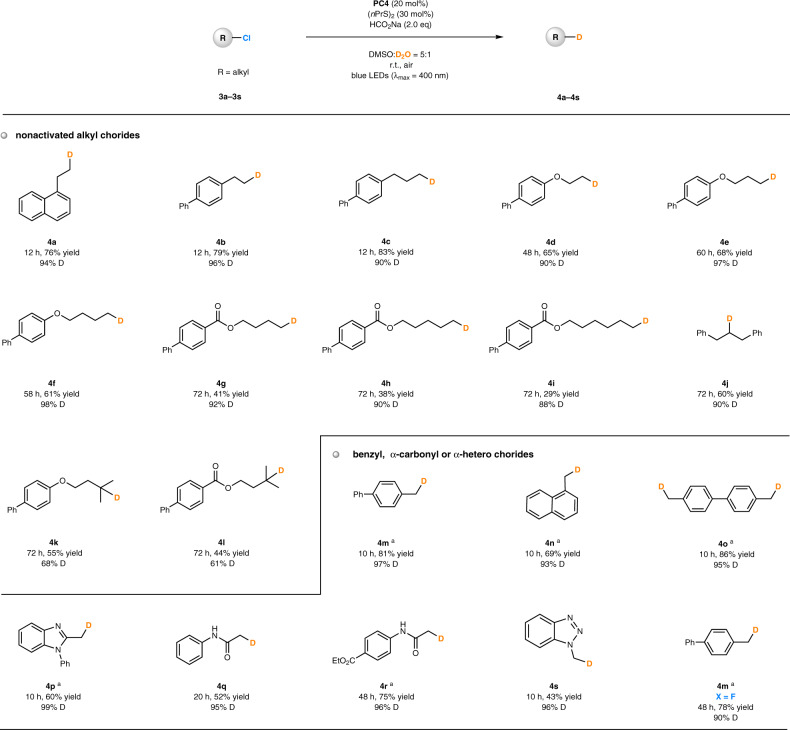
Deuterodehalogenation of alkyl chlorides
Unactivated alkyl chlorides are particularly resistant to deuterodehalogenation. The traditional transition-metal catalytic approaches are not applicable due to the possibility of side reactions such as β-elimination. The highly negative reduction potentials of C(sp3)-Cl bonds and the reactive intermediates eliminate them from the scope of radical-mediated methods65. For these reasons, deuterodehalogenation of unactivated alkyl chlorides has not been reported to date.
To our delight, the photochemical deuterodehalogenation reaction of 3a, a terminal alkyl chloride, gave the desired deuterated product (4a) in 76% yield and with 94% deuterium incorporation within 12 h in the presence of PC4 (E*ox = −2.42 to −2.60 V vs SCE) as the photocatalyst (see more details for reinvestigation of reaction conditions in Supplementary Table 2). A number of unactivated alkyl chlorides were examined under the developed reaction conditions (Fig. 5). Primary alkyl chlorides bearing a remote aromatic ring (products 4a–4c), an ether bond (products 4d–4f) or an ester linkage (products 4g–4i) all provided the deuterated products in reasonable yields (29–83%) and with high deuterium incorporation (88–98%). The secondary alkyl chloride also reacted, delivering product 4j in 60% yield and with 90% D-inc. In comparison, the tertiary alkyl chlorides were less effective substrates, whose reactions proceeded much more slowly and provided 4k, 4l with 68 and 61% D-inc., respectively. Relatively active C(sp3)-Cl precursors containing a benzyl group (products 4m–4p), an α-carbonyl moiety (products 4q, 4r) and α-hetero substitutent (products 4s) were found to be excellent substrates in photocatalytic deuterodehalogenation. These reactions proceeded more rapidly (typically at a lower catalyst loading) and resulted in higher deuterium incorporation (93–99%). The neighboring effects even enabled the reductive cleavage of C-F followed by deuteration, affording product 4m from a fluorine-containing starting material.
Fig. 5. Scope of photocatalytic deuterodehalogenation of alkyl chlorides.
aPC4 (10 mol%). PC, photocatalyst; r.t., room temperature; eq, equivalent; DMSO, dimethyl sulfoxide.
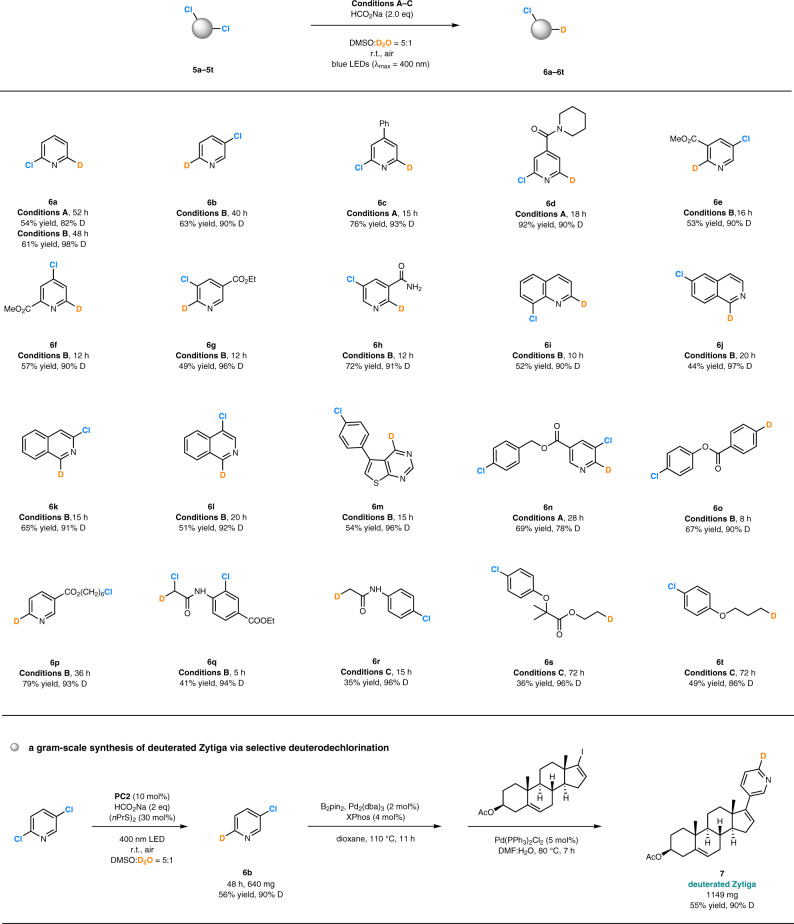
Site-selective deuterodehalogenation of polychlorinated compounds
Selective funtionalization of carbon-halogen bonds with little or no difference in polarity and other features in polyhalogenated compounds is a highly desirable attribute in multi-step organic synthesis but remains highly challenging66–69. It was found that this photocatalytic system could selectively install one deuterium atom in dichlorinated or polychlorinated compounds through choice of a photocatalyst with appropriate reducing ability, that is important for rapid construction of deuterated molecule arrays through diverse functionalization of the residual C-Cl bonds (Fig. 6). For example, under the standard conditions and in the presence of PC1 or PC2 as the photocatalyst, the reactions of symmetric dichlorinated pyridine derivatives (5a−5d) only afforded monodeuterated compounds (6a−6d) in 61‒92% yield and with 90‒98% D-inc. Prolonged reaction time did not lead to the formation of dideuterated products. Excellent site-selectivity could be observed for a number of unsymmetric (hetero)aryl chlorides, all of which provided monodeuterated products (6e–6o) with high deuterium incorporation (78‒97%). Typically, a C-Cl bond at α-positions next to the nitrogen atom in pyridines, diazines or quinolines was more readily to be reacted, which is a hot spot for aldehyde oxidase (AOX) metabolism70. Assemble of deuterium atoms at these positions in drugs would be useful for pharmacokinetic investigations. Moreover, selective deuterodehalogenation of compounds bearing both C(sp2)-Cl and C(sp3)-Cl bonds could be accessed as well. For example, photocatalyst PC2 enabled attachment of one deuterium atom to the pyridyl moiety of 5p and delivered product 6p with 93% D-inc., while photocatalyst PC4 offered an opportunity to introduce deuterium atoms on the alkyl chains instead of on the electron-rich phenyl rings in 5r−5t (86‒96% D-inc.). Such a transformation has not been realized by other reported methods, and will provide a useful access to selectively incorporate deuterium atoms in complex halogen-containing biomolecules as well as drugs. For example, Zytiga is a specific medicine for the treatment of patients with metastatic castration-resistant prostate cancer or high-risk castration-sensitive prostate cancer71, and was the 46th drug in retail sales in 201972. A gram-scale synthesis of D-labeled Zytiga 7 (1149 mg, 90% D-inc.) could be readily developed based on the selective deuterodehalogenation of 2,5-dichoropyridine (product 6b) followed by Pd-catalyzed coupling reactions.
Fig. 6. Site-selective deuterodehalogenation of polychlorides.
Conditions A: PC1 (10 mol%), (MeS)2 (30 mol%). Conditions B: PC2 (10 mol%), (nPrS)2 (30 mol%). Conditions C: PC4 (20 mol%), (nPrS)2 (30 mol%). PC, photocatalyst; r.t., room temperature; eq, equivalent; DMSO, dimethyl sulfoxide; DMF, N,N-dimethylformamide; B2pin2, Bis(pinacolato)diboron.
Mechanistic studies
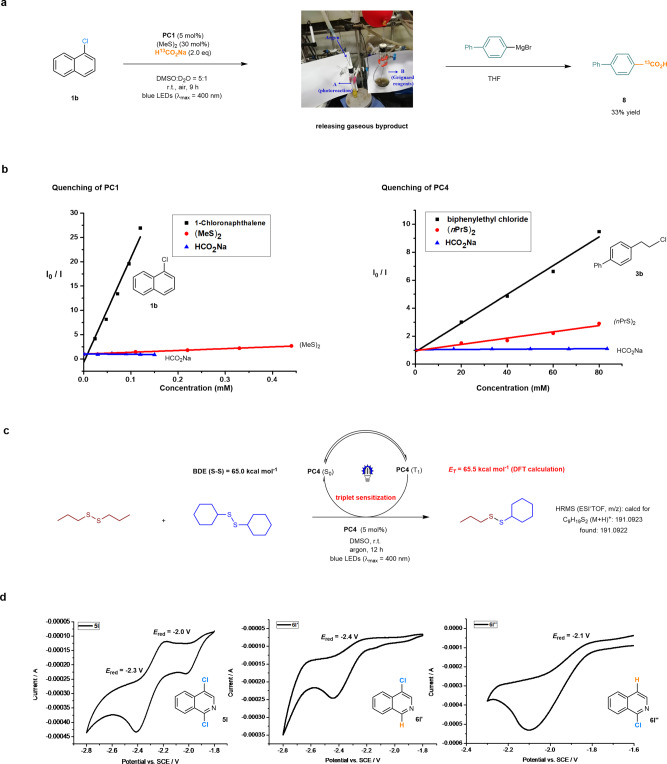
Several control experiments were conducted to gain some insight into the mechanism of this reaction (Fig. 7). The photochemical reaction 1b→2b under the standard conditions with the 13C-labeled sodium formate (H13CO2Na) led to the formation of 13CO2, which was trapped by a Grignard reagent to afford the 13C-containing p-phenylbenzoic acid (8) in 33% yield (Fig. 7a). This observation confirmed that sodium formate serves as an essential electron donor (a reductant) in the catalytic cycles with release of CO2 as a byproduct. Luminescence quenching experiments revealed that the aryl chloride (1b) was capable of obviously quenching the excited state of PC1 (Fig. 7b, left). The disulfide cocatalyst (MeS)2 somewhat quenched its excited state as well while sodium formate failed to do so, revealing the possible interactions between the photocatalyst and other reaction components. The similar results were observed in a system of photocatalyst PC4, alkyl chloride 3b, 1,2-dipropyldisulfane and sodium formate (Fig. 7b, right).
Fig. 7. Mechanistic studies.
a CO2 trapping experiments. b Stern–Volmer quenching experiments. c Thiyl radical scrambling experiments. d Cyclic voltammetry of the dichloride and the possible mono reduction products. PC, photocatalyst; r.t., room temperature; eq, equivalent; THF, tetrahydrofuran; DMSO, dimethyl sulfoxide; DFT, density functional theory; BDE, bond dissociation energy; SCE, saturated calomel electrode.
A radical scrambling experiment was performed to probe the existence of thiyl radical intermediates in the photochemical reaction. Under the standard conditions and in the presence of the photocatalyst PC4, irradiation of dipropyl disulfide and dicyclohexyl disulfide with blue light led to the observation by HRMS of cyclohexyl propyl disulfane (Fig. 7c). The formation of cyclohexyl propyl disulfane probably involved thiyl radicals as intermediates and saw their radical recombination or radical addition into another disulfide molecule. Density functional theory calculations further established that an energy transfer between alkyl disulfides (BDE (S–S) = 65.0 kcal mol−1) and the photocatalyst (ET (PC4) = 65.5 kcal mol−1) was energetically favorable in the reaction (see more details in Supplementary Method 4.8)73.
Electrochemical analysis of one dichlorinated starting material (5l), H-analogs of the possible regioisomeric products (6l′, 6l′′) was performed (Fig. 7d). Two reversible reduction/oxidation signals were observed at Ered = −2.3 V and −2.0 V for 5l. The reversible reduction peaks of 6l′ and 6l′′ were found at −2.4 V and −2.1 V, respectively. These outcomes suggested that C-Cl bond at α-position of the isoquinoline moiety was more active than that at the other position, and 6l′ was more resistant to further reductive deuterodehalogenation. However, the electronic properties and bond dissociation energies (BDE (C-Cl) = 88.7 vs 84.3 kcal mol−1) are close to each other, demonstrating that this photocatalytic system has precise recognition ability toward these similar C-Cl bonds (see more details in Supplementary Method 4.9).
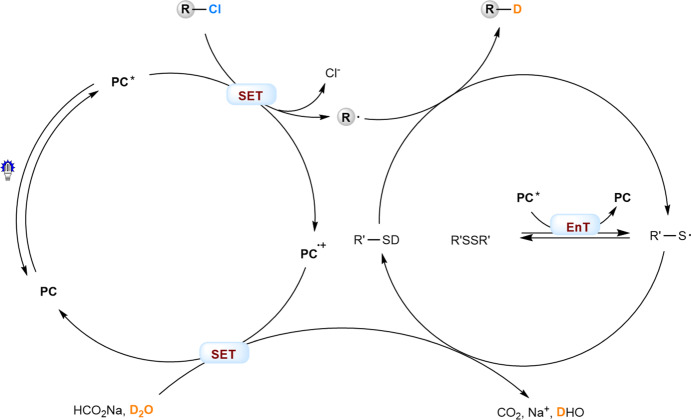
Mechanistic proposal
On basis of the initial experiments and these mechanistic studies, a plausible mechanism for the photocatalytic deuterodehalogenation of organochlorides is shown in Fig. 8. The photoexcited catalyst (PC*, E*ox = −1.61 to −2.71 V vs SCE) undergoes single electron transfer with the organic chloride (Ered = −1.8 to −3.0 V) to give rise to PC∙+ and a carbon radical (R•). Meanwhile, PC* serve as an energy transfer catalyst (ET (PC4) = 65.5 kcal mol−1) to induce homolysis of the disulfides (BDE (S-S) = 65.0 kcal mol−1) affording a thiyl radical (R’S•). In combination with sodium formate as the electron donor and hydrogen donor, R’S• performs effective deuterium atom transfer, and close both the photocatalytic and organocatalytic cycles.
Fig. 8. Proposed reaction mechanism.
The photocatalysts are introduced as strongly reducing photoredox catalysts to initiate cleavage of C-Cl bonds, at the same time as energy transfer catalysts to induce homolysis of the disulfide cocatalysts. PC, photocatalyst; SET, single electron transfer; EnT, energy transfer.
Synthetic application to D-labeled bioactive molecules and drugs
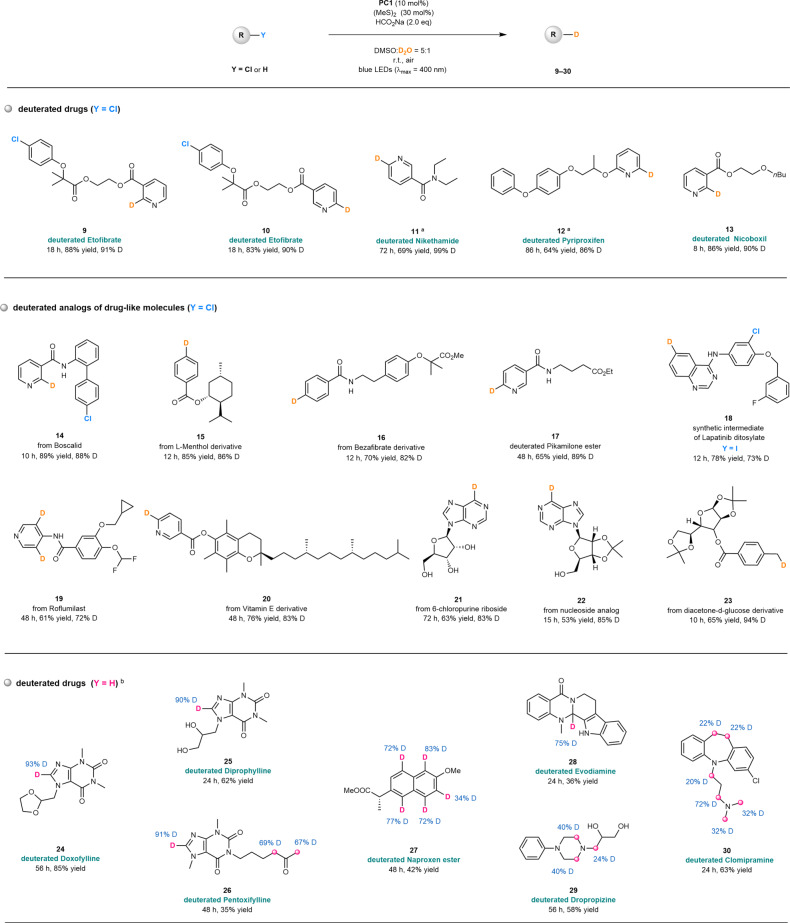
Since the developed photocatalytic system exhibits excellent functional group compatibility and broad substrate scope, we questioned whether it could be used for deuterium-labeling of more complex bioactive molecules and drug derivatives (Fig. 9). For example, Etofibrate is a compound produced by the combination of clofibrate ester linked to niacin, and is used to treat hyperlipemia74. Photocatalytic deuterodehalogenation of its chlorinated derivatives with PC1 as the photocatalyst under the standard conditions led to selective incorporation of deuterium atoms into Etofibrate at specific positions of the pyridyl moiety without affecting the chlorine atoms on the electronically rich phenyl ring, and delivered deuterated products (9, 10) with 91 and 90% D-inc., respectively. Using a similar strategy, deuterated Nikethamide (11), Pyriproxifen (12), and Nicoboxil (13) were obtained in good yields (64−86%) and with high deuterium incorporation (86−99%). Boscalid from BASF is a nicotinamide germicide, which has a broad spectrum of bactericidal activity and has a preventative effect, being active against many types of fungal diseases75. Under the standard conditions, the C(sp2)-Cl bond located on the pyridyl moiety of Boscalid was selectively converted into a C-D bond, affording a product (14) in 89% yield and with 88% deuterium incorporation. Deuterodehalogenation of an L-menthol derivative (product 15), Bezafibrate derivative (product 16), Pikamilone ester (product 17), a synthetic intermediate of Lapatinib ditosylate (Y = I, product 18), Roflumilast (product 19), Vitamin E derivative (product 20), 6-chloropurine riboside (product 21), and nucleoside analog (product 22) all proceeded smoothly, leading to the formation of deuterated products in 53−89% yield and with 72−88% D-inc. The diacetone-d-glucose derivative, an aliphatic chlorinated biomolecule, was also found to be well tolerated in the reaction and provided product 23 in 65% yield and 94% D-inc.
Fig. 9. Synthetic application to D-labeled bioactive molecules and drugs.
aPC4 (20 mol%), (nPrS)2 (30 mol%), in argon. bPC2 (10 mol%), (nPrS)2 (60 mol%), HCO2Na (4.0 eq). PC, photocatalyst; eq, equivalent; r.t., room temperature; DMSO, dimethyl sulfoxide.
H/D exchange of C(sp3)-H or C(sp2)-H bonds is a appealing access to deuterated molecules, but its application in D-labeling of pharmaceutical compounds remains a formidably difficult problem25. Interestingly, this photocatalytic system could be used for direct H/D exchange of some drug and drug-like molecules. Under modified conditions in the presence of PC2 as the photocatalyst, the C(sp2)-H bond located on the imidazole moiety of Doxofyline, an injectable drug for treatment of asthma, was selectively deuterated, delivering product 24 in 85% yield and 93% D-inc. within 56 h. The reactions of its derivatives, Diprophylline and Pentoxifylline, gave similar results and afforded product 25 and 26 with good deuterium incorporation, respectively. Such H/D exchange phenomena was observed in the photochemical reactions of Naproxen ester (product 27), Evodiamine (product 28), Dropropizine (product 29), and Chomipramine (product 30), in which one or more C(sp2)-H/C(sp3)-H bonds were deuterated. These outcomes further demonstrated synthetic utility of this method in D-labeling of structurally complex biomolecules.
Discussion
We have designed an organophotocatalytic system by merging aryl-amine-based photocatalysis and disulfide catalysis. The conjugate aryl amines, such as PC1 and PC2 which have not been used as photocatalysts in previous studies, are introduced as strongly reducing photoredox catalysts to initiate cleavage of C-Cl bonds, at the same time as energy transfer catalysts to induce homolysis of the disulfide cocatalysts. Common alkyl disulfides are involved to enable a high level of deuterium incorporation. Sodium formate is employed as a mild electron and hydrogen sacrificial agent to promote the radical transformations and to close the catalytic cycles. This strategy allows us establish an efficient channel for deuterium transfer, and suppressing side reactions such as self-coupling and elimination. Under very mild reaction conditions (visible light, D2O/DMSO, room temperature, in air, benign byproducts such as CO2), a wide range of aryl chlorides, alkyl chlorides, and other halides including many bioactive molecules (>90 examples) can be converted to deuterated products in good yields and with up to 99% D-incorporation. Moreover, precise site-selectivity toward C-Cl bonds of polychlorinated compounds that have little or no difference in polarity or other features has been observed. The successful production to deuterated drugs and other bioactive molecules as well as application to H/D exchange of C(sp2)-H or C(sp3)-H bonds with D2O demonstrate considerable potential of this method in pharmaceutical chemistry.
Methods
General procedure for deuterodehalogenation of aryl chlorides
A dried 5 mL glass vial was charged with aryl chlorides (0.20 mmol), photocatalyst PC1 (8.7 mg, 0.020 mmol), (MeS)2 (6 μL, 0.060 mmol), HCO2Na (27.2 mg, 0.40 mmol), D2O (200 μL) and DMSO (1.0 mL) under air and then performed in a sealed vessel. The glass vial was positioned approximately 3 cm away from a 50 W blue LEDs lamp (λmax = 400 nm). After being stirred at room temperature (~30 °C under irradiation) for the indicated time, the reaction mixture was purified by flash chromatography on silica gel to afford product.
General procedure for deuterodehalogenation of alkyl chlorides
A dried 5 mL glass vial was charged with alkyl chlorides (0.20 mmol), photocatalyst PC4 (11.3 mg, 0.040 mmol), (nPrS)2 (10 μL, 0.060 mmol), HCO2Na (27.2 mg, 0.40 mmol), D2O (200 μL) and DMSO (1.0 mL) under air and then performed in a sealed vessel. The glass vial was positioned ~3 cm away from a 50 W blue LEDs lamp (λmax = 400 nm). After being stirred at room temperature (~30 °C under irradiation) for the indicated time, the reaction mixture was purified by flash chromatography on silica gel to afford product.
Supplementary information
Acknowledgements
We gratefully acknowledge funding from the National Natural Science Foundation of China (grant no. 22071209, 22071206), the Natural Science Foundation of Fujian Province of China (grant no. 2017J06006), and the Fundamental Research Funds for the Central Universities (grant no. 20720190048).
Author contributions
Y.L. and L.G. designed and conceived the project. Y.L. and Z.Y. conducted all the synthetic and catalytic reactions. Y.L., Z.Y., Y.-M.L., and L.G. analyzed and interpreted the experimental data. Z.Y. designed and performed the theoretical calculations. Y.L. and Y.Z. performed the HRMS experiments and analyzed the data. L.G. prepared the manuscript. Y.L. and Z.Y. prepared the Supplementary Information.
Data availability
The authors declare that the data supporting the findings of this study are available within this article and its Supplementary Information file, or from the corresponding author upon reasonable request. The experimental procedures and characterization of all new compounds are provided in Supplementary Information.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Huiying Zeng and the other anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-23255-0.
References
- 1.Wiberg KB. The deuterium isotope effect. Chem. Rev. 1955;55:713–743. doi: 10.1021/cr50004a004. [DOI] [Google Scholar]
- 2.Simmons EM, Hartwig JF. On the interpretation of deuterium kinetic isotope effects in C-H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 2012;51:3066–3072. doi: 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
- 3.Lukin S, et al. Isotope labeling reveals fast atomic and molecular exchange in mechanochemical milling reactions. J. Am. Chem. Soc. 2019;141:1212–1216. doi: 10.1021/jacs.8b12149. [DOI] [PubMed] [Google Scholar]
- 4.McAtee RC, Noten EA, Stephenson CRJ. Arene dearomatization through a catalytic N-centered radical cascade reaction. Nat. Commun. 2020;11:2528. doi: 10.1038/s41467-020-16369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plesniak MP, et al. Samarium(II) folding cascades involving hydrogen atom transfer for the synthesis of complex polycycles. Nat. Commun. 2018;9:4802. doi: 10.1038/s41467-018-07194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watile RA, et al. Intramolecular substitutions of secondary and tertiary alcohols with chirality transfer by an iron(III) catalyst. Nat. Commun. 2019;10:3826. doi: 10.1038/s41467-019-11838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JH, Greßies S, Glorius F. Cooperative Lewis Acid/Cp*CoIII catalyzed C-H bond activation for the synthesis of isoquinolin-3-ones. Angew. Chem. Int. Ed. 2016;55:5577–5581. doi: 10.1002/anie.201601003. [DOI] [PubMed] [Google Scholar]
- 8.Miyashita M, Sasaki M, Hattori I, Sakai M, Tanino K. Total synthesis of norzoanthamine. Science. 2004;305:495–499. doi: 10.1126/science.1098851. [DOI] [PubMed] [Google Scholar]
- 9.Atzrodt J, Derdau V, Fey T, Zimmermann J. The renaissance of H/D exchange. Angew. Chem. Int. Ed. 2007;46:7744–7765. doi: 10.1002/anie.200700039. [DOI] [PubMed] [Google Scholar]
- 10.Emmert MH, Gary J, Villalobos J, Sanford M. Platinum and palladium complexes containing cationic ligands as catalysts for arene H/D exchange and oxidation. Angew. Chem. Int. Ed. 2010;49:5884–5886. doi: 10.1002/anie.201002351. [DOI] [PubMed] [Google Scholar]
- 11.Pieters G, et al. Regioselective and stereospecific deuteration of bioactive aza compounds by the use of ruthenium nanoparticles. Angew. Chem. Int. Ed. 2014;53:230–234. doi: 10.1002/anie.201307930. [DOI] [PubMed] [Google Scholar]
- 12.Quasdorf KW, Huters AD, Lodewyk MW, Tantillo DJ, Garg NK. Total synthesis of oxidized welwitindolinones and (-)-N-methylwelwitindolinone C isonitrile. J. Am. Chem. Soc. 2012;134:1396–1399. doi: 10.1021/ja210837b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheppele SE. Kinetic isotope effects as a valid measure of structure-reactivity relations. Isotope effects and nonclassical theory. Chem. Rev. 1972;72:511–532. doi: 10.1021/cr60279a004. [DOI] [Google Scholar]
- 14.Wacker SA, Kashyap S, Li X, Kapoor TM. Examining the mechanism of action of a kinesin inhibitor using stable isotope labeled inhibitors for crosslinking (SILIC) J. Am. Chem. Soc. 2011;133:12386–12389. doi: 10.1021/ja204561q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Q, et al. Large anisotropy and effect of deuteration on permittivity in an olefin copper(I) complex. Angew. Chem. Int. Ed. 2007;46:6852–6856. doi: 10.1002/anie.200700629. [DOI] [PubMed] [Google Scholar]
- 16.Katsnelson A. Heavy drugs draw heavy interest from pharma backers. Nat. Med. 2013;19:656. doi: 10.1038/nm0613-656. [DOI] [PubMed] [Google Scholar]
- 17.Cress BF, et al. Heavy heparin: a stable isotope enriched, chemoenzymatically-synthesized, poly-component drug. Angew. Chem. Int. Ed. 2019;58:5962–5966. doi: 10.1002/anie.201900768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palazzolo A, et al. Efficient access to deuterated and tritiated nucleobase pharmaceuticals and oligonucleotides using hydrogen-isotope exchange. Angew. Chem. Int. Ed. 2019;58:4891–4895. doi: 10.1002/anie.201813946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmore CS, Bragg RA. Isotope chemistry; a useful tool in the drug discovery arsenal. Bioorg. Med. Chem. Lett. 2015;25:167–171. doi: 10.1016/j.bmcl.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Isin EM, Elmore CS, Nilsson GN, Thompson RA, Weidolf L. Use of radiolabeled compounds in drug metabolism and pharmacokinetic studies. Chem. Res. Toxicol. 2012;25:532–542. doi: 10.1021/tx2005212. [DOI] [PubMed] [Google Scholar]
- 21.Mullard A. Deuterated drugs draw heavier backing. Nat. Rev. Drug Discov. 2016;15:219–221. doi: 10.1038/nrd.2016.63. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt C. First deuterated drug approved. Nat. Biotechnol. 2017;35:493–494. doi: 10.1038/nbt0617-493. [DOI] [PubMed] [Google Scholar]
- 23.Gant TG. Using deuterium in drug discovery: leaving the label in the drug. J. Med. Chem. 2014;57:3595–3611. doi: 10.1021/jm4007998. [DOI] [PubMed] [Google Scholar]
- 24.Meanwell NA. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 2011;54:2529–2591. doi: 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]
- 25.Loh YY, et al. Photoredox catalyzed deuteration and tritiation of pharmaceutical compounds. Science. 2017;358:1182–1187. doi: 10.1126/science.aap9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pony Yu,R, Hesk D, Rivera N, Pelczer I, Chirik PJ. Iron catalysed tritiation of pharmaceuticals. Nature. 2016;529:195–199. doi: 10.1038/nature16464. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, et al. Mesoionic carbene (MIC)-catalyzed H/D exchange at formyl groups. Chem. 2019;5:2484–2494. doi: 10.1016/j.chempr.2019.08.011. [DOI] [Google Scholar]
- 28.Geng H, et al. Practical synthesis of C1 deuterated aldehydes enabled by NHC catalysis. Nat. Catal. 2019;2:1071–1077. doi: 10.1038/s41929-019-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Yuan X-A, Zhu C, Xie J. Deoxygenative deuteration of carboxylic acids with D2O. Angew. Chem. Int. Ed. 2019;58:312–316. doi: 10.1002/anie.201811522. [DOI] [PubMed] [Google Scholar]
- 30.Chang Y, et al. Catalytic deuterium incorporation within metabolically stable β-Amino C-H bonds of drug molecules. J. Am. Chem. Soc. 2019;141:14570–14575. doi: 10.1021/jacs.9b08662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou R, et al. Visible-light-mediated deuteration of silanes with deuterium oxide. Chem. Sci. 2019;10:7340–7344. doi: 10.1039/C9SC02818H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Espinal-Viguri M, Neale SE, Coles NT, Macgregor SA, Webster RL. Room temperature iron-catalyzed transfer hydrogenation and regioselective deuteration of carbon-carbon double bonds. J. Am. Chem. Soc. 2019;141:572–582. doi: 10.1021/jacs.8b11553. [DOI] [PubMed] [Google Scholar]
- 33.Codolà Z, et al. Design of iron coordination complexes as highly active homogenous water oxidation catalysts by deuteration of oxidation-sensitive sites. J. Am. Chem. Soc. 2019;141:323–333. doi: 10.1021/jacs.8b10211. [DOI] [PubMed] [Google Scholar]
- 34.Kurimoto A, Sherbo RS, Cao Y, Loo NWX, Berlinguette CP. Electrolytic deuteration of unsaturated bonds without using D2. Nat. Catal. 2020;3:719–726. doi: 10.1038/s41929-020-0488-z. [DOI] [Google Scholar]
- 35.Koniarczyk JL, Hesk D, Overgard A, Davies IW, McNally A. A general strategy for site-selective incorporation of deuterium and tritium into pyridines, diazines, and pharmaceuticals. J. Am. Chem. Soc. 2018;140:1990–1993. doi: 10.1021/jacs.7b11710. [DOI] [PubMed] [Google Scholar]
- 36.Puleo TR, Strong AJ, Bandar JS. Catalytic α-selective deuteration of styrene derivatives. J. Am. Chem. Soc. 2019;141:1467–1472. doi: 10.1021/jacs.8b12874. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Han S, Li M, Chong X, Zhang B. Electrocatalytic deuteration of halides with D2O as the deuterium source over a copper nanowire arrays cathode. Angew. Chem. Int. Ed. 2020;59:18527–18531. doi: 10.1002/anie.202009155. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Zhu M-H, Liu W-B. Potassium alkoxide/disilane-mediated dehalogenative deuteration. Synlett. 2019;30:1003–1007. doi: 10.1055/s-0037-1610699. [DOI] [Google Scholar]
- 39.Yu Y, Zhang B. Photocatalytic deuteration of halides using D2O over CdSe porous nanosheets: a mild and controllable route to deuterated molecules. Angew. Chem. Int. Ed. 2018;57:5590–5592. doi: 10.1002/anie.201801541. [DOI] [PubMed] [Google Scholar]
- 40.Soulard V, Villa G, Vollmar DP, Renaud P. Radical deuteration with D2O: catalysis and mechanistic insights. J. Am. Chem. Soc. 2018;140:155–158. doi: 10.1021/jacs.7b12105. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, et al. General and practical potassium methoxide/disilane-mediated dehalogenative deuteration of (hetero) arylhalides. J. Am. Chem. Soc. 2018;140:10970–10974. doi: 10.1021/jacs.8b07597. [DOI] [PubMed] [Google Scholar]
- 42.Liu C, et al. Controllable deuteration of halogenated compounds by photocatalytic D2O splitting. Nat. Commun. 2018;9:80. doi: 10.1038/s41467-017-02551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia A, Xie X, Hu X, Xu W, Liu Y. Dehalogenative deuteration of unactivated alkyl halides using D2O as the deuterium source. J. Org. Chem. 2019;84:13841–13857. doi: 10.1021/acs.joc.9b02026. [DOI] [PubMed] [Google Scholar]
- 44.Shao T, et al. Photoredox-catalyzed enantioselective α-deuteration of azaarenes with D2O. iScience. 2019;16:410–419. doi: 10.1016/j.isci.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong Y, et al. Plasmon-enhanced deuteration under visible-light irradiation. ACS Nano. 2019;13:10754–10760. doi: 10.1021/acsnano.9b05523. [DOI] [PubMed] [Google Scholar]
- 46.Kuriyama M, et al. Deuterodechlorination of aryl/heteroaryl chlorides catalyzed by a Palladium/unsymmetrical NHC system. J. Org. Chem. 2016;81:8934–8946. doi: 10.1021/acs.joc.6b01609. [DOI] [PubMed] [Google Scholar]
- 47.Lambert, F. L. & Ingall, G. B. Voltammetry of organic halogen compounds. IV. The reduction of organic chlorides at the vitreous (glassy) carbon electrode. Tetrahedron Lett. 3231–3234 (1974).
- 48.Yin H, et al. The hexachlorocerate(III) anion: a potent, benchtop stable, and readily available ultraviolet a photosensitizer for aryl chlorides. J. Am. Chem. Soc. 2016;138:16266–16273. doi: 10.1021/jacs.6b05712. [DOI] [PubMed] [Google Scholar]
- 49.Yin H, Carroll PJ, Anna JM, Schelter EJ. Luminescent Ce(III) complexes as stoichiometric and catalytic photoreductants for halogen atom abstraction reactions. J. Am. Chem. Soc. 2015;137:9234–9237. doi: 10.1021/jacs.5b05411. [DOI] [PubMed] [Google Scholar]
- 50.Cybularczyk-Cecotka M, Szczepanik J, Giedyk M. Photocatalytic strategies for the activation of organic chlorides. Nat. Catal. 2020;3:872–886. doi: 10.1038/s41929-020-00515-8. [DOI] [Google Scholar]
- 51.Pagire SK, Föll T, Reiser O. Shining visible light on vinyl halides: expanding the horizons of photocatalysis. Acc. Chem. Res. 2020;53:782–791. doi: 10.1021/acs.accounts.9b00615. [DOI] [PubMed] [Google Scholar]
- 52.Kim H, Kim H, Lambert T, Lin S. Reductive electrophotocatalysis: merging electricity and light to achieve extreme reduction potentials. J. Am. Chem. Soc. 2020;142:2087–2092. doi: 10.1021/jacs.9b10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh I, Ghosh T, Bardagi JI, König B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science. 2014;346:725–728. doi: 10.1126/science.1258232. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Lei M, Gong L. Photocatalytic regio- and stereoselective C(sp3)-H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes. Nat. Catal. 2019;2:1016–1026. doi: 10.1038/s41929-019-0357-9. [DOI] [Google Scholar]
- 55.Han B, Li Y, Yu Y, Gong L. Photocatalytic enantioselective α-aminoalkylation of acyclic imine derivatives by a chiral copper catalyst. Nat. Commun. 2019;10:3804. doi: 10.1038/s41467-019-11688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y, et al. Copper(II)–catalyzed asymmetric photoredox reactions: enantioselective alkylation of imines driven by visible light. J. Am. Chem. Soc. 2018;140:15850–15858. doi: 10.1021/jacs.8b09251. [DOI] [PubMed] [Google Scholar]
- 57.Shen X, et al. A chiral nickel DBFOX complex as a bifunctional catalyst for visible-light-promoted asymmetric photoredox reactions. Chem. Sci. 2018;9:4562–4568. doi: 10.1039/C8SC01219A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou K, Yu Y, Lin Y-M, Li Y, Gong L. Copper-catalyzed aerobic asymmetric cross-dehydrogenative coupling of C(sp3)-H bonds driven by visible light. Green. Chem. 2020;22:4597–4603. doi: 10.1039/D0GC00262C. [DOI] [Google Scholar]
- 59.McCarthy BG, et al. Structure-property relationships for tailoring phenoxazines as reducing photoredox catalysts. J. Am. Chem. Soc. 2018;140:5088–5101. doi: 10.1021/jacs.7b12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noto N, Koike T, Akita M. Visible light-triggered monofluoromethylation of alkenes by strongly reducing 1,4-bis(diphenylamino)naphthalene photoredox catalysis. ACS Catal. 2019;9:4382–4387. doi: 10.1021/acscatal.9b00473. [DOI] [Google Scholar]
- 61.Vishnoi P, Sen S, Patwari GN, Murugavel R. Charge transfer aided selective sensing and capture of picric acid by triphenylbenzenes. N. J. Chem. 2015;39:886–892. doi: 10.1039/C4NJ01093K. [DOI] [Google Scholar]
- 62.Constantin T, et al. Aminoalkyl radicals as halogen-atom transfer agents for activation of alkyl and aryl halides. Science. 2020;367:1021–1026. doi: 10.1126/science.aba2419. [DOI] [PubMed] [Google Scholar]
- 63.Boyington AJ, Seath CP, Zearfoss AM, Xu Z, Jui NT. Catalytic strategy for regioselective arylethylamine synthesis. J. Am. Chem. Soc. 2019;141:4147–4153. doi: 10.1021/jacs.9b01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patehebieke Y. An overview on disulfide-catalyzed and -cocatalyzed photoreactions. Beilstein J. Org. Chem. 2020;16:1418–1435. doi: 10.3762/bjoc.16.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giedyk M, et al. Photocatalytic activation of alkyl chlorides by assembly-promoted single electron transfer in microheterogeneous solutions. Nat. Catal. 2020;3:40–47. doi: 10.1038/s41929-019-0369-5. [DOI] [Google Scholar]
- 66.Golding WA, Pearce-Higgins R, Phipps RJ. Site-selective cross-coupling of remote chlorides enabled by electrostatically directed palladium catalysis. J. Am. Chem. Soc. 2018;140:13570–13574. doi: 10.1021/jacs.8b08686. [DOI] [PubMed] [Google Scholar]
- 67.Golding WA, Schmitt HL, Phipps RJ. Systematic variation of ligand and cation parameters enables site-selective C-C and C-N cross-coupling of multiply chlorinated arenes through substrate-ligand electrostatic interactions. J. Am. Chem. Soc. 2020;142:21891–21898. doi: 10.1021/jacs.0c11056. [DOI] [PubMed] [Google Scholar]
- 68.Desaintjean A, et al. Regioselective bromine/magnesium-exchange for selective functionalization of polyhalogenated aromatics and heterocycles. Angew. Chem. Int. Ed. 2021;60:1513–1518. doi: 10.1002/anie.202012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandfort F, Knecht T, Pinkert T, Daniliuc CG, Glorius F. Site-selective thiolation of (multi)halogenated heteroarenes. J. Am. Chem. Soc. 2020;142:6913–6919. doi: 10.1021/jacs.0c01630. [DOI] [PubMed] [Google Scholar]
- 70.Xu Y, et al. Aldehyde oxidase mediated metabolism in drug-like molecules: a combined computational and experimental study. J. Med. Chem. 2017;60:2973–2982. doi: 10.1021/acs.jmedchem.7b00019. [DOI] [PubMed] [Google Scholar]
- 71.Li Z, et al. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature. 2016;533:547–551. doi: 10.1038/nature17954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGrath NA, Brichacek M, Njardarson JT. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 2010;87:1348–1349. doi: 10.1021/ed1003806. [DOI] [Google Scholar]
- 73.Teders M, et al. The energy-transfer-enabled biocompatible disulfide-ene reaction. Nat. Chem. 2018;10:981–988. doi: 10.1038/s41557-018-0102-z. [DOI] [PubMed] [Google Scholar]
- 74.Proctor RSJ, Davis HJ, Phipps RJ. Catalytic enantioselective minisci-type addition to heteroarenes. Science. 2018;360:419–422. doi: 10.1126/science.aar6376. [DOI] [PubMed] [Google Scholar]
- 75.Nagaki A, Ichinari D, Yoshida J. Three-component coupling based on flash chemistry. Carbolithiation of benzyne with functionalized aryllithiums followed by reactions with electrophiles. J. Am. Chem. Soc. 2014;136:12245–12248. doi: 10.1021/ja5071762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within this article and its Supplementary Information file, or from the corresponding author upon reasonable request. The experimental procedures and characterization of all new compounds are provided in Supplementary Information.