Abstract
Objective: To evaluate the diagnostic value of ultrasound detection of the fetal middle cerebral artery, umbilical artery blood flow and fetal movement reduction in fetal distress. Methods: A total of 30 cases of pregnant women with fetal distress (FIUD) in our hospital were selected as the observation group, and 60 cases of normal pregnant women in the same period were selected as the control group. The fetal umbilical artery, middle cerebral artery resistance index (RI), pulsatility index (PI), the ratio of peak systolic blood flow velocity to end-diastolic blood flow velocity (S/D) and fetal movement reduction were detected. The diagnostic value of the above indicators alone and in combination for fetal distress was observed. Results: The RI, PI and S/D of umbilical artery in the observation group were higher than those in the control group (P<0.001). The S/D, RI and PI of the middle cerebral artery in the observation group were lower than those in the control group (P<0.01). The number of cases with decreased fetal heart rate in the observation group was significantly higher than that in the control group (P<0.001). The area under the curve (AUC) of umbilical artery S/D, umbilical artery RI, umbilical artery PI, middle cerebral artery S/D, middle cerebral artery PI, middle cerebral artery RI, fetal movement reduction and combined above indexes were 0.788, 0.870, 0.847, 0.852, 0.802, 0.658, 0.750 and 1.000 respectively (all P<0.05). The combined diagnosis has a higher diagnostic value for fetal distress. Conclusion: Umbilical artery, middle cerebral artery hemodynamics combined with fetal movement has a specific value in diagnosing fetal distress, which is worth of clinical application.
Keywords: Umbilical artery, middle cerebral artery, fetal distress, the diagnostic value
Introduction
Fetal intrauterine distress (FIUD) refers to the fetus’s acute and chronic hypoxic symptoms under the influence of various factors in the uterus. It is the primary factor of intrauterine fetal death [1]. The incidence of fetal intrauterine distress is 2.7%-38.5% [2]. The occurrence of FIUD can lead to changes in fetal heart rate and metabolism and can cause neonatal asphyxia. Cerebral ischemia and hypoxia may affect the development of the neonatal nervous system and continuous ischemia and hypoxia can cause nervous system damage. In serious cases, continuous ischemia and hypoxia can lead to death and seriously affect newborns’ prognosis and quality of life [3,4]. Therefore, early accurate diagnosis of FIUD is of great significance for the prognosis of newborns. The gold standard for the diagnosis of FIUD is to detect the pH value of umbilical artery blood. Still, the examination is invasive and not suitable for detection and diagnosis before delivery, which brings difficulties to the diagnosis of FIUD [5]. Clinical use of fetal heart rate monitoring before delivery can reflect placental function and oxygen reserve, but false positive or false negative often occursdue to external factors, which affects clinical judgment [6]. Color Doppler ultrasound is a non-invasive and repeatable examination and is an important tool for prenatal diagnosis [7]. The middle cerebral artery (MCA) is one of the main arteries supplying blood to the brain, which can directly reflect the cerebral blood flow and indirectly reflect fetus’s situation in the uterine cavity [8]. Studies have shown that umbilical artery (UA) blood flow is also closely related to fetal cerebral ischemia and hypoxia [9]. At present, there lack of studies on the combined diagnosis of FIUD with the indicators of the umbilical artery, middle cerebral artery hemodynamics and fetal movement reduction. This study combined ultrasonic monitoring of the middle cerebral artery, umbilical artery and fetal heart monitoring to study the diagnostic value of FIUD.
Materials and methods
Clinical data
This study was approved by the Ethics Committee of Linyi Women’s and Children’s Hospital. A total of 30 cases of pregnant women with FIUD admitted to our hospital from January 2018 to October 2020 were selected, and 60 of 646 pregnant women with normal childbirth in the same period were randomly selected as the control group. All the patients were 20-34 years old, with an average age of 27.3±2.9 years old. All the pregnant women included in this study have signed informed consent.
Inclusion and exclusion criteria
Inclusion criteria: 1) About 72 suspected FIUD pregnant women were included with cesarean section, and 30 cases were under the diagnostic criteria of FIUD after cesarean section: 1. fetal movement decreased, fetal movement <6 time/2 hours or reduced by 50%, disappeared; 2. fetal heart rate monitoring, fetal heart rate baseline >160 time/min or fetal heart rate baseline <110 time/min, or late deceleration, severe variation deceleration, sine wave; 3. amniotic fluid III degree pollution; 4. neonatal asphyxia, 1-5 minutes Apgar score 7 points, and the pH value of umbilical artery blood was less than 7.2 [10]; 2) the results showed that the age of pregnant women was more than 18 years old; 3) FIUD first occurred in a pregnant women; 4) the detection of middle cerebral artery, umbilical artery and fetal heart rate was carried out and completed for all the included fetuses; 5) all the included pregnant women were determined the PH value of umbilical artery blood after delivery and finally diagnosed as FIUD.
Exclusion criteria: 1) pregnant women with multiple pregnancies; 2) those with abnormal fetal position and unable to display normal blood flow; 3) those with chromosomal abnormalities or diagnosed as genetic diseases; 4) patients with vascular malformations detected by fetal ultrasound.
Ultrasonic testing method
Middle cerebral artery detection method
Color Doppler ultrasound (GE voluson E8, USA) was used. Under the positioning of two-dimensional color Doppler ultrasound, the fetal head was positioned and the biparietal part of the fetus was displayed. Then the probe was moved down to the cerebral angle to display the middle cerebral artery. The sampling line of the middle cerebral artery sample was selected to keep consistent with the blood flow direction That is, the included angle was less than 30°. Three values were continuously measured to record the middle cerebral artery resistance index (RI), pulsation index (PI) and the ratio of peak-systolic blood flow velocity and end-diastolic blood flow velocity (S/D) and the average value was taken.
Umbilical artery detection method
Color Doppler ultrasound (GE voluson E8, USA) was used to detect the umbilical cord’s route, and the location where the umbilical cord entered the placenta was found to show the blood flow of the umbilical artery. The sampling point of the umbilical artery was taken at the blood flow filling place, and the sampling line was consistent with the blood flow direction. That is, the included angle was less than 30° and three values were continuously measured. The values of RI, PI and S/D of the umbilical artery were recorded respectively and the average value was taken.
Fetal heart rate detection
Fetal heart rate was detected by fetal heart rate detector (Edan ultrasonic Doppler fetal monitor) in the semi-decubitus position of pregnant women for 30 minutes.
Outcome measures
The hemodynamic indexes of the umbilical artery: RI, PI and S/D of umbilical artery were compared between the two groups; the hemodynamic indexes of fetal middle cerebral artery: RI, PI and S/D were compared between the two groups; the incidence of decreased fetal movement was compared between the two groups; the incidence of decreased fetal movement = (number of cases of decreased fetal movement/total number of cases) * 100%. The diagnostic value of umbilical artery, middle cerebral artery and fetal movement reduction index and the combination of the above three indexes for FIUD.
Statistical indicators
The data were analyzed by SPSS 17.0 statistical software. Continuous variables were expressed as mean ± standard deviation (x̅ ± sd), which accorded with normal distribution and homogeneity of variance. A T-test of independent samples was used for comparison between groups. The count data were expressed by % and the Pearson chi-square test was performed. The ROC curve was drawn by SPSS 17.0 statistical software, and the area under the ROC curve (AUROC) was calculated, including a 95% confidence interval. The ROC diagnostic curve was used to evaluate the diagnostic value. Multiple logistic regression models were established by combining multiple variables. The ROC curve was drawn by medical software, and the area under the curve was calculated. The differences between different ROC curves were compared by the Z test.
Results
Comparison of general data between the two groups of pregnant women
There was no difference in general data between the two groups (P>0.05). See Table 1.
Table 1.
Comparison of general information between the two groups (n)
| Groups | Observation group (n=30) | Control group (n=60) | χ2/t | P |
|---|---|---|---|---|
| Age (year) | 27.4±2.7 | 27.1±2.3 | 0.550 | 0.584 |
| Gestational age (week) | 38.2±1.5 | 38.4±1.6 | 0.571 | 0.570 |
| Number of pregnancies | 2.537 | 0.111 | ||
| The first time | 24 | 55 | ||
| ≥2 times | 6 | 5 | ||
| History of abortion | 6 | 8 | 0.667 | 0.411 |
| BMI (kg/m2) | 24.23±2.61 | 23.92±2.83 | 0.502 | 0.617 |
| Comorbidities | ||||
| Gestational hypertension | 6 | 13 | 0.033 | 0.855 |
| Gestational diabetes | 8 | 10 | 0.478 | 0.489 |
Note: BMI: body mass index.
Comparison of umbilical artery blood flow between the two groups
The S/D, RI and PI of the umbilical artery in the observation group were higher than those in the control group (P<0.001), as shown in Table 2.
Table 2.
Comparison of umbilical artery blood flow between two groups
| Groups | Observation group (n=30) | Control group (n=60) | t | P |
|---|---|---|---|---|
| S/D | 4.16±1.46 | 2.76±0.62 | 6.398 | <0.001 |
| RI | 0.74±0.07 | 0.61±0.09 | 6.926 | <0.001 |
| PI | 1.84±0.31 | 1.07±0.29 | 11.601 | <0.001 |
Note: S/D: systolic blood flow velocity to end-diastolic blood flow velocity; RI: resistance index; PI: pulsatility index.
Comparison of blood flow of the middle cerebral artery between the two groups of pregnant women
S/D, RI and PI of the middle cerebral artery in the observation group were lower than those in the control group (P<0.01), as shown in Table 3 and Figure 1.
Table 3.
Comparison of the blood flow of middle cerebral artery between the two groups
| Groups | Observation group (n=30) | Control group (n=60) | t | P |
|---|---|---|---|---|
| S/D | 3.43±0.73 | 5.26±1.52 | 6.232 | <0.001 |
| RI | 0.67±0.08 | 0.78±0.10 | 5.241 | <0.001 |
| PI | 1.53±0.38 | 1.87±0.59 | 2.869 | 0.005 |
Note: S/D: systolic blood flow velocity to end-diastolic blood flow velocity; RI: resistance index; PI: pulsatility index.
Figure 1.
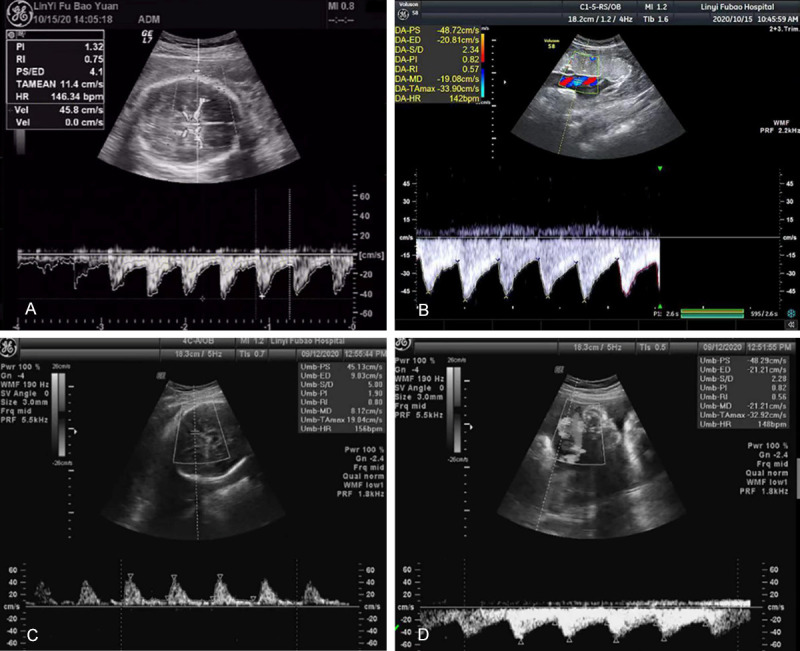
Comparison of umbilical artery and middle cerebral artery blood flow by ultrasound in two groups of pregnant women. A. The middle cerebral artery flow diagram of normal pregnant women, PI is 1.32, RI is 0.75, S/D is 4.1; B. Umbilical artery flow diagram of normal pregnant women, PI is 0.82, RI is 0.57, S/D is 2.34; C. Middle cerebral artery flow diagram of pregnant women with fetal distress, PI is 0.62, RI is 0.42, S/D is 3.32; D. Umbilical artery flow diagram of pregnant women with fetal distress, PI is 0.76, RI is 0.86, S/D is 2.65. RI: resistance index; PI: pulsatility index; S/D: systolic blood flow velocity to end-diastolic blood flow velocity.
Comparison of the number of cases of fetal heart rate reduction between the two groups
The number of cases with decreased fetal heart rate in the observation group was significantly higher than that in the control group (P<0.001). See Table 4.
Table 4.
Comparison of cases of fetal heart rate reduction between the two groups
| Groups | Observation group (n=30) | Control group (n=60) | χ2 | P |
|---|---|---|---|---|
| Fetal heart rate reduction (n %) | 21 (70.00) | 11 (18.33) | 23.300 | <0.001 |
Diagnostic value of different indicators for fetal distress
Logistic regression was performed in the combined diagnosis. The best diagnostic model equation was obtained by logistic regression: Logit (P) = -59.084 + 26.760 * umbilical artery S/D + 16.736 * umbilical artery RI + 53.843 * umbilical artery PI + (-3.539) * middle cerebral artery S/D + 45.465 * umbilical artery RI + 59.768 * umbilical artery PI + 7.473 * fetal movement reduction. The risk probability value of fetal distress was established. Risk probability value refers to the probability of predicting the occurrence of disease based on risk factors (P= +e-(-59.084 + 26.760 × umbilical artery S/D + 16.736 × umbilical artery RI + 53.843 × umbilical artery PI + (-3.539) × middle cerebral artery S/D + 45.465 × umbilical artery RI + 59.768 × umbilical artery PI + 7.473 × fetal movement reduction)). According to the predicted value of the joint diagnostic index obtained by regression analysis, ROC curve diagnosis was performed. The combined diagnosis of different indexes had the highest value for fetal distress. See Table 5 and Figure 2.
Table 5.
Diagnostic value of different indexes for fetal distress
| Groups | Sensitivity | Specificity | Yoden index | Area under the curve (AUC) | Cutoff value | P | 95% CI | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Upper limitation | Lower limitation | |||||||
| Umbilical artery S/D | 0.533 | 0.975 | 0.508 | 0.788 | 3.771 | <0.001 | 0.674 | 0.902 |
| Umbilical artery RI | 0.867 | 0.725 | 0.592 | 0.870 | 0.665 | <0.001 | 0.789 | 0.952 |
| Umbilical artery PI | 0.767 | 0.800 | 0.567 | 0.847 | 1.595 | <0.001 | 0.759 | 0.936 |
| Middle cerebral artery S/D | 1.000 | 0.625 | 0.625 | 0.852 | 4.801 | <0.001 | 0.766 | 0.937 |
| Middle cerebral artery PI | 0.933 | 0.500 | 0.433 | 0.802 | 0.802 | <0.001 | 0.705 | 0.904 |
| Middle cerebral artery RI | 0.467 | 0.800 | 0.267 | 0.658 | 1.292 | 0.025 | 0.526 | 0.790 |
| Fetal movement reduction | 0.700 | 0.800 | 0.500 | 0.750 | <0.001 | 0.630 | 0.870 | |
| Combined indexes | 1.000 | 1.000 | 1.000 | 1.000a,b,c,d,e,f,g | 1.000 | <0.001 | 1.000 | 1.000 |
Note: Compared with umbilical artery S/D;
P<0.05.
Compared with umbilical artery RI;
P<0.05.
Compared with umbilical artery PI;
P<0.05.
Compared with middle cerebral artery S/D;
P<0.05.
Compared with middle cerebral artery PI;
P<0.05.
Compared with middle cerebral artery RI;
P<0.05.
Compared with fetal movement reduction;
P<0.05.
RI: resistance index; PI: pulsatility index; S/D: systolic blood flow velocity to end-diastolic blood flow velocity.
Figure 2.
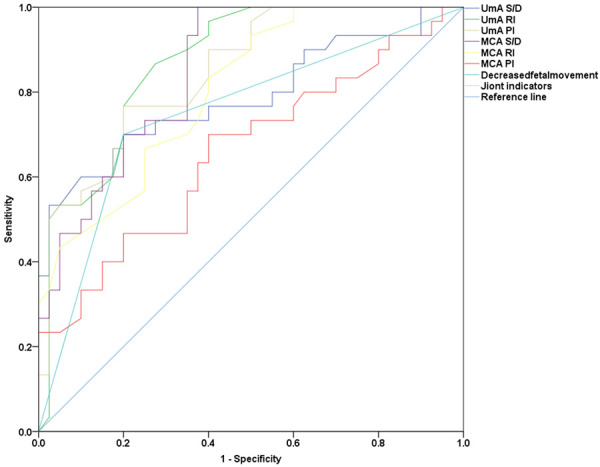
Diagnostic value of different indexes for fetal distress. RI: resistance index; PI: pulsatility index; S/D: systolic blood flow velocity to end-diastolic blood flow velocity.
Discussion
Fetal distress (FIUD) in late pregnancy leads to poor pregnancy outcomes [11]. There are many factors that cause the occurrence of FIUD, including reduction of maternal blood oxygen supply, fetal dysplasia and oxygen supply disorder between the mother and fetus, etc. [12]. The early diagnosis of FIUD plays an important role in improving the pregnancy outcomes of pregnant women. Ultrasound is one of the primary means of prenatal diagnosis [13]. Previous studies have shown that fetal umbilical artery and middle cerebral artery hemodynamics often appear abnormal changes in the state of ischemia and hypoxia [9,14]. Studies have shown that the umbilical artery mainly plays a role in connecting the fetus and placenta to transport blood flow and oxygen, which comes with a good response to the early fetal ischemia and hypoxia [15]. With the increase of gestational weeks in norma pregnancy, the umbilical artery blood flow should show a downward trend [16]. The resistance index (RI), pulsatility index (PI), systolic-peak blood flow velocity and end-diastolic blood flow velocity ratio (S/D) in the arterial blood flow of patients can better reflect the situation of blood flow resistance [17]. In this study, the RI, PI and S/D values of umbilical artery of FIUD pregnant women were higher than those of normal pregnant women, indicating the presence of ischemia and hypoxia symptoms. The results of previous studies are consistent [16]. However, some studies have pointed out that due to the influence of maternal body temperature and pulse on umbilical artery blood flow, hemodynamic changes occur, and there are limitations in judging FIUD only from the umbilical artery [18]. The middle cerebral artery (MCA) is the continuation of the carotid artery entering the brain and is the blood vessel with high oxygen content during the fetal period. Previous studies have shown that after FIUD, the fetal blood supply to the brain is guaranteed, and the systemic circulation blood supply is decreased. The expansion resistance of the middle cerebral artery is reduced to ensure the blood supply to the brain [19]. In this study, we also showed that the blood flow resistance of the middle cerebral artery decreased in FIUD, which was consistent with the above results. Fetal heart rate monitoring is a commonly used means to monitor fetal health. In the early stage of FIUD, due to the short duration of hypoxia, the fetus often has no obvious change or the fetal heart rate is not accelerated to meet the fetal blood and oxygen supply. With the aggravation of hypoxia, the vagus nerve was stimulated and the heart rate of fetus gradually slows down [20]. This study also showed that the number of cases of slow fetal heart rate in late pregnancy increased significantly, which may be consistent with the mechanism mentioned above.
In this study, the above indicators were further combined for diagnosis. The combined diagnosis of indicators can increase the sensitivity and specificity of FIUD, and the combined diagnosis of different indicators can complement each other. Previous studies on umbilical artery related indicators and middle cerebral artery related indicators have good value for the diagnosis of FIUD, and the joint diagnosis effect is better, which is consistent with the results of this study [21]. Studies have shown that single index diagnosis is more limited. Combined diagnosis can learn from each other’s strengths and make up for weaknesses, which is conducive to the early diagnosis of the disease and can make a more comprehensive judgment on the situation of the fetus [22].
Limitations: the sample size of this study is small, and the follow-up time is short. We can further expand the sample size for a multi-center study and increase the follow-up time to observe the diagnostic value of the above indicators.
In conclusion: The umbilical artery, middle cerebral artery combined with fetal movement has a certain value in diagnosing fetal distress, which is worth of clinical application.
Disclosure of conflict of interest
None.
References
- 1.Sandman CA. Prenatal CRH: An integrating signal of fetal distress. Dev Psychopathol. 2018;30:941–952. doi: 10.1017/S0954579418000664. [DOI] [PubMed] [Google Scholar]
- 2.Xie X. In: Obstetrics and Gynecology. 9th editior. Xie X, editor. Beijing: People’s Medical Publishing House; 2018. pp. 138–140. [Google Scholar]
- 3.Straface G, Scambia G, Zanardo V. Does ST analysis of fetal ECG reduce cesarean section rate for fetal distress? J Matern Fetal Neonatal Med. 2017;30:1799–1802. doi: 10.1080/14767058.2016.1226794. [DOI] [PubMed] [Google Scholar]
- 4.Pharande P, Balegar Virupakshappa KK, Mehta B, Badawi N. Fetal/Neonatal pericardial effusion in down’s syndrome: case report and review of literature. AJP Rep. 2018;8:e301–e306. doi: 10.1055/s-0038-1675337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbasi H, Drury PP, Lear CA, Gunn AJ, Davidson JO, Bennet L, Unsworth CP. EEG sharp waves are a biomarker of striatal neuronal survival after hypoxia-ischemia in preterm fetal sheep. Sci Rep. 2018;8:16312. doi: 10.1038/s41598-018-34654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ZL, Chen HT, Wang J. Application of electronic fetal heart rate monitoring during labor. J Prac Obste Gynecol. 2019;035:10–12. [Google Scholar]
- 7.Castelijn B, Hollander K, Hensbergen JF, IJzerman RG, Valkenburg-van den Berg AW, Twisk J, De Groot C, Wouters M. Peripartum fetal distress in diabetic women: a retrospective case-cohort study. BMC Pregnancy Childbirth. 2018;18:228. doi: 10.1186/s12884-018-1880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero R, Hernandez-Andrade E. Doppler of the middle cerebral artery for the assessment of fetal well-being. Am J Obstet Gynecol. 2015;213:1. doi: 10.1016/j.ajog.2015.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin W, Wang JH. Fetal middle cerebral artery blood flow characteristics of gestational hypertension combined with fetal distress in uterus as well as their correlation with hypoxia. J Hainan Med Univ. 2017;23:143–146. [Google Scholar]
- 10.Warmerdam GJJ, Vullings R, Van Laar JOEH, Van der Hout-Van der Jagt MB, Bergmans JWM, Schmitt L, Oei SG. Detection rate of fetal distress using contraction-dependent fetal heart rate variability analysis. Physiol Meas. 2018;39:025008. doi: 10.1088/1361-6579/aaa925. [DOI] [PubMed] [Google Scholar]
- 11.Cateriano-Alberdi MP, Palacios-Revilla CD, Segura ER. Survey of diagnostic criteria for fetal distress in latin american and african countries: over diagnosis or under diagnosis? J Clin Diagn Res. 2017;11:Sl01–Sl02. doi: 10.7860/JCDR/2017/27374.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andriessen P, Zwanenburg A, van Laar J, Vullings R, Hermans BJM, Niemarkt HJ, Jellema RK, Ophelders D, Wolfs T, Kramer BW, Delhaas T. ST waveform analysis for monitoring hypoxic distress in fetal sheep after prolonged umbilical cord occlusion. PLoS One. 2018;13:e0195978. doi: 10.1371/journal.pone.0195978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan Z, Yang Y, Zhan Y, Chen D, Liang L, Yang X. Fetal outcomes and associated factors of adverse outcomes of pregnancy in southern Chinese women with systemic lupus erythematosus. PLoS One. 2017;12:e0176457. doi: 10.1371/journal.pone.0176457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yazawa H, Takiguchi K, Ito F, Fujimori K. Uterine rupture at 33rd week of gestation after laparoscopic myomectomy with signs of fetal distress. A case report and review of literature. Taiwan J Obstet Gynecol. 2018;57:304–310. doi: 10.1016/j.tjog.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 15.DeFreitas MJ, Mathur D, Seeherunvong W, Cano T, Katsoufis CP, Duara S, Yasin S, Zilleruelo G, Rodriguez MM, Abitbol CL. Umbilical artery histomorphometry: a link between the intrauterine environment and kidney development. J Dev Orig Health Dis. 2017;8:349–356. doi: 10.1017/S2040174417000113. [DOI] [PubMed] [Google Scholar]
- 16.Alfirevic Z, Stampalija T, Dowswell T. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev. 2017;6:Cd007529. doi: 10.1002/14651858.CD007529.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Güven D, Altunkaynak BZ, Altun G, Alkan I, Kocak I. Histomorphometric changes in the placenta and umbilical cord during complications of pregnancy. Biotech Histochem. 2018;93:198–210. doi: 10.1080/10520295.2017.1410993. [DOI] [PubMed] [Google Scholar]
- 18.Vicente Bertagnolli T, Souza Rangel Machado M, Ferreira CJH, Machado JSR, Duarte G, Cavalli RC. Safety of a physical therapy protocol for women with preeclampsia: a randomized controlled feasibility trial. Hypertens Pregnancy. 2018;37:59–67. doi: 10.1080/10641955.2018.1439059. [DOI] [PubMed] [Google Scholar]
- 19.Kutuk MS, Dolanbay M, Gokmen Karasu AF, Ozgun MT. Relationship between fetal peak systolic velocity in Middle cerebral artery and umbilical blood gas values and hemoglobin levels in diabetic pregnant women. J Clin Ultrasound. 2018;46:391–396. doi: 10.1002/jcu.22593. [DOI] [PubMed] [Google Scholar]
- 20.Kanda T, Iizuka T, Yamazaki R, Iwadare J, Ono M, Fujiwara H. Giant fetal hydrometrocolpos associated with cloacal anomaly causing postnatal respiratory distress. J Obstet Gynaecol Res. 2017;43:1769–1772. doi: 10.1111/jog.13433. [DOI] [PubMed] [Google Scholar]
- 21.Ye FL, Xie N, Wang Y. The diagnostic value of fetal umbilical artery, renal artery, middle cerebral artery blood flow in fetal distress. The Chin J Hum Sexuality. 2020;29:85–88. [Google Scholar]
- 22.Migda M, Gieryn K, Migda B, Migda MS, Maleńczyk M. Utility of Doppler parameters at 36-42 weeks’ gestation in the prediction of adverse perinatal outcomes in appropriate-for-gestational-age fetuses. J Ultrason. 2018;18:22–28. doi: 10.15557/JoU.2018.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]


