Abstract
Objective: This research was designed to probe into the regulatory mechanism of long non-coding RNA (LncRNA) differentiation antagonizing non-protein coding RNA (DANCR) in potential applications and molecular mechanisms of prostate carcinoma (PC). Methods: The DANCR and miR-214-5p levels in PC tissues and cell lines were tested via real-time PCR, and those of transforming growth factor-β (TGF-β) signaling pathway related proteins were evaluated via Western Blot (WB). Cell proliferation, migration, apoptosis and the regulatory relationship between target genes were assessed via MTT method, scratch test, flow cytometry, dual-luciferase report, RNA co-immunoprecipitation and RNA pull-down test, respectively. Results: DANCR was up-regulated in PC patients’ serum and cell lines, while miR-214-5p was opposite, showing negative correlation. Besides, DANCR was significantly correlated with PSA, Gleason score and T stage in PC patients. The area under the curve (AUC) of DANCR and miR-214-5p for diagnosing PC was not less than 0.850, while the AUC for predicting poor prognosis was more than 0.800. Cox analysis results also revealed that the two might be prognostic indicators of PC patients. We found that DANCR high levels or miR-214-5p low levels were related to PC patients’ poor prognosis. Up-regulating DANCR or down-regulating miR-214-5p could promote PC cells’ malignant proliferation and migration, prevent apoptosis, and activate TGF-β signaling pathway, while reverse treatment of DANCR or miR-214-5p can reverse the above results. DANCR regulates miR-214-5p in a targeted manner, and DANCR over-expression can reduce the cancer inhibitory effect of miR-214-5p on PC cells. Conclusion: DANCR-miR-214-5p-TGF-β axis regulatory network plays a key regulatory part in PC progression. It may provide new strategies for the screening and treatment of patients.
Keywords: DANCR, miR-214-5p, TGF-β signaling pathway, prostate cancer
Introduction
Prostate cancer (PC) is a common reproductive tumor in men all over the world, and metastatic castration-resistant PC still has very high cure difficulty [1,2]. According to the epidemiological data, at least 650,000 men are diagnosed as PC every year, which accounts for 10% of the new cases of male cancer in the world, and up to 30% of patients relapse after surgery [3]. At the moment, PC faces a dilemma of highly heterogeneous molecular phenotype and no recognition of early symptoms, which is very unfavorable for early curative treatment of PC patients [4]. The early treatment methods of PC include radical resection, radiotherapy and androgen deprivation therapy, which are harmful to men to varying degrees [5]. In addition, due to the high heterogeneity of tumors, high-risk patients often need multiple repeated biopsies to receive treatment, which brings great harm to the body of PC patients and puts forward great demands for high-efficiency non-invasive serum biological indicators [6]. Therefore, it quite remarkable to explore highly sensitive molecular indicators and related molecular mechanisms in PC progression for improving the screening methods and prognosis of patients.
Long non-coding RNA (LncRNA) adjusts gene expression in multiple stages such as transcription, post-transcription, post-translation, etc., thus playing a regulatory part in malignant development mechanisms such as tumor development, progression, metastasis, etc. Therefore, finding LncRNA highly correlated with PC will help us to explore the development mechanism of PC [7-9]. LncRNA GAS5 is reported to regulate PC progression by mediating AKT/mTOR signaling pathway and sponge miR-103 [10]. LncRNA HOXD-AS1 was found to be associated with PC proliferation and chemical resistance; and it was effective through recruitment of WDR5 [11]. Differentiation antagonist non-protein coding RNA (DANCR) is a carcinogenic LncRNA that is highly correlated with tumors. Its abnormal imbalance is manifested in various cancer scenarios such as hepatocellular carcinoma, breast cancer, glioma, etc. [12]. A recent study has shown that DANCR is also relevant to PC invasion and can be effective by epigenetic negative regulation of TIMP2/3 expression [13]. In addition, it can also act as a molecular sponge of miR-214-5p to play an oncogene role in pancreatic cancer [14]. It is reported that miR-214-5p acts as a cancer suppressor to suppress PC cell progression through targeted regulation of CRMP5 [15]. Transforming growth factor-β (TGF-β) signal pathway is a highly correlated signal regulatory pathway in tumor microenvironment. Its regulatory range not only involves the occurrence and apoptosis of early tumors, but also includes the proliferation, infiltration and angiogenesis of late cancers [16]. Song [17] and others pointed out that activating TGF-β signal pathway was beneficial to castration-resistant PC progression, suggesting that research on targeted regulation of this signal pathway was beneficial to finding anti-PC molecular regulatory axis. We further explored the potential relationship between TGF-β signal pathway and DANCR, miR-214-5p. Cao et al. [18] confirmed that DANCR regulated the activity and metastasis of tumor cells through ERK-SMAD pathway, which coincided with the related response molecules of TGF-β signal pathway (TGF-β R1, p-SMAD3, SMAD3, etc.); it suggested that there was a secret relationship between DANCR and TGF-β signal pathway. In addition, Qi et al. [19] confirmed that miR-214-5p played a part in the adipogenic differentiation of bone marrow stem cells in postmenopausal osteoporosis patients by regulating TGF-β signal pathway; and it can be seen that miR-214-5p was also related to this pathway.
We suspect that DANCR-miR-214-5p-TGF-β axis regulatory network is involved in PC progression. The specific research and report were as follows.
Materials and methods
Collection of tissue samples
From February 2012 to February 2015, 53 PC patients (PC group) and 47 healthy persons (normal group) were included. They were (45.62±6.23) years old on average, and their ages were comparable (P<0.05). All patients and their families were willing to take part in the experiment, and they signed an informed consent form. It was approved by the Hospital Ethics Committee of the Second Xiangya Hospital, Central South University, and tissue samples were collected in accordance with the Declaration of Helsinki. Inclusion criteria were as follows: those confirmed by pathological diagnosis as PC [20]; first treatment; those who had not taken hormone or other drugs within half a year; those with normal cognition and no communication problems. Exclusion criteria were as follows: those with other prostate diseases; those with other malignancies; those with serious organ or systemic diseases; those with severe infectious diseases.
Follow-up
We conducted a 5-year follow-up visit to patients by telephones, visiting and inquiring medical records, once every three months. The overall survival (OS) was from the day of diagnosis to the death of the patients or the end of the last follow-up.
Cell culture and transfection
We purchased human PC cell lines DU145, 22Rv1, RC-92a, PC-3M and normal prostate epithelial cells RWPE-1 (Xuanke Biotechnology Co., Ltd., Shanghai, China, XK-XB-1390, XK-XB-2018, XK-XB-2864, XK-XB-2441, XK-XB-1339). They were cultivated at 37°C, 5% CO2 in DMEM medium (Beinuo Biotechnology Co., Ltd., Shanghai, China, Gibco12800-017) with 10% PBS, 100 units/ml penicillin and 100 μg/ml streptomycin (Xiangsheng Xingye Technology Co., Ltd., Beijing, China, 15140148).
The cells were transfected by targeted inhibition of DANCR (si-DANCR) or over-expression of DANCR (DANCR), negative control RNA (si-NC), miR-214-5p over-expression sequence (miR-214-5p), miR-214-5p inhibition sequence (anti-miR-214-5p), miR negative control (miR-NC) and anti-miR negative control (anti-miR-NC) with Lipofectamine™ 2000 kit (BioMag Biotechnology Co., Ltd., Wuxi, China, 11668019).
Real-time quantitative PCR
Firstly, the total RNA in tissues and cells was extracted with Trizol reagent (Shanran Biotechnology Co., Ltd., Shanghai, China, 10296-010). Then, 5 μg total RNA was employed to transcribe cDNA based on the reverse transcription kit instructions (Yuduo Biotechnology Co., Ltd., Shanghai, China, YDJ2547); 1 μL synthesized cDNA was employed in the amplification system: cDNA 1 μL, upstream and downstream primers 0.4 μL each, 2× TPCnsScript® Tip Green qPCR SuperMix 10 μL, Passive Reference Dye (50×) 0.4 μL, Nuclease-free Water added to 20 μL. mRNA used GADPH as internal reference, miRNA used U6 as internal reference. All data were assessed via 2-ΔΔct.
WB test
The cultured PC cells were extracted via RIPA lysis method, and the concentration was tested via BCA kit (Junrui Biotechnology Co., Ltd., Shanghai, China, LCB004). The dilution ratio of primary antibodies such as TGF-β, TGF-β R1, SMAD3, p-SMAD3, β-Actin, etc. were all 1:1000. All primary antibodies were detected with rabbit polyclonal antibody. The antibodies were bought from Shanghai Qiming Biotech Co., Ltd. Each membrane was potched 3 times with PBS (Yanjin Biotech Co., Ltd., Shanghai, China, SH30256.01B), 15 min each time, and then cultivated with horseradish peroxidase labeled goat anti-rabbit secondary antibody. Excess liquid was absorbed by Filter paper. The protein bands were developed in a dark room under ECL reagent and the gray value was evaluated.
Cell viability is tested by MTT assay
Cell proliferation was tested via MTT kit (Yiyan Biotechnology Co., Ltd., Shanghai, China, EY-19002). Cells were inoculated on 96-well plates 24 h after transfection, and the density was changed to 4×103 cells/well. They were incubated 0, 24, 48 and 72 h at 37°C. Next, 20 μL MTT solution (5 μmg/mL) was added at each time point and cultured 4 h at 37°C, and then 200 μL dimethyl sulfoxide was added to each well. The OD values of cells were measured at 450 mm wavelength using Q-drop spectrophotometer (Houze Biotechnology Co., Ltd., Hangzhou, China, XY-381-07341).
Cell migration is detected by scratch test
We diluted the cells to 3×105 cells/ml and inoculated them in a 6-well plate. After observing that they grew to 85%, we divided them into a cell-free area in the center culture plate by using a 200 μl sterile loading gun. We washed the divided cells with PBS and added a new medium. About 0 h (W0) and 24 h (W24) later, the cell migration was assessed by microscope for scratches at three different positions.
Apoptosis is tested by flow cytometry
Transfected cells were digested with 0.25% trypsin and washed twice with PBS after digestion. Next, 100 μL binding buffer was added. It was prepared into 1×106/mL suspension, sequentially added with AnnexinV-FITC and PI 10 μL each, and finally cultivated 5 min at room temperature. The results were tested with FACSCalibur flow cytometry (Shiwei Experimental Instrument Technology Co., Ltd., Shanghai, China) and averaged over 3 times.
Dual-luciferase report assay
Complementary DNA fragments consisting of DANCR wild-type (DANCR-Wt) or DANCR mutant (DANCR-Mut) fragments were subcloned downstream of the luciferase gene in psi-CHECK2 luciferase reporting vector. The miR-214-5p mimetic was co-transfected with the DANCR-Wt or DANCR-Mut report vector using transfection reagents. Forty-eight hours after transfection, fireflies and renin luciferase activities in cell lysates were continuously tested with a dual-luciferase reporter kit (Qunji Biotechnology Co., Ltd., Shanghai, China, KA3784).
RNA immunoprecipitation
Based on RNA immunoprecipitation (RIP), we confirmed whether DANCR and miR-214-5p could interact or bind with the potential binding protein Ago2. It was strictly in view of the instructions of EZMagna RIP kit (Taizejiaye Technology Development Co., Ltd., Beijing, China, 17-701). Cells were lysed and cultivated with protein A magnetic beads conjugated to antibodies at 4°C. Six hours later, the beads were cleaned with washing buffer, and then cultivated 30 min with 0.1% SDS/0.5 mg/ml protease K at 55°C. In the end, the immunoprecipitated RNA was analyzed via qRT-PCR to prove the existence of DANCR and miR-214-5p using specific primers.
RNA pull-down experiment
PC cells were transfected with biotinylated miR-214-5p-Wt, miR-214-5p-Mut and negative control Bio-NC, respectively. Forty-eight hours later, the cell lysate was cultivated with Streptomyces M-280 magnetic beads in line with the production instructions; the DANCR level in the RNA complex bound to the beads was tested via qRT-PCR.
Statistical methods
All data were analyzed and figures were drawn by GraphPad 6. The inter-group comparison was evaluated by independent-samples t test. The comparison among multiple groups was assessed by one-way analysis of variance (ANOVA) and represented as F. The post-event comparison was performed by LSD-t test. Multi-time point indicators were compared by repeated measures ANOVA and represented as F, and Bonferroni was employed for back testing. AUC for PC identification was evaluated through receiver operating characteristic (ROC) curve. The correlation was assessed through Pearson test. P<0.05 was statistically different.
Results
DANCR may be carcinogenic in PC patients
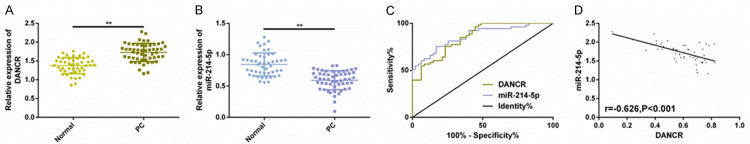
The DANC and miR-214-5p expression in serum of PC patients (n=53) and healthy subjects (n=47) were detected, so as to explore their potential relationship with PC development and progression. The DANCR expression in serum of PC patients was higher than that of normal group, while the miR-214-5p expression was different from the former. The AUCs of the two discrimination PC were 0.852 and 0.863, respectively, and they also had negative correlation (r=-0.626, P<0.001). The above results had statistical significance (P<0.05) (Figure 1).
Figure 1.
DANCR may be carcinogenic in PC patients. A, B. The levels of DANCR and miR-214-5p in serum of PC patients (n=53) and healthy subjects (n=47) are detected by RT-PCR. C. ROC curve was drawn to evaluate the diagnostic value of DANCR and miR-214-5p for PC. D. The correlation between the two is analyzed by Pearson correlation coefficient. Note: **P<0.01.
DANCR is significantly correlated with PSA, Gleason score and T stage in PC patients
The relationship between DANCR and pathological data of PC patients (n=53) were analyzed, so as to probe into the potential relationship between DANCR and PC pathological parameters. It manifested that DANCR was obviously related to PSA, Gleason score, T stage, N stage and M stage of PC patients (P<0.05). Among them, PSA and Gleason scores were positively correlated with the degree of PC malignancy or disease risk [21,22] (Table 1).
Table 1.
Relationship between DANCR and pathological data of PC patients [n (%), mean ± SD]
| Factors | n=53 | DANCR | t/F value | P value |
|---|---|---|---|---|
| Age (year) | 1.379 | 0.174 | ||
| <45 | 24 | 1.70±0.12 | ||
| ≥45 | 29 | 1.75±0.14 | ||
| PSA (ng/mL) | 11.164 | <0.001 | ||
| <4 | 10 | 1.59±0.08 | ||
| 4-10 | 16 | 1.68±0.13 | ||
| >10 | 27 | 1.80±0.14 | ||
| Gleason score (point) | 4.800 | <0.001 | ||
| <7 | 25 | 1.63±0.15 | ||
| ≥7 | 28 | 1.85±0.18 | ||
| T stage | 5.522 | <0.001 | ||
| T1/T2 | 27 | 1.62±0.14 | ||
| T3/T4 | 26 | 1.84±0.15 | ||
| N stage | 8.146 | <0.001 | ||
| N0 | 21 | 1.51±0.12 | ||
| N1 | 32 | 1.89±0.19 | ||
| M stage | 10.271 | <0.001 | ||
| M0 | 30 | 1.55±0.17 | ||
| M1 | 23 | 1.96±0.10 |
Note: DANCR, differentiation antagonizing non-protein coding RNA; PC, prostate cancer; PSA, prostate-specific antigen; T, tumor; N, node; M, metastasis.
DANCR and miR-214-5p may be prognostic indicators for PC patients
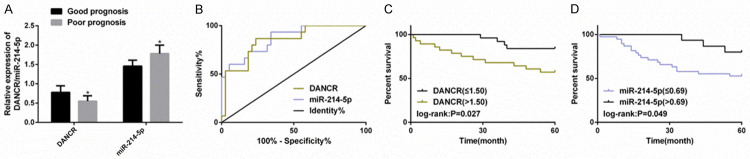
Based on the expression data of serum DANCR and miR-214-5p in PC patients (n=53), their relationship with patients’ prognosis was analyzed to verify whether they were prognostic indicators. Fifty-three PC patients were followed up for 5 years. Thereinto, those survived were taken as a good prognosis group (GPG) (n=38), while those died were regarded as a poor prognosis group (PPG) (n=15). OS in this study was 71.70% (38/53) in 5 years. In the PPG, there were higher levels of DANCR and lower levels of miR-214-5p, and AUCs for predicting poor prognosis of PC patients were 0.846 and 0.868, respectively. We took the best cut-off value of ROC parameters for predicting poor prognosis as the cut-off point for high and low expression, and plotted the survival curve. We found that DANCR high level or miR-214-5p low level were associated with lower 5-year OS in PC patients. Cox regression analysis found that N stage, M stage, pathological differentiation degree, DANCR and miR-214-5p were their independent prognostic factors. All results were statistically remarkable (P<0.05) (Figure 2; Table 2).
Figure 2.
Low level of DANCR or high level of miR-214-5p is associated with poor prognosis of PC patients. A. The levels of DANCR and miR-214-5p are markedly higher in patients with poor prognosis (n=15) and those with good prognosis (n=38) confirmed by RT-PCR. B. ROC curve is used to evaluate the value of the two methods in predicting poor prognosis of PC patients. C, D. The effect of DANCR and miR-214-5p on 5-year OS of PC patients is evaluated by survival curve. Note: *P<0.05 v.s. good prognosis group.
Table 2.
Univariate and multivariate Cox regression analysis
| Indicators | Single factors | Multiple factors | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender | 1.214 (0.790-2.172) | 0.220 | ||
| Age | 1.176 (0.441-3.025) | 0.759 | ||
| T stages | 1.791 (1.213-2.658) | 0.022 | 1.101 (0.887-1.542) | 0.096 |
| N stages | 1.962 (2.008-2.163) | 0.017 | 1.644 (1.447-2.968) | 0.012 |
| M stages | 2.308 (1.397-3.798) | 0.001 | 1.830 (1.084-3.057) | 0.024 |
| Degree of pathological differentiation | 0.577 (0.189-8.274) | 0.001 | 0.352 (1.210-6.447) | 0.006 |
| DANCR | 3.655 (0.859-12.349) | <0.001 | 7.052 (1.547-9.244) | 0.003 |
| miR-214-5p | 2.214 (1.205-3.781) | 0.009 | 5.098 (1.196-3.781) | 0.026 |
Note: T, tumor; N, node; M, metastasis; DANCR, differentiation antagonizing non-protein coding RNA; miR, microRNA.
Up-regulating DANCR can promote PC cell progression
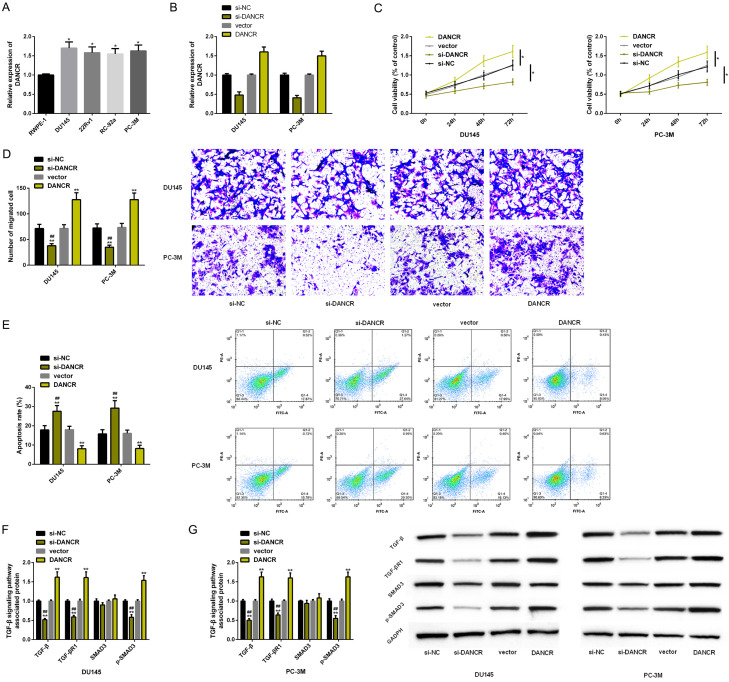
DANCR’s effect on the proliferation, migration, apoptosis and other biological functions of PC cell lines, and TGF-β signal pathway were analyzed, in order to explore the molecular effects of DANCR on PC progression. First of all, the DANCR expression in PC cell lines was tested; it in DU145 and PC-3M was dramatically higher. Then, these two PC cells were further analyzed. Through transfection, we respectively carried out up-regulation and down-regulation of DANCR expression. Cell behavior analysis manifested that PC cell proliferation and migration were enhanced, apoptosis level reduced, and TGF-β, TGF-β R1, and p-SMAD3 protein levels increased after DANCR up-regulation. However, the down-regulation of DANCR was different from the above results. The above results had statistical remarkable (P<0.05) (Figure 3).
Figure 3.
Up-regulating DANCR can promote PC cell progression. A, B. The expression and transfection efficiency of DANCR are detected by RT-PCR. C-E. The effects of DANCR on proliferation, migration and apoptosis of PC cells are evaluated through MTT assay, scratch test and flow cytometry. F, G. The effect of DANCR on TGF-β signal pathway in PC cells is evaluated by Western blot analysis. Note: *P<0.05, **P<0.01 v.s. RWPE-1 cells/si-NC; ##P<0.01 v.s. DANCR.
DANCR and miR-214-5p have target control relationship
The molecular mechanism of DANCR in PC was assessed through biological analysis. Specifically, there was a targeted binding site between miR-214-5p and DANCR through Starbase (http://starbase.sysu.edu.cn/index.php). Dual-luciferase activity signified that up-regulation of miR-214-5p only reduced the luciferase activity of DANCR 3’UTR-Wt (instead of DANCR 3’UTR-Mut). In RIP experiment, the DANCR and miR-214-5p levels precipitated by Ago2 antibody were dramatically higher than IgG. In RNA pull-down experiment, DANCR was only pulled down by biotin-labeled miR-214-5p-Wt. WB analysis found that the DANCR expression in PC cells was inhibited after miR-214-5p was up-regulated. The above results had statistical significance (P<0.05) (Figure 4).
Figure 4.
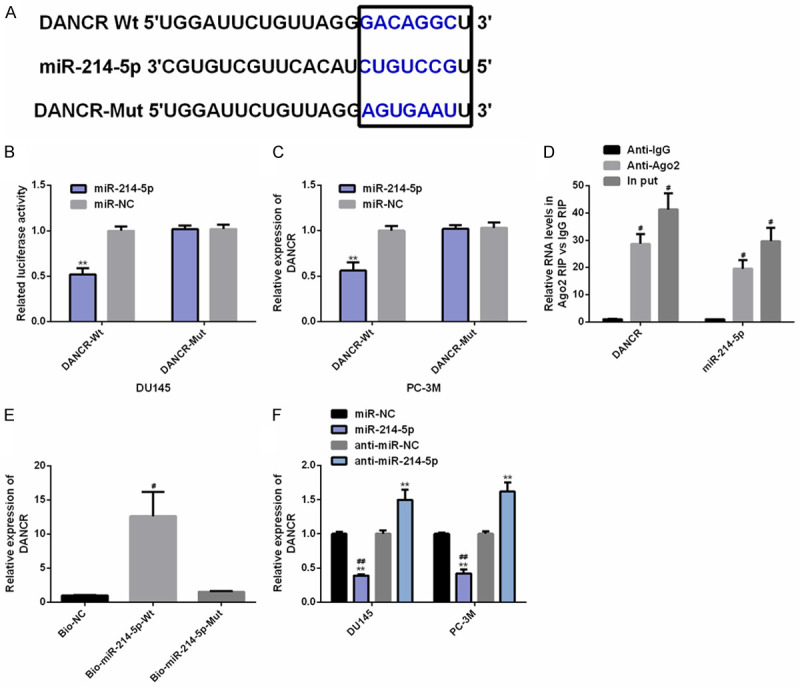
DANCR has target-controlled relationship with miR-214-5p. A. The target sites of DANCR and miR-214-5p are discovered through Starbase (http://starbase.sysu.edu.cn/index.php). B-E. The target relationship between the two is verified by relative luciferase activity-dual luciferase report, rip and RNA pull-down tests. F. The effect of miR-214-5p on DANCR expression in PC cells is assessed by RT-PCR. Note: **P<0.01 v.s. miR-NC; #P<0.05, ##P<0.01 v.s. Anti-IgG/Bio-NC/anti-miR-214-5p.
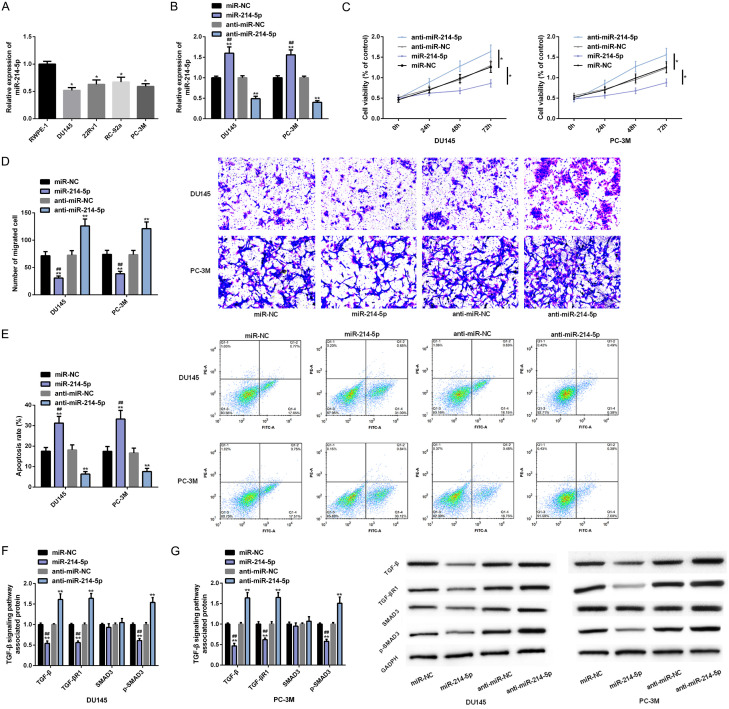
Down-regulating miR-214-5p promotes PC cell progression
In view of the targeted relationship between DANCR and miR-214-5p, we also further analyzed DANCR’s effects on proliferation, migration, apoptosis and other biological functions of PC cells, as well as TGF-β signal pathway, so as to explore the relationship between DANCR and PC progress. Similarly, we found lower levels of miR-214-5p in DU145 and PC-3M. We further analyzed these two PC cells. And cell function analysis manifested that after miR-214-5p was down-regulated, PC cell proliferation and migration levels increased, while apoptosis levels decreased, and TGF-β, TGF-β R1, and p-SMAD3 protein levels increased. However, up-regulation of miR-214-5p was different from the above results. The above results had statistical significance (P<0.05) (Figure 5).
Figure 5.
Down-regulating miR-214-5p can promote PC cell progression. A, B. The expression and transfection efficiency of miR-214-5p are analyzed by RT-PCR. C-E. The effects of miR-214-5p on proliferation, migration and apoptosis of PC cell lines are analyzed by MTT assay, scratch test and flow cytometry. F, G. The effect of miR-214-5p on TGF-β signal pathway in PC cell lines is analyzed via Western blot analysis. Note: *P<0.05, **P<0.01 v.s. RWPE-1 cells/si-NC; ##P<0.01 v.s. DANCR.
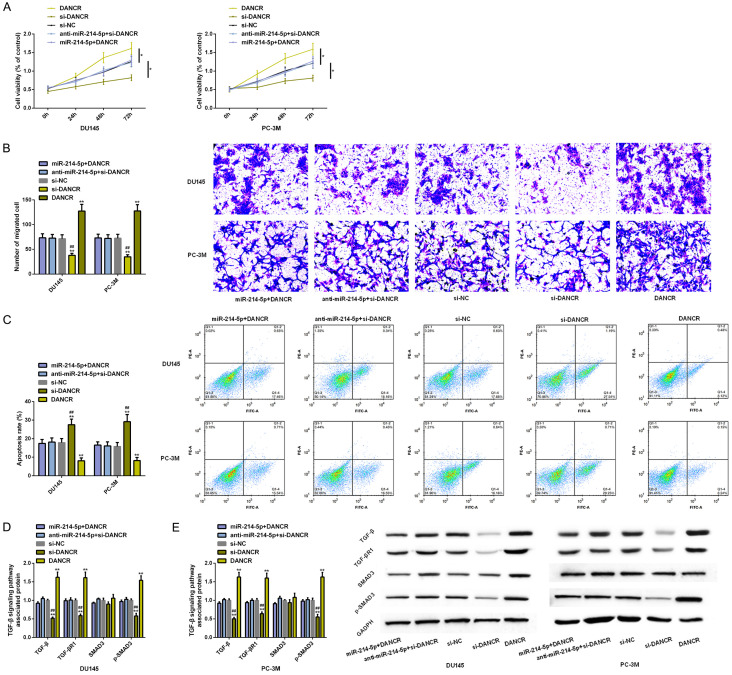
Up-regulating or down-regulating DANCR and miR-214-5p can eliminate this promotion effect
To further verify the molecular regulatory mechanism of DANCR-miR-214-5p axis in PC development, we conducted a co-transfection study. After up-regulating or down-regulating DANCR and miR-214-5p at the same time, the proliferation, migration, apoptosis and TGF-β signal pathway related protein levels of PC cells had no remarkable difference compared with si-NC (P>0.05). However, compared with those cells that up-regulated DANCR, the proliferation and migration as well as TGF-β, TGF-β R1 and p-SMAD3 protein levels were obviously inhibited, and the apoptosis was enhanced. Compared with down-regulation of DANCR, the above results were all contrary, and the difference was statistically remarkable (P<0.05) (Figure 6).
Figure 6.
Co-transfection experiment. A-C. The effects of DANCR and miR-214-5p co-expression on proliferation, migration and apoptosis of PC cell lines are analyzed by MTT assay, scratch test and flow cytometry. D, E. The effects of DANCR and miR-214-5p co-expression on TGF-β signal pathway in PC cell lines are analyzed by Western blot analysis. Note: *P<0.05, **P<0.01 v.s. si-NC; ##P<0.01 v.s. DANCR.
Discussion
PC is the main cause of human mortality and morbidity in the world. Although the mortality has shown a downward trend, the morbidity has increased [23]. LncRNA is regarded as an anti-cancer target due to its close participation in human physiological and pathological processes [24]. While we pay close attention to the potential clinical application value and regulation mechanism of DANCR, hoping to supply new directions for prevention and treatment.
More and more researchers are interested in regulating DANCR in PC. For example, Ma and others [25] pointed out that DANCR mediated PC chemical sensitivity by regulating miR-34a-5p/JAG1 molecular signal. However, Lu [26] and others studied that DANCR was abnormally up-regulated in PC patients; knocking down the DANCR expression was helpful to prevent PC cell lines’ survival. DANCR was also up-regulated in various diseases, playing a potential carcinogenicity. For example, in osteosarcoma, it can promote tumor progression by adjusting miR-33a-5p-AXL-Akt molecular signal; in breast cancer, it can mediate the inflammatory phenotype and malignant behavior of tumor cells by regulating EZH2-SOCS3 axis; in hepatic cellular cancer (HCC), the positive feedback signal of IL-6-STAT3 can be activated to enhance the resistance of sorafenib, so as to exert carcinogenicity [27-29]. Besides, the previously mentioned DANCR molecular target miR-214-5p has also been found to have potential targeted binding sites in Starbase online target gene prediction website, suggesting that miR-214-5p may be a downstream tumor regulator of DANCR. Liu and others [30] also pointed out that miR-214-5p could mediate the progression of ovarian cancer, which was also a reproductive tumor, and it undertook cancer suppression work. In addition, it has been reported that miR-214-5p has cancer suppressive function in HCC, cervical cancer, glioma and other tumors [31-33].
In this research, we first evaluated the DANCR and miR-214-5p levels in PC patients’ serum, and found that DANCR was up-regulated while miR-214-5p was down-regulated; it indicated that the former might play a carcinogenic part in PC progression, while the latter might inhibit cancer. In addition, the AUC of both diagnosis PC was not less than 0.850, indicating that both might be biological molecular screening indicators. Correlation analysis signified that there was a remarkable negative correlation between the two, showing that the two might play an antagonistic part in PC progression. We found that DANCR was significantly correlated with PSA, Gleason score, T stage, N stage and M stage of PC patients, suggesting that it had a certain value in the above clinical parameters. Prognostic results manifested that high-level of DANCR or low-level of miR-214-5p existed in the PPG. The AUC for predicting poor prognosis of PC patients by both of them exceeded 0.800, and high-level of DANCR or low-level of miR-214-5p were dramatically correlated with lower 5-year OS, suggesting that both might be prognostic indicators of PC. Cox analysis results showed that N stage, M stage, pathological differentiation degree, DANCR, and miR-214-5p were independent prognostic indicators for PC patients. Research showed that the 5-year OS of PC patients was 58.5-81.5%, and married men had an average higher level. In this study, the 5-year OS was 71.70% [34].
We then analyzed the PC molecular mechanism of DANCR and miR-214-5p. It manifested that there were also up-regulated DANCR and down-regulated miR-214-5p in PC cells, and the expression was most remarkable in PC cell lines DU145 and PC-3M. We will carry out subsequent transfection experiments with DU145 and PC-3M. Functional analysis of PC cells identified that up-regulating DANCR or down-regulating miR-214-5p was helpful to boost PC cell proliferation and migration, activate TGF-β signaling pathway, and inhibit apoptosis, while reverse treatment (down-regulation of DANCR or up-regulation of miR-214-5p) reverses the above results; it indicated that down-regulating DANCR or up-regulating miR-214-5p expression was helpful to improve its progression, and both might have carcinogenicity and carcinogenicity in PC respectively by mediating TGF-β signal pathway. The key members of TGF-β signal pathway are TGF-β, TGF-β R1, SMAD3, etc. In PC progression, this signal pathway is often activated, which is manifested by increased levels of TGF-β, TGF-β R1, and p-SMAD3 proteins, which are consistent with the results of this study [35]. Jin [36] and others reported that TGF-β signal pathway could reduce PC cell invasiveness under negative regulation of miR-15a/miR-16 clusters, suggesting that inhibiting this pathway was helpful to improve PC. Lowering DANCR or increasing miR-214-5p also inhibits TGF-β signal pathway and reverses PC progression. What’s more, inhibitng TGF-β signal pathway has therapeutic significance in various tumor diseases, such as glioma, breast cancer, PC bone metastasis [37-39].
LncRNA usually plays a negative regulatory part as a molecular sponge of miRNA, and exerts influence on tumor development by the crosstalk structure of LncRNA-miRNA [40]. Chen et al. [41] reported that miR-214-5p could be regulated by DANCR and reverse the carcinogenic effect of DANCR in NSCLC by combining with downstream factor CIZ1. We have proved whether DANCR in PC miRNA’s molecular sponge. Dual-luciferase report, RIP and RNA pull-down experiments have all confirmed that DANCR and miR-214-5p have a target control relationship; it suggests that DANCR, as endogenous RNA, participates in the pathological mechanism of PC through sponge miR-214-5p. We also conducted co-transfection experiments. The results showed that up-regulating or down-regulating DANCR and miR-214-5p simultaneously would eliminate the promotion of PC progression and activation of TGF-β signal pathway when the two were up-regulated or down-regulated. This proved once again that the existence of DANCR-miR-214-5p-TGF-β molecular regulatory axis had regulatory effect on PC progression. The above research results inspire us that the development of DANCR inhibitor or miR-214-5p promoter may have extraordinary clinical value.
This study reported that DANCR competitively binds miR-214-5p and activates TGF-β signal pathway to promote PC progression. However, there is still room for improvement. Firstly, we can investigate whether DANCR has the value of diagnosing the clinical pathological parameters of PC patients. Secondly, we can supplement whether DANCR influences PC chemical resistance and clarifies the potential regulation mechanism on its chemical sensitivity. Moreover, we can add DANCR’s potential value in predicting PC recurrence.
Overall, up-regulating DANCR in PC has certain value for PC screening and prognosis prediction, and down-regulating it can inhibit the molecular progression. We also first propose that DANCR-miR-214-5p-TGF-β molecular regulatory axis is involved in proliferation, migration and apoptosis of PC cells, which may provide a new scheme of clinical application for treatment.
Disclosure of conflict of interest
None.
References
- 1.Liu HT, Fang L, Cheng YX, Sun Q. LncRNA PVT1 regulates prostate cancer cell growth by inducing the methylation of miR-146a. Cancer Med. 2016;5:3512–3519. doi: 10.1002/cam4.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goto Y, Kurozumi A, Arai T, Nohata N, Kojima S, Okato A, Kato M, Yamazaki K, Ishida Y, Naya Y, Ichikawa T, Seki N. Impact of novel miR-145-3p regulatory networks on survival in patients with castration-resistant prostate cancer. Br J Cancer. 2017;117:409–420. doi: 10.1038/bjc.2017.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karatas OF, Wang J, Shao L, Ozen M, Zhang Y, Creighton CJ, Ittmann M. miR-33a is a tumor suppressor microRNA that is decreased in prostate cancer. Oncotarget. 2017;8:60243–60256. doi: 10.18632/oncotarget.19521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres-Ferreira J, Ramalho-Carvalho J, Gomez A, Menezes FD, Freitas R, Oliveira J, Antunes L, Bento MJ, Esteller M, Henrique R, Jeronimo C. MiR-193b promoter methylation accurately detects prostate cancer in urine sediments and miR-34b/c or miR-129-2 promoter methylation define subsets of clinically aggressive tumors. Mol Cancer. 2017;16:26. doi: 10.1186/s12943-017-0604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly RS, Vander Heiden MG, Giovannucci E, Mucci LA. Metabolomic biomarkers of prostate cancer: prediction, diagnosis, progression, prognosis, and recurrence. cancer epidemiol biomarkers. Cancer Epidemiol Biomarkers Prev. 2016;25:887–906. doi: 10.1158/1055-9965.EPI-15-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boegemann M, Stephan C, Cammann H, Vincendeau S, Houlgatte A, Jung K, Blanchet JS, Semjonow A. The percentage of prostate-specific antigen (PSA) isoform [-2] proPSA and the prostate health index improve the diagnostic accuracy for clinically relevant prostate cancer at initial and repeat biopsy compared with total PSA and percentage free PSA in men aged ≤65 years. BJU Int. 2016;117:72–79. doi: 10.1111/bju.13139. [DOI] [PubMed] [Google Scholar]
- 7.Wan X, Huang W, Yang S, Zhang Y, Pu H, Fu F, Huang Y, Wu H, Li T, Li Y. Identification of androgen-responsive lncRNAs as diagnostic and prognostic markers for prostate cancer. Oncotarget. 2016;7:60503–60518. doi: 10.18632/oncotarget.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smolle MA, Bauernhofer T, Pummer K, Calin GA, Pichler M. Current insights into long non-coding RNAs (LncRNAs) in prostate cancer. Int J Mol Sci. 2017;18:2. doi: 10.3390/ijms18020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitobe Y, Takayama KI, Horie-Inoue K, Inoue S. Prostate cancer-associated lncRNAs. Cancer Lett. 2018;418:159–166. doi: 10.1016/j.canlet.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Xue D, Zhou C, Lu H, Xu R, Xu X, He X. LncRNA GAS5 inhibits proliferation and progression of prostate cancer by targeting miR-103 through AKT/mTOR signaling pathway. Tumour Biol. 2016;37:16187–16197. doi: 10.1007/s13277-016-5429-8. [DOI] [PubMed] [Google Scholar]
- 11.Gu P, Chen X, Xie R, Han J, Xie W, Wang B, Dong W, Chen C, Yang M, Jiang J, Chen Z, Huang J, Lin T. lncRNA HOXD-AS1 regulates proliferation and chemo-resistance of castration-resistant prostate cancer via recruiting WDR5. Mol Ther. 2017;25:1959–1973. doi: 10.1016/j.ymthe.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thin KZ, Liu X, Feng X, Raveendran S, Tu JC. LncRNA-DANCR: a valuable cancer related long non-coding RNA for human cancers. Pathol Res Pract. 2018;214:801–805. doi: 10.1016/j.prp.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Jia J, Li F, Tang XS, Xu S, Gao Y, Shi Q, Guo W, Wang X, He D, Guo P. Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget. 2016;7:37868–37881. doi: 10.18632/oncotarget.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Z, Chen Q, Ni Z, Zhou L, Wang Y, Yang Y, Huang H. Long non-coding RNA differentiation antagonizing nonprotein coding RNA (DANCR) promotes proliferation and invasion of pancreatic cancer by sponging miR-214-5p to regulate E2F2 expression. Med Sci Monit. 2019;25:4544–4552. doi: 10.12659/MSM.916960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng C, Guo K, Chen B, Wen Y, Xu Y. miR-214-5p inhibits human prostate cancer proliferation and migration through regulating CRMP5. Cancer Biomark. 2019;26:193–202. doi: 10.3233/CBM-190128. [DOI] [PubMed] [Google Scholar]
- 16.Seoane J, Gomis RR. TGF-beta family signaling in tumor suppression and cancer progression. Cold Spring Harb Perspect Biol. 2017;9:a022277. doi: 10.1101/cshperspect.a022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song B, Park SH, Zhao JC, Fong KW, Li S, Lee Y, Yang YA, Sridhar S, Lu X, Abdulkadir SA, Vessella RL, Morrissey C, Kuzel TM, Catalona W, Yang X, Yu J. Targeting FOXA1-mediated repression of TGF-beta signaling suppresses castration-resistant prostate cancer progression. J Clin Invest. 2019;129:569–582. doi: 10.1172/JCI122367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao L, Jin H, Zheng Y, Mao Y, Fu Z, Li X, Dong L. DANCR-mediated microRNA-665 regulates proliferation and metastasis of cervical cancer through the ERK/SMAD pathway. Cancer Sci. 2019;110:913–925. doi: 10.1111/cas.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu J, Huang G, Na N, Chen L. MicroRNA-214-5p/TGF-beta/Smad2 signaling alters adipogenic differentiation of bone marrow stem cells in postmenopausal osteoporosis. Mol Med Rep. 2018;17:6301–6310. doi: 10.3892/mmr.2018.8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohler JL, Armstrong AJ, Bahnson RR, D’Amico AV, Davis BJ, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Kane CJ, Kawachi MH, Kuettel M, Lee RJ, Meeks JJ, Penson DF, Plimack ER, Pow-Sang JM, Raben D, Richey S, Roach M 3rd, Rosenfeld S, Schaeffer E, Skolarus TA, Small EJ, Sonpavde G, Srinivas S, Strope SA, Tward J, Shead DA, Freedman-Cass DA. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 21.Kryvenko ON, Wang Y, Sadasivan S, Gupta NS, Rogers C, Bobbitt K, Chitale DA, Rundle A, Tang D, Rybicki BA. Potential effect of anti-inflammatory drug use on PSA kinetics and subsequent prostate cancer diagnosis: risk stratification in black and white men with benign prostate biopsy. Prostate. 2019;79:1090–1098. doi: 10.1002/pros.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, Vickers AJ, Parwani AV, Reuter VE, Fine SW, Eastham JA, Wiklund P, Han M, Reddy CA, Ciezki JP, Nyberg T, Klein EA. A contemporary prostate cancer grading system: a validated alternative to the gleason score. Eur Urol. 2016;69:428–435. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong MC, Goggins WB, Wang HH, Fung FD, Leung C, Wong SY, Ng CF, Sung JJ. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. 2016;70:862–874. doi: 10.1016/j.eururo.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 24.Fan L, Li H, Zhang Y. LINC00908 negatively regulates microRNA-483-5p to increase TSPYL5 expression and inhibit the development of prostate cancer. Cancer Cell Int. 2020;20:10. doi: 10.1186/s12935-019-1073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, Fan B, Ren Z, Liu B, Wang Y. Long noncoding RNA DANCR contributes to docetaxel resistance in prostate cancer through targeting the miR-34a-5p/JAG1 pathway. Onco Targets Ther. 2019;12:5485–5497. doi: 10.2147/OTT.S197009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y, Hu Z, Mangala LS, Stine ZE, Hu X, Jiang D, Xiang Y, Zhang Y, Pradeep S, Rodriguez-Aguayo C, Lopez-Berestein G, DeMarzo AM, Sood AK, Zhang L, Dang CV. MYC targeted long noncoding RNA DANCR promotes cancer in part by reducing p21 levels. Cancer Res. 2018;78:64–74. doi: 10.1158/0008-5472.CAN-17-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang N, Wang X, Xie X, Liao Y, Liu N, Liu J, Miao N, Shen J, Peng T. lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 2017;405:46–55. doi: 10.1016/j.canlet.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Zhang KJ, Tan XL, Guo L. The long non-coding RNA DANCR regulates the inflammatory phenotype of breast cancer cells and promotes breast cancer progression via EZH2-dependent suppression of SOCS3 transcription. Mol Oncol. 2020;14:309–328. doi: 10.1002/1878-0261.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Chen L, Yuan H, Guo S, Wu G. LncRNA DANCR promotes sorafenib resistance via activation of IL-6/STAT3 signaling in hepatocellular carcinoma cells. Onco Targets Ther. 2020;13:1145–1157. doi: 10.2147/OTT.S229957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Zhou H, Ma L, Hou Y, Pan J, Sun C, Yang Y, Zhang J. MiR-214 suppressed ovarian cancer and negatively regulated semaphorin 4D. Tumour Biol. 2016;37:8239–8248. doi: 10.1007/s13277-015-4708-0. [DOI] [PubMed] [Google Scholar]
- 31.Hu M, Han Y, Zhang Y, Zhou Y, Ye L. lncRNA TINCR sponges miR-214-5p to upregulate ROCK1 in hepatocellular carcinoma. BMC Med Genet. 2020;21:2. doi: 10.1186/s12881-019-0940-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Guo M, Lin B, Li G, Lin J, Jiang X. LncRNA TDRG1 promotes the proliferation, migration, and invasion of cervical cancer cells by sponging miR-214-5p to target SOX4. J Recept Signal Transduct Res. 2020;40:281–293. doi: 10.1080/10799893.2020.1731537. [DOI] [PubMed] [Google Scholar]
- 33.Qiao J, Liu M, Tian Q, Liu X. Microarray analysis of circRNAs expression profile in gliomas reveals that circ_0037655 could promote glioma progression by regulating miR-214/PI3K signaling. Life Sci. 2020;245:117363. doi: 10.1016/j.lfs.2020.117363. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Xia Q, Xia J, Zhu H, Jiang H, Chen X, Zheng Y, Zhang F, Li S. The impact of marriage on the overall survival of prostate cancer patients: a surveillance, epidemiology, and end results (SEER) analysis. Can Urol Assoc J. 2019;13:E135–E139. doi: 10.5489/cuaj.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang LL, Sun BF, Huang LR, Yuan HB, Zhang S, Chen J, Yu ZJ, Luo H. Potent inhibition of miR-34b on migration and invasion in metastatic prostate cancer cells by regulating the TGF-beta pathway. Int J Mol Sci. 2017;18:12. doi: 10.3390/ijms18122762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin W, Chen F, Wang K, Song Y, Fei X, Wu B. miR-15a/miR-16 cluster inhibits invasion of prostate cancer cells by suppressing TGF-beta signaling pathway. Biomed Pharmacother. 2018;104:637–644. doi: 10.1016/j.biopha.2018.05.041. [DOI] [PubMed] [Google Scholar]
- 37.Yan Y, Liu X, Gao J, Wu Y, Li Y. Inhibition of TGF-beta signaling in gliomas by the flavonoid diosmetin isolated from dracocephalum peregrinum L. Molecules. 2020;25:192. doi: 10.3390/molecules25010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmadalizadeh Khanehsar M, Hoseinbeyki M, Fakhr Taha M, Javeri A. Repression of TGF-beta signaling in breast cancer cells by miR-302/367 cluster. Cell J. 2020;21:444–450. doi: 10.22074/cellj.2020.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang C, Dai Y, Wu Z, Yang Q, He S, Zhang X, Guo W, Lai Y, Du H, Wang H, Ren D, Peng X. SMAD3/SP1 complex-mediated constitutive active loop between lncRNA PCAT7 and TGF-beta signaling promotes prostate cancer bone metastasis. Mol Oncol. 2020;14:808–828. doi: 10.1002/1878-0261.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing miRNA-LncRNA interactions. Methods Mol Biol. 2016;1402:271–286. doi: 10.1007/978-1-4939-3378-5_21. [DOI] [PubMed] [Google Scholar]
- 41.Chen YR, Wu YS, Wang WS, Zhang JS, Wu QG. Upregulation of lncRNA DANCR functions as an oncogenic role in non-small lung cancer by regulating miR-214-5p/CIZ1 axis. Eur Rev Med Pharmacol Sci. 2020;24:2539–2547. doi: 10.26355/eurrev_202003_20521. [DOI] [PubMed] [Google Scholar]