Abstract
The objective was to design a scaffold that could continuously deliver nerve growth factor (NGF) combined with neurally differentiated bone marrow mesenchymal stem cells (BMSCs) to promote better recovery of spinal cord injury (SCI) in rats. BMSCs were induced to differentiate into neurons for 6 days in vitro, and then seeded on a NGF persistent delivery scaffold, both were transplanted to SCI rats in combination. Relevant extensive tests were conducted 1, 4 and 8 weeks after transplantation. The results showed that the scaffold had a stable ability to continuously release NGF and that the BMSCs on the scaffold could successfully differentiate into nerve cells. The results of Bacco, Beattie and Bresnahan (BBB) scores, inclined plane tests and electrophysiological investigations revealed that the rats in the combined regimen had better locomotor functional recovery. The results of H&E/Nissl staining, Golgi staining and immunofluorescence showed that the rats in the combined regimen retained the most neurons and had the least cavities and more formations of dendritic spines. Similarly, the positive rate was high for MAP2, NeuN and MBP, and low for GFAP. The graft of the NGF persistent delivery scaffold seeded with neurally differentiated BMSCs significantly reduced the formation of cavities and glial scars at the SCI sites and promoted neuronal survival, axonal regeneration and locomotor function recovery. Compared with the single graft of NGF persistent delivery scaffold or the single graft of neurally differentiated BMSCs, this combined scheme had a better effect in promoting the recovery of SCI.
Keywords: Spinal cord injury, nerve growth factor, scaffold, neural differentiation, bone mesenchymal stem cells, transplantation
Introduction
Spinal cord injury (SCI) caused by trauma not only directly causes the loss of sensory, motor and autonomic nerve functions below the injured site, but also causes dysfunctions of multiple organs such as those involved in respiration, urine production and digestion [1,2]. The reason is that the injury leads to apoptosis in the spinal cord, the fracture of axons and stimulation of the inflammatory response, and the presence of glial scarring and cavity hinders recovery [3,4]. Although there are many treatment methods, such as surgery, drugs, rehabilitation and so on, most have limited efficacy [5,6]. To improve the nerve function of the damaged spinal cord, it is necessary to carry out structural reconstruction, promote the regeneration of neurons and restore axon conduction. Because acute SCI is a multistage, multifactorial injury process, there is currently no single protocol that can be used to effectively treat it [7,8]. Tissue engineering grafts brings new possibilities for the recovery of SCI through the effective combination of scaffolds, seed cells and cytokines.
Bone marrow mesenchymal stem cells (BMSCs) are commonly used in tissue engineering implants. They are also ideal seed cells, which have the advantages of less ethical controversy, easy access, rapid proliferation and low immunogenicity [9]. Previous studies have shown that BMSCs can be transformed into nerve cells in vitro and then transplanted in rats with transected SCI to effectively promote axonal regeneration and spinal cord functional recovery [10]. Other research has found that compared with ordinary BMSCs, BMSCs with pre-neuronal differentiation can be transformed into more neurons and axons after implantation in rats with SCI, and that their locomotor functions are better restored [11].
Recent studies have reported that neurotrophic factors can inhibit inflammation, reduce the formation of glial scarring, protect neurons and promote axon regeneration, and have a good repair effect on SCI [12]. However, the most common route of administration is intravenous [13] or intrathecal injection [14], the former is not effective, however, because of the blood-brain barrier, while the latter cannot produce high concentration at the local injury site. In addition, various scaffolds have also been used to load neurotrophic factors to solve the problem of crossing the blood-brain barrier, but scaffolds are usually prepared as tubes and implanted after half or whole segments of the spinal cord have been removed in animal models [15,16]. However, complete dissociation is rare in clinical patients with spinal cord injuries and tubular scaffolds cannot be inserted by removing a segment of the spinal cord during treatment. Thus, we constructed a new thin-film tissue engineering scaffold with chitosan (CS) as the inner layer, polylactic acid (PLA) as the outer layer and polyglycolic acid (PLGA) microspheres loaded with nerve growth factor (NGF) in the middle layer, which can be directly applied to the surface of an injured spinal cord to promote spinal cord recovery by continuously releasing NGF (Figure 1A).
Figure 1.
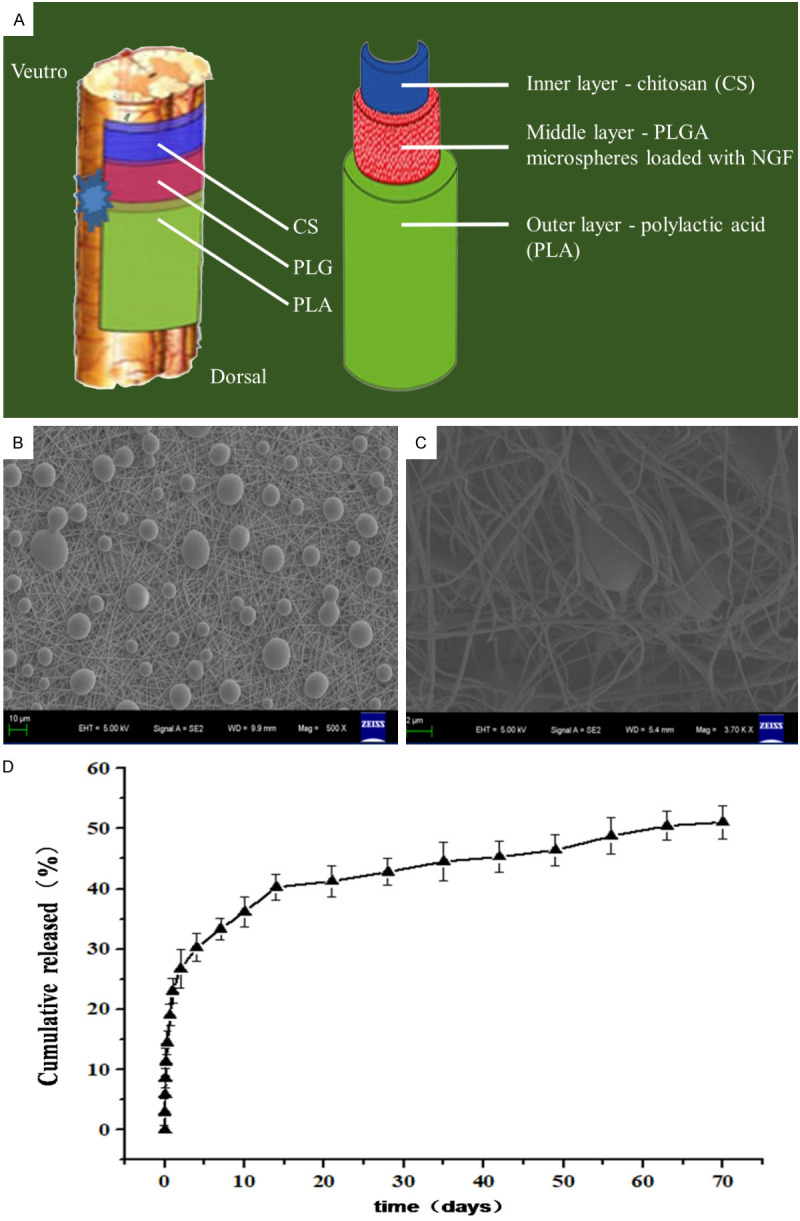
Schematic diagram of persistent release of NGF scaffold and its related characteristics. (A) is the schematic diagram of persistent release of NGF scaffold, which is composed of PLGA microspheres in the middle layer, CS in the inner layer and PLA in the outer layer. Its thin layer design allows it to be applied directly to the site of SCI. (B and C) are the electron microscope observation images of the scaffold, and the spherical structure in the figure is PLGA microsphere loaded with NGF. (D) is the release curve of NGF in vitro, from which it can be seen that the scaffold can release NGF continuously and stably.
In the present study, BMSCs were first induced to differentiate into nerve cells in vitro, and then transferred onto a scaffold that persistently delivered NGF before being transplanted into rats to investigate its effects on SCI.
Material and methods
Preparation of scaffolds
Briefly, PLA solution (7wt%) was first prepared by dissolving polylactic acid (PLA, Shanghai Tianyu Trading Co., Ltd.) in hexafluoroisopropanol (Shanghai Tianyu Trading Co., Ltd.). For CS solution (6.5wt%), Chatasan (CS, Lianyungang Biologicals LNC.) was dissolved in a 2:1 volume mixture of trifluoroaceticacid (Shanghai Tianyu Trading Co.,Ltd.) and dichlormethane (Shanghai Tianyu Trading Co., Ltd.). For preparation of PLGA organic solution, PLGA (50:50 LA:GA, Jinan Daigang Biomaterial Co., Ltd.) was dissolved in trichloromethane (Shanghai Tianyu Trading Co., Ltd). Rat nerve growth factor (NGF, Shutaishen Biopharmaceutical Co., Ltd.) was dissolved in deionized water to prepare an aqueous phase solution. The aqueous solution containing NGF (0.5 mg/mL) was then added to 7wt% PLGA organic solution to prepare the drug mixture. The aluminum foil was coated on the surface of the receiving plate as a collector and the PLA solution was spun by blunt needle electrospinning for 6 h, then the PLGA mixed solution containing NGF was electro-sprayed by blunt needle for 1 h, and finally a layer of CS fibres was deposited on the receiving plate for 1 h by using the electrostatic spinning device again. The film scaffold containing NGF prepared by the above method was freeze-dried to maintain its structure. The scaffold loaded with NGF was placed in a dialysis bag with an intercept molecular weight of 50,000, and added into PBS at pH 7.4. Two mL samples were taken out regularly (at 0.5, 1, 2, 4, 8, 16 and 24 h) to measure the absorbance of NGF at a wavelength of 450 nm by ELISA. The measured absorbance was calculated according to the standard curve. The in vitro release curve of NGF was obtained by taking time as the abscissa coordinate and the cumulative release percentage of NGF as the ordinate. For the control group, we designed a scaffold without NGF in the same way (vide supra). It consisted of PLA/PLGA/CS, and the PLGA layer in the middle did not contain mixed NGF and was called a blank scaffold.
Neural differentiation of BMSCs on scaffolds
After anesthesia, the 1-week-old SD rats were sacrificed, and the ends of the femur and tibia were dissociated and cut off. The bone marrow cavity was rinsed with DMEM (Gibco) and a cell suspension was collected and cultured in an incubator with a 5% CO2 atmosphere at 37°C. The culture medium was first changed after 8 h to remove unattached cells and then the culture medium was replaced every 2 days, when the cells occupied about 80-90% of the whole bottom of the culture bottle for passage. Third generation BMSCs were planted in 24-well plates at 1×105 cells per plate and placed in an incubator with a 5% CO2 atmosphere at 37°C overnight. After incubation, 2% N2 (Gibco), 2% B27 (Gibco), 25 ng/mL bFGF (PeproTech), 25 ng/mL BDNF (PeproTech) and 40 ng/mL NGF (PeproTech) were added to the culture medium. When the solution was changed every 3 days, the above cytokines were added again to continue the induction. Cells were digested with trypsin (Hyclone) on day 6 and transferred to a scaffold. After 12 days of further induction, the culture medium was removed, 4% paraformaldehyde (Alladin) was added for fixation at room temperature and then the medium rinsed with pre-cooled PBS. A primary antibody (microtubule-associated protein 2 (MAP2), Alexa Fluor) was added and incubated at room temperature for 2 h. The primary antibody was removed, followed by fluorescence secondary antibody and an anti-fluorescence quenching agent (Solarbio). Images were captured using a laser confocal fluorescence microscope (Nikon).
Preparation of cells and scaffold grafts
The scaffold size was trimmed to the required size (8 mm long, 5 mm wide). The scaffolds were divided into experimental scaffolds and blank scaffolds groups. After being disinfected with ultraviolet light for 30 min, the scaffolds were placed in petri dishes, with the chitosan layer upwards. According to the experimental requirements, BMSCs that were not induced and BMSCs that were induced in vitro for 6 days were uniformly seeded on the chitosan side of the scaffold with a total of 1×105 cells and implanted into rats with SCI 24 h later.
SCI and transplantation
All experimental protocols and animal handling procedures were approved by the Animal Care and Use Committee of Southeast University, and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Briefly, adult male SD rats (300-350 g weight, n=75, supplied by the Experimental Animal Center of South East University) were anesthetized with 1% pentobarbital sodium (40 mg/kg, i.p.) and attached to an animal stereotactic apparatus. The skin was disinfected and cut, the paravertebral muscles were separated, and a laminectomy performed at 1 segment of each upper and lower segment centering on T10 to expose the dura. Allen’s impactor was used to prepare the SCI model. During the strike, the spine was fixed and a 20 g batting stick was allowed to free-fall from a height of 30 mm to hit the dural sac at T10, the batting stick was allowed to remain in this position for 3 min. The successful standard of beating was spasmodic tail wagging, retractive flapping of the lower limbs and delayed paralysis.
The rats were divided into 5 groups as follows. The control group was a Sham operation group (Group Sham) that did not undergo surgery. The operation group (Group OG) was directly sutured after the SCI model was prepared. In the neural differentiated BMSCs group (Group P), the spinal dural membrane of rats was incised, and the blank scaffold with neural differentiated BMSCs was applied to the surface of the injured spinal cord. In the scaffold group (Group S), the spinal dural membrane was incised and the NGF persistent delivery scaffold seeded with ordinary BMSCs was applied to the surface of the SCI. In the neural differentiated BMSCs + scaffold (Group PS), the spinal dural membrane was incised and the NGF persistent delivery scaffold seeded with neural differentiated BMSCs applied to the surface of the SCI. The rats in each group were sutured layer by layer after operation, fed in a single cage, exposed to natural light, and fed with soft feed at a fixed time and quantities. The feeding environment temperature was 20-23°C and the humidity 50-60%. Intraperitoneal injections of penicillin 80,000 U/pill and gentamicin 2,000 U/pill were administered daily for 7 days after the operation to prevent postoperative infections. Postoperative artificial urination was elicitedtwice a day. Rats in the groups were tested at the 3 time points of 1 week, 4 weeks and 8 weeks, with 3 rats examined at each time point.
Assessment of locomotor function
All rats underwent the inclined plane test and Basso, Beattie and Bresnahan (BBB) scoring on the first day after surgery, and then assessment of their locomotor functions was performed once a week for 8 weeks [17,18]. In the inclined plane test, the rats were placed horizontally on a modified Rivilin inclined board, with their heads faced to the left and the angle gradually increased from the horizontal position (0 degrees). The rats were graded according to the maximum angle at which they could stay on the board for 5 s without falling off. The experiment was repeated 3 times and the average value calculated. For BBB scoring, rats were individually placed in an open area with a non-slippery surface and allowed to move freely for 5 min. The BBB scores ranged from 0 to 21, where 0 meant no hind limb movement was observed and 21 meant normal movement. If the score was <8, the rats could only move their hind limbs without supporting their weight. Scores ranging from 9 to 13 indicated that the rats were able to support their weight without coordination. A score between 14-21 inclusively indicated that a rat could stabilize its trunk and coordinate its movement. In the present study, locomotor functions of the hind limbs were evaluated strictly according to objective criteria. The motion was recorded at each point by a digital camera and analyzed by two researchers who did not know the purpose of the experiments.
Electrophysiological studies
At 8 weeks after SCI, somatosensory evoked potentials (SEP) and motor evoked potentials (MEP) were measured in each group (3 rats in each group). Rats were randomly divided into SEP or MEP recording groups. For SEP, the stimulating electrodes were positioned in close proximity to the median and posterior tibial nerves of anesthetized rats. The stimulation intensity was 2.0 mA, pulse duration 20 ms and a stimulation frequency of 3.43 Hz. The recording electrode was inserted at the midpoint of the connection between the two ears in the parietal skull. The reference electrode was inserted into the subcutaneous area of the forehead. MEP was used to place the stimulation electrode under the scalp (cortical motor area) 2 mm in front of the craniocerebral coronal suture and 2 mm in front of the sagittal suture, and the recording electrode was placed in the middle abdominal region of the triceps brachii and gastrectomy. The stimulation intensity was 30 V, and the number of stimulation trains was 5. A key-point evoked potential/electromyography (Medtronic, US) was used to assess the MEP and SEP of the experimental rats. The latency and amplitude of the 2 evoked potentials were recorded and analyzed.
Tissue processing
At 1, 4 and 8 weeks after surgery, 3 rats in each group were anesthetized and their hearts exposed. After left ventricular-aortic cannulation, 200 mL of normal saline was injected, followed by 200 mL of 4% paraformaldehyde (Alladin). After 2 h perfusion and fixation, the transplanted spinal cord and the 3 segments of the cephalic and caudate were removed and fixed using the same fixative solution for a further 24 h. Paraffin-embedded coronal tissues were cut into 4 µm sections for analysis. All sections were divided into 3 groups: H&E/Nissl staining for Group 1, Golgi staining for Group 2 and immunofluorescence staining for Group 3.
H&E staining
The slices were heated for 2 h and dehydrated in xylene for 10 min. Then, they were successively soaked in 95% ethanol, 80% ethanol, 60% ethanol, 30% ethanol and pure water. The specimens were stained with hematoxylin and eosin solution and then washed in distilled water for 30 s. After washing with 80% ethanol, 95% ethanol, anhydrous ethanol and xylene, the samples were sealed and viewed with a microscope and photographed.
Nissl staining
Sections of specimens were placed in xylene, then transferred to anhydrous ethanol after dewaxing and immersed in alcohol at different concentrations (95%, 90%, 70%) successively. After washing, the sections were immersed in 1% toluidine blue staining solution for 20 min, and then dehydrated with different concentrations (90%, 95%, 100%) of ethanol successively, and then sealed for microscopic viewing. The number of Nissellites with a normal shape were counted under the field of vision. Five random images in ×40 fields were selected for each group and the counts averaged.
Golgi staining
Rats were humanely killed after anaesthesia, spinal laminectomy was performed, the spinal cord was exposed, carefully extracted and placed in cold artificial cerebrospinal fluid, arachnoid and pia meninges removed and the injured graft segment (T10) left 3 cm above and 3 cm below the relevant region. Tissues were rinsed with double-distilled water and then immersed in a solution containing potassium dichromate and mercury chloride. After dyeing, tissues were immersed in double-distilled water containing 15% glycerin (volume ratio) and 20% sucrose (mass ratio) for 24 h. Tissues were sectioned into coronal slices with a thickness of 120 μm. The slices were sequentially attached to gelatin-hanging slides and dried naturally at room temperature. The specimens were dehydrated with 50%, 75% and 95% anhydrous ethanol successively, then sealed with neutral gum and observed under a microscope and photographed using a digital camera. Image J software was used to count and compare the number of dendritic spines in each group.
Immunofluorescence staining
After conventional treatment, a primary antibody was added. The primary antibodies included rabbit anti-GFAP (Abcam) to label astrocytes, rabbit anti-MAP2 (Sigma) to label mature neurons, rabbit anti-NeuN (Sigma) label neurons, rabbit anti-myelin basic protein (MBP, Santa Cruz) label myelin sheath. The primary antibody was added over night at 4°C and then reheated, followed by the secondary antibody, DAPI staining, and finally the anti-fluorescence quenching agent (Solarbio) was added, sections placed in the dark and subsequently viewed using a fluorescence microscope (Nikon).
Statistical analysis
All data are presented as the mean ± SD. Statistical differences between two groups were analyzed using Student’s t-test. Statistical comparison of the BBB scores were performed using a repeated ANOVA followed by a post hoc Bonferroni comparison using SPSS (ver. 23.0). The latencies and amplitudes of SEPs or MEPs among groups were also analyzed using a one-way ANOVA with post hoc comparisons. Values of P<0.05 were deemed to be statistically significant.
Results
Neural differentiation of BMSCs on the scaffold
The results showed that the thin-film scaffold had a stable ability to continuously release NGF (Figure 1B-D). We successfully extracted and cultured BMSCs (Figure 2A), and they were neurally induced in vitro for 6 days before being transferred to the scaffold for further neural induction for 12 more days. After 18 days of induction in vitro, about 70% of the BMSCs showed obvious neuronal cell morphology, with long spindle shapes and synaptic structures (Figure 2B). Immunofluorescence detection revealed that the BMSCs were mainly MAP2 positive cells, indicating that the BMSCs on the scaffold could successfully differentiate into neurons (Figure 2C).
Figure 2.
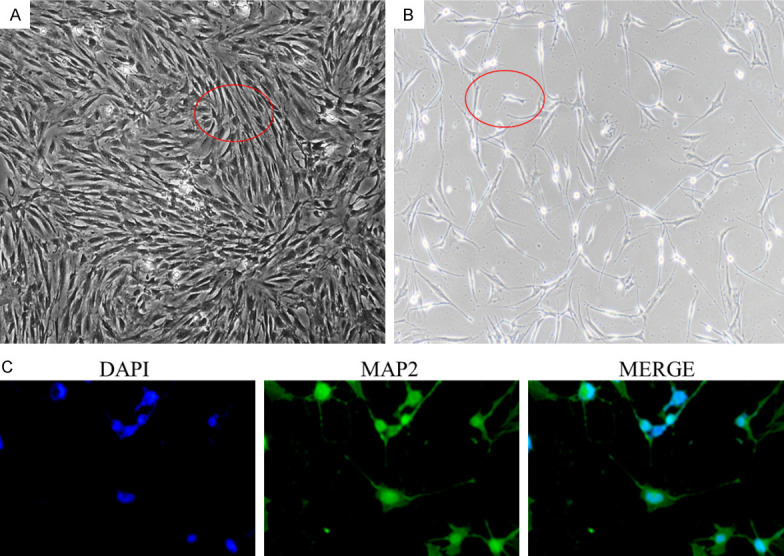
BMSCs were successfully extracted and induced into nerve cells. (A) is BMSCs extracted and cultured by whole bone marrow adherent method. They show a long fusiform appearance. (B) is the BMSCs induced to nerve cells for 18 days, it can be seen that the cell morphology changes, with partial spindle shape and synaptic structure of some cells. (C) is the BSMCs that continued to be induced on the scaffold for 12 days, mainly MAP2 positive cells, indicating that these cells had been successfully induced as neuro-like cells.
Functional recovery
In order to evaluate the improvement of locomotor functions in rats, BBB scores and the inclined plane test were used for postoperative evaluations. The BBB scores of the rats were zero immediately after the operation in all 4 operated groups. In the PS group, the BBB scores of the rats were significantly higher than the scores in the other groups. To evaluate the coordination of the hindlimbs and the involvement of major joints, the inclined plane test was conducted. In the PS group, coordination between the forelimbs and hindlimbs was often observed. In contrast, rats in groups P and S climbed the plane with their forelimbs, and the hindlimbs were simply dragged along without a spontaneous placing reflex. In both BBB and the inclined plane test, it was found that the motor function of group S was significantly improved about 5 weeks after injury and was significantly better than for group P, while the result was just the opposite previously found (Figure 3A, 3B).
Figure 3.
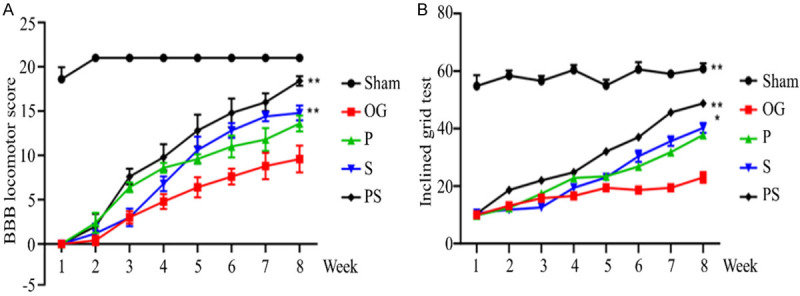
Locomotor functional detection after spinal cord injury in each group. (A) is the BBB score within 8 weeks after injury. (B) shows the inclined plane test results within 8 weeks after injury. It was found that the locomotor functional improvement of Group PS was the best at all stages, while Group OG was the worst. Within 5 weeks of injury, Group P improvements were better than for Group B, and then Group B was better than for Group P.
Electrophysiology
Because the peak and width of evoked potentials (EP) may reflect the number of regenerated axons and the conduction velocity of the nerves, we measured EP to evaluate further any recovery in sensory and locomotor functions. The amplitude and latency of SEP and MEP waveforms in each group were analyzed at the 8th week after the operation. Compared with the control group, the amplitude and latency of SEP and MEP waveforms in each operation group were significantly reduced, and the latency was significantly prolonged. Compared with group OG, the amplitudes of SEP and MEP in the other groups were significantly increased and latency significantly shortened. The amplitude of group PS was significantly higher than that of groups P and S, and the latency was significantly shorter than for these 2 groups. There was no significant difference in SEP and MEP between groups P and S (Figure 4A-C).
Figure 4.
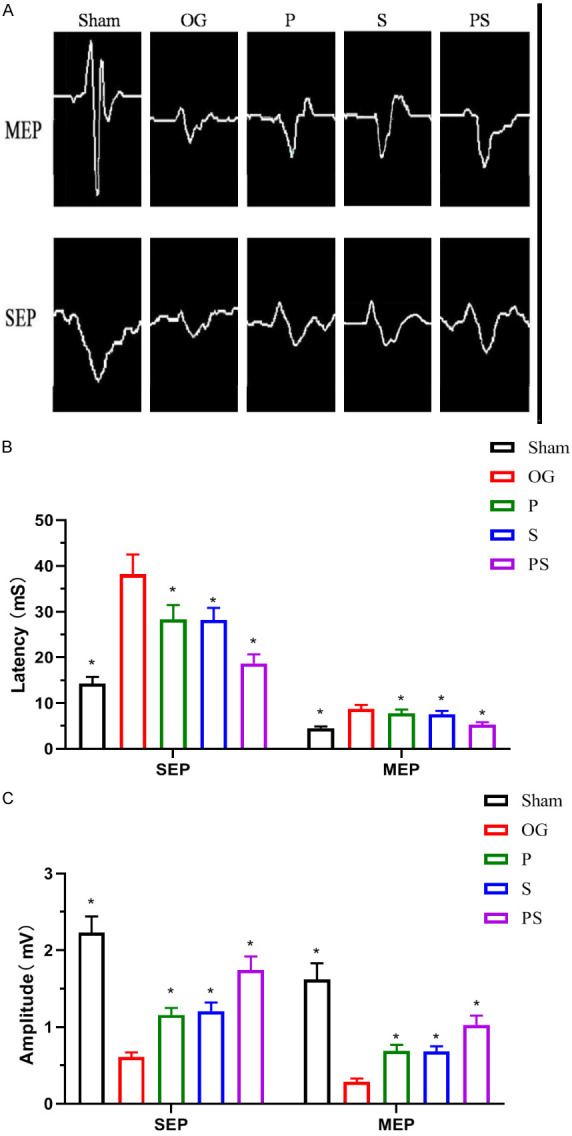
Neuroelectrophysiological detection after spinal cord injury in each group. (A) shows the electrophysiological results of rats in each group recorded at the 8th week after surgery. (B and C) shows that the amplitude of SEP and MEP in group PS was significantly higher than that in group P and S, and the latency was significantly shorter than that in these two groups. There was no significant difference in SEP and MEP between group P and S.
Nerve regeneration
The morphology of the SCI sites in each group was examined 1, 4 and 8 weeks after injury. In group OG, the spinal cord structure was disordered, nerve cells extensively disrupted, fewer neurons and more large cavities. In contrast, the group PS retained the most neurons and had the least cavities, and this change was significantly better than for groups P and S (Figure 5A-C). In order to detect synapse formation and function between neurons at the damaged site, Golgi staining was performed, and the quantitative results showed that compared with group Sham, dendritic spines were seriously lost in each operated group, while group PS had more dendritic spines formation compared with the other 3 groups (Figure 5D, 5E). To clarify further the recovery of neurological functions, we detected the positive expression levels of MAP2 and NeuN in the spinal cord tissues in each group by an immunofluorescence method. Compared with group Sham, the positive expression rates of MAP2 and NeuN were significantly decreased in other spinal tissues. Compared with the other surgery groups, the positive expression rates of MAP2 and NeuN in group PS were significantly increased. The positive expression rate of NeuN in group P was higher than in group S (Figure 6A-C).
Figure 5.
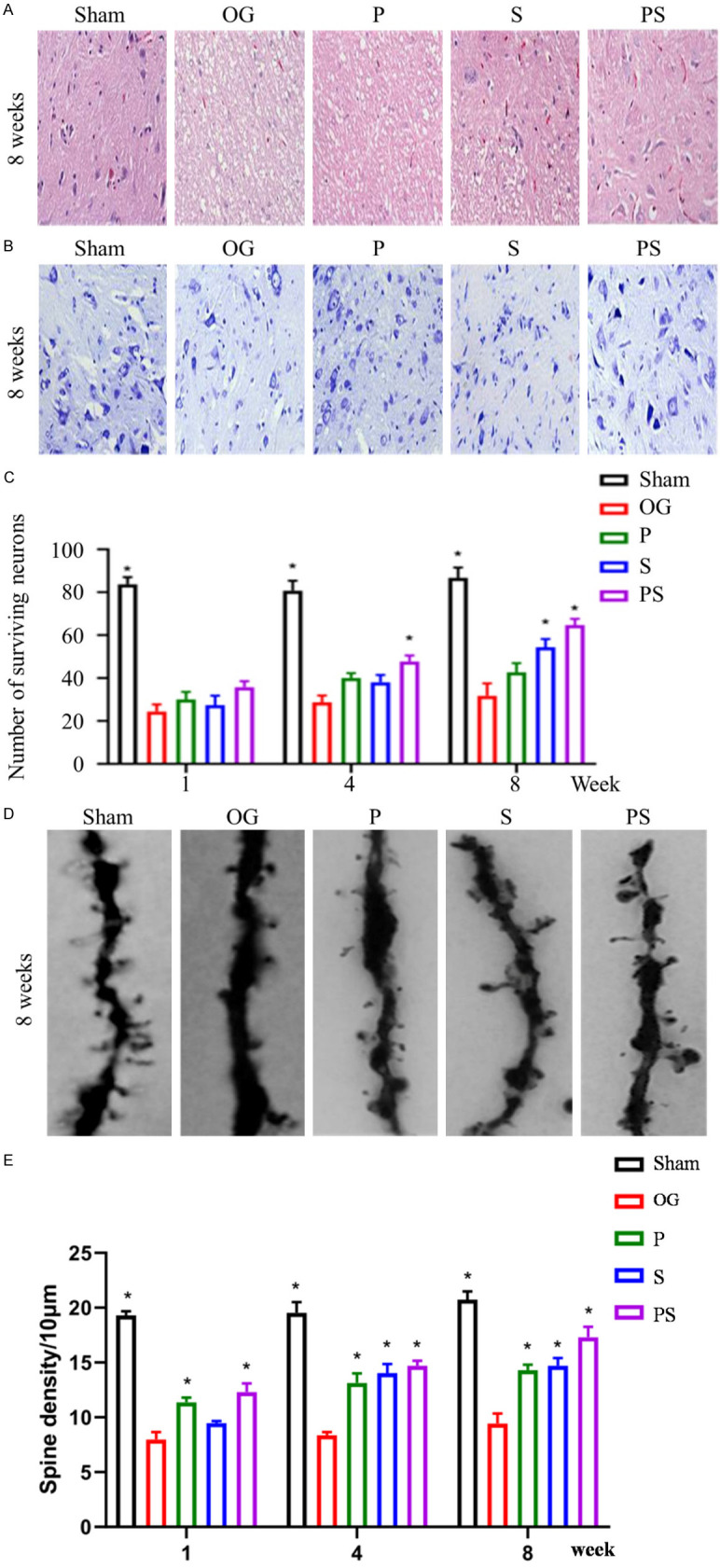
Neuron survival and cavity after spinal cord injury in each group. (A) shows H&E staining in each group at 8 weeks, the cavity in group PS was the least and significantly better than in groups P and S. (B and C) shows Nissl staining in each group, the number of neurons remaining in group PS was the largest and more than in groups P and S after 1, 4 and 8 weeks. (D and E) shows the Golgi staining of each group at week 8, dendritic spines of neurons in group PS were the most numerous and more than in groups P and S.
Figure 6.
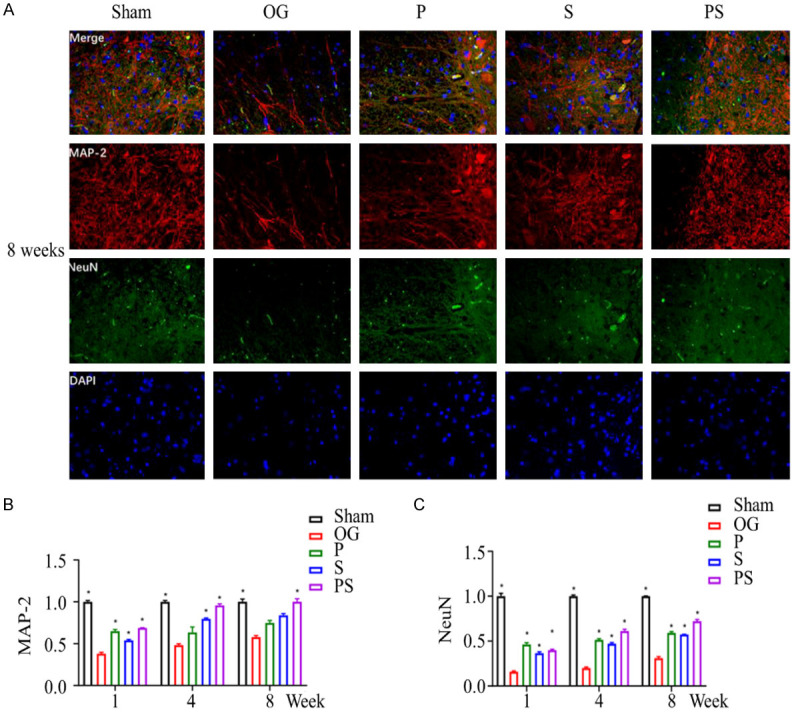
The neuronal survival after spinal cord injury in each group was detected by immunofluorescence staining. (A) shows the positive cells of MAP2 and NeuN in SCI site cells at the 8th week. (B and C) show the highest expression levels in group PS, which was significantly higher than that in groups P and S.
Inhibition of glial scar formation
Studies have shown that SCI can lead to astrocyte proliferation, which can produce a glial scar, that along with the damaged cavity, creates a physical barrier to axon regeneration [19]. GFAP was used to label and analyze astrocytes. In group OG, large numbers of astrocytes were found to proliferate, and their number gradually increased with time. However, the positive rate of GFAP in the spinal cord of group PS was significantly lower than that of the other groups, and by week 8, the value in group PS had decreased to a level close to the group Sham. Compared with group P, there was a brief decrease in the early post-injury period (4 weeks) in group S, and then a gradual increase. By 8 weeks, there was no significant difference between the 2 groups (Figure 7A, 7B).
Figure 7.
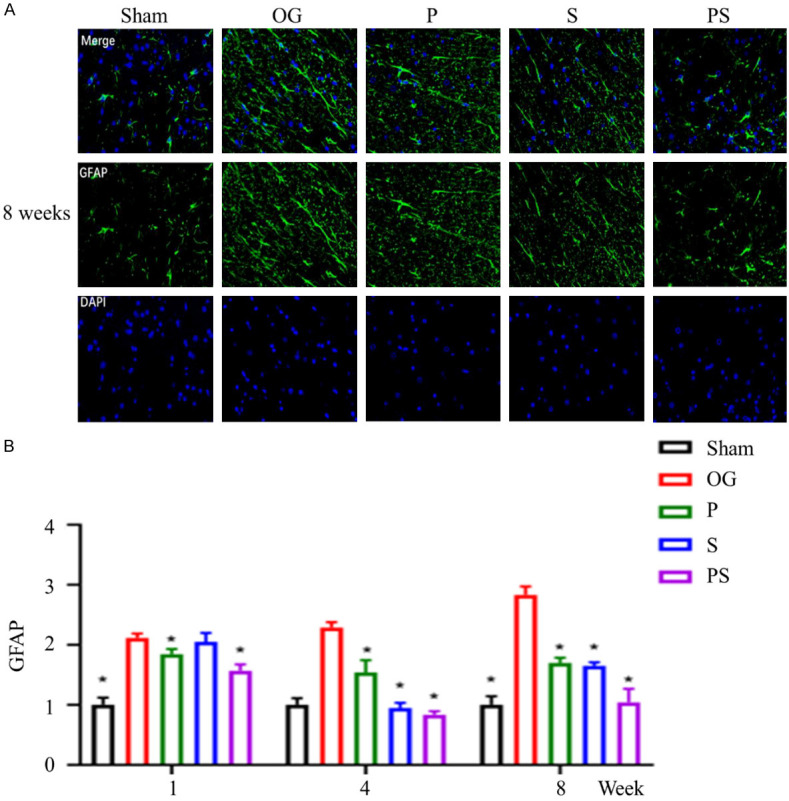
The formation of glial scar after spinal cord injury in each group. (A) shows the number of GFAP positive cells in each group at week 8. (B) shows the number of GFAP positive cells in each group at week 1, 4, and 8, from which it could be seen that the positive rate of GFAP in the spinal cord of group PS is significantly lower than that of the other groups. By week 8, the number of GFAP positive cells had decreased to a level close to that of the control group.
Myelination of axons
Axon regeneration is the basis of functional recovery after SCI, and the myelin sheath is one of the most important factors ensuring efficient axon conduction. Myelin basic protein (MBP), a protein on the myelin sheath, is necessary for impulse conduction along axons. Our study found that the MBP positive rate in group OG was the lowest, indicating the lowest myelination of axons in group OG. Among all surgery groups, the MBP positive rate in group PS was the highest, which was close to the level of group Sham at week 8. However, there was no significant difference between groups P and S (Figure 8A, 8B).
Figure 8.
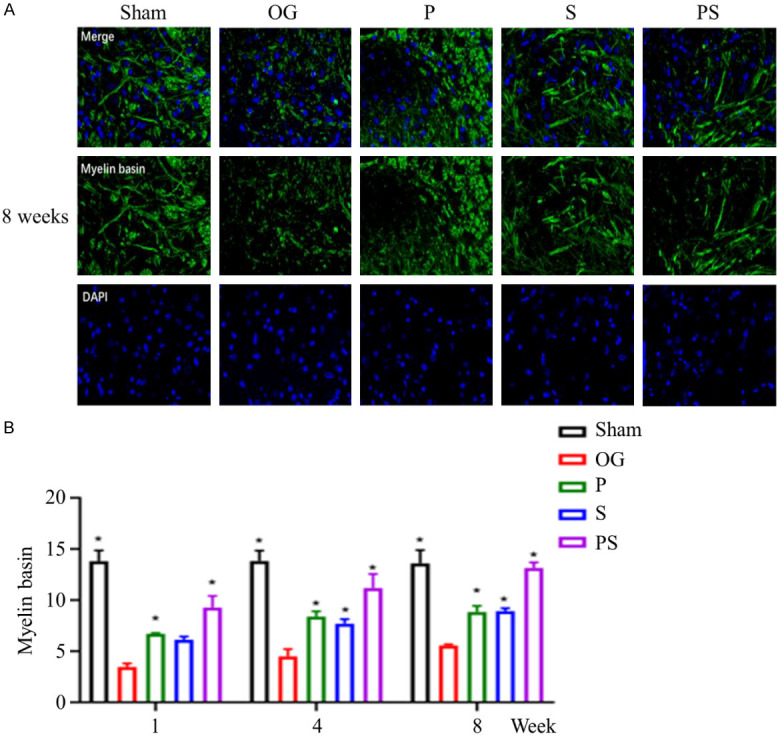
The formation of axonal myelin sheath after spinal cord injury in each group. (A) shows the MBP positive cells in each group at week 8, and (B) shows the changes in the number of MBP positive cells in each group at week 1, 4, and 8. It can be seen that the positive rate of MBP in the spinal cord in group PS was significantly higher than that in the other groups, and by week 8 the number of MBP positive cells approached the level of the control group.
Discussion
The incidence of SCI is very high. Unlike other tissues in the human body, it is difficult for the spinal cord to regain its original functions after traumatic injury. The pathological processes are very complex, and the underlying mechanisms remain unclear. Previous studies have shown that the loss of neurons at the site of SCI, glial scars and cavities that prevent axon regeneration, are important reasons for the failure of spinal cord functions [19,20]. Therefore, various intervention methods including cell transplantation, bridging scaffold, bioactive factors and suppression of inflammation, have been applied to alleviate SCI [21,22]. Here, we adopted a comprehensive strategy that first induced neuronal differentiation of BMSCs in vitro and then combined them with the NGF persistent delivery scaffold to promote the recovery of SCI in rats. The BMSCs used were not genetically modified or transfected. They are easy to obtain, abundant in sources and can be used for clinical autologous transplantation without the risk of an immune response [23,24]. Sung-Rae Cho et al. showed that compared with ordinary BMSCs, implantation of neural-differentiated BMSCs into rats with SCI could better promote functional recovery [25]. NGF plays an important role in promoting neuronal survival, differentiation, axon growth, post-demyelination regeneration and neuroplasticity. After injury, neurons will undergo apoptosis, degeneration and necrosis due to the loss of NGF supply [26,27]. If exogenous NGF can be supplemented in time, the initiation of “suicide mechanism” can be inhibited and the survival of neurons improved [28]. In order to increase NGF in the region of SCI microenvironment and make it more sustainable, we mixed NGF into PLGA, which is a commonly used material in tissue engineering technology. The continuous release of NGF can be achieved through the gradual degradation of PLGA. At the same time, the two sides of PLGA microspheres are covered with chitosan and PLA. As the inner layer of the scaffold, chitosan is conducive to the adhesion and proliferation of BMSCs. Our study showed that BMSCs had good biocompatibility with the scaffold, it could adhere and proliferate well on the scaffold and successfully differentiate into neurons.
Previous studies have shown that scaffolds that continuously release neurotrophic factors protect neurons at the site of injury better than conventional scaffolds [29]. Compared with normal BMSCs, the neutrally differentiated BMSCs implanted into rats with SCI can better promote the survival of neurons and replace dead neurons [11,25]. One intriguing question is whether a combination of the two would protect neurons better. In order to understand the numbers and functions of neurons surviving at the injury site, Nissl and Golgi staining were performed, and the positive expression levels of MAP2 and NeuN in the spinal cord tissues in each group were detected by an immunofluorescence method. Our results revealed that in each period after injury, the rats in the graft of the NGF persistent delivery scaffold seeded with neurally differentiated BMSCs had the most viable neurons at the injured sites and more synaptic connections were formed between neurons. The positive expression levels of the neuronal markers MAP2 and NeuN were highest, and were significantly higher than those of the BMSCs group and the NGF persistent delivery scaffold group alone. These findings indicate that the combination of the two schemes is more beneficial to promoting the survival and functional expression of neurons than a single one.
In this study, we chose the contusion model of the spinal cord rather than the animal model with half or complete spinal cord amputation. This is because complete spinal cord amputation is rarely found in clinical practice and it is impossible to treat by removing a section of the spinal cord. We applied the NGF persistent delivery scaffold seeded with neurally differentiated BMSCs directly to the SCI site. The results showed that the number of cavities and astrocytes at the site was lowest in the rats treated with this scheme, indicating that it could better inhibit the formation of cavities and glial scars, thus promoting axonal regeneration. Axonal regeneration is the tissue basis of nerve function recovery, and myelin sheath is one of the keys to ensuring axonal conduction. We used an immunofluorescence method to detect the key protein MBP in myelin sheath. The results showed that the amount of MBP in the surgery group without any intervention was much lower than that of the other groups, while the MBP amount in the above comprehensive regimen was the largest at all stages, and the amount gradually increased with time. We also conducted electrophysiological tests on each group at the 8th week after operation and found similar results. The amplitude of sensory and motor evoked potentials was significantly increased, and the latency was significantly shortened in rats treated with the combination regimen. These results went a long way toward explaining why the rats in this group showed better BBB scores and inclined plane test results than the other groups. It also showed that a combination of the two schemes was more beneficial in promoting the regeneration and myelination of axons and locomotor functional recovery than a single regimen.
In the present study, BMSCs that underwent neuronal differentiation for 6 days were selected for implantation into rats with SCI. This was because during induction in vitro, about 40% of the BMSCs showed neuronal morphology at 6 days. After 18 days, the proportion reached about 70-80%, and the MAP2 staining of these cells was positive, suggesting that they were mainly induced as neurons. In the case of SCI, both neurons and axons are damaged, and the implanted cells should play a role in promoting the recovery of both types. Therefore, we chose to induce some BMSC into becoming neurons and implanted them into the body, in the hope that they could be transformed into neurons earlier and faster in the microenvironment of SCI. Other uninduced cells differentiated into other types of neurons in the microenvironment, allowing them to secrete various types of neurotransmitters. Studies have shown that during the recovery process from SCI, transplanted cells do not function by transforming and replacing damaged neurons, but rather by secreting various neurotransmitters [9,30]. Similar results were also confirmed in the present study. We found that 8 weeks after injury, rats in the group receiving the NGF persistent delivery scaffold had better motor function recovery, numbers of neurons and dendritic spines surviving at the site of injury, and reduced cavitation and glial scars in the group that received neurally differentiated BMSCs. Interestingly, however, the opposite was true in the early post injury period (up to 4 weeks). Couldn’t neural differentiated BMSCs release neurotransmitters well after transplantation? Whether NGF plays a more important role in the later recovery of SCI needs further studies.
The results of the study have shown that the combination strategy is a potentially promising treatment option for complex SCIs. Furthermore, the survival of neurons, axon regeneration and locomotor functional recovery in rats treated with combination therapy were still lower than those in the control group. This finding indicates that the recovery process after SCI is obviously very complex. We need to improve further the scaffold composition, increase the numbers of important neurotrophic factors, such as brain derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), and further explore the relevant mechanisms involved.
In conclusion, we successfully prepared an NGF persistent delivery scaffold. The graft of this scaffold seeded with neurally differentiated BMSCs significantly reduced the formation of cavities and glial scars at SCI sites, and promoted neuronal survival, axonal regeneration and motor function recovery. Compared with the single graft of an NGF persistent delivery scaffold or a single graft of neurally differentiated BMSCs, this scheme had a better effect in promoting the recovery of SCI.
Acknowledgements
Supported by Clinical Medical Science and Technology Development Fund of Jiangsu University (JLY20180027), General Project of Jiangsu Health Commission (H2018023), Social development science and technology Demonstration Project of Wuxi (N20192001).
Disclosure of conflict of interest
None.
References
- 1.Holmes D. Spinal-cord injury: spurring regrowth. Nature. 2017;552:S49. doi: 10.1038/d41586-017-07550-9. [DOI] [PubMed] [Google Scholar]
- 2.Abbas WA, Ibrahim ME, El-Naggar M, Abass WA, Abdullah IH, Awad BI, Allam NK. Recent advances in the regenerative approaches for traumatic spinal cord injury: materials perspective. ACS Biomater Sci Eng. 2020;6:6490–6509. doi: 10.1021/acsbiomaterials.0c01074. [DOI] [PubMed] [Google Scholar]
- 3.Soares S, von BY, Nothias F. Repair strategies for traumatic spinal cord injury, with special emphasis on novel biomaterial-based approaches. Rev Neurol (Paris) 2020;176:252–260. doi: 10.1016/j.neurol.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23:371–83. doi: 10.1089/neu.2006.23.371. [DOI] [PubMed] [Google Scholar]
- 5.Koffler J, Zhu W, Qu X, Platoshyn O, Dulin JN, Brock J, Graham L, Lu P, Sakamoto J, Marsala M, Chen S, Tuszynski MH. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med. 2019;25:263–269. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi F, Papa S, Perale G, Veglianese P. How can nanovectors be used to treat spinal cord injury. Nanomedicine (Lond) 2019;14:3123–3125. doi: 10.2217/nnm-2019-0355. [DOI] [PubMed] [Google Scholar]
- 7.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26(Suppl):S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 8.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 9.Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 10.Brock JH, Graham L, Staufenberg E, Collyer E, Koffler J, Tuszynski MH. Bone marrow stromal cell intraspinal transplants fail to improve motor outcomes in a severe model of spinal cord injury. J Neurotrauma. 2016;33:1103–14. doi: 10.1089/neu.2015.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang EZ, Zhang GW, Xu JG, Chen S, Wang H, Cao LL, Liang B, Lian XF. Multichannel polymer scaffold seeded with activated Schwann cells and bone mesenchymal stem cells improves axonal regeneration and functional recovery after rat spinal cord injury. Acta Pharmacol Sin. 2017;38:623–637. doi: 10.1038/aps.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodgetts SI, Harvey AR. Neurotrophic factors used to treat spinal cord injury. Vitam Horm. 2017;104:405–457. doi: 10.1016/bs.vh.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Zaviskova K, Tukmachev D, Dubisova J, Vackova I, Hejcl A, Bystronova J, Pravda M, Scigalkova I, Sulakova R, Velebny V, Wolfova L, Kubinova S. Injectable hydroxyphenyl derivative of hyaluronic acid hydrogel modified with RGD as scaffold for spinal cord injury repair. J Biomed Mater Res A. 2018;106:1129–1140. doi: 10.1002/jbm.a.36311. [DOI] [PubMed] [Google Scholar]
- 14.Liu D, Chen J, Jiang T, Li W, Huang Y, Lu X, Liu Z, Zhang W, Zhou Z, Ding Q, Santos HA, Yin G, Fan J. Biodegradable spheres protect traumatically injured spinal cord by alleviating the glutamate-induced excitotoxicity. Adv Mater. 2018;30:e1706032. doi: 10.1002/adma.201706032. [DOI] [PubMed] [Google Scholar]
- 15.Jiao G, Pan Y, Wang C, Li Z, Li Z, Guo R. A bridging SF/Alg composite scaffold loaded NGF for spinal cord injury repair. Mater Sci Eng C Mater Biol Appl. 2017;76:81–87. doi: 10.1016/j.msec.2017.02.102. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Che MT, Zeng X, Qiu XC, Feng B, Lai BQ, Shen HY, Ling EA, Zeng YS. Neurotrophin-3 released from implant of tissue-engineered fibroin scaffolds inhibits inlammation, enhances nerve fiber regeneration, and improves motor function in canine spinal cord injury. J Biomed Mater Res A. 2018;106:2158–2170. doi: 10.1002/jbm.a.36414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson AR, Hook MA, Garcia G, Bresnahan JC, Beattie MS, Grau JW. A simple post hoc transformation that improves the metric properties of the BBB scale for rats with moderate to severe spinal cord injury. J Neurotrauma. 2004;21:1601–13. doi: 10.1089/neu.2004.21.1601. [DOI] [PubMed] [Google Scholar]
- 18.Rejc E, Angeli CA. Spinal cord epidural stimulation for lower limb motor function recovery in individuals with motor complete spinal cord injury. Phys Med Rehabil Clin N Am. 2019;30:337–354. doi: 10.1016/j.pmr.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Kjell J, Olson L. Rat models of spinal cord injury: from pathology to potential therapies. Dis Model Mech. 2016;9:1125–1137. doi: 10.1242/dmm.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Cheng T, Chen Y, Gao F, Guan F, Yao M. A chitosan-based thermosensitive scaffold loaded with bone marrow-derived mesenchymal stem cells promotes motor function recovery in spinal cord injured mice. Biomed Mater. 2020;15:035020. doi: 10.1088/1748-605X/ab785f. [DOI] [PubMed] [Google Scholar]
- 21.Tseng TC, Tao L, Hsieh FY, Wei Y, Chiu IM, Hsu SH. An injectable, self-healing hydrogel to repair the central nervous system. Adv Mater. 2015;27:3518–24. doi: 10.1002/adma.201500762. [DOI] [PubMed] [Google Scholar]
- 22.Chedly J, Soares S, Montembault A, von Boxberg Y, Veron-Ravaille M, Mouffle C, Benassy MN, Taxi J, David L, Nothias F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials. 2017;138:91–107. doi: 10.1016/j.biomaterials.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Satti HS, Waheed A, Ahmed P, Ahmed K, Akram Z, Aziz T, Satti TM, Shahbaz N, Khan MA, Malik SA. Autologous mesenchymal stromal cell transplantation for spinal cord injury: a phase I pilot study. Cytotherapy. 2016;18:518–22. doi: 10.1016/j.jcyt.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Oh SK, Choi KH, Yoo JY, Kim DY, Kim SJ, Jeon SR. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem Cell therapy for spinal cord injury. Neurosurgery. 2016;78:436–47. doi: 10.1227/NEU.0000000000001056. discussion 447. [DOI] [PubMed] [Google Scholar]
- 25.Cho SR, Kim YR, Kang HS, Yim SH, Park CI, Min YH, Lee BH, Shin JC, Lim JB. Functional recovery after the transplantation of neurally differentiated mesenchymal stem cells derived from bone marrow in a rat model of spinal cord injury. Cell Transplant. 2016;25:1423. doi: 10.3727/096368916X692078. [DOI] [PubMed] [Google Scholar]
- 26.Zhao YZ, Jiang X, Xiao J, Lin Q, Yu WZ, Tian FR, Mao KL, Yang W, Wong HL, Lu CT. Using NGF heparin-poloxamer thermosensitive hydrogels to enhance the nerve regeneration for spinal cord injury. Acta Biomater. 2016;29:71–80. doi: 10.1016/j.actbio.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wada N, Shimizu T, Shimizu N, de Groat WC, Kanai AJ, Tyagi P, Kakizaki H, Yoshimura N. The effect of neutralization of nerve growth factor (NGF) on bladder and urethral dysfunction in mice with spinal cord injury. Neurourol Urodyn. 2018;37:1889–1896. doi: 10.1002/nau.23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown A, Ricci MJ, Weaver LC. NGF mRNA is expressed in the dorsal root ganglia after spinal cord injury in the rat. Exp Neurol. 2007;205:283–6. doi: 10.1016/j.expneurol.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Li G, Che MT, Zhang K, Qin LN, Zhang YT, Chen RQ, Rong LM, Liu S, Ding Y, Shen HY, Long SM, Wu JL, Ling EA, Zeng YS. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials. 2016;83:233–48. doi: 10.1016/j.biomaterials.2015.11.059. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Wu J, Sun R, Zhao Y, Li Y, Pan J, Chen Y, Wang X. Tubular scaffold with microchannels and an H-shaped lumen loaded with bone marrow stromal cells promotes neuroregeneration and inhibits apoptosis after spinal cord injury. J Tissue Eng Regen Med. 2020;14:397–411. doi: 10.1002/term.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]


