Abstract
Background: Cardiorenal syndrome (CRS) is a condition that defines disorders of the heart and kidneys whereby “acute or chronic dysfunction in one organ may induce acute or chronic dysfunction of the other”. Early diagnosis of its biomarkers has a significant impact on the treatment and prognosis of the CRS. Elevated serum NGAL and NT-proBNP levels are independent risk factors for predicting heart and kidney disease. Therefore, we proposed early detection of type 1 CRS using serum NGAL in combination with NT-proBNP. Objective: This study intended to investigate the clinical value of serum NGAL in combination with NT-proBNP in the early diagnosis of type 1 CRS. Methods: In this paper, 80 patients with type 1 CRS and 80 healthy controls admitted to our hospital from January 2019 to August 2020 were retrospectively included, and the predictive value of single index and combined indices for predicting CRS were judged by calculating the correlation between serum NGAL, NT-proBNP and the creatinine levels and plotting receiver operating characteristic (ROC) curves. Results: There was no difference in baseline data between the control and patient groups. Serum NGAL and NT-proBNP in the patient group were significantly higher than those in the control group, and were positively correlated with changes in blood creatinine. The ROC curves showed that serum NGAL and NT-proBNP independently had a high predictive value for CRS, and the combination of the two had a better predictive value. Conclusion: Serum NGAL in combination with NT-proBNP is of high clinical value for the early diagnosis of type 1 CRS.
Keywords: Cardiorenal syndrome, neutrophil gelatinase-associated lipocalin, N-terminal pro b-type natriuretic peptide, prognosis
Introduction
In clinical diseases, kidney and heart, as two important organs of the human body, are often damaged in the pathological process. The two organs have close relationship, including the cross-talk in terms of hemodynamics, neurohormones, and inflammatory factors. Cardiorenal syndrome (CRS), defined as a large group of pathophysiological disorders involving both heart and kidney, in which acute or chronic failure of one organ could cause acute or chronic dysfunction of the other [1]. Five subtypes of CRS are recognized on basis of the speed of disease progression and the order of organs involved [2] and patients may switch between different subtypes as the disease progresses. Cardiorenal syndrome type 1 (CRS1), also known as acute heart and kidney syndrome (acute CRS), refers to acute renal failure caused by heart failure, such as cardiogenic shock and acute renal failure caused by acute coronary syndrome and acute renal failure caused by acute heart failure. Approximately 14%-40% of hospitalized patients with acute decompensated heart failure (ADHF) progress to occur acute renal injury (AKI) [3].
Currently, the diagnostic criteria for CRS1 are mainly the presence of an increase in serum creatinine of ≥0.3 mg/dl in the setting of acute heart failure. However, the sensitivity and specificity of serum creatinine are unsatisfactory and have a delayed effect on the determination of renal function, since the elevated creatinine has already indicated severe renal damage. Therefore, we tried to seek effective markers for the early diagnosis of cardiac and renal damage to achieve the best possible preservation of cardiac and renal function. Neutrophil gelatinase-associated lipocalin (NGAL) is a 25 kD protein secreted by the renal tubular epithelium cells, cardiomyocytes and other specific tissues. The predictive value of NGAL for AKI was evidenced in a variety of pathological settings, such as patients following cardiac surgery and critically illed patients. Meanwhile, blood AKI and urine AKI exhibited the same diagnostic accuracy [4]. Brain natriuretic peptide (BNP) was first isolated from porcine brain tissue, hence named BNP, but was subsequently found to be synthesized and secreted primarily by ventricular myocytes in response to ventricular overload or dilatation, and thus could indicate altered ventricular function [5]. Compared to BNP, N-terminal prohormone of brain natriuretic peptide (NT-proBNP) is relatively stable (half-life of 120 min) and more clinically accessible [6]. It has been found that NT-proBNP levels are usually high in the elderly, and they are also elevated in patients with tachycardia, myocardial ischemia, hypoxemia, renal insufficiency, cirrhosis, sepsis and infections. Also, NT-proBNP is helpful in the diagnosis of heart failure and is considered to be a gold standard biomarker for heart failure similar to BNP [7]. Therefore, we proposed to use serum NGAL combined with NT-proBNP as biomarkers for early diagnosis of patients with CRS1.
Materials and methods
Patients
A total of 80 patients with type 1 CRS who were admitted to our hospital from January 2019 to August 2020 were retrospectively included in this study. Inclusion criteria: (1) age ≥18 years; (2) admission with acute decompensated heart failure (ADHF) as the primary diagnosis; Eligible patients were to have a left ventricular ejection fraction ≤40% and signs and symptoms of HF along with BNP concentration ≥400 pg/mL. (3) with increase in serum creatinine ≥0.3 mg/dl after admission; and (4) patient signed the informed consent. Exclusion criteria: patients with primary renal disease, endocrine disease (diabetes mellitus, thyroid disease, primary aldosteronism, etc.), malignancy, acute cerebrovascular disease, severe anemia; without laboratory measurements or medical records; pregnant patients. In addition, 80 healthy volunteers were recruited during the same period. This study was approved by ethics committee of the Second Affiliated Hospital of Nanchang University, and all volunteers signed the informed consent.
Records and assays
Once written informed consent had been obtained, baseline demographic data were recorded and blood samples were obtained for measurements of creatinine (Cr), NGAL, and NT-proBNP within 24 h from hospitalization. These were repeated daily for up to seven days or until discharge. The serum creatine testing was performed by Creatinine (serum) Assay Kit Data (Cayman Chemical, Ann Arbor, Michigan, USA). The NT-proBNP testing was performed by One Step Test for NT-proBNP (Colloidal Gold) (GeteinBiotech, Nanjing, Jiangsu, China). As for the NGAL testing, The Triage NGAL Test (Alere Inc., San Diego, CA, USA) achieved an immunoassay in a single-use plastic cartridge that contains a fluorescently labelled monoclonal antibody against NGAL. The data for the control group were collected from physical examination and questionnaires.
Statistical analysis
Statistical analysis was performed using the SPSS 24.0 (SPSS Inc., Chicago IL, United States). Continuous variables were presented as mean and standard deviation (x̅ ± s). Qualitative variables were reported as frequencies or percentages. The differences in baseline data between the two groups were evaluated by the student-t test for quantitative variables and the Chi-squared test for qualitative variables. Pearson correlation analysis was performed for correlation analysis. ROC curves were plotted for evaluating the predictive value of NGAL/NT-proBNP for CRS, and the best diagnostic value was evaluated by the Youden index. P<0.05 indicated significant differences.
Results
Clinical characteristics of the study population
The clinical characteristics of the patient and control groups were summarized in Table 1, indicating that the baseline was comparable between two groups. The NGAL and NT-proBNP in the patient group were significantly higher than those in the control group (P<0.05).
Table 1.
Demographic parameters between the control group and the patient group
| Demographic parameter | Control group | Patient group | P-value |
|---|---|---|---|
| Number | 80 | 80 | |
| Age (years) | 49.29±8.969 | 49.53±9.461 | 0.871 |
| Gender (M/F) | 38/42 | 42/38 | 0.527 |
| BMI (kg/m2) | 21.79±2.70 | 21.91±2.87 | 0.77 |
| Baseline Cr (mg/dL) | 0.70±0.19 | 0.68±0.18 | 0.37 |
| NGAL (ng/mL) | 109.40±87.80 | 243.37±129.72 | <0.001 |
| NT-proBNP (ng/L) | 296.23±309.65 | 878.33±473.26 | <0.001 |
BMI: Body mass index; Baseline Cr: baseline serum creatinine; NGAL: Neutrophil gelatinase-associated lipocalin; NT-proBNP: N-terminal prohormone of brain natriuretic peptide.
Correlation analysis
The outcome measures of serum Cr were 0.70±0.19 (mg/dL) and 1.81±0.52 (mg/dL) respectively for the control group and patient group. The ΔCr were significantly higher in the patient group than the control group (P<0.05). We correlated NGAL/NT-proBNP and ΔCr in the patient group. The results showed a significant positive correlation between NGAL/NT-proBNP and ΔCr (Figures 1, 2; Table 2).
Figure 1.
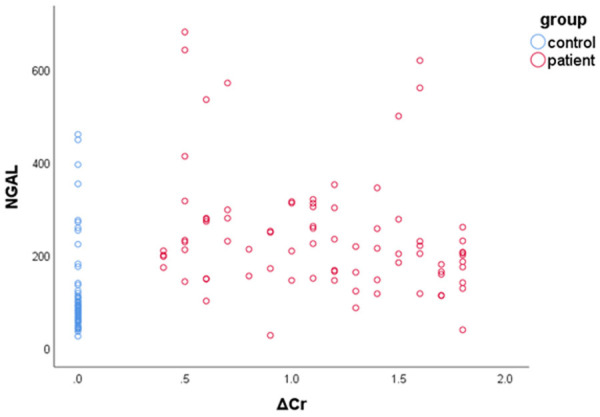
Scatter plot showing the relationship between NGAL and ΔCr.
Figure 2.
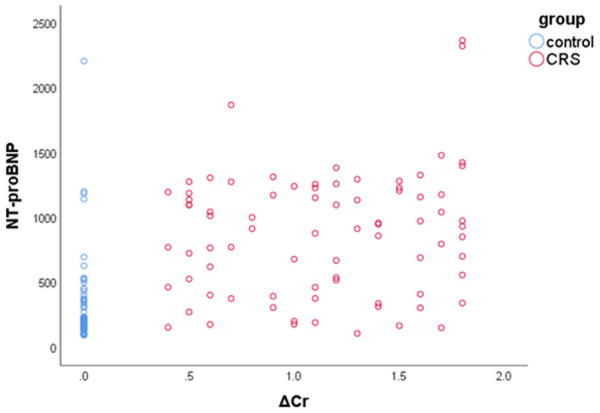
Scatter plot showing the relationship between NT-proBNP and ΔCr.
Table 2.
Analysis of the correlation between serum NGAL, NT-proBNP with ΔCr
| NGAL | NT-proBNP | ||
|---|---|---|---|
| ΔCr | r | 0.371 | 0.565 |
| P-value | <0.001 | <0.001 |
ROC curves of NGAL/NT-proBNP for predicting CRS1
AUC of NGAL predicting type 1 CRS was 0.875 and the optimal diagnostic value was 114.37 ng/mL, with a sensitivity of 0.95 and a specificity of 0.812. AUC of NT-proBNP predicting type 1 CRS was 0.872 and the optimal diagnostic value was 376.72 ng/mL, with a sensitivity of 0.825 and a specificity of 0.825. The AUC of two joint indicators was 0.917, with a sensitivity of 0.925 and a specificity of 0.8, which was significantly higher than the predictive value of index alone (Figure 3; Tables 3, 4).
Figure 3.
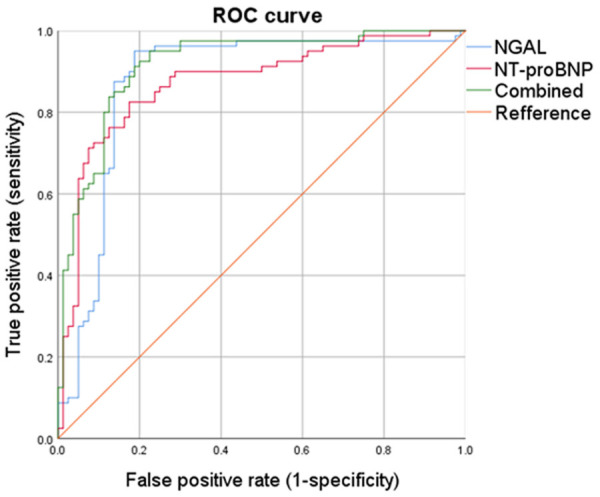
Receive operating characteristic curve analysis of the prediction of type 1 CRS by serum NGAL, NT-proBNP and combined NGAL with NT-proBNP.
Table 3.
Parameters of receive operating characteristic curves
| Parameter | AUC | SD | P-value | 95% Confidence interval | |
|---|---|---|---|---|---|
|
| |||||
| Lower limit | Upper limit | ||||
| NGAL | 0.875 | 0.032 | <0.001 | 0.813 | 0.937 |
| NT-proBNP | 0.872 | 0.029 | <0.001 | 0.815 | 0.929 |
| Combined | 0.917 | 0.023 | <0.001 | 0.872 | 0.961 |
Table 4.
Cut-off values for NGAL and NT-proBNP
| Cut off | Sensitivity | Specificity | Youden index | |
|---|---|---|---|---|
| NGAL | 114.3700 | 0.950 | 0.813 | 0.138 |
| 112.8000 | 0.950 | 0.800 | 0.150 | |
| 115.0000 | 0.938 | 0.813 | 0.125 | |
| 117.1300 | 0.925 | 0.813 | 0.113 | |
| 142.1400 | 0.875 | 0.863 | 0.013 | |
| NT-proBNP | 376.72 | 0.825 | 0.825 | 0.000 |
| 538.30 | 0.713 | 0.925 | -0.213 | |
| 374.96 | 0.825 | 0.813 | 0.013 | |
| 378.26 | 0.813 | 0.825 | -0.013 | |
| 459.12 | 0.763 | 0.875 | -0.113 | |
| Combined | 0.925 | 0.800 | 0.125 | |
| 0.950 | 0.775 | 0.175 | ||
| 0.913 | 0.813 | 0.100 | ||
| 0.938 | 0.775 | 0.163 | ||
| 0.925 | 0.788 | 0.138 |
Discussion
The pathogenesis of CRS is complex, involving the heart and kidney, two major organs of the body, which interacts with each other to promote disease progression through a variety of pathways and mechanisms. Among them, overactivation of the neurohormonal system and elevated venous pressure are the main contributing factors [8]. For example, the RAAS, the sympathetic nervous system, and the arginine-vasopressin system, which are initiated at the beginning of the course as a protective response to maintain steady state, are instead involved in degradation and decompensation, causing further damage to the heart and kidneys [9]. The treatment option of CRS is mainly targeted at primary organ failure, and the prognosis of patients is poor [10]. Therefore, early diagnostic options, early intervention, and timely protective treatment of the heart and kidneys are crucial for patient prognosis.
NGAL, first discovered in mature neutrophil granules, is also widely expressed, including in kidney cells, liver cells, cardiomyocytes, neurons, many immune cells and smooth muscle cells [11,12]. In models of acute kidneyinjury, NGAL is primarily derived from renal cells; smaller amounts are derived from extrarenal sources, such as circulating immune cells. NGAL has been shown to be a small secreted glycoprotein. It was evidenced that NGAL is involved in iron transport and plays a bacteriostatic and chemotactic role in immune function [13]. NGAL is also a growth factor that promotes tissue proliferation and differentiation. Pathologically, NGAL has been widely recognized as a biomarker for acute kidney injury [14,15]. Compared with traditional markers of renal injury, such as creatinine and blood urea nitrogen levels, NGAL is more sensitive to changes in NGAL [16]. In an animal model of ischemia-reperfusion, a significant increase in NGAL levels was observed within 3 hours of ischemia when changes in blood creatinine levels remain insignificant [17]. Moreover, changes in NGAL levels caused by renal injury persist over a long period of time, leaving a long window for clinical diagnosis. In one animal study on renal injury, both urinary cystatin C and NGAL were elevated following renal damage, but cystatin C levels recovered more rapidly, while NGAL levels remained consistently high [18]. In addition to being a biomarker for kidney injury, NGAL has also been shown to be associated with the development of cardiovascular disease. Serum NGAL levels were found to be higher in patients with acute myocardial infarction and chronic heart failure than in healthy subjects [19]. In conclusion, NGAL is involved in the pathology of renal and cardiac diseases through multiple mechanisms and is a sensitive biomarker of renal injury and myocardial damage.
BNP belongs to the natriuretic peptide family, which also includes atrial natriuretic peptide (ANP) and c-type natriuretic peptide (CNP), of which CNP is mainly secreted by the vascular endothelium [20]. ANP and BNP are mainly produced by cardiomyocytes, and ventricular wall stress is the main stimulus for the synthesis and secretion of BNP-related peptide, which is involved in the regulation of cardiovascular homeostasis [21]. The secretion of natriuretic peptide is accompanied by an increase in the severity of left ventricular dysfunction [22], and in patients with acute coronary syndromes, BNP levels are also increased without significant ventricular dilation [23]. In addition, BNP levels are also elevated in patients with renal failure or pulmonary hypertension [24]. In summary, the synthesis and secretion of BNP in cardiomyocytes are regulated by a multifaceted interaction of mechanical, neurohormonal, and immune factors. Heart failure and myocardial infarction can lead to upregulation of NT-proBNP levels in local ventricular cells, which subsequently flow into the systemic circulation. Compared with BNP, NT-proBNP has a longer half-life, is more stable in the blood, and has a higher plasma concentration [25]. Also, NT-proBNP is mainly metabolized by renal clearance, while BNP is only metabolized in small amounts by the kidney, making NT-proBNP more advantageous in the assessment of renal function. NT-proBNP is effective in terms of diagnosis and prognosis of heart failure [26].
In conclusion, serum NGAL and NT-proBNP are key factors in the pathology of heart failure and kidney injury, and both can independently indicate the severity of heart or renal failure. In type 1 CRS, both indicators could predict the occurrence of CRS, while the combined testing of both indicators could significantly improve the predictive accuracy and timely prevent permanent damage to the heart and kidneys. However, the conclusions of this study have some limitations such as limited sample size and short follow-up time. A large multicenter cohort clinical study is needed to confirm the conclusions.
Acknowledgements
Special clinical research project of the Second Affiliated Hospital of Nanchang University (Grant No. 2014YNLC12018).
Disclosure of conflict of interest
None.
References
- 1.Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation. 2018;138:929–944. doi: 10.1161/CIRCULATIONAHA.117.028814. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagshaw SM, Cruz DN, Aspromonte N, Daliento L, Ronco F, Sheinfeld G, Anker SD, Anand I, Bellomo R, Berl T, Bobek I, Davenport A, Haapio M, Hillege H, House A, Katz N, Maisel A, Mankad S, McCullough P, Mebazaa A, Palazzuoli A, Ponikowski P, Shaw A, Soni S, Vescovo G, Zamperetti N, Zanco P, Ronco C. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25:1406–1416. doi: 10.1093/ndt/gfq066. [DOI] [PubMed] [Google Scholar]
- 4.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 5.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–322. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 6.Kerkelä R, Ulvila J, Magga J. Natriuretic peptides in the regulation of cardiovascular physiology and metabolic events. J Am Heart Assoc. 2015;4:e002423. doi: 10.1161/JAHA.115.002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panagopoulou V, Deftereos S, Kossyvakis C, Raisakis K, Giannopoulos G, Bouras G, Pyrgakis V, Cleman MW. NTproBNP: an important biomarker in cardiac diseases. Curr Top Med Chem. 2013;13:82–94. doi: 10.2174/1568026611313020002. [DOI] [PubMed] [Google Scholar]
- 8.Hadjiphilippou S, Kon SP. Cardiorenal syndrome: review of our current understanding. J R Soc Med. 2016;109:12–17. doi: 10.1177/0141076815616091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yogasundaram H, Chappell MC, Braam B, Oudit GY. Cardiorenal syndrome and heart failure-challenges and opportunities. Can J Cardiol. 2019;35:1208–1219. doi: 10.1016/j.cjca.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, Lerma EV, Mezue K, Molitch M, Mullens W, Ronco C, Tang WHW, McCullough PA American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the american heart association. Circulation. 2019;139:e840–e878. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 11.Eilenberg W, Stojkovic S, Piechota-Polanczyk A, Kaun C, Rauscher S, Gröger M, Klinger M, Wojta J, Neumayer C, Huk I, Demyanets S. Neutrophil gelatinase-associated lipocalin (NGAL) is associated with symptomatic carotid atherosclerosis and drives pro-inflammatory state in vitro. Eur J Vasc Endovasc Surg. 2016;51:623–631. doi: 10.1016/j.ejvs.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Yang H, Chen H, Zhang M, Ma Q. High expression of neutrophil gelatinase-associated lipocalin (NGAL) in the kidney proximal tubules of diabetic rats. Adv Med Sci. 2015;60:133–138. doi: 10.1016/j.advms.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Shao S, Cao T, Jin L, Li B, Fang H, Zhang J, Zhang Y, Hu J, Wang G. Increased lipocalin-2 contributes to the pathogenesis of psoriasis by modulating neutrophil chemotaxis and cytokine secretion. J Invest Dermatol. 2016;136:1418–1428. doi: 10.1016/j.jid.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Di Grande A, Giuffrida C, Carpinteri G, Narbone G, Pirrone G, Di Mauro A, Calandra S, Noto P, Le Moli C, Alongi B, Nigro F. Neutrophil gelatinase-associated lipocalin: a novel biomarker for the early diagnosis of acute kidney injury in the emergency department. Eur Rev Med Pharmacol Sci. 2009;13:197–200. [PubMed] [Google Scholar]
- 15.Kanagasundaram NS. New biomarkers of acute kidney injury: promise for the future but beware the lure of novelty. Crit Care Med. 2009;37:766–767. doi: 10.1097/CCM.0b013e318194dfd0. [DOI] [PubMed] [Google Scholar]
- 16.Beker BM, Corleto MG, Fieiras C, Musso CG. Novel acute kidney injury biomarkers: their characteristics, utility and concerns. Int Urol Nephrol. 2018;50:705–713. doi: 10.1007/s11255-017-1781-x. [DOI] [PubMed] [Google Scholar]
- 17.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 18.Woodson BW, Wang L, Mandava S, Lee BR. Urinary cystatin C and NGAL as early biomarkers for assessment of renal ischemia-reperfusion injury: a serum marker to replace creatinine? J Endourol. 2013;27:1510–1515. doi: 10.1089/end.2013.0198. [DOI] [PubMed] [Google Scholar]
- 19.Nakada Y, Kawakami R, Matsui M, Ueda T, Nakano T, Takitsume A, Nakagawa H, Nishida T, Onoue K, Soeda T, Okayama S, Watanabe M, Kawata M, Okura H, Saito Y. Prognostic value of urinary neutrophil gelatinase-associated lipocalin on the first day of admission for adverse events in patients with acute decompensated heart failure. J Am Heart Assoc. 2017;6:e004582. doi: 10.1161/JAHA.116.004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa Y, Nishikimi T, Kuwahara K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides. 2019;111:18–25. doi: 10.1016/j.peptides.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Forte M, Madonna M, Schiavon S, Valenti V, Versaci F, Zoccai GB, Frati G, Sciarretta S. Cardiovascular pleiotropic effects of natriuretic peptides. Int J Mol Sci. 2019;20:3874. doi: 10.3390/ijms20163874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rullman E, Melin M, Mandić M, Gonon A, Fernandez-Gonzalo R, Gustafsson T. Circulatory factors associated with function and prognosis in patients with severe heart failure. Clin Res Cardiol. 2020;109:655–672. doi: 10.1007/s00392-019-01554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clerico A, Recchia FA, Passino C, Emdin M. Cardiac endocrine function is an essential component of the homeostatic regulation network: physiological and clinical implications. Am J Physiol Heart Circ Physiol. 2006;290:H17–29. doi: 10.1152/ajpheart.00684.2005. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto R, Ali Y, Hashizume R, Suzuki N, Ito M. BNP as a major player in the heart-kidney connection. Int J Mol Sci. 2019;20:3581. doi: 10.3390/ijms20143581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santaguida PL, Don-Wauchope AC, Oremus M, McKelvie R, Ali U, Hill SA, Balion C, Booth RA, Brown JA, Bustamam A, Sohel N, Raina P. BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart Fail Rev. 2014;19:453–470. doi: 10.1007/s10741-014-9442-y. [DOI] [PubMed] [Google Scholar]
- 26.Srisawasdi P, Vanavanan S, Charoenpanichkit C, Kroll MH. The effect of renal dysfunction on BNP, NT-proBNP, and their ratio. Am J Clin Pathol. 2010;133:14–23. doi: 10.1309/AJCP60HTPGIGFCNK. [DOI] [PubMed] [Google Scholar]


