Abstract
Background: The present study aimed to investigate the clinical outcomes of percutaneous transforaminal endoscopic discectomy (PTED) and microendoscopic discectomy (MED) in the treatment of upper lumbar disc herniation (ULDH). Methods: A total of 62 ULDH patients treated with PTED or MED were enrolled in this study and were randomly divided into group A (PTED, n=31) and group B (MED, n=31). The characteristics, surgical duration, incision length, blood loss, volume of drainage, length of hospital stay, and the complications and recurrences of patients were recorded and compared between the two groups. The Japanese Orthopedic Association (JOA), Oswestry Disability Index (ODI), and visual analogue scale (VAS) scores were compared preoperatively, postoperatively, and at the final follow-up between group A and group B. The postoperatively clinical outcomes of patients were evaluated according to the modified MacNab criterion. Results: The incision length, the duration of surgery, intraoperative blood loss, volume of drainage, and length of hospital stay in group A were less than those in group B (P<0.01). Compared with group B, the JOA scores of the patients in group A were significantly enhanced at 1 month (P<0.01), 3 months (P<0.01), and 6 months (P<0.01), the VAS scores were significantly improved at 1 month (P<0.01), 3 months (P<0.01), 6 months (P<0.05), and 12 months (P<0.05), and the ODI scores exhibited significant improvements at 1 month (P<0.01) and 3 months (P<0.05). Conclusion: PTED provides better results in the treatment of ULDH compared with MED. It is beneficial to improve the quality of life of patients and is worthy of promotion in clinical application.
Keywords: Percutaneous transforaminal endoscopic discectomy, microendoscopic discectomy, upper lumbar disc herniation, minimally invasive spine surgery, clinical outcome
Introduction
Upper lumbar disc herniation (ULDH), generally defined as L1-L2 and L2-L3 levels, is rare in lumbar disc herniation with the incidence less than 5% [1,2]. The definition of upper lumbar in the spectrum of lumbar disc herniation is still controversial. Most scholars define the discs as L1-L2 and L2-L3 levels [3,4], and some expand it to include T12-L1, L1-L2, L2-L3, and L3-4 levels [5-7]. Due to specific characteristics of L1-L2 and L2-L3 levels of lumbar disc herniation, the postoperatively clinical outcome of ULDH was less favorable than lower lumbar disc herniation (LLDH) [1]. In addition, compared with LLDH, ULDH has unique anatomical features, including a narrow spinal canal, short nerve roots, less distance between the dura and nerve roots, and a location adjacent to the lumbosacral enlargement area of the spinal cord [8]. Therefore, surgical decompression of ULDH is more necessary than that of LLDH, despite the higher risks and bigger challenges of surgery [9].
With the recent advances of surgical techniques, the percutaneous spinal endoscopic technique has been widely used in minimally invasive spine surgery (MISS) and has been proved to be safe and feasible. Percutaneous endoscopic transforaminal discectomy (PETD) is reported to be an appropriate and viable choice for ULDH without dural traction and laminectomy [10,11]. Microendoscopic discectomy (MED), another well-known conventional MISS technique, has been widely used in the past few years [12-14]. However, as far as we know, there are limited studies on PETD for ULDH, and few studies have compared the surgical outcomes of PETD with MED in the treatment of ULDH. Therefore, we designed a retrospective comparative study to evaluate the advantages and clinical outcomes of PETD and MED as two different surgical techniques, so as to describe the technical strategies of PETD for ULDH.
Materials and methods
Patient characteristics
From June 2014 to June 2019, a total of 62 patients with monosegment ULDH in Shanxi Provincial People’ Hospital were treated with PTED and MED, respectively. All the patients were informed in advance and signed a consent form. This study was approved by the Shanxi Provincial People’ Hospital Ethics Committee. All the patients were randomly divided into group A (PTED, n=31) and group B (MED, n=31). There were 17 males and 14 females in group A, aged 30-66 years, with an average age of (51.32±8.99) years. There were 16 males and 15 females in group B, aged 27-69 years, with an average age of (50.75±9.36) years.
Inclusion and exclusion criteria
Preoperative indicators included medical history collection, lumbar positive side, neurological examinations, X-ray, CT and magnetic resonance imaging (MRI) examination. All of these patients were diagnosed according to inclusion and exclusion criteria as followed: The inclusion criteria were a single segment of central or lateral ULDH without segmental instability observed by CT and MRI, conservative treatment failed over three months and unilateral radicular lower limb pain identified with the radiographic findings. The exclusion criteria were the recurrence of ULDH after previous surgeries, tumor or tuberculosis discitis, ankylosing spondylitis, severe central spinal stenosis, fracture of lumbar vertebra, intervertebral disc calcification, and cauda equina syndrome.
Surgical techniques
All patients in group A were performed a local anesthetic (1% lidocaine) and placed in the lateral position with the affected side at the top and the healthy side at the bottom. The skin entry point and puncture needle path were determined by the guidance of C-arm X-ray machine. The puncture needle entry point was located around 6 to 9 cm from the midline, and a steep needle trajectory angle of about 35° to 45° was generally selected in order to avoid damage to the nerve roots and dural sac. An 18-gauge spinal needle was guided and inserted into the annular fibrosus surface of the affected target disc, and local infiltration anesthesia with 1% lidocaine was performed in the puncture path. The needle tip was pointed at the midline of the pedicle on the anteroposterior view, and located at the posterior margin of vertebral bodyline and intervertebral disc on lateral view. A guidewire as a substitute for the spinal needle was then inserted in it after the nucleus pulposus was stained with contrast agent. A 7-mm incision was cut, and a tapered dilating obturator was passed through the guidewire. Finally, a bevel-ended working cannula was inserted, and the herniated disc was resected with endoscopic forceps.
All patients in group B were performed spinal-epidural anesthesia and placed in the prone position. The operating level was determined using C-arm fluoroscopy. A 2-cm incision was made from 1.5 to 2.0 cm of the posterior midline. After exposing the ligamentum flavum and lamina of the targeted level, a minimally invasive tubular retractor with microendoscope was inserted. Subsequently, a portion of the ligamentum flavum and lamina were removed using a Kerrison rongeur under microscopic visualization. Next, relative tissues including nerve root, dura mater, and disc were exposed in the surgical field, and the loose and protruded disc tissue was removed using disc forceps until the nerve root was decompressed completely.
Outcome measures
The patients were followed up at 1, 3, 6, and 12 months postoperatively by telephone or clinical visits. According to patient’s recovery, the follow-up was performed every 1 to 2 years. The general clinical data and surgical information were collected for all patients, and postoperative complications and recurrences were recorded. The clinical efficacy was evaluated using preoperative and postoperative scores as well as modified MacNab criterion. The Japanese Orthopedic Association (JOA), Oswestry Disability Index (ODI), and visual analogue scale (VAS) scores were recorded preoperatively, postoperatively (1, 3, 6, and 12 months), and at the final follow-up.
VAS was applied to measure pain degrees of the patients, ranging from 0 to 10, with higher scores indicating more severe pain [15]. A 10-cm line was draw on the paper, the beginning and end of the line were marked 0 and 10 points, and then the patient were asked to point the level of pain on it. According to the pain of patients in varying degrees, specific VAS scores criteria was classified as follow: 0 point: painlessness; 1 to 3 points: slight pain; 4 to 6 points: tolerable moderate pain; 7 to 10 points: unbearable intense pain.
Similarly, ODI was used to evaluate the degrees of dysfunction, ranging from 0 to 100, with higher scores manifesting greater dysfunction related to pain [16]. Combined with the clinical symptoms and the doctor’s guidance, the patient’s actual degree of dysfunction was scored using Oswestry disability questionnaire, which was consisted of 10 questions, including pain intensity, personal care, lifting, walking, sitting, standing, sleeping, sex life, social life, and travelling. ODI scores criteria as follow: 0 to 20 points: mild dysfunction; 20 to 40 points: moderate dysfunction; 40 to 60 points: severe dysfunction; 60 to 80 points: severer dysfunction; 80 to 100 points: extremely severe dysfunction.
Besides, neurological function of the patients was assessed by JOA scores [17], which was inversely proportional to the degree of pain, lower score, and lesser pain. The total JOA scores, ranging from 0 to 29, were divided into four parts: subjective symptoms were accounted for 9 points in the total scores including low back pain, pain or numbness of lower limb and gait; clinical signs (0 to 6 points) such as Lasegue sign, sensory disorder, and dyskinesia; activities daily limitation (0 to 14 points), and bladder function (-6 to 0 points).
The clinical outcomes of patients were evaluated for one year postoperatively based on the modified MacNab criterion as follow [18,19]: Excellent: the symptoms completely disappear, and the original work and life return; Good: with mild symptoms, mildly limited activity, no impact on work and life; Fair: the symptom are alleviated, and the activity is restricted, affecting the normal work and the life; Poor: no difference before and after treatment, and even worse.
Statistical analysis
Data were analyzed via SPSS 13.0 software (IBM, USA). Univariate analyses were performed for characteristics of patients, surgical information, complications and recurrences, clinical efficacy by unpaired student’s t-test and the Chi-square test. The enumeration data were shown as [n (%)] and tested by χ2 between the two groups; and the independent sample t test was used for comparison between group A and group B. Additionally, JOA, VAS, and ODI scores preoperatively, postoperatively (at different time points), and at the final follow-up were evaluated with one-way analysis of variance (ANOVA) followed by Tukey test analyzed using ANOVA analysis. The measurement data of results were expressed as mean ± standard deviation. P<0.05 and P<0.01 was considered statistically significant difference.
Results
Comparison of baseline data between the two groups
The characteristics of ULDH patients including gender, age, body mass index (BMI), symptomatic duration, affected level, types of lumbar disc herniation, medical history, clinical signs and symptoms were compared between the two groups. Among the 62 patients who met the inclusion criteria, half of them underwent PTED and the others received MED for ULDH. The mean symptomatic duration in group A was (7.94±4.77) months and that in group B was (8.58±5.26) months. Eighteen patients underwent the surgery at the L1-L2 level, and 44 patients underwent the surgery at the L13-L3 level. The zones of disc herniation were central in 7, lateral (including posterolateral and foraminal) in 55. The preoperative clinical signs in group A were positive Lasègue sign in 10 patients (32.26%), positive Bragard sign in 7 patients (22.58%), lower limb paresthesia in 6 patients (19.35%), and lower extremity weakness in 9 patients (29.03%). More detailed general clinical information was clearly listed (Table 1). Statistical analysis showed that group A and group B had no significant differences in terms of the general materials (P>0.05) before surgery, which were comparable.
Table 1.
Characteristics of patients with upper lumbar disc herniation
| Variable | Group A (PTED, n=31) | Group B (MED, n=31) | t/χ 2 | P value |
|---|---|---|---|---|
| Gender | 0.065 | 0.799 | ||
| Male | 17 (54.84%) | 16 (51.61%) | ||
| Female | 14 (45.16%) | 15 (48.39%) | ||
| Age (years) | 51.32±8.99 | 50.19±9.36 | 0.485 | 0.630 |
| Age range (years) | 30~66 | 27~69 | ||
| BMI (kg/m2) | 23.06±2.63 | 22.08±2.47 | 1.506 | 0.137 |
| Symptomatic duration (months) | 7.94±4.77 | 8.58±5.26 | 0.506 | 0.615 |
| Affected level | 0.313 | 0.576 | ||
| L1-L2 | 10 (32.26%) | 8 (25.81%) | ||
| L2-L3 | 21 (67.74%) | 23 (74.19%) | ||
| Zone of disc herniation | 0.161 | 0.688 | ||
| Central | 3 (9.68%) | 4 (12.90%) | ||
| Lateral | 28 (90.32%) | 27 (87.10) | ||
| Contained disc herniation | 0.097 | 0.755 | ||
| Yes | 6 (19.35) | 7 (22.58) | ||
| No | 25 (80.65) | 24 (77.42) | ||
| Migrated disc herniation | 0.369 | 0.544 | ||
| Yes | 8 (25.81%) | 6 (19.35) | ||
| No | 23 (74.19%) | 25 (80.65) | ||
| Smoking | 0.076 | 0.783 | ||
| Yes | 9 (29.03%) | 10 (32.26%) | ||
| No | 22 (70.97%) | 21 (67.74%) | ||
| Drinking | 0.069 | 0.793 | ||
| Yes | 11 (35.48%) | 12 (38.71%) | ||
| No | 20 (64.52%) | 19 (61.29%) | ||
| Diabetes | 0.218 | 0.641 | ||
| Yes | 3 (9.68%) | 2 (6.45%) | ||
| No | 28 (90.32%) | 29 (93.54%) | ||
| Hypertension | 0.088 | 0.767 | ||
| Yes | 7 (22.58%) | 8 (25.81%) | ||
| No | 24 (77.42%) | 23 (74.19%) | ||
| Clinical signs and symptoms | 0.282 | 0.569 | ||
| Lasègue sign (+) | 10 (32.26%) | 12 (38.71%) | ||
| Bragard sign (+) | 7 (22.58%) | 11 (35.48%) | ||
| Lower extremity weakness | 9 (29.03%) | 8 (25.81%) | ||
| Paresthesia in lower limbs | 6 (19.35%) | 7 (22.58%) |
Comparison of surgical information between the two groups
The incision length in group A was (0.73±0.18) cm (Table 2), whereas the incision length in group B was (1.8±0.19) cm (P<0.01). In addition, the duration of surgery, intraoperative blood loss, volume of drainage, and length of hospital stay in group A were less than those in group B (P<0.01). Comparison of surgical information indicated that the PTED for ULDH could provide a smaller incision, reduce intraoperative blood loss and volume of drainage, and even reduce the time of surgery and hospital stay.
Table 2.
Comparison of surgical information between patients in group A and group B
| Variable | Group A (PTED, n=31) | Group B (MED, n=31) | t | P value |
|---|---|---|---|---|
| Length of incision (cm) | 0.73±0.18 | 1.8±0.19 | 23.119 | <0.001 |
| Duration of surgery (min) | 49.94±13.70 | 61.68±11.93 | 3.599 | 0.001 |
| Intraoperative blood loss (mL) | 20.93±2.59 | 30.63±2.57 | 14.824 | <0.001 |
| Drainage (mL) | 42.05±12.11 | 76.99±9.28 | 12.750 | <0.001 |
| Length of hospital stay (days) | 5.03±0.98 | 9.03±1.14 | 14.800 | <0.001 |
Comparison of complications and recurrences between the two groups
The complications and recurrences of group A were recorded and compared with group B (Table 3). The complications included neural injury, cerebrospinal fluid leak, postoperative dysesthesia, infection, and unhealed wound. Group B had 1 patient (3.23%) with recurrence, and group A had 2 patients (6.45%) with residue and recurrence (P>0.05). Comparison of complications and recurrences in THE two groups indicated that the clinical complications and recurrences were not related to the specific MISS system.
Table 3.
Comparison of complications and recurrences in each group
| Variable | Group A (PTED, n=31) | Group B (MED, n=31) | t | P value |
|---|---|---|---|---|
| Neural injury | 2 (6.45%) | 1 (3.23%) | 0.350 | 0.554 |
| Cerebrospinal fluid leak | 2 (6.45%) | 1 (3.23%) | 0.350 | 0.554 |
| Postoperative dysesthesia | 1 (3.23%) | 3 (9.68%) | 1.069 | 0.301 |
| Infection | 0 (0.00%) | 1 (3.23%) | 1.016 | 0.313 |
| Poor wound healing | 0 (0.00%) | 1 (3.23%) | 1.016 | 0.313 |
| Persistent aggravated pain | 1 (3.23%) | 0 (0.00%) | 1.016 | 0.313 |
| Residue/recurrence | 2 (6.45%) | 1 (3.23%) | 0.350 | 0.554 |
Comparison of clinical efficacy between the two groups
Clinical efficacy was evaluated by JOA, VAS, ODI scores, and modified MacNab criterion (Table 4). The mean of JOA scores enhanced from (12.29±2.09) to (27.71±1.92) in group A and from (12.26±1.90) to (27.26±1.53) in group B. However, compared with group B, the JOA scores of the patients in group A were significantly enhanced at 1 month (P<0.01), 3 months (P<0.01), and 6 months (P<0.01) (Figure 1). The mean of VAS scores improved from (8.32±0.95) to (1.10±0.60) in group A and from (8.39±1.02) to (1.06±0.68) in group B, manifesting that there was no significant difference in VAS scores between the two groups at preoperative and the final follow-up (P>0.05). Nevertheless, compared with group B, the VAS scores in group A were significantly improved at 1 month (P<0.01), 3 months (P<0.01), 6 months (P<0.05), and 12 months (P<0.05) (Figure 2). Furthermore, both groups showed no significant difference in the ODI scores for the degrees of dysfunction related to pain at preoperative, 6 months, 12 months, and the final follow-up (P>0.05), whereas the ODI scores in group A exhibited significant improvements at 1 month (P<0.01) and 3 months (P<0.05), respectively (Figure 3).
Table 4.
Clinical efficacy and score and modified MacNab criterion of patients with upper lumbar disc herniation
| Variable | Group A (PTED, n=31) | Group B (MED, n=31) | t/χ 2 | P value |
|---|---|---|---|---|
| JOA scores | ||||
| Preoperative | 12.29±2.09 | 12.26±1.90 | 0.064 | 0.949 |
| Final follow-up | 27.71±1.92 | 27.26±1.53 | 1.026 | 0.309 |
| VAS scores | ||||
| Preoperative | 8.32±0.95 | 8.39±1.02 | 0.258 | 0.797 |
| Final follow-up | 1.10±0.60 | 1.06±0.68 | 0.198 | 0.843 |
| ODI scores | ||||
| Preoperative | 51.10±8.17 | 51.13±9.88 | 0.014 | 0.989 |
| Final follow-up | 4.84±2.45 | 5.39±2.26 | 0.916 | 0.363 |
| Modified MacNab criterion | 0.300 | 0.861 | ||
| Excellence | 21 (67.74%) | 19 (61.29%) | ||
| Good | 9 (29.03%) | 11 (35.48%) | ||
| Fair | 1 (3.23%) | 1 (3.23%) | ||
| Poor | 0 (0.00%) | 0 (0.00%) |
Figure 1.
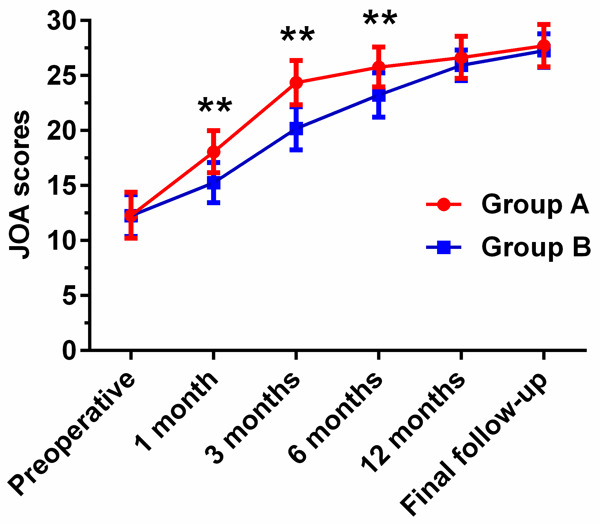
Comparison of preoperative and postoperative JOA scores between group A (PTED) and group B (MED) at different time points. *P<0.05, **P<0.01: JOA scores of patients in group A compared with group B.
Figure 2.
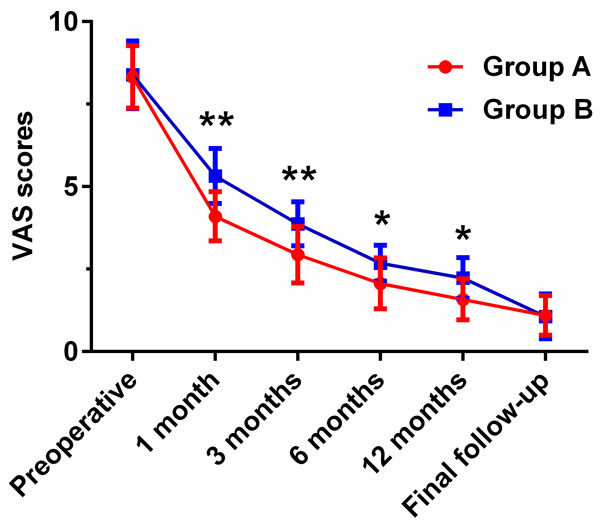
Comparison of preoperative and postoperative VAS scores between group A (PTED) and group B (MED) at different time points. *P<0.05, **P<0.01: JOA scores of patients in group A compared with group B.
Figure 3.
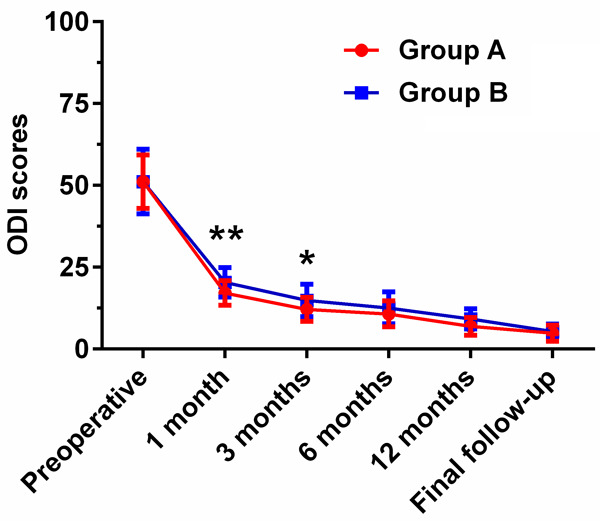
Comparison of preoperative and postoperative ODI scores between group A (PTED) and group B (MED) at different time points. *P<0.05, **P<0.01: JOA scores of patients in group A compared with group B.
Based on modified MacNab criterion, the clinical outcome in group A was excellent in 21 cases, good in 9 cases, and fair in one case, with an excellence or good rate of 96.77%. The clinical outcome in group B was excellent in 19 cases, good in 11 cases, and fair in 1 case.
Discussion
It is well known that the upper lumbar spine is made up of a larger dural sac but a narrower spinal canal compared with lower lumbar spine, and it also has other structures such as cauda equinus and lumbar nerve roots, and these anatomical features cause the disordered and compressed upper lumbar disc [20,21]. Besides, the nerve roots without any innervated specific muscles lead to neurological findings and nonspecific clinical symptoms, which can cause the misdiagnosis of ULDH [22,23]. Due to an anatomical complexity, low incidence, and high misdiagnosis rate, the surgical outcome of ULDH is less satisfactory compared with LLDH. With the development and progress of MISS system, a variety of anterior and posterior approaches and increasing surgical techniques can be selected for ULDH to achieve more satisfactory clinical outcomes [9,24-26]. MED is used for the treatment of lumbar spinal stenosis and lumbar disc herniation with the fewer learning cycles for the surgeons. There is smaller incision length with the MED technique compared with open decompression, but the incidence of ligament and lamina damage is similar to that of open decompression [27]. Additionally, excessive bone removal may lead to segment spinal instability and iatrogenic spinal osteolysis [28]. In order to avoid the abovementioned problems in the treatment of ULDH, minimally invasive PTED has become a promising alternative technique.
In the present study, we analyzed the related information of ULDH patients underwent PTED or MED during the surgery. The duration of surgery, intraoperative blood loss, volume of drainage, and length of hospital stay in group A were less than those in group B. Group B not only had a longer duration of surgery and larger intraoperative blood loss, but also had longer incision length and more severe paraspinal muscle damage than group A. Furthermore, MED also brings damage to soft tissue, vertebral plate, ligamentum flavum, nerve root, and spinal stability, which are harmful to the rehabilitation [29]. Therefore, it is considered that PTED has reduced duration of surgery, less bleeding amount, less wound drainage, and shorter hospital stay, which is more conducive to the recovery and prognosis of ULDH patients compared with MED. Besides, Ren et al. reported that lumbar disc herniation patients who received PTED had a significantly shorter length of incision, shorter operation time, and shorter length of hospital stay compared with MED [30]. Similarly, Wang et al. drew a conclusion that compared with MED, PTED had less trauma, less blood loss, and faster recovery after surgery in treatment of lumbar disc herniation [31]. Liu et al. also demonstrated that PETD for lumbar disc herniation provided most satisfactory operation data about incision length, duration of the operation, blood loss, length of hospital stay compared with MED and microdiscectomy [27]. These results are consistent with this study, which probably on account of smaller invasive operation and less damage of the ligamentum flavum and lamina in PTED. PTED is an excellent combination of currently endoscopic surgical technique and conventional open discectomy with unique technological superiorities, and can remove the nucleus pulposus from the lesion intervertebral space without injuring the nerve and paraspinal muscles and disordering the spinal stability [32,33]. The advantages of PETD for ULDH maybe attribute to the following factors: First, PETD can shorten surgical duration, reduce blood loss and drainage volume, reduce postoperative spinal instability and wound complications, because the smaller incision skin leads to less iatrogenic tissue damage, reduced paravertebral muscle trauma, and the preservation of bone and posterior ligament. Second, the local anesthesia of PETD is usually performed under conscious sedation, which is conducive to reducing anesthesia-related complications and accelerating rehabilitation. Third, PETD for the treatment of ULDH preserves the segmental motion without dural retraction. Therefore, PETD for ULDH could reduce unnecessary implants. Besides, there was no significant difference in complications and recurrences between group A and group B. Reportedly, PTED improved patients’ quality of life by reducing and even avoiding adverse complications in traditional discectomy [34].
The comparisons of preoperatively and postoperatively various scores were evaluated to compare the clinical outcomes between group A and group B. Frist of all, compared with the MED, the JOA scores of the patients with PTED for ULDH were significantly enhanced at 1, 3, and 6 months. Next, compared with group B, the VAS scores in group A were significantly improved at 1, 3, 6, and 12 months, respectively. Then, the ODI scores in group A exhibited significant improvements at 1 and 3 months. Finally, in accordance with the modified MacNab criteria, the clinical outcome was equally excellent/good in 96.77% of patients in both of the groups. The JOA scores were up-regulated, and the VAS scores and the ODI scores were down-regulated in ULDH patients of PTED group, indicating that the pain of patients was relieved and the clinical outcomes were improved. In consequence, we considered that ULDH patient with PTED could gain less pain but better clinical outcomes and faster rehabilitation with a shorter inpatient stay. However, it seems to be controversial about clinical efficacy of PTED after surgery, For instance, Chen et al. manifested that PTED neither show superior clinical outcomes nor be a safer procedure for patients with lumbar disc herniation compared with MED [35,36]. On the contrary, Wang et al. reported that PTED is conducive to faster recovery with better VAS score after surgery compared with MED [31]. Similar to this study, Liu et al. also confirmed that PETD can result in better clinical outcomes and rapid recovery after at least 2 years of follow-up [27]. Therefore, according to abovementioned research, PTED for ULDH can be conducive to the postoperative recovery and clinical efficacy, probably due to less anatomically structural damage on upper lumbar.
Nevertheless, there are still certain limitations in this study. One was the relatively small sample sizes in PTED group and MED group, which may have weakened the conclusion. The other was relatively short duration of follow-up, which result in the long-term outcomes of the two different surgical technique were indistinct. Comprehensive investigation of long-term clinical outcomes among ULDH patients treated with PTED or MED are required due to relatively short follow-up duration and small number of cases. Moreover, a randomized controlled trial or prospective study with more participants and longer duration of follow-up is extremely necessary for further research, because of the limitation of retrospective study.
Conclusion
In summary, this comparative study compares PTED with MED in the treatment of ULDH for the first time. We conclude that PETD for ULDH can improve postoperative healing, reduce the risk of iatrogenic injury, alleviate pain, accelerate ambulation recovery and achieve satisfactory surgical outcome compared with MED. It is beneficial to improve the quality of life of patients and is worthy of promotion in clinical application.
Disclosure of conflict of interest
None.
References
- 1.Sanderson S, Houten J, Errico T, Forshaw D, Bauman J, Cooper P. The unique characteristics of “upper” lumbar disc herniations. Neurosurgery. 2004;55:385–389. doi: 10.1227/01.neu.0000129548.14898.9b. discussion 389. [DOI] [PubMed] [Google Scholar]
- 2.Albert T, Balderston R, Heller J, Herkowitz H, Garfin S, Tomany K, An H, Simeone F. Upper lumbar disc herniations. J Spinal Disord. 1993;6:351–359. doi: 10.1097/00002517-199306040-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bosacco S, Berman A, Raisis L, Zamarin R. High lumbar disk herniations. Case reports. Orthopedics. 1989;12:275–278. doi: 10.3928/0147-7447-19890201-11. [DOI] [PubMed] [Google Scholar]
- 4.Bartolomei L, Carbonin C, Cagnin G, Toso V. Unilateral swelling of the lower abdominal wall. Unusual clinical manifestation of an upper lumbar disc herniation. Acta Neurochir (Wien) 1992;117:78–79. doi: 10.1007/BF01400642. [DOI] [PubMed] [Google Scholar]
- 5.Yüce I, Kahyaoğlu O, Mertan P, Çavuşoğlu H, Aydın Y. Analysis of clinical characteristics and surgical results of upper lumbar disc herniations. Neurochirurgie. 2019;65:158–163. doi: 10.1016/j.neuchi.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Gutterman P, Shenkin H. Syndromes associated with protrusion of upper lumbar intervertebral discs. Results of surgery. J Neurosurg. 1973;38:499–503. doi: 10.3171/jns.1973.38.4.0499. [DOI] [PubMed] [Google Scholar]
- 7.Hsu K, Zucherman J, Shea W, Kaiser J, White A, Schofferman J, Amelon C. High lumbar disc degeneration. Incidence and etiology. Spine (Phila Pa 1976) 1990;15:679–682. doi: 10.1097/00007632-199007000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Lee D, Park K, Park M. The comparative analysis of clinical characteristics and surgical results between the upper and lower lumbar disc herniations. J Korean Neurosurg Soc. 2013;54:379–383. doi: 10.3340/jkns.2013.54.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn Y, Lee S, Lee J, Kim J, Liu W. Transforaminal percutaneous endoscopic lumbar discectomy for upper lumbar disc herniation: clinical outcome, prognostic factors, and technical consideration. Acta Neurochir (Wien) 2009;151:199–206. doi: 10.1007/s00701-009-0204-x. [DOI] [PubMed] [Google Scholar]
- 10.Shin M, Bae J, Cho H, Jang I. Extradiscal epiduroscopic percutaneous endoscopic discectomy for upper lumbar disc herniation a technical note. Clin Spine Surg. 2019;32:98–103. doi: 10.1097/BSD.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 11.Oyelese A, Fridley J, Choi D, Telfeian A, Gokaslan Z. Minimally invasive direct lateral, retroperitoneal transforaminal approach for large L1-2 disc herniations with intraoperative CT navigational assistance: technical note and report of 3 cases. J Neurosurg Spine. 2018;29:46–53. doi: 10.3171/2017.11.SPINE17509. [DOI] [PubMed] [Google Scholar]
- 12.Hubbe U, Franco-Jimenez P, Klingler J, Vasilikos I, Scholz C, Kogias E. Minimally invasive tubular microdiscectomy for recurrent lumbar disc herniation. J Neurosurg Spine. 2016;24:48–53. doi: 10.3171/2015.4.SPINE14883. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Cruet M, Foley K, Isaacs R, Rice-Wyllie L, Wellington R, Smith M, Fessler R. Microendoscopic lumbar discectomy: technical note. Neurosurgery. 2002;51(Suppl):S129–136. [PubMed] [Google Scholar]
- 14.Tsutsumimoto T, Yui M, Uehara M, Ohta H, Kosaku H, Misawa H. A prospective study of the incidence and outcomes of incidental dural tears in microendoscopic lumbar decompressive surgery. Bone Joint J. 2014;96-B:641–645. doi: 10.1302/0301-620X.96B5.32957. [DOI] [PubMed] [Google Scholar]
- 15.Bailey C, Rasoulinejad P, Taylor D, Sequeira K, Miller T, Watson J, Rosedale R, Bailey S, Gurr K, Siddiqi F, Glennie A, Urquhart J. Surgery versus conservative care for persistent sciatica lasting 4 to 12 months. N Engl J Med. 2020;382:1093–1102. doi: 10.1056/NEJMoa1912658. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg H, Firtch W, Tyburski M, Pressman A, Ackerson L, Hamilton L, Smith W, Carver R, Maratukulam A, Won L, Carragee E, Avins A. Oral steroids for acute radiculopathy due to a herniated lumbar disk: a randomized clinical trial. JAMA. 2015;313:1915–1923. doi: 10.1001/jama.2015.4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Zhang W, Lian L, Xu J, Ding W. Transforaminal endoscopic discectomy for treatment of central disc herniation: surgical techniques and clinical outcome. Pain physician. 2018;21:E113–E123. [PubMed] [Google Scholar]
- 18.Kang T, Park S, Park G, Lee S, Park J, Suh S. Biportal endoscopic discectomy for high-grade migrated lumbar disc herniation. J Neurosurg Spine. 2020:1–6. doi: 10.3171/2020.2.SPINE191452. [DOI] [PubMed] [Google Scholar]
- 19.Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am. 1971;53:891–903. [PubMed] [Google Scholar]
- 20.Wiltse L, Berger P, McCulloch J. A system for reporting the size and location of lesions in the spine. Spine (Phila Pa 1976) 1997;22:1534–1537. doi: 10.1097/00007632-199707010-00023. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Zhou Y, Zhang Z, Li C, Zheng W, Huang B. Disc herniation in the thoracolumbar junction treated by minimally invasive transforaminal interbody fusion surgery. J Clin Neurosci. 2014;21:431–435. doi: 10.1016/j.jocn.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Kido T, Okuyama K, Chiba M, Sasaki H, Seki N, Kamo K, Miyakoshi N, Shimada Y. Clinical diagnosis of upper lumbar disc herniation: pain and/or numbness distribution are more useful for appropriate level diagnosis. J Orthop Sci. 2016;21:419–424. doi: 10.1016/j.jos.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Bae J, Lee S, Shin S, Seo J, Kim K, Jang J. Radiological analysis of upper lumbar disc herniation and spinopelvic sagittal alignment. Eur Spine J. 2016;25:1382–1388. doi: 10.1007/s00586-016-4382-y. [DOI] [PubMed] [Google Scholar]
- 24.Lin T, Wang Y, Chang C, Wong C, Cheng Y, Fu T. Surgical outcomes for upper lumbar disc herniation: decompression alone versus fusion surgery. J Clin Med. 2019;8:1435. doi: 10.3390/jcm8091435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son S, Lee S, Kim W, Ahn Y. Advantages of a microsurgical translaminar approach (Keyhole Laminotomy) for upper lumbar disc herniation. World Neurosurg. 2018;119:e16–e22. doi: 10.1016/j.wneu.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Jha R, Syed H, Catalino M, Sandhu F. Contralateral approach for minimally invasive treatment of upper lumbar intervertebral disc herniation: technical note and case series. World Neurosurg. 2017;100:583–589. doi: 10.1016/j.wneu.2017.01.059. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Yuan S, Tian Y, Wang L, Gong L, Zheng Y, Li J. Comparison of percutaneous endoscopic transforaminal discectomy, microendoscopic discectomy, and microdiscectomy for symptomatic lumbar disc herniation: minimum 2-year follow-up results. J Neurosurg Spine. 2018;28:317–325. doi: 10.3171/2017.6.SPINE172. [DOI] [PubMed] [Google Scholar]
- 28.Vazan M, Gempt J, Meyer B, Buchmann N, Ryang Y. Minimally invasive transforaminal lumbar interbody fusion versus open transforaminal lumbar interbody fusion: a technical description and review of the literature. Acta Neurochir (Wien) 2017;159:1137–1146. doi: 10.1007/s00701-017-3078-3. [DOI] [PubMed] [Google Scholar]
- 29.Shin M, Kim J, Ryu K, Hur J. Bilateral decompression via Microscopic Tubular Crossing Laminotomy (MTCL) for lumbar spinal stenosis: technique and early surgical result. Neurol Med Chir (Tokyo) 2015;55:570–577. doi: 10.2176/nmc.oa.2014-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren C, Qin R, Li Y, Wang P. Microendoscopic discectomy combined with annular suture versus percutaneous transforaminal endoscopic discectomy for lumbar disc herniation: a prospective observational study. Pain Physician. 2020;23:E713–E721. [PubMed] [Google Scholar]
- 31.Wang F, Guo D, Sun T, Guan K. A comparative study on short-term therapeutic effects of percutaneous transforaminal endoscopic discectomy and microendoscopic discectomy on lumbar disc herniation. Pak J Med Sci. 2019;35:426–431. doi: 10.12669/pjms.35.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee D, Shim C, Ahn Y, Choi Y, Kim H, Lee S. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for recurrent disc herniation. J Korean Neurosurg Soc. 2009;46:515–521. doi: 10.3340/jkns.2009.46.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn S, Kim S, Kim D, Lee B. Comparison of outcomes of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for young adults: a retrospective matched cohort study. World Neurosurg. 2016;86:250–258. doi: 10.1016/j.wneu.2015.09.047. [DOI] [PubMed] [Google Scholar]
- 34.Sairyo K, Chikawa T, Nagamachi A. State-of-the-art transforaminal percutaneous endoscopic lumbar surgery under local anesthesia: discectomy, foraminoplasty, and ventral facetectomy. J Orthop Sci. 2018;23:229–236. doi: 10.1016/j.jos.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Zhang L, Dong J, Xie P, Liu B, Wang Q, Chen R, Feng F, Yang B, Shu T, Li S, Yang Y, He L, Pang M, Rong L. Percutaneous transforaminal endoscopic discectomy compared with microendoscopic discectomy for lumbar disc herniation: 1-year results of an ongoing randomized controlled trial. J Neurosurg Spine. 2018;28:300–310. doi: 10.3171/2017.7.SPINE161434. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, Zhang L, Dong J, Xie P, Liu B, Wang Q, Chen R, Shu T, Li S, Feng F, Yang B, He L, Yang Y, Liu Z, Pang M, Rong L. Percutaneous transforaminal endoscopic discectomy versus microendoscopic discectomy for lumbar disc herniation: two-year results of a randomized controlled trial. Spine (Phila Pa 1976) 2020;45:493–503. doi: 10.1097/BRS.0000000000003314. [DOI] [PubMed] [Google Scholar]


