Abstract
Objective: To analyze the efficacy of hydroxyethyl starch (HES) combined with Ulinastatin (Uti) in the treatment of newborns with capillary leak syndrome (CLS). Methods: A total of 60 newborns with CLS admitted to four hospitals were selected as the study subjects, and were randomly divided into the control group (n = 30) and the observation group (n = 30) in accordance with the random number table. The control group was treated with HES alone, while the observation group was treated with Uti combined with HES. Results: At 5 d after treatment, the incidence rates of systemic edema and pulmonary edema, the levels of CRP, NE, and BUN, and the duration for the improvement of systemic edema, pulmonary edema and NICU hospital stay in the control group were superior to those in the observation group, while the 24-h urine output, PaO2 and MAP levels, the levels of A, SCr, ALT, and IL-10 in the observation group were superior to those in the control group (P < 0.05). After 3 months of follow-up after treatment, the mortality rate of newborns in the observation group (13.33%) was lower than that in the control group (36.67%) (P < 0.05). Conclusion: HES combined with Uti can effectively alleviate edema, control inflammatory levels, and improve hepatic and renal functions and neonatal survival rate of newborns with CLS.
Keywords: Newborns, capillary leak syndrome, hydroxyethyl starch, ulinastatin, treatment, degree of edema
Introduction
Capillary leak syndrome (CLS) is a clinical syndrome characterized by the remarkable increase in the levels of plasma protein and the infiltration of fluid into tissue space after an increase in vascular permeability. The main symptoms of CLS are hypoproteinemia, hypotension, hypoxemia, acute renal ischemia and systemic edema [1]. CLS is highly severe after its onset, and it progresses rapidly. In severe cases, multiple organ failure may occur, endangering the life of patients with CLS [2].
Since newborns are not well developed and have a weak immunity, they are easily susceptible to hypoxia, shock and septicemia, easily leading to systemic capillary leakage [3]. A study has indicated that CLS has become an important factor leading to the poor prognosis and even deaths of newborns [4]. Continuous renal replacement therapy (CRRT) has been proven to be an effective treatment option for CLS. However, due to special physiological characteristics of newborns, CRRT cannot be tolerated [5]. Clinically, the conventional therapy is often implemented for the treatment of newborns with CLS, including primary disease treatment, anti-shock treatment, anti-infection treatment, and mechanical ventilation. However, conventional therapy can insignificantly improve the degree of edema of newborns with CLS and cannot effectively improve the living conditions of newborns [3,6]. Therefore, clinical studies on the treatment of newborns with CLS have been extensively explored in order to seek safer and more effective options.
Hydroxyethyl starch (HES) is a class of synthetic colloids, and Uti is a urinary trypsin inhibitor. A study suggested that Uti had a satisfactory effect on systemic inflammatory response syndrome, acute pulmonary injury and acute pancreatitis [7]. A study exhibited that HES could quickly restore the blood volume of patients, and play a role in blocking the leakage of albumin in capillaries [8]. There were few studies on HES and Uti in the treatment of newborns with CLS, and there have been no studies on the clinical implementation of HES combined with Uti in the treatment of newborns with CLS. For this reason, this study analyzed the clinical value of HES combined with Uti in 60 newborns.
Materials and methods
Data
A total of 60 newborns with CLS admitted to four hospitals (Women’s and Children’s Hospital, Pingxiang, Jiangxi Maternal and Child Health Hospital, Jiujiang City Maternal and Child Health Care and the First Affiliated Hospital of Nanchang University) from January 2017 to December 2019 were selected as the study subjects, and were randomly divided into the control group (n = 30) and the observation group (n = 30) in accordance with the random number table. Inclusion criteria: Newborns delivered in the Department of Obstetrics and Gynaecology of our hospital who were in line with the diagnostic criteria for CLS [9]; those with typical systemic progressive edema, complicated with hydropericardium, pleural effusion and increased body weight; parents who voluntarily signed the informed consent form. This study was reviewed and approved by the ethics committee of four hospitals (Women’s and Children’s Hospital, Pingxiang, Jiangxi Maternal and Child Health Hospital, Jiujiang City Maternal and Child Health Care and the First Affiliated Hospital of Nanchang University). Exclusion criteria: Newborns complicated with simple renal insufficiency; those with edema caused by other definite causes or unknown causes; coagulation dysfunction; those complicated with acute kidney injury; those complicated with other severe diseases.
Methods
All newborns received the active treatment for the primary diseases, and symptomatic treatment (e.g., mechanical ventilation, anti-shock and anti-infection treatment). Albumin [specification: 5 g (20%, 25 ml)/vial, SFDA approval number: S10960052, manufacturer: Shenzhen Weiguang Biological Products Co., Ltd.] was administrated (1 g/kg each time), followed by administrating 1 g/kg albumin again after 2 h. Whether to continue the administration of albumin was then determined based on the assessments on neonatal’s conditions. Immediately after administration of albumin, a dose of 0.5-1.0 mg/kg furosemide (specification: 2 ml:20 mg, SFDA approval number: H37021056, manufacturer: Shandong Fangming Pharmaceutical Group Co., Ltd.) was administrated (0.5-1.0 mg/kg each time), and then an appropriate amount of furosemide was administrated based on the intake and output volume of newborns. The intake and output volume of newborns was strictly controlled. If the 24-h urine output of newborns exceeded the 24-h physiological demand, the fluid replacement amount on the next day was determined as the physiological demand. If the 24-h urine output of newborns was lower than the physiological demand, the fluid replacement amount on the next day was determined as the 24-hour urine output in the previous day.
The control group was treated with HES [trade name: Zemu, specification: vial for polypropylene injection, 500 ml:30 g: 4.5 g/vial, SFDA approval number: 20066740, manufacturer: Huaren Pharmaceutical (Rizhao) Co., Ltd.], once every morning and evening, a dose of 50 ml/kg each time for 5 d. The observation group was treated with HES combined with Uti (trade name: ulinastatin, specification: 50,000 units, SFDA approval number: H19990133, manufacturer: Guangdong Tianpu Biochemical Pharmaceutical Co., Ltd.). The usage and dosage of HES in the observation group were the same as those in the control group, and Uti was administrated in the observation group once every morning and evening, a dose of 20,000 U/kg each time for 5 d.
Observational indices
Improvement of edema: the incidence rates of systemic and pulmonary edema were compared between the two groups before treatment and at 5 d after treatment.
Improvement of symptoms: the duration for the improvement of general and pulmonary edema and NICU hospital stay were compared between the two groups.
Sign levels: 24-h urine output, partial pressure of oxygen (PaO2) and mean arterial pressure (MAP) were measured before treatment and at 5 d after treatment. 24-h urine output was measured by container method, and PaO2 and MAP were measured by fully automatic biochemical analyzer (Shenzhen Icubio Biomedical Technology Co., Ltd.) in strict accordance with operating procedures.
Hepatic and renal functions: albumin (A), serum creatinine (SCr), alanine aminotransferase (ALT) and blood urea nitrogen (BUN) were measured before treatment and at 5 d after treatment. At the two time points, 5 ml of fasting venous blood was taken as samples in the two groups in the morning, and the samples were detected by fully automatic biochemical analyzer in accordance with the operating procedure.
Inflammatory level: The levels of, C-reaction protein (CRP), neutrophilic granulocyte (NE) and interleukin-10 (IL-10) were measured in the two groups before treatment and at 5 d after treatment. 3 ml of venous blood was taken at the two time points respectively, followed by centrifugation at 3000 rpm for 10 min. The upper serum was used for enzyme-linked immunosorbent assay (ELISA) using the matching kit, and the detection was completed in strict accordance with the kit instructions.
Prognosis: after treatment, the newborns of the two groups were followed up for 3 months, the mortality rate of newborns during the follow-up period was compared between the two groups, and the 3-month survival curve was drawn.
Statistical method
SPSS23.0 was used for statistical analysis. The enumeration data were expressed as [n (%)], and detected using X2 test. The measurement data were expressed as mean ± standard deviation (x̅ ± sd), and detected using t test. The multi-point comparison was performed using ANVOA analysis, and detected using F test. The graphics were made using Graphpad Prism 8. P < 0.05 indicated a statistically significant difference.
Results
General data
There was no significant difference in the ratios of male newborns to female newborns and multiple causes of diseases between the two groups (P > 0.05). There was no statistical difference in the comparison of mean birth time and mean gestational weeks of delivery between the two groups (P > 0.05) (Table 1).
Table 1.
Comparison of general data between the two groups (x̅ ± sd)/[n (%)]
| Data | Observation group (n = 30) | Control group (n = 30) | t/X2 | P | |
|---|---|---|---|---|---|
| Gender | M | 19 (63.33) | 17 (56.67) | 0.278 | 0.598 |
| F | 11 (36.67) | 13 (43.33) | |||
| Birth time (d) | 3.56 ± 2.29 | 3.61 ± 2.41 | 0.082 | 0.935 | |
| Gestational weeks of delivery | 39.42 ± 0.67 | 39.51 ± 0.58 | 0.556 | 0.580 | |
| Causes of disease | Infection | 8 (26.67) | 9 (30.00) | 1.085 | 0.319 |
| Trauma | 3 (10.00) | 5 (16.67) | |||
| Shock | 12 (40.00) | 10 (33.33) | |||
| Hypoxemia | 7 (23.33) | 6 (20.00) | |||
Combination treatment alleviated edema
There was no significant difference in the incidence rates of systemic and pulmonary edema between the two groups before treatment (P > 0.05). At 5 d after treatment, the incidence rates of systemic and pulmonary edema in the two groups were lower than those before treatment, and the incidence rates of systemic and pulmonary edema in the observation group were lower than those in the control group (P < 0.05) (Table 2).
Table 2.
Comparison of degrees of edema between the two groups before and after treatment [n (%)]
| Group | Number of cases | Before treatment | 5 d after treatment | ||
|---|---|---|---|---|---|
|
|
|
||||
| Systemic edema | Pulmonary edema | Systemic edema | Pulmonary edema | ||
| Observation group | 30 | 28 (93.33) | 26 (86.67) | 4 (13.33) | 2 (6.67) |
| Control group | 30 | 26 (86.67) | 27 (90.00) | 11 (36.67) | 8 (26.67) |
| X2 | 0.741 | 0.162 | 4.356 | 4.320 | |
| P | 0.389 | 0.688 | 0.037 | 0.038 | |
Combination treatment controlled symptoms
The duration for the improvement of systemic edema and pulmonary edema was 3.42 ± 1.16 d and 6.82 ± 1.54 d in the observation group, and 5.13 ± 1.84 d and 8.91 ± 2.11 d in the control group, respectively. The NICU hospital stay was 27.46 ± 5.16 d in the observation group and 36.83 ± 6.71 d in the control group, respectively. After treatment, the duration for the improvement of systemic and pulmonary edema and NICU hospitalization time in the observation group were shorter than those in the control group (P < 0.05) (Figure 1).
Figure 1.
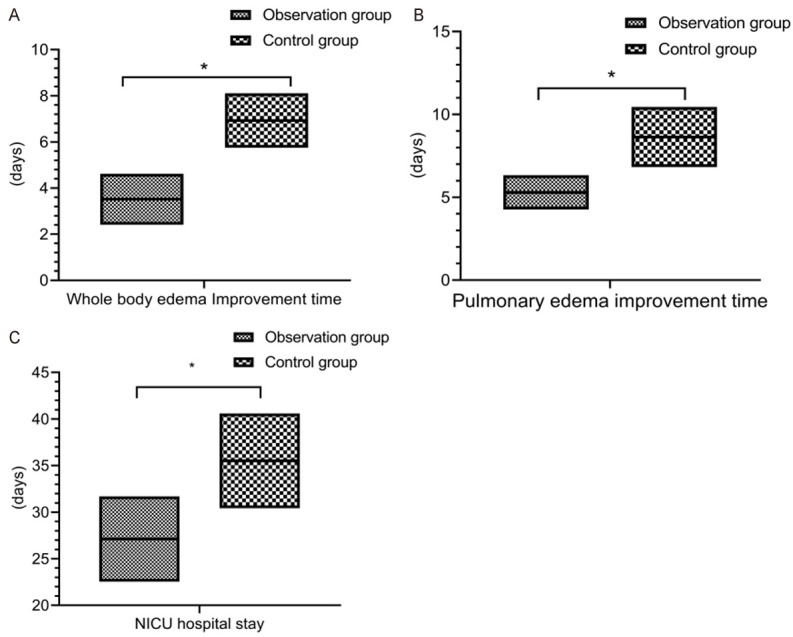
Improvement of symptoms. The duration for the improvement of systemic and pulmonary edema (A and B) and NICU hospital stay (C) in the observation group were shorter than those in the control group (P < 0.05). *indicates P < 0.05 for the comparison between groups.
Combination treatment improved signs
Before treatment, there was no statistical difference in 24-h urine output, and the levels of PaO2 and MAP between the two groups (P > 0.05). At 5 d after treatment, the 24-h urine output and the levels of PaO2 and MAP were increased in the two groups, and there was a significance difference in the comparison of the 24-h urine output and the levels of PaO2 and MAP between the two groups before and after treatment (P < 0.05). The urine output and the levels of PaO2 and MAP in the observation group were higher than those in the control group (P < 0.05) (Figure 2).
Figure 2.
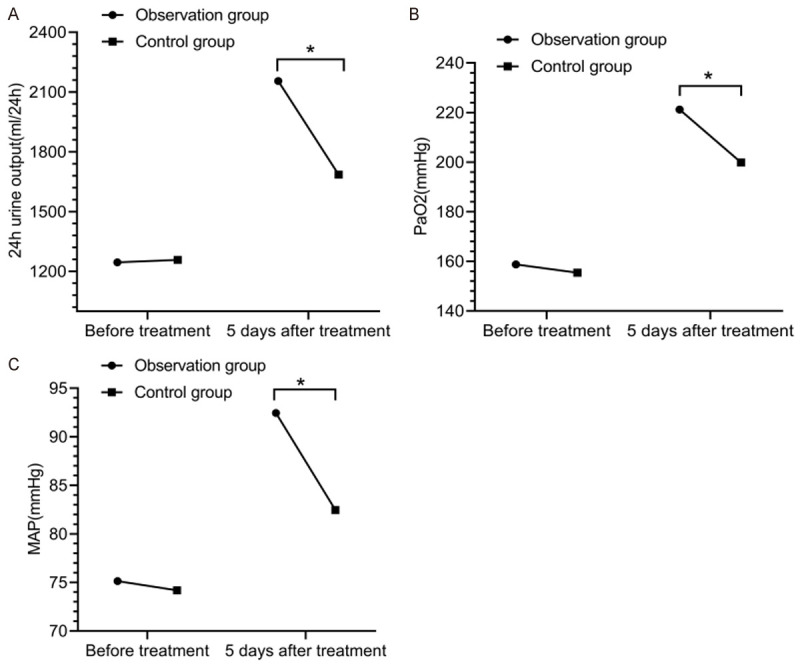
Sign levels. There was no significant difference in the comparison of 24-h urine output (A) and the levels of PaO2 (B) and MAP (C) between the two groups (P > 0.05). At 5 d after treatment, 24-h urine output and the levels of PaO2 and MAP in the observation group were lower than those in the control group (P < 0.05). *indicates P < 0.05 for the comparison between groups.
Combination treatment improved hepatic and renal functions
There was no statistically significant difference in the levels of A, SCr, ALT and BUN between the two groups before treatment (P > 0.05). At 5 d after treatment, the levels of A, SCr and ALT in the observation group were higher than those in the control group, while the levels of BUN in the observation group were lower than those in the control group (P < 0.05) (Figure 3).
Figure 3.
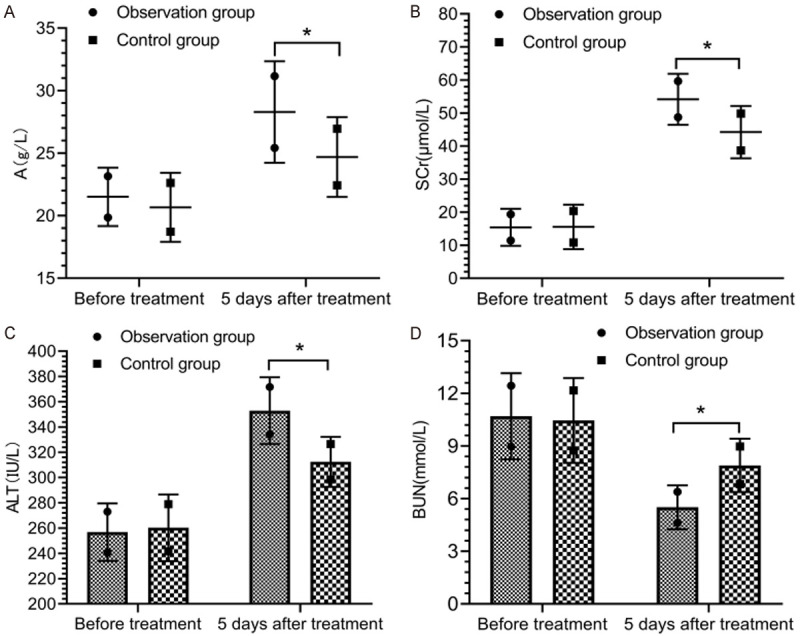
Hepatic and renal functions. Before treatment, there was no remarkable difference in the comparison of the levels of A (A), SCr (B), ALT (C) and BUN (D) between the two groups (P > 0.05). At 5 d after treatment, the levels of A, SCr and ALT in the observation group were higher than those in the control group, while the levels of BUN in the observation group were lower than those in the control group (P < 0.05). *indicates P < 0.05 for the comparison between groups.
Combination treatment controlled inflammatory levels
Before treatment, there was no statistically significant difference in the levels of CRP, NE and IL-10 between the two groups (P > 0.05). At 5 d after treatment, the levels of CRP and NE in the two groups decreased, while the levels of IL-10 were elevated. There was no statistically significant difference in levels of CRP, NE and IL-10 between the two groups before and after treatment (P > 0.05). At 5 d after treatment, the levels of CRP and NE in the observation group were lower than those in the control group, while the levels of IL-10 in the observation group were higher than those in the control group (P < 0.05) (Figure 4).
Figure 4.
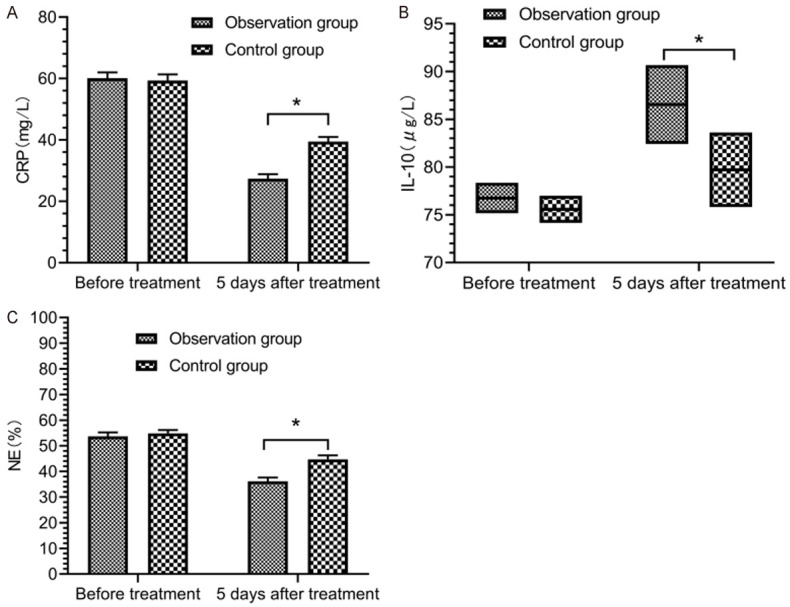
Inflammatory levels. Before treatment, there was no marked difference in the comparison of the levels of CRP (A), IL-10 (B) and NE (C) between the two groups (P > 0.05). At 5 d after treatment, the levels of CRP, IL-10 and NE in the observation group were higher than those in the control group (P < 0.05). *indicates P < 0.05 for the comparison between groups.
Combination treatment improved prognosis
After 3 months of follow-up after treatment, the mortality rate of newborns in the observation group (13.33%) was lower than that in the control group (36.67%), while the survival rate in the observation group (86.67%) was higher than that in the control group (63.33%) (P < 0.05) (Table 3; Figure 5).
Table 3.
Comparison of mortality rates of newborns between the two groups after 3 months of follow-up after treatment [n (%)]
| Group | Number of cases | Survival | Death |
|---|---|---|---|
| Observation group | 30 | 26 (86.67) | 4 (13.33) |
| Control group | 30 | 19 (63.33) | 11 (36.67) |
| X2 | 4.356 | ||
| P | 0.037 | ||
Figure 5.
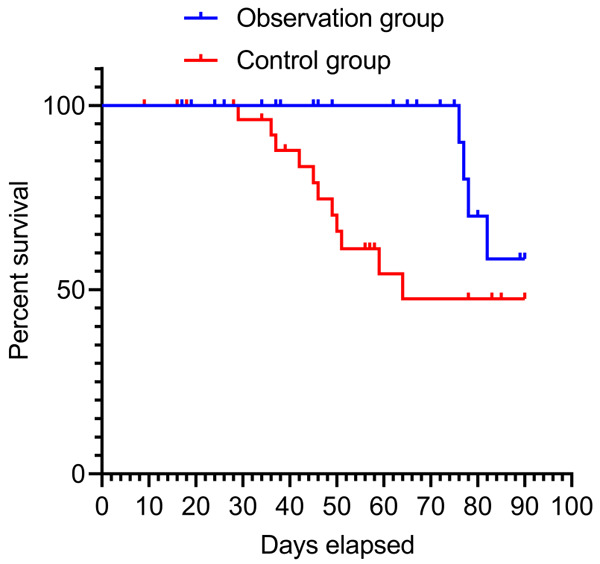
Survival curve. Hazard Ratio (Mantel-Haenszel): Ratio (and its reciprocal) O/C = 0.2870, C/O = 3.484, 95% CI of ratio O/C = 0.1024 to 0.8047, C/O = 1.243 to 9.768. Hazard Ratio (logrank): Ratio (and its reciprocal) O/C = 0.2772, C/O = 3.608, 95% CI of ratio O/C = 009981 to 0.7699, C/O = 1.299 to 10.02. O = Observation group, C = Control group.
Discussion
CLS is a pediatric critical disease that can lead to multiple organ dysfunctions. It has complicated clinical manifestations, poor prognosis and high mortality rate [10]. The clinical studies have suggested that there are multiple causes leading to CLS in newborns. Among them, sepsis is the main cause, and there is a risk of CLS after cardiopulmonary bypass (CPB) [11,12]. A study on the risk factors of CLS in newborns revealed that severe sepsis, shock and low PRISM III scores were the independent risk factors [13]. In this study, the main causes of CLS in 60 newborns were infection, trauma, shock and hypoxemia. The basic principle for the treatment of such newborns lied in actively treating the primary causes. Therefore, conventional treatment (e.g., anti-infection and symptomatic treatment) can hardly improve the prognosis of the newborns.
For newborns with CLS, it is crucial to actively expand and restore blood volume. HES is an amylopectin substituted with hydroxy-ethyl groups, and its relative molecular weight is between 100×103 and 200×103 [14]. Numerous studies have proved that HES has a good application value in the treatment of CLS [15,16]. There are two viewpoints on the mechanism of HES in treating CLS; one is that HES plays a role in biophysics, and the other is that HES plays a role in biochemistry [17,18]. However, a study has exhibited that HES may exert adverse effects on renal function and coagulation function, and harmful free radicals may be generated in the body after medication, which may affect the prognosis of children to a certain extent, especially that of newborns. Therefore, caution should be exercised with the use of HES [19]. Uti is a broad-spectrum protease inhibitor that is involved in the regulation of inflammatory response and inhibits the excessive activation of leukocytes [20]. A study indicated that Uti could protect basement membrane and vascular endothelial cells, inhibit the elevation of vascular permeability, and thus effectively improve the dysfunction of microvascular endothelial cells [21]. In this study, the observation group received the combination treatment of HES and Uti, and the results showed that the incidence rates of systemic and pulmonary edema in the observation group were lower than those in the control group treated with HES alone at 5 d after treatment. The duration for the improvement of systemic and pulmonary edema and NICU hospital stay in the observation group were shorter than those in the control group (P < 0.05), exhibiting that the combination treatment of HES and Uti played a more effective role in controlling the edema of newborns with CLS. At 5 d after treatment, the 24-h urine output and the levels of PaO2, MAP, A, SCr and ALT in the observation group were higher than those in the control group, while the levels of BUN in the observation group were lower than those in the control group (P < 0.05). This exhibited that HES combined with Uti could more effectively improve blood oxygen status and hepatic and renal functions of newborns with CLS. The degree of edema can reflect the degree of renal injury, and abnormal renal function can lead to the increased risk of water retention [22]. In this study, the hepatic and renal functions in the observation group were remarkably improved after treatment. The previous studies revealed that the observation group exhibited better improvement rate and duration for the systemic and pulmonary edema than the control group, exhibiting that the hepatic and renal functions were related to edema. Uti could alleviate the fluid load of the body through improving the hepatic and renal functions of newborns, thereby improving edema.
At 5 d after treatment, the levels of CRP and NE in the observation group were lower than those in the control group, while the levels of IL-10 in the observation group were higher than those in the control group (P < 0.05). A study exhibited that Uti could effectively control the inflammatory level in the treatment of CLS [23]. This is due to the fact that Uti can inhibit the formation of Elastase, α-chymotrypsin, hyaluronidase and trypsin and improve the stability of lysosomal membrane and suppress its release, thus playing a good anti-inflammatory effect [24]. A study showed that Uti could effectively reduce the levels of CRP and NE and increase the level of IL-10 [25]. Uti can block the over-activation of inflammatory responses through regulating the balance between pro-inflammatory and anti-inflammatory responses, thus controlling the inflammatory levels. During the 3-month follow-up period after treatment, the mortality rate of newborns in the observation group (13.33%) was lower than that in the control group (36.67%) (P < 0.05). After treatment, the survival rate in the observation group after treatment was higher than that in the control group, suggesting a better prognosis when HES combined with Uti was used. Similar studies indicated that the survival rate of newborns with CLS receiving Uti treatment was over 75%, which was significantly higher than that in the control group (58%) [26]. A study showed that the mortality rate of newborns with CLS was positively correlated with the degree of endothelial cell injury, and Uti could improve the vascular endothelial function, thereby improving the mortality rate [27]. It is possible that compared with the single medication, the combined medication can give full play to the effects of HES and Uti, and the overall efficacy can be elevated through their synergistic effects.
In summary, HES combined with Uti can effectively alleviate edema, control inflammatory levels, and improve hepatic and renal functions and neonatal survival rate of newborns with CLS, exhibiting a satisfactory application value. However, there are limitations in this study. Fpr example, the age range of enrolled subjects is too narrow, and there are very few studies on the mechanism of action of HES and Uti. Therefore, the future studies with a larger sample size and a wider age range of subjects are needed to elaborate the mechanism of Uti in the treatment of CLS.
Disclosure of conflict of interest
None.
References
- 1.Baloch NU, Bikak M, Rehman A, Rahman O. Recognition and management of idiopathic systemic capillary leak syndrome: an evidence-based review. Expert Rev Cardiovasc Ther. 2018;16:331–340. doi: 10.1080/14779072.2018.1456920. [DOI] [PubMed] [Google Scholar]
- 2.Druey KM, Parikh SM. Idiopathic systemic capillary leak syndrome (Clarkson disease) J Allergy Clin Immunol. 2017;140:663–670. doi: 10.1016/j.jaci.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulihova K, Prochazkova M, Semberova J, Janota J. Fatal primary capillary leak syndrome in a late preterm newborn. Indian J Pediatr. 2016;83:1197–1199. doi: 10.1007/s12098-016-2134-y. [DOI] [PubMed] [Google Scholar]
- 4.Asmundsson ASE, Bjorklund AR, Fisher GA. Diagnosis of systemic capillary leak syndrome in a young child treated with intravenous immunoglobulin in the acute phase. J Pediatr Intensive Care. 2018;7:94–96. doi: 10.1055/s-0037-1607342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw AR, Mueller BA. Antibiotic dosing in continuous renal replacement therapy. Adv Chronic Kidney Dis. 2017;24:219–227. doi: 10.1053/j.ackd.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Pierce R, Ji W, Chan EC, Xie Z, Long LM, Khokha M, Lakhani S, Druey KM. Whole-exome sequencing of adult and pediatric cohorts of the rare vascular disorder systemic capillary leak syndrome. Shock. 2019;52:183–190. doi: 10.1097/SHK.0000000000001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abhyankar SV, Vartak AM. Impact of ulinastatin on outcomes in acute burns patients. J Burn Care Res. 2018;39:109–116. doi: 10.1097/BCR.0000000000000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paleos CM, Sideratou Z, Tsiourvas D. Drug delivery systems based on hydroxyethyl starch. Bioconjug Chem. 2017;28:1611–1624. doi: 10.1021/acs.bioconjchem.7b00186. [DOI] [PubMed] [Google Scholar]
- 9.Siddall E, Khatri M, Radhakrishnan J. Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int. 2017;92:37–46. doi: 10.1016/j.kint.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Leung KKY, Rosa Duque JS, Yu KM, Cheong KN, Chong PC, Ho MH, Chow PC. Myocardial oedema in an 8-year-old Chinese boy with Idiopathic systemic capillary leak syndrome. BMC Pediatr. 2019;19:28. doi: 10.1186/s12887-019-1401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce RW, Merola J, Lavik JP, Kluger MS, Huttner A, Khokha MK, Pober JS. A p190BRhoGAP mutation and prolonged RhoB activation in fatal systemic capillary leak syndrome. J Exp Med. 2017;214:3497–3505. doi: 10.1084/jem.20162143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayakrishnan MP, Geeta MG, Krishnakumar P, Rajesh TV, George B. Snake bite mortality in children: beyond bite to needle time. Arch Dis Child. 2017;102:445–449. doi: 10.1136/archdischild-2016-311142. [DOI] [PubMed] [Google Scholar]
- 13.Bozzini MA, Milani GP, Bianchetti MG, Fossali EF, Lava SAG. Idiopathic systemic capillary leak syndrome (Clarkson syndrome) in childhood: systematic literature review. Eur J Pediatr. 2018;177:1149–1154. doi: 10.1007/s00431-018-3189-8. [DOI] [PubMed] [Google Scholar]
- 14.Priebe HJ. Should hydroxyethyl starch be banned? Lancet. 2018;392:117–118. doi: 10.1016/S0140-6736(18)31172-3. [DOI] [PubMed] [Google Scholar]
- 15.James MF, Richards GA, Gopalan PD, Levin AI, Lundgren AC. Should hydroxyethyl starch be banned? Lancet. 2018;392:119. doi: 10.1016/S0140-6736(18)31176-0. [DOI] [PubMed] [Google Scholar]
- 16.Bilotta F, Giordano G, Caroletti F, Pugliese F. Hydroxyethyl starch: a half-century enigma. Acta Anaesthesiol Scand. 2019;63:128–130. doi: 10.1111/aas.13257. [DOI] [PubMed] [Google Scholar]
- 17.Roberts I, Shakur H, Bellomo R, Bion J, Finfer S, Hunt B, Myburgh J, Perner A, Reinhart K. Hydroxyethyl starch solutions and patient harm. Lancet. 2018;391:736. doi: 10.1016/S0140-6736(18)30255-1. [DOI] [PubMed] [Google Scholar]
- 18.Jung DM, Ahn HJ, Yang M, Kim JA, Kim DK, Lee SM, Park JH. Hydroxyethyl starch is associated with early postoperative delirium in patients undergoing esophagectomy. J Thorac Cardiovasc Surg. 2018;155:1333–1343. doi: 10.1016/j.jtcvs.2017.10.077. [DOI] [PubMed] [Google Scholar]
- 19.Sevcikova S, Vymazal T, Durila M. Effect of balanced crystalloid, gelatin and hydroxyethyl starch on coagulation detected by rotational thromboelastometry in vitro. Clin Lab. 2017;63:1691–1700. doi: 10.7754/Clin.Lab.2017.170505. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Cheng ZY, Chen XJ, Xue HZ. Ulinastatin protects rats with myocardial infarction by activating Nrf2/NOS pathway. Eur Rev Med Pharmacol Sci. 2018;22:8990–8998. doi: 10.26355/eurrev_201812_16670. [DOI] [PubMed] [Google Scholar]
- 21.Long Y, Zhang Y, Cai SS, Sun DM, Li YH. Ulinastatin inhibits high glucose-induced cardiomyocyte apoptosis through activating Akt signaling. Eur Rev Med Pharmacol Sci. 2018;22:4691–4697. doi: 10.26355/eurrev_201807_15530. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Lee J, Johnson AE, Mark RG, Celi LA, Danziger J. Right ventricular function, peripheral edema, and acute kidney injury in critical illness. Kidney Int Rep. 2017;2:1059–1065. doi: 10.1016/j.ekir.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P, Guo P, Lin C, He M, Zhu X, Liu C, Tang J, Wang W, Liang W. The synergistic effect of propofol and ulinastatin suppressed the viability of the human lung adenocarcinoma epithelial A549 cell line. Oncol Lett. 2018;16:5191–5199. doi: 10.3892/ol.2018.9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Q, Yan Q, Chen S. Ulinastatin is effective in reducing mortality for critically ill patients with sepsis: a causal mediation analysis. Sci Rep. 2018;8:14360. doi: 10.1038/s41598-018-32533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang G, Liu Y, Zhou SF, Qiu P, Xu L, Wen P, Wen J, Xiao X. Effect of somatostatin, ulinastatin and gabexate on the treatment of severe acute pancreatitis. Am J Med Sci. 2016;351:506–512. doi: 10.1016/j.amjms.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Peng C, Zhang Z, Shi J, Lin Y, Gu L, Ma X, Li H. Intravenous infusion of ulinastatin attenuates acute kidney injury after cold ischemia/reperfusion. Int Urol Nephrol. 2019;51:1873–1881. doi: 10.1007/s11255-019-02204-3. [DOI] [PubMed] [Google Scholar]
- 27.Han D, Shang W, Wang G, Sun L, Zhang Y, Wen H, Xu L. Ulinastatin- and thymosin α1-based immunomodulatory strategy for sepsis: a meta-analysis. Int Immunopharmacol. 2015;29:377–382. doi: 10.1016/j.intimp.2015.10.026. [DOI] [PubMed] [Google Scholar]


