Abstract
Objective: This study aimed at exploring the diagnostic values and pathological conditions of serum HGF and CA199 in endometriosis (EMT). Methods: From May 2017 to January 2019, 86 patients with EMT, who were admitted to our hospital, were grouped as Group B, whereas 78 healthy women undergoing physical examinations during the same period were grouped as Group A. Serum HGF and CA199 levels in both groups were detected to analyze their diagnostic values for EMT and their relationships with clinical data. Results: Compared with those in Group A, both serum HGF and CA199 expression elevated significantly in Group B (P<0.05). Their expression was correlated with the age, course of disease, number of nodules and infiltration depth of the patients (P<0.05). Besides, their expression was remarkably higher in patients with stages III ± IV than that in patients with stages I ± II (P<0.05). According to the Kaplan-Meier curves for tumor-free survival, the high expression of HGF and CA199 was related to the poor prognosis of the patients (P<0.05). According to the COX regression analysis for risk factors, the number of nodules, infiltration depth, American Fertility Society (AFS) staging, HGF and CA199 were the independent risk factors of EMT recurrence (P<0.05). Conclusion: HGF and CA199 increase in patients with EMT, and can be used as the biomarkers for diagnosing the disease and predicting its prognosis.
Keywords: Endometriosis, HGF, CA199, diagnosis
Introduction
As a common and challenging gynecological disease among women of childbearing age, endometriosis (EMT) is difficult to cure and has a high risk of recurrence after drug discontinuance, and surgery remains the best treatment option [1]. According to statistics, EMT affects 10.8% of premenopausal women in the world, and 30-50% of them develop typical symptoms [2]. The symptoms include dysmenorrhea, chronic pelvic pain, dyspareunia and infertility, which seriously affect the quality of life of patients and their families, so the diagnosis and treatment of EMT are important measures to solve this problem [3]. At present, the diagnosis of EMT is only performed by surgery, and no consistent biomarker has been found [4]. As an invasive diagnosis method, surgery causes certain damage to the patient’s body. Compared with the detection of pathological sections, serological detection has the characteristics of low costs and short time [5]. Therefore, in this study, the serological diagnostic markers for EMT were explored to provide a valuable clinical basis for the early diagnosis of the disease.
With the development of cytokine-related research, many EMT-related biomarkers (such as serum long-chain non-coding RNAs) have been discovered, and their diagnostic values have been verified by numerous studies [6]. HGF is a pleiotropic cell growth factor that promotes mitosis and tissue morphogenesis, induces the migration and invasion of epithelial cells and angiogenesis, and is an important biomarker for the progression of many cancers [7-9]. As reported by Arablou et al., this factor plays a pivotal role in the development of EMT, and its high expression indicates the progression of the disease, but the treatment with resveratrol remarkably reduces its expression in patients with EMT [10]. According to the team of Zeng, serum CA199 is not only highly expressed in patients with pancreatic cancer and other cancers, but also abnormally expressed in non-cancerous diseases [11]. In the study by Khodaverdi et al., CA199 can be used as a diagnostic biomarker of ovarian cancer because of its high expression in this cancer [12]. All of the above studies suggest that both HGF and CA199 may be used as the biomarkers of EMT, but there are few clinical studies on these two indicators in the disease, and their clinical significance remains unclear.
Therefore, in this study, HGF and CA199 expressions in patients with EMT were detected, so as to study the diagnostic and prognostic values of the two indicators, and provide reference for the clinical diagnosis and treatment of the disease.
Materials and methods
Collection of clinical data
Admitted to Dongying Second People’s Hospital of Shandong province from May 2017 to January 2019, 86 patients with EMT were enrolled in Group B, and 78 healthy women undergoing physical examinations were enrolled in Group A. These women were older than 18 years, with normal laboratory parameters and without congenital immune deficiency. Inclusion criteria for the patients: All patients were diagnosed with EMT by laparoscopic surgery and pathological examinations, with the diagnostic criteria referring to the relevant guidelines of the Society of Obstetrics and Gynecology, Chinese Medical Association [13]; patients who were aged 18-45; patients who had not been treated with hormone drugs in recent 6 months; patients who cooperated with the follow-ups; patients who were informed of this study and signed the informed consent form. Exclusion criteria for the patients: Those with the dysfunction of important organs; those complicated with malignant tumors or other gynecological diseases; those with communication disorders; pregnant and lactating women. This study has been approved by the Medical Ethics Committee of Dongying Second People’s Hospital of Shandong province.
Sample collection and detection
Enzyme-linked immunosorbent assay (ELISA) was applied to detect the expression of HGF and CA199 in serum, with the kits provided by MSK Biotechnology Co., Ltd., Wuhan. Fasting venous blood (5 mL) was collected from all research objects, allowed to stand at room temperature for 30 minutes, and then centrifuged (3000×g at 4°C for 10 min) to obtain the supernatant, which was placed in a refrigerator at -80°C for cryopreservation and later use. The standard substances with the known concentration of the substance to be detected and the samples with unknown concentrations were added into the microwells of the enzyme-labeled plate for detection. At first, the substances to be detected and the biotin-labeled antibodies were incubated at the same time. After being cleaned, they were added with avidin-labeled horse radish peroxidase (HRP). After incubation and washing, the unbound enzyme conjugates were removed, and then substrates A and B were added to act with the conjugates at the same time, so as to produce a color. The color was proportional to the concentration of the substance to be detected in the samples. The optical density (OD) values at the wavelength of 450 nm were measured with a microplate reader, and the corresponding concentration was calculated through the standard curve.
HE staining
The EMT tissues were fixed with formaldehyde, dehydrated with ethanol gradient, and after the use of xylene for transparency, the sections were embedded with paraffin. Then, the tissues were dewaxed with xylene, underwent ethanol until water-washing. Besides, they were stained with hematoxylin aqueous solution, dehydrated with ethanol, and dyed with eosin staining solution. At last, they were dehydrated with ethanol, transparent with xylene, and sealed for observation.
Follow-ups
The tumor lesions of all patients were cleared after laparoscopic surgery. Through telephones, internet and outpatient reexaminations, patients were followed up for one year for their tumor-free survival status, with the recurrence of EMT considered to be the follow-up outcome.
Outcome measures
Main outcome measures: Serum HGF and CA199 levels and their diagnostic values (analyzed by receiver characteristics operating curve, ROC curve); the correlation of serum HGF and CA199 expression with clinical data; the correlation of tumor-free survival status with serum HGF and CA199; the independent risk factors of EMT recurrence (analyzed by multivariate Cox regression analysis).
Secondary outcome measures: The histological morphology of EMT cases; the correlation of serum HGF and CA199 with American Fertility Society (AFS) staging.
Statistical analysis
In this study, SPSS23.0 (SPSS Inc., Chicago, IL, USA) was used to statistically analyze the collected data. GraphPad Prism 8 (GraphPad Software, Inc., San Diego CA, USA) was used to plot figures. Count data were expressed as rate (%), compared by chi-square test, and represented by X2. Measurement data were expressed as mean ± standard deviation (mean ± SD), compared between two groups by independent samples t test, and represented by t. P<0.05 indicated a statistically significant difference.
Results
Comparison of general clinical data
According to the general clinical data of both groups, there were no statistical differences between Groups A and B in age, body mass index (BMI), history of smoking, history of alcoholism, place of residence, course of disease, number of nodules, infiltration depth and AFS staging [14] (P>0.05), which indicates comparability (Table 1).
Table 1.
General information sheet
| Factors | Group A (n=78) | Group B (n=86) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (Years) | 28.46±2.42 | 29.16±2.35 | 1.878 | 0.062 |
| BMI (kg/m2) | 21.64±1.76 | 22.03±2.08 | 1.289 | 0.199 |
| History of smoking | ||||
| Yes | 32 (41.03) | 45 (52.33) | 2.097 | 0.148 |
| No | 46 (58.97) | 41 (47.67) | ||
| History of alcoholism | ||||
| Yes | 41 (52.56) | 52 (60.47) | 1.040 | 0.308 |
| No | 37 (47.44) | 34 (39.53) | ||
| Place of residence | ||||
| City | 35 (44.87) | 46 (53.49) | 1.215 | 0.270 |
| Countryside | 43 (55.13) | 40 (46.51) | ||
| Course of disease | 0 (0.00) | 4.72±2.14 | ||
| Number of nodules | ||||
| 1 | 0 (0.00) | 64 (74.42) | ||
| ≥2 | 0 (0.00) | 22 (25.58) | ||
| Infiltration depth | ||||
| <5 mm | 0 (0.00) | 60 (69.77) | ||
| ≥5 mm | 0 (0.00) | 26 (30.23) | ||
| AFS staging | ||||
| Stages I ± II | 0 (0.00) | 55 (63.95) | ||
| Stages III ± IV | 0 (0.00) | 31 (36.05) |
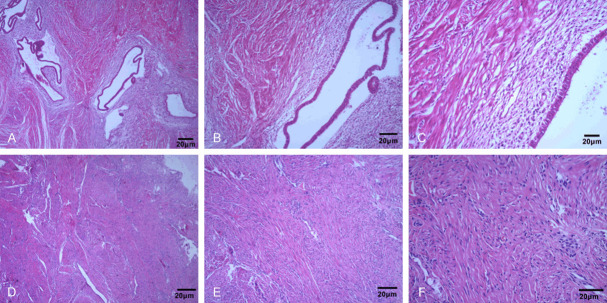
Histological images under a microscope for EMT
According to the histological microscopy, the EMT lesions were round or oval in shape, and showed cystic growth and enlargement. They were filled with yellowish, dark red or white fluid, surrounded by a large number of blood vessels with inflammatory cell infiltration and obvious hyperplasia, and some of the cysts were covered by a little connective tissue. In normal subjects, the endometrial smooth muscle tissue was intact, and the glandular epithelium was a single layer of columnar or cuboidal epithelial cells, with intact glandular lumen and no abnormal secretions, and uniform distribution of interstitial cells in the lamina propria of the mucosa (Figure 1).
Figure 1.
Histological images of endometrial tissue of EMT patients and healthy subjects under microscope. A-C. Histological micrographs of EMT patients at 40/100/200 magnification. D-F. Histological micrographs of healthy subjects at 40/100/200 magnification.
Diagnostic values of serum HGF and CA199 in EMT
According to the ELISA, serum HGF and CA199 expression elevated significantly in Group B compared with that in Group A (P<0.001). Their expression was collected from both groups and the ROC curves were plotted. The areas under the curves (AUCs) of HGF and CA199 were 0.904 and 0.907, respectively, and the 95% CI was 0.847-0.962 and 0.855-0.959, respectively (Figure 2).
Figure 2.
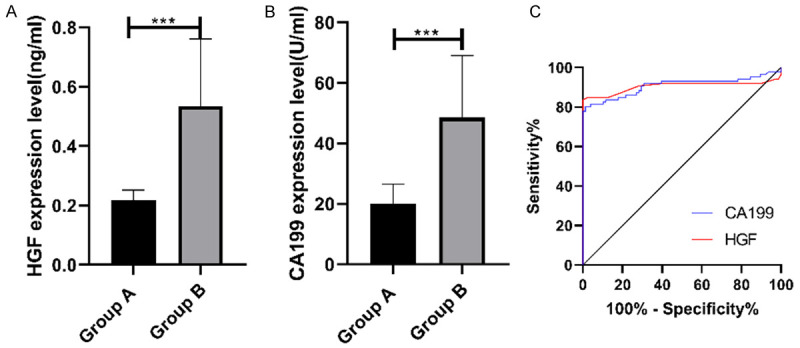
Diagnostic values of serum HGF and CA199 in EMT. A. Serum HGF expression in Group B is remarkably higher than that in Group A. B. Serum CA199 expression in Group B is remarkably higher than that in Group A. C. The red line indicates the AUC of HGF, with the AUC of 0.904 and the 95% CI of 0.847-0.962. The blue line indicates the AUC of CA199, with the AUC of 0.907 and a 95% CI of 0.855-0.959, respectively. *** indicates P<0.001.
Correlation of serum HGF and CA199 expression with clinical data
The expression of HGF and CA199 in serum was tested. According to the median expression of the two indicators, the patients were divided into two high expression groups and two low expression groups, and their clinical data were collected for univariate analysis. There were significant differences in age, course of disease, number of nodules and infiltration depth between the high and low expression groups of HGF and CA199 (P<0.05) (Tables 2 and 3).
Table 2.
Correlation of serum HGF expression with clinical data
| Factors | Low expression group (n=43) | High expression group (n=43) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (Years) | 26.83±1.85 | 32.15±1.92 | 18.03 | <0.001 |
| BMI (kg/m2) | 22.26±2.04 | 22.17±1.97 | 0.287 | 0.774 |
| History of smoking | ||||
| Yes (n=45) | 19 (44.19) | 26 (60.47) | 2.284 | 0.131 |
| No (n=41) | 24 (55.81) | 17 (39.53) | ||
| History of alcoholism | ||||
| Yes (n=52) | 30 (69.77) | 22 (51.16) | 3.113 | 0.078 |
| No (n=34) | 13 (30.23) | 21 (48.84) | ||
| Place of residence | ||||
| City (n=46) | 25 (58.14) | 21 (48.84) | 0.748 | 0.387 |
| Countryside (n=40) | 18 (41.86) | 22 (51.16) | ||
| Course of disease | 3.51±1.68 | 5.62±1.59 | 5.982 | <0.001 |
| Number of nodules | ||||
| 1 (n=64) | 37 (86.05) | 27 (62.79) | 6.108 | 0.014 |
| ≥2 (n=22) | 6 (13.95) | 16 (37.21) | ||
| Infiltration depth | ||||
| <5 mm (n=60) | 36 (83.72) | 24 (55.81) | 7.938 | 0.005 |
| ≥5 mm (n=26) | 7 (16.28) | 19 (44.19) | ||
| AFS staging | ||||
| Stages I ± II (n=55) | 35 (81.40) | 20 (46.51) | 11.350 | 0.001 |
| Stages III ± IV (n=31) | 8 (18.60) | 23 (53.49) |
Table 3.
Correlation of serum CA199 expression with clinical data
| Factors | Low expression group (n=43) | High expression group (n=43) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (Years) | 27.25±1.77 | 31.86±1.87 | 11.74 | <0.001 |
| BMI (kg/m2) | 21.96±2.11 | 22.14±2.06 | 0.400 | 0.690 |
| History of smoking | ||||
| Yes (n=45) | 22 (51.16) | 23 (53.49) | 0.047 | 0.829 |
| No (n=41) | 21 (48.84) | 20 (46.51) | ||
| History of alcoholism | ||||
| Yes (n=52) | 27 (62.79) | 25 (58.14) | 0.195 | 0.659 |
| No (n=34) | 16 (37.21) | 18 (41.86) | ||
| Place of residence | ||||
| City (n=46) | 19 (44.19) | 27 (62.79) | 2.991 | 0.084 |
| Countryside (n=40) | 24 (55.81) | 16 (37.21) | ||
| Course of disease | 3.44±1.73 | 5.56±1.67 | 5.781 | <0.001 |
| Number of nodules | ||||
| 1 (n=64) | 39 (90.70) | 25 (58.14) | 11.97 | <0.001 |
| ≥2 (n=22) | 4 (9.30) | 18 (41.86) | ||
| Infiltration depth | ||||
| <5 mm (n=60) | 37 (86.05) | 23 (53.49) | 10.81 | 0.001 |
| ≥5 mm (n=26) | 6 (13.95) | 20 (46.51) | ||
| AFS staging | ||||
| Stages I ± II (n=55) | 36 (83.72) | 19 (44.19) | 14.58 | <0.001 |
| Stages III ± IV (n=31) | 7 (16.28) | 24 (55.81) |
Correlation of serum HGF and CA199 with AFS staging
Serum HGF and CA199 expression in patients with stages I ± II and III ± IV was detected, and the two were remarkably higher in those with stages III ± IV (P<0.001) (Figure 3).
Figure 3.
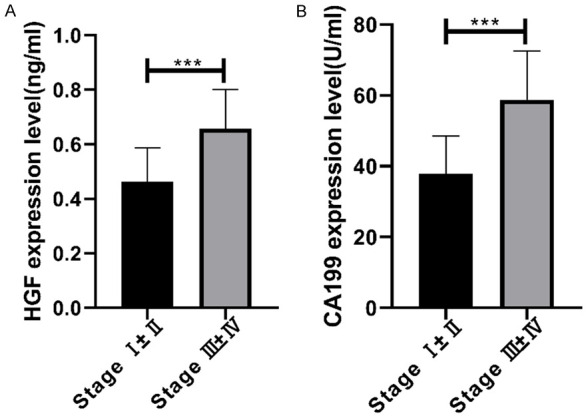
Correlation of serum HGF and CA199 with AFS staging. A. Serum HGF expression in patients with stages III ± IV is remarkably higher than that in those with stages I ± II. B. Serum CA199 expression in patients with stages III ± IV is remarkably higher than that in those with stages I ± II. *** indicates P<0.001.
Correlation of tumor-free survival with serum HGF and CA199
The patients were followed up for one year for the tumor-free survival status, with the recurrence of EMT considered to be the follow-up outcome. Among them, all 86 patients were followed up successfully and no one was lost to follow up; 15 cases developed the recurrence of EMT, with a recurrence rate of 17.44%. According to the median expression of HGF and CA199, the patients were divided into low and high expression groups, and the tumor-free survival rate was compared between the two groups. The rate in the low HGF expression group was remarkably higher than that in the high HGF expression group (P=0.041), and the rate in the low CA199 expression group was remarkably higher than that in the high CA199 expression group (P=0.009) (Figure 4).
Figure 4.
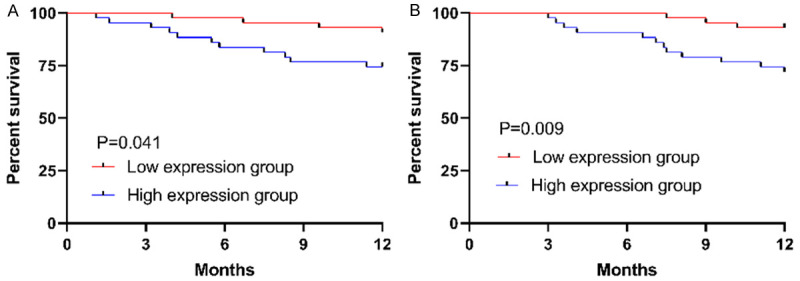
Tumor-free survival status. A. The tumor-free survival rate in the low HGF expression group is remarkably higher than that in the high HGF expression group (P=0.041). B. The tumor-free survival rate in the low CA199 expression group is remarkably higher than that in the high HGF expression group (P=0.009).
Independent risk factors of EMT recurrence
According to the univariate Cox regression analysis, age, course of disease, the number of nodules, infiltration depth, AFS staging, HGF and CA199 were the independent risk factors of EMT recurrence. According to the multivariate Cox regression analysis, the number of nodules, infiltration depth, AFS stage, HGF and CA199 were the independent risk factors (Table 4).
Table 4.
Independent risk factors of EMT recurrence
| Factors | Univariate Cox | Multivariate Cox | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Exp (B) | 95% CI | Sig. | Exp (B) | 95% CI | Sig. | |
| Age | 0.146 | 0.061-0.351 | 0.000 | |||
| BMI | 0.892 | 0.433-1.837 | 0.757 | |||
| History of smoking | 1.279 | 0.668-2.654 | 0.527 | |||
| History of alcoholism | 1.146 | 0.583-3.527 | 0.430 | |||
| Place of residence | 0.781 | 0.379-1.607 | 0.501 | |||
| Course of disease | 0.254 | 0.127-0.509 | 0.000 | |||
| Number of nodules | 3.187 | 1.328-7.524 | 0.005 | 2.682 | 0.889-7.935 | 0.045 |
| Infiltration depth | 20.483 | 8.764-47.623 | 0.000 | 6.891 | 2.418-18.216 | 0.000 |
| AFS staging | 5.721 | 2.539-12.764 | 0.000 | 4.238 | 1.549-12.752 | 0.004 |
| HGF expression (median) | 3.194 | 1.856-5.494 | 0.000 | 2.677 | 1.453-4.933 | 0.002 |
| CA199 expression (median) | 5.304 | 2.745-10.247 | 0.000 | 3.195 | 1.636-6.236 | 0.001 |
Discussion
As a common gynecological disease among adolescent females and women of childbearing age, EMT is characterized by the existence of endometrial tissues outside the uterus, an early menarche age, a short menstrual period, and high height [15]. EMT may also be a major cause of infertility and metastatic cancers, so clinical research on its diagnosis and treatment is of great significance to improve the prognosis and quality of life of patients with EMT [16]. Serological biomarkers can be used as early and non-invasive in vitro diagnostic tools to minimize the serious consequences caused by delayed diagnosis and improve the reproductive health of patients. Therefore, in this study, the diagnostic and prognostic values of HGF and CA199 for EMT were explored through detecting their expression in patients with EMT.
The pathogenesis of EMT, which is a heterogeneous disease, is caused by many factors [17]. In our study, the histological morphology of EMT was observed firstly. According to the microscope, EMT lesions showed cystic growth and enlargement, with angiogenesis in ectopic cysts. Laparoscopy is the gold standard for diagnosing EMT; however, the delay in diagnosis and the high rate of complications usually result from the onset of symptoms to surgical examination [18]. In this study, HGF and CA199 expression in the serum of healthy people and EMT patients was detected, and the expression was remarkably higher in the healthy people. This indicates that the two indicators are abnormally expressed in patients with EMT, and that they might be used for distinguishing healthy people from the patients. The AUCs of HGF and CA199 were 0.904 and 0.907, respectively, suggesting that the two indicators have relatively high values for diagnosing EMT and might be potential biological indicators of the diagnosis. HGF as an angiogenesis factor is highly expressed in patients with EMT, which contributes to the angiogenesis and progression of the disease [19]. In the studies by the teams of Jørgensen [20] and Rokhgireh [21], compared with the control group, HGF and CA199 expression remarkably rises in patients with EMT, indicating that the two indicators may be used as serological diagnostic indicators of the disease, which finding is similar to the result of our research. As for the correlations of HGF and CA199 with clinical data, there were significant differences in age, course of disease, number of nodules and infiltration depth between the high and low expression groups of HGF and CA199. Such results reveal that the high expression of HGF and CA199 is related to the older age, the long course of disease, more than one nodule, the large infiltration depth and the high AFS staging. As for the correlations of the indicators with AFS staging, the expression of serum HGF and CA199 in patients with stages III ± IV was remarkably higher than that in those with stages I ± II. This finding suggests that HGF and CA199 might be used as the observational indicators of EMT severity, so we can predict the severity by detecting their expression and thereby seek the best treatment plan.
Although surgery is the first choice for EMT, incomplete surgical resection is an independent risk factor for the recurrence of the disease; this leads to a relatively high postoperative recurrence rate, and the postoperative recurrence cannot be controlled by drug treatment alone [22]. One study supported that the recurrence rate of EMT is between 5% and 60%, which is related to comprehensive treatment schemes and surgical skills [23]. At present, there is no relevant study on HGF and CA199 in the tumor-free survival of patients with EMT. Therefore, we followed up the patients for one year with recurrence as the follow-up outcome, and found that the recurrence rate of EMT was 17.44%. In this study, the one-year tumor-free survival rates in the high HGF and CA199 expression groups were remarkably lower than those in the low expression groups, indicating that HGF and CA199 expression is also closely related to the postoperative quality of life of patients. According to the Cox regression analysis, the number of nodules, infiltration depth, AFS staging, HGF and CA199 were the independent risk factors of EMT recurrence. Therefore, monitoring the expression of HGF and CA199 can help predict the progression of EMT, and the two can be used as an independent prognostic index of the disease, thus providing reference for treating the disease. In the study by Selcuk et al., the age over 35 years old and the deep infiltration are risk factors for the postoperative recurrence of EMT [24], which is similar to our research result. In a study of metastasis-associated gene 1 (MTA1), hang et al. [25] showed that MTA1 is aberrantly expressed in EMT and correlates with the severity of EMT, and that its overexpression and late AFS staging are risk factors for postoperative recurrence and can be used as a new indicator of EMT [25]. This finding is similar to our research result. CA125 is currently recognized as a single diagnostic biomarker of EMT, while CA199 has significant correlation with it and can also be used as a biological indicator of diagnosing the disease [26]. At present, there are few clinical studies on HGF and CA199 in the postoperative recurrence of EMT.
There are still some shortcomings in this study. First, the expression of serum HGF and CA199 in the patients after treatment was not detected, so it is unclear whether the expression can be used as a predictor of efficacy. Second, the pathogenesis of EMT is complex, and the molecular mechanisms of the two indicators in the disease remain unknown. Therefore, we hope to make up for these shortcomings in future studies to verify our research results.
In summary, HGF and CA199 increase in patients with EMT, and can be used as the biomarkers for diagnosing the disease and predicting its prognosis.
Disclosure of conflict of interest
None.
References
- 1.Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557–571. doi: 10.1097/AOG.0000000000002469. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg VH, Weil C, Chodick G, Shalev V. Epidemiology of endometriosis: a large population-based database study from a healthcare provider with 2 million members. BJOG. 2018;125:55–62. doi: 10.1111/1471-0528.14711. [DOI] [PubMed] [Google Scholar]
- 3.Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4:9. doi: 10.1038/s41572-018-0008-5. [DOI] [PubMed] [Google Scholar]
- 4.Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction. 2016;152:R63–78. doi: 10.1530/REP-16-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montani F, Marzi MJ, Dezi F, Dama E, Carletti RM, Bonizzi G, Bertolotti R, Bellomi M, Rampinelli C, Maisonneuve P, Spaggiari L, Veronesi G, Nicassio F, Di Fiore PP, Bianchi F. miR-test: a blood test for lung cancer early detection. J Natl Cancer Inst. 2015;107:djv063. doi: 10.1093/jnci/djv063. [DOI] [PubMed] [Google Scholar]
- 6.Ahn SH, Singh V, Tayade C. Biomarkers in endometriosis: challenges and opportunities. Fertil Steril. 2017;107:523–532. doi: 10.1016/j.fertnstert.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 8.Sakai K, Aoki S, Matsumoto K. Hepatocyte growth factor and Met in drug discovery. J Biochem. 2015;157:271–284. doi: 10.1093/jb/mvv027. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto K, Umitsu M, De Silva DM, Roy A, Bottaro DP. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017;108:296–307. doi: 10.1111/cas.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arablou T, Delbandi AA, Khodaverdi S, Arefi S, Kolahdouz-Mohammadi R, Heidari S, Mohammadi T, Aryaeian N. Resveratrol reduces the expression of insulin-like growth factor-1 and hepatocyte growth factor in stromal cells of women with endometriosis compared with nonendometriotic women. Phytother Res. 2019;33:1044–1054. doi: 10.1002/ptr.6298. [DOI] [PubMed] [Google Scholar]
- 11.Zeng P, Li H, Chen Y, Pei H, Zhang L. Serum CA199 levels are significantly increased in patients suffering from liver, lung, and other diseases. Prog Mol Biol Transl Sci. 2019;162:253–264. doi: 10.1016/bs.pmbts.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Khodaverdi S, Amini-Moghaddam S, Almassi Nokiani F, Hashemi N, Mohammad Beigi R. Adnexal mass with extremely high levels of CA-125 and CA19-9 but normal Human Epididymis Protein 4 (HE4) and Risk of Ovarian Malignancy Algorithm (ROMA): endometriosis or ovarian malignancy? A case report. Int J Reprod Biomed. 2018;16:413–416. [PMC free article] [PubMed] [Google Scholar]
- 13.Cooperative Group of Endometriosis, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. Guideline for the diagnosis and treatment of endometriosis. Zhonghua Fu Chan Ke Za Zhi. 2015;50:161–169. [PubMed] [Google Scholar]
- 14.Hirata T, Nakazawa A, Fukuda S, Hirota Y, Izumi G, Takamura M, Harada M, Koga K, Wada-Hiraike O, Fujii T, Osuga Y. Four cases of postoperative pneumothorax among 2814 consecutive laparoscopic gynecologic surgeries: a possible correlation between postoperative pneumothorax and endometriosis. J Minim Invasive Gynecol. 2015;22:980–984. doi: 10.1016/j.jmig.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6:34–41. doi: 10.1007/s13669-017-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai Y, Li X, Shi J, Leng J. A review of the risk factors, genetics and treatment of endometriosis in Chinese women: a comparative update. Reprod Health. 2018;15:82. doi: 10.1186/s12978-018-0506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laganà AS, Garzon S, Götte M, Viganò P, Franchi M, Ghezzi F, Martin DC. The pathogenesis of endometriosis: molecular and cell biology insights. Int J Mol Sci. 2019;20:5615. doi: 10.3390/ijms20225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, Singh SS, Taylor HS. Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol. 2019;220:354.e1–354.e12. doi: 10.1016/j.ajog.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Yerlikaya G, Balendran S, Pröstling K, Reischer T, Birner P, Wenzl R, Kuessel L, Streubel B, Husslein H. Comprehensive study of angiogenic factors in women with endometriosis compared to women without endometriosis. Eur J Obstet Gynecol Reprod Biol. 2016;204:88–98. doi: 10.1016/j.ejogrb.2016.07.500. [DOI] [PubMed] [Google Scholar]
- 20.Jørgensen H, Hill AS, Beste MT, Kumar MP, Chiswick E, Fedorcsak P, Isaacson KB, Lauffenburger DA, Griffith LG, Qvigstad E. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil Steril. 2017;107:1191–1199. e1192. doi: 10.1016/j.fertnstert.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Rokhgireh S, Mehdizadeh Kashi A, Chaichian S, Delbandi AA, Allahqoli L, Ahmadi-Pishkuhi M, Khodaverdi S, Alkatout I. The diagnostic accuracy of combined enolase/Cr, CA125, and CA19-9 in the detection of endometriosis. Biomed Res Int. 2020;2020:5208279. doi: 10.1155/2020/5208279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ianieri MM, Mautone D, Ceccaroni M. Recurrence in deep infiltrating endometriosis: a systematic review of the literature. J Minim Invasive Gynecol. 2018;25:786–793. doi: 10.1016/j.jmig.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Alkatout I, Wedel T, Maass N. Combined treatment of endometriosis: radical yet gentle. Aktuelle Urol. 2018;49:60–72. doi: 10.1055/s-0043-122175. [DOI] [PubMed] [Google Scholar]
- 24.Selcuk S, Cam C, Koc N, Kucukbas M, Ozkaya E, Eser A, Karateke A. Evaluation of risk factors for the recurrence of ovarian endometriomas. Eur J Obstet Gynecol Reprod Biol. 2016;203:56–60. doi: 10.1016/j.ejogrb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Wang H, Meng Q, Chen J, Wang J, Huang S. Expression of MTA1 in endometriosis and its relationship to the recurrence. Medicine (Baltimore) 2018;97:e12115. doi: 10.1097/MD.0000000000012115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiala L, Bob P, Raboch J. Oncological markers CA-125, CA19-9 and endometriosis. Medicine (Baltimore) 2018;97:e13759. doi: 10.1097/MD.0000000000013759. [DOI] [PMC free article] [PubMed] [Google Scholar]