Abstract
Objective: To investigate the effects and possible mechanism of prevention and treatment of digestive tract reactions to postoperative adjuvant chemotherapy for pancreatic cancer (PC) using terra flava usta. Methods: A total of 75 PC patients with postoperative adjuvant chemotherapy who were admitted to our hospital from July 2016 to August 2019 were selected and randomly divided into a control group (CG) (n=37) and an observation group (OG) (n=38). Thirty min before chemotherapy, the CG received 8 mg of ondansetron via an intravenous drip, while the OG received 8 mg of ondansetron via an intravenous drip and terra flava usta. The grades of acute nausea and vomiting and delayed nausea and vomiting were compared between the two groups. The levels of gastrin (GAS), motilin (MTL), vasoactive peptide (VIP), hemoglobin (Hb), prealbumin (PA), and albumin (ALB), the proportion of CD4+, CD8+ and NK cells in T cell subsets, and the levels of 5-hydroxytryptamine (5-HT) and substance P (SP) were compared between the two groups. Results: The grades of delayed nausea and vomiting in the OG were superior to those in the CG (P < 0.05). The OG had higher levels of GAS and MTL and lower level of VIP than the CG after chemotherapy (P < 0.05). After chemotherapy, the levels of Hb, PA and ALB in the OG were higher than those in the CG (P < 0.05). After chemotherapy, the proportion of CD4+ and NK cells in the OG were higher than those in the CG, while the proportion of CD8+ in the OG was lower than that in the CG (P < 0.05). The serum 5-HT level in the acute phase and the delayed phase was higher than that before chemotherapy (P < 0.05), and the serum 5-HT level in the delayed phase was lower than that in the acute phase (P < 0.05). There was no significant difference in serum 5-HT level between the two groups in the acute phase and the delayed phase (P > 0.05). Compared with that before chemotherapy, SP levels in the OG decreased in the acute phase and the delayed phase, while SP levels in the CG did not change significantly, and SP levels in the OG were lower than those in the CG (P < 0.05). Conclusion: Terra flava usta can effectively prevent and treat digestive tract reactions (e.g., delayed nausea and vomiting) induced by postoperative adjuvant chemotherapy for PC and protect gastrointestinal function, nutritional status and immune functions of PC patients. The mechanism of prevention and treatment of delayed digestive tract reactions after chemotherapy with terra flava usta may be related to the regulation of SP content. Therefore, terra flava usta is worthy of promotion and implementation.
Keywords: Terra Flava Usta, pancreatic cancer, adjuvant chemotherapy, digestive tract reactions, nutritional status, immune functions, mechanism
Introduction
Pancreatic cancer (PC) is a highly malignant tumor of the digestive system, which is insidious and difficult to detect, and most PC patients are diagnosed in the advanced stages and the prognosis is poor [1,2]. To date, radical resection combined with chemotherapy based on gemcitabine is commonly adopted to treat PC, but the 5-year survival rate is merely 20% approximately [3]. Digestive tract reactions, manifested as loss of appetite and nausea and vomiting, are common toxic side effects of postoperative chemotherapy for PC, which hinder the successful completion of chemotherapy [4]. Clinically, 5-hydroxytryptamine (5-HT) receptor inhibitors are primarily adopted to prevent nausea and vomiting after chemotherapy, but the efficacy is not satisfactory, diminishing the confidence of patients in chemotherapy treatment [5,6]. In recent years, Chinese medicine adjuvant therapy has been extensively explored for the prevention and treatment of digestive tract reactions after chemotherapy. Doctors of traditional Chinese medicine (TCM) believe that the spleen and stomach are closely related to human digestive function, and chemotherapy drugs lead to dysfunction of the spleen and stomach, failure of stomach qi to descend, and thus gastrointestinal reactions. Terra flava usta can warm up the spleen and stomach, stop nausea and vomiting, and treat nausea and vomiting and anorexia induced by deficient cold of the spleen and stomach. In this study, terra flava usta was adopted to investigate the effects and possible mechanisms of prevention and treatment of digestive tract reactions induced by postoperative adjuvant chemotherapy for PC, and the influences of terra flava usta on gastrointestinal secretion function, body nutrition and immune function of PC patients were observed. Results are reported as follows.
Material and methods
General data
A total of 75 PC patients receiving postoperative adjuvant chemotherapy for PC who were admitted to our hospital from July 2016 to August 2019 were selected as study subjects. Inclusion criteria: (1) PC diagnosed by spiral CT, color ultrasound and clinical manifestations, and confirmed by postoperative pathological examination. (2) Locally advanced PC. (3) The digestive tract reactions were in accord with grade 1-3 of acute radiation injury grading standard of the upper digestive tract. (4) Karnofsy score ≥ 70 points. (5) Clear consciousness. (6) The estimated overall survival (OS) is > 6 months. (7) Voluntary signing of an informed consent form. Exclusion criteria: (1) Those with craniocerebral diseases. (2) Those with intestinal obstructions, intussusception and other digestive system diseases affecting observation indexes. (3) Those who were undergoing enteral or parenteral nutrition. (4) Those who do not tolerate Chinese medicine decoction. (5) Smokers and alcoholics.
According to a random number table, the subjects were divided into a control group (CG) (37 cases) and an observation group (OG) (38 cases). This study was approved by the Medical Ethics Committee. (6) The levels of 5-HT and substance P (SP) in the two groups were compared: 5-HT was detected by enzyme-linked immunosorbent assay (ELISA) and SP was detected by radioimmunoassay (RIA) before chemotherapy, in the acute phase and the delayed phase.
Methods
Chemotherapy method: At 4-6 weeks after surgery, gemcitabine for injection (EliLillyand Company, registration number: H20160225) was given via an intravenous drip at 1.0 g/m2 (body surface area), the duration was 30 min, on d 1, d 8 and d 15. Four weeks was a course of treatment, chemotherapy lasted for 4 courses of treatment.
At 30 min before chemotherapy, the CG received 8 mg of ondansetron (Changzhou Lanling Pharmaceutical Production Co., Ltd., SFDA Approval Number: H19980013) via an intravenous drip once a day for 14 d before chemotherapy.
Based on the regimen of the CG, the OG was additionally treated with terra flava usta: 100 g of terra flava usta (decocted medicine water used in place of water as solvent), 15 g of codonopsis pilosula, 12 g of poria cocos, 10 g of pinellia ternata, 10 g of atractylodes macrocephala koidz, 10 g of hawthorn coke, 10 g of coke divine comedy, 10 g of coke malt, 6 g of ginger, 6 g of liquorice, 3 g of aucklandia lappa decne and 3 g of semen alpiniae katsumadai. One dose a day, decoction (400 ml), taken twice a day, treated continuously for 14 d.
Assessment standard
(1) The incidence of acute nausea and vomiting was observed after chemotherapy (within 24 h). (2) The incidence of delayed nausea and vomiting was observed after chemotherapy (after 24 h). Nausea: Grade 1: Loss of appetite without changes in eating habits. Grade 2: reduced food intake without weight loss, dehydration or malnutrition. Grade 3: Insufficient food intake, nasal feeding, total parenteral nutrition or hospitalization required. Vomiting: Grade 1: the number of vomiting episodes within 24 hours is ≤ 2. Grade 2: vomiting episodes occurring 3-5 times within 24 h. Vomiting episodes ≥ 6 times within 24 h, nasal feeding, parenteral nutrition or hospitalization required. Grade 4: life-threatening, emergency treatment required. Grade 5: Death. (3) Comparison of gastrointestinal hormone indexes between the two groups: 3 ml of venous blood was collected before and after chemotherapy, centrifuged at 3000 r/min for 10 min, and the levels of gastrin (GAS), motilin (MTL) and vasoactive peptide (VIP) were detected using enzyme-linked immunosorbent assay (ELISA). (4) Comparison of nutritional indexes between the two groups: 3 ml of venous blood was collected before and after chemotherapy, the serum was separated by centrifugation at 3000 r/min for 10 min, and the levels of hemoglobin (Hb), prealbumin (PA) and albumin (ALB) were detected using the full-automatic analyzer. (5) Comparison of immune function between the two groups: 3 ml of venous blood was collected before and after chemotherapy, and the proportions of CD4+, CD8+ and NK cells were detected by flow cytometry (FCM).
Statistical analysis
SPSS 25.0 was adopted for statistical analysis. The measurement data were expressed using x̅±sd, and detected using t test. The enumeration data were expressed using %, and detected using the χ2 test. P < 0.05 indicated a statistically significant difference.
Results
Comparison of clinical data between the two groups
There was no significant difference in general data between the two groups (P > 0.05), as such the groups were comparable (Table 1).
Table 1.
Comparison of general data between the two groups (x̅±s, n)
| Parameter | Observation group (n=38) | Control group (n=37) | χ2/t | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 20 | 21 | 0.129 | 0.720 |
| Female | 18 | 16 | ||
| Age (years) | 56.87±5.91 | 57.06±6.24 | 0.135 | 0.893 |
| Tumor diameter (cm) | 4.67±0.95 | 4.71±0.91 | 0.186 | 0.853 |
| Time of operation (min) | 353.72±27.39 | 350.18±30.45 | 0.530 | 0.598 |
| Amount of bleeding (ml) | 506.82±93.54 | 513.65±97.02 | 0.310 | 0.757 |
| Lesion site | ||||
| Head of pancreas | 19 | 20 | ||
| Body of pancreas | 11 | 10 | 0.127 | 0.939 |
| Tail of pancreas | 7 | 7 | ||
| Staging | ||||
| III | 16 | 17 | 0.112 | 0.738 |
| IV | 22 | 20 |
Comparison of the incidence of acute nausea and vomiting between the two groups
There was no statistically significant difference in the grades of acute nausea and vomiting between the two groups (P > 0.05), suggesting that the terra flava usta exerted no negative effects in treating acute digestive tract reactions induced by postoperative adjuvant chemotherapy for PC (Table 2).
Table 2.
Comparison of acute nausea and vomiting between the two groups [n (%)]
| Parameter | Observation group (n=38) | Control group (n=37) | χ2 | P |
|---|---|---|---|---|
| Nausea | ||||
| Grade 1 | 22 (57.89) | 17 (45.95) | ||
| Grade 2 | 12 (31.58) | 15 (40.54) | 1.072 | 0.585 |
| Grade 3 | 4 (10.53) | 5 (13.51) | ||
| Vomiting | ||||
| Grade 1 | 25 (65.79) | 20 (54.05) | ||
| Grade 2 | 11 (28.95) | 13 (35.14) | 1.376 | 0.503 |
| Grade 3 | 2 (5.26) | 4 (10.81) |
Comparison of the incidence of delayed nausea and vomiting between the two groups
The grades of delayed nausea and vomiting in the OG were superior to those in the CG (P < 0.05), exhibiting that terra flava usta exerted marked effects in preventing and treating delayed digestive tract reactions induced by postoperative adjuvant chemotherapy for PC, and significantly mitigated the incidence of delayed nausea and vomiting (Table 3).
Table 3.
Comparison of the incidence of delayed nausea and vomiting between the two groups [n (%)]
| Parameter | Observation group (n=38) | Control group (n=37) | χ2 | P |
|---|---|---|---|---|
| Nausea | ||||
| Grade 1 | 28 (73.68) | 11 (29.73) | ||
| Grade 2 | 10 (26.32) | 22 (59.46) | 15.900 | 0.004 |
| Grade 3 | 0 (0.00) | 4 (10.81) | ||
| Vomiting | ||||
| Grade 1 | 23 (60.53) | 13 (35.14) | ||
| Grade 2 | 15 (39.47) | 21 (56.76) | 6.766 | 0.034 |
| Grade 3 | 0 (0.00) | 3 (8.11) |
Comparison of gastrointestinal hormone indexes between the two groups
After chemotherapy, the levels of GAS and MTL in the two groups decreased significantly, while and level of VIP increased markedly, and the differences were statistically significant (P < 0.001). However, after chemotherapy, the levels of GAS and MTL in the OG were higher than those in the CG, while the level of VIP in the OG was lower than that in the CG (P < 0.001), signaling that terra flava usta effectively improved the gastrointestinal secretion disorders induced by postoperative adjuvant chemotherapy for PC (Figure 1).
Figure 1.
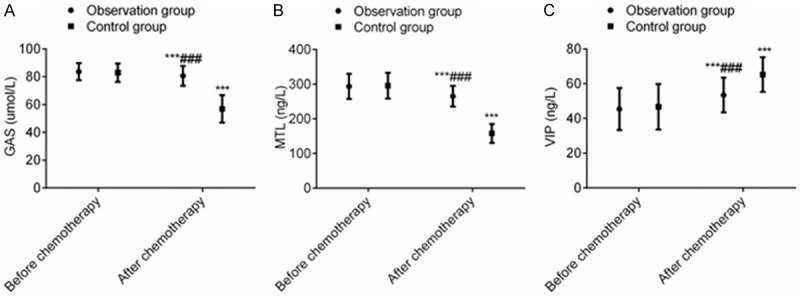
Terra flava usta can effectively maintain the levels of gastrointestinal secretion of PC patients receiving postoperative chemotherapy. Note: Compared with before chemotherapy, ***P < 0.001. Compared with control group, ###P < 0.001.
Comparison of nutritional indexes between the two groups
The levels of Hb, PA and ALB decreased significantly after chemotherapy, and the differences were statistically significantly different (P < 0.001). However, after chemotherapy, the levels of Hb, PA and ALB in the OG were lower than those in the CG (P < 0.001), indicating that terra flava usta effectively improved the nutritional status of PC patients receiving postoperative chemotherapy (Figure 2).
Figure 2.
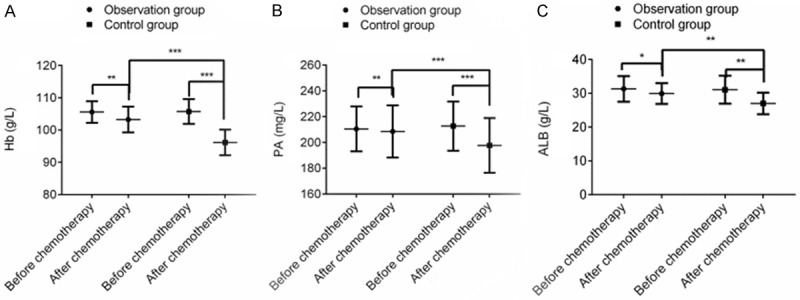
Terra flava usta can effectively improve the nutritional status of PC patients receiving postoperative chemotherapy. Note: *P < 0.05, **P < 0.01, ***P < 0.001.
Comparison of immune functions between the two groups
Before chemotherapy, there was no statistically significant difference in the proportions of CD4+, CD8+ and NK cells between the two groups (P > 0.05). After chemotherapy, the proportions of CD4+ and NK cells in the OG were higher than those in the CG, while the proportion of CD8+ was lower than that in the CG (P < 0.05), exhibiting that terra flava usta remarkably improved the immune functions of PC patients receiving postoperative chemotherapy (Figure 3).
Figure 3.
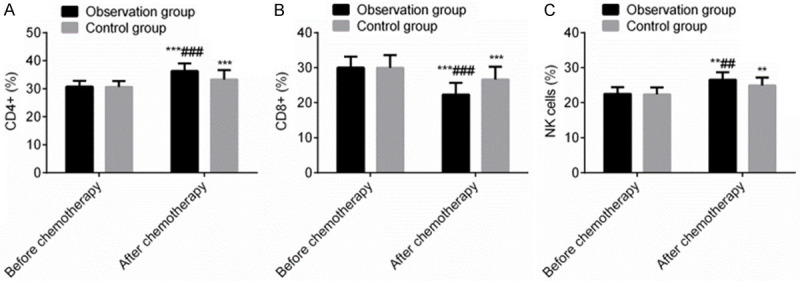
Terra flava usta can markedly improve the immune functions of PC patients receiving postoperative chemotherapy (%). Note: Compared with before chemotherapy, **P < 0.01, ***P < 0.001. Compared with control group, ##P < 0.01, ###P < 0.001.
Comparison of serum 5-HT and SP levels between the two groups
The serum 5-HT level in the acute phase and the delayed phase was higher than that before chemotherapy (P < 0.05), and the serum 5-HT level in the delayed phase was lower than that in the acute phase (P < 0.05). There was no significant difference in the serum 5-HT level in the acute phase and the delayed phase between the two groups (P > 0.05). Compared with those before chemotherapy, SP levels in the OG decreased in the acute phase and delayed phase, while those in the CG showed no significant changes. SP levels in the acute phase and delayed phase of the OG were lower than those of in the CG (P < 0.05), as shown in Table 4.
Table 4.
Comparison of serum 5-HT and SP levels between the two groups (x̅±s, pg/mL)
| Parameter | Observation group (n=38) | Control group (n=37) | t | P |
|---|---|---|---|---|
| 5-HT | ||||
| Before chemotherapy | 31.29±7.51 | 31.52±8.37 | 0.125 | 0.901 |
| Acute phrase | 67.37±10.36* | 72.24±11.23* | 1.953 | 0.055 |
| Delayed phrase | 43.65±8.79*,Δ | 47.35±9.56*,Δ | 1.746 | 0.085 |
| SP | ||||
| Before chemotherapy | 19.02±3.59 | 19.13±3.30 | 0.138 | 0.891 |
| Acute phrase | 15.26±3.47* | 20.95±3.65 | 6.921 | 0.000 |
| Delayed phrase | 10.72±2.73*,Δ | 20.15±2.11 | 16.706 | 0.000 |
indicates compared with before chemotherapy, P < 0.05;
compared with acute phrase, P < 0.05.
Discussion
Chemotherapy-induced nausea and vomiting symptoms are related to the digestive tract and central nervous system [7]. Chemotherapy can damage the gastrointestinal mucosa, stimulate chromaffin cells in the intestinal mucosa to release a large amount of 5-HT, activate 5-HT receptors on neurons, causing changes in intestinal motility and visceral sensitivity and then leading to nausea and vomiting [8,9]. In addition, chemotherapy drugs enter the central nervous system through the cerebrospinal fluid barrier, and act on the relevant sites of nerve cells to release a massive amount of 5-HT. 5-HT binds with the 5-HT3 receptor in the the chemoreceptor trigger zone (CTZ) for emesis, causing excitement of vomiting center and thus resulting in vomiting [10]. Ondansetron, a 5-HT receptor inhibitor, is a common drug for preventing and treating chemotherapy-induced nausea and vomiting [11]. The mechanisms of action of ondansetron are to inhibit 5-HT introduced into the vagus nerve and central chemoreceptors in the upper digestive tract, and reduce the level of 5-HT, thus preventing and treating nausea and vomiting symptoms as a result of excessive release of 5-HT [12,13].
Doctors of Chinese medicine believe that the spleen is in charge of transportation and transformation, while the stomach is in charge of storage. Chemotherapy drugs are highly toxic drugs. Chemotherapy consumes healthy qi of the human body, damages the spleen and stomach, and leads to dysfunction of the spleen in in transportation and transformation, failure of stomach qi to descend, deficiency of cold of the spleen and stomach, resulting in nausea and vomiting. Calcined yellow earth, also called terra flava usta, is clean calcined yellow earth in small lumps formed in the center of the hearth of an old firewood stove. Terra flava usta can warm up the spleen and stomach, stop nausea and vomiting and diarrhea, and it mainly treats vomiting and anorexia caused by deficiency of cold of the spleen and stomach. Codonopsis pilosula can invigorate spleen and lung qi. Modern pharmacology exhibits that codonopsis pilosula can resist cancer, regulate immune functions and gastrointestinal motility, and inhibit gastric acid secretion. Poria cocos is conducive to strengthening spleen and eliminating dampness. Modern pharmacology [14] indicates that poria cocos has many biological activities, such as resisting tumors, enhancing immunity and regulating intestinal flora. Pinellia ternata can help to lower adverse qi and stop vomiting. Atractylodes macrocephala koidz can invigorate spleen and supplement qi. Modern pharmacology [15] suggests that atractylodes macrocephala koidz can resist tumors and regulate gastrointestinal functions. Jiao Sanxian, namely, hawthorn coke, coke divine comedy, coke malt, can promote digestion and remove stagnated food. Ginger can help to dispel stomach cold, reduce adverse qi and stop vomiting. Liquorice has the detoxifying effects, and can relieve pain and moderate the property of herbs. Aucklandia lappa decne can regulate middle energizers and guide out stagnation. Semen alpiniae katsumadai can dispel cold, relieve diarrhea, strengthen stomach and stop vomiting. The combination of the aforementioned herbs can invigorate the spleen and stomach, stop vomiting and diarrhea, and reduce adverse qi. Terra flava usta combined with ondansetron can reduce toxicity and improve efficiency. The results of this study exhibit that there is no statistically significant difference in the grades of acute nausea and vomiting between the two groups, and the grades of delayed nausea and vomiting in the OG are superior to those in the CG. This suggests that terra flava usta can prevent and treat delayed nausea and vomiting induced by postoperative adjuvant chemotherapy for PC, and digestive tract reactions during chemotherapy. This may be due to the reason that ondansetron inhibits gastrointestinal reactions, and terra flava usta invigorates spleen and stomach, stops vomiting and diarrhea, and alleviates adverse reactions, so that improved synergistic efficacies and reduced chemotherapy toxicity are achieved, and thus nausea and vomiting symptoms are effectively prevented and treated after chemotherapy.
Chemotherapy drugs damage the spleen and stomach, resulting in gastrointestinal dysfunction and thus affecting gastrointestinal hormone secretion [16]. GAS and MTL, which are of the gastrin families, can coordinate the contraction of esophageal and gastric sphincter, promote the secretion of gastric acid and pancreatic juice, and regulate the gastrointestinal motility [17,18]. VIP, a kind of neurotransmitter, it can promote the synthesis of nitric oxide by target cells, thereby facilitating smooth muscle relaxation, reducing gastric secretions, inhibit smooth muscle contraction and regulate gastrointestinal motility [19,20]. The results of this study suggest that after chemotherapy, the levels of GAS and MTL in the OG were higher than those in the CG, while the level of VIP was lower than that in the CG. This may be due to the reason that chemotherapy causes gastrointestinal secretion disorders, resulting in a rise in the levels of GAS and MTL and a fall in the level of VIP. However, terra flava usta can effectively reduce chemotherapy toxicity, maintain the levels of gastrointestinal secretions and relieve gastrointestinal secretion disorders. Meanwhile, codonopsis pilosula, poria cocos, and atractylodes macrocephala koidz can regulate gastrointestinal functions and improve gastrointestinal secretions [21].
After chemotherapy, nausea and vomiting symptoms directly affect patients’ nutrition intake, resulting in a fall in the levels of Hb, PA and ALB [22,23]. The decrease in nutrition intake affects the immune function. Meanwhile, chemotherapy drugs directly inhibit the cellular immune functions of PC patients, causing changes in the proportion of CD4+, CD8+ and NK cells [24]. The results of this study indicate that after chemotherapy, the levels of Hb, PA and ALB in the OG were lower than those in the CG, and the proportions of CD4+ and NK cells in the OG were higher than those in the CG, while the proportion of CD8+ in the OG was lower than that in the CG. This result is similar to that in related reports [25]. It signals that terra flava usta can effectively improve the nutritional status and immunosuppression of PC patients receiving postoperative chemotherapy. This may be due to the reason that terra flava usta, which is adopted to prevent and treat nausea and vomiting symptoms, can improve the diet and immune function, nutrition intake, and physical fitness of PC patients. Additionally, codonopsis pilosula can regulate immune function, gastrointestinal motility and gastric acid secretions. Poria cocos can enhance immunity and moderate intestinal flora. Atractylodes macrocephala can regulate gastrointestinal functions, and improve gastrointestinal function and immune function through multiple channels and multi-target pharmacological effects of multiple active ingredients of Chinese herbs, thus promoting nutrition intake and immunity [26]. Various neurotransmitters, such as 5-HT and SP, are believed to be closely related to the occurrence of nausea and vomiting after chemotherapy, among which 5-HT plays an important role in the occurrence of acute digestive tract reactions, while SP plays an important role in the occurrence of delayed digestive tract reactions [27]. The results of this study showed that the serum 5-HT of the OG was slightly lower than that of the CG after chemotherapy, but the difference was not statistically significant (P > 0.05), and the SP level of the OG in the acute phase and the delayed phase was significantly lower than that of the CG (P < 0.05), suggesting that the mechanism of terra flava usta in the prevention and treatment of delayed digestive tract reactions induced by postoperative adjuvant chemotherapy may be related to the regulation of SP content.
In summary, terra flava usta can effectively prevent and treat digestive tract reactions (e.g., delayed nausea and vomiting) induced by postoperative adjuvant chemotherapy for PC, and protect gastrointestinal functions, nutritional status and immune functions of PC patients. The mechanism of prevention and treatment of delayed digestive tract reaction after chemotherapy with terra flava usta may be related to the regulation of SP content. However, there are some shortcomings in this study, such as a single center study and small sample size. Therefore, the future studies with a greater sample size should be conducted so as to further verify the clinical value of terra flava usta in preventing and treating digestive tract reactions induced by malignant tumor chemotherapy.
Disclosure of conflict of interest
None.
References
- 1.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 2.Springfeld C, Jäger D, Büchler MW, Strobel O, Hackert T, Palmer DH, Neoptolemos JP. Chemotherapy for pancreatic cancer. Presse Med. 2019;48:e159–e174. doi: 10.1016/j.lpm.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 3.McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, Honda G, Matsumoto I, Wada K, Furuse J, Matsuyama Y, Unno M. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05) Jpn J Clin Oncol. 2019;49:190–194. doi: 10.1093/jjco/hyy190. [DOI] [PubMed] [Google Scholar]
- 5.Saung MT, Zheng L. Current standards of chemotherapy for pancreatic cancer. Clin Ther. 2017;39:2125–2134. doi: 10.1016/j.clinthera.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinrich S, Lang H. Neoadjuvant therapy of pancreatic cancer: definitions and benefits. Int J Mol Sci. 2017;18:1622. doi: 10.3390/ijms18081622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, Marszalek JR, Maitra A, Yee C, Rezvani K, Shpall E, LeBleu VS, Kalluri R. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3:e99263. doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S, Yu X. Selecting chemotherapy for pancreatic cancer: far away or so close? Semin Oncol. 2019;46:39–47. doi: 10.1053/j.seminoncol.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Gupta R, Amanam I, Chung V. Current and future therapies for advanced pancreatic cancer. J Surg Oncol. 2017;116:25–34. doi: 10.1002/jso.24623. [DOI] [PubMed] [Google Scholar]
- 10.Dreyer SB, Chang DK, Bailey P, Biankin AV. Pancreatic cancer genomes: implications for clinical management and therapeutic development. Clin Cancer Res. 2017;23:1638–1646. doi: 10.1158/1078-0432.CCR-16-2411. [DOI] [PubMed] [Google Scholar]
- 11.Vennin C, Murphy KJ, Morton JP, Cox TR, Pajic M, Timpson P. Reshaping the tumor stroma for treatment of pancreatic cancer. Gastroenterology. 2018;154:820–838. doi: 10.1053/j.gastro.2017.11.280. [DOI] [PubMed] [Google Scholar]
- 12.Klaiber U, Leonhardt CS, Strobel O, Tjaden C, Hackert T, Neoptolemos JP. Neoadjuvant and adjuvant chemotherapy in pancreatic cancer. Langenbecks Arch Surg. 2018;403:917–932. doi: 10.1007/s00423-018-1724-8. [DOI] [PubMed] [Google Scholar]
- 13.Ducreux M, Seufferlein T, Van Laethem JL, Laurent-Puig P, Smolenschi C, Malka D, Boige V, Hollebecque A, Conroy T. Systemic treatment of pancreatic cancer revisited. Semin Oncol. 2019;46:28–38. doi: 10.1053/j.seminoncol.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Wu J, Liu S, Zhang X, Zhang B. Network meta-analysis of Chinese herbal injections combined with the chemotherapy for the treatment of pancreatic cancer. Medicine (Baltimore) 2017;96:e7005. doi: 10.1097/MD.0000000000007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11–26. doi: 10.1038/s41571-018-0112-1. [DOI] [PubMed] [Google Scholar]
- 16.Conroy T, Ducreux M. Adjuvant treatment of pancreatic cancer. Curr Opin Oncol. 2019;31:346–353. doi: 10.1097/CCO.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 17.Abreu FB, Liu X, Tsongalis GJ. miRNA analysis in pancreatic cancer: the Dartmouth experience. Clin Chem Lab Med. 2017;55:755–762. doi: 10.1515/cclm-2017-0046. [DOI] [PubMed] [Google Scholar]
- 18.Hajatdoost L, Sedaghat K, Walker EJ, Thomas J, Kosari S. Chemotherapy in pancreatic cancer: a systematic review. Medicina (Kaunas) 2018;54:48. doi: 10.3390/medicina54030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Geus SWL, Eskander MF, Kasumova GG, Ng SC, Kent TS, Mancias JD, Callery MP, Mahadevan A, Tseng JF. Stereotactic body radiotherapy for unresected pancreatic cancer: a nationwide review. Cancer. 2017;123:4158–4167. doi: 10.1002/cncr.30856. [DOI] [PubMed] [Google Scholar]
- 20.Badiyan SN, Molitoris JK, Chuong MD, Regine WF, Kaiser A. The role of radiation therapy for pancreatic cancer in the adjuvant and neoadjuvant settings. Surg Oncol Clin N Am. 2017;26:431–453. doi: 10.1016/j.soc.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Sohal DPS, Kennedy EB, Khorana A, Copur MS, Crane CH, Garrido-Laguna I, Krishnamurthi S, Moravek C, O’Reilly EM, Philip PA, Ramanathan RK, Ruggiero JT, Shah MA, Urba S, Uronis HE, Lau MW, Laheru D. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 2018;36:2545–2556. doi: 10.1200/JCO.2018.78.9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunner M, Wu Z, Krautz C, Pilarsky C, Grützmann R, Weber GF. Current clinical strategies of pancreatic cancer treatment and open molecular questions. Int J Mol Sci. 2019;20:4543. doi: 10.3390/ijms20184543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altman AM, Wirth K, Marmor S, Lou E, Chang K, Hui JYC, Tuttle TM, Jensen EH, Denbo JW. Completion of adjuvant chemotherapy after upfront surgical resection for pancreatic cancer is uncommon yet associated with improved survival. Ann Surg Oncol. 2019;26:4108–4116. doi: 10.1245/s10434-019-07602-6. [DOI] [PubMed] [Google Scholar]
- 24.Luo XM, Niu LZ, Chen JB, Xu KC. Advances in cryoablation for pancreatic cancer. World J Gastroenterol. 2016;22:790–800. doi: 10.3748/wjg.v22.i2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erkan M, Kurtoglu M, Kleeff J. The role of hypoxia in pancreatic cancer: a potential therapeutic target? Expert Rev Gastroenterol Hepatol. 2016;10:301–316. doi: 10.1586/17474124.2016.1117386. [DOI] [PubMed] [Google Scholar]
- 26.Ma J, Wang J, Ge L, Long B, Zhang J. The impact of diabetes mellitus on clinical outcomes following chemotherapy for the patients with pancreatic cancer: a meta-analysis. Acta Diabetol. 2019;56:1103–1111. doi: 10.1007/s00592-019-01337-2. [DOI] [PubMed] [Google Scholar]
- 27.Lorusso V, Russo A, Giotta F, Codega P. Management of chemotherapy-induced Nausea and Vomiting (CINV): a short review on the role of Netupitant-Palonosetron (NEPA) Core Evid. 2020;15:21–29. doi: 10.2147/CE.S203634. [DOI] [PMC free article] [PubMed] [Google Scholar]


