Abstract
Apigenin (APG), a natural flavonoid with anti-inflammatory and anti-fibrosis properties, has been shown to play a protective role in diabetic nephropathy (DN), but their molecular protection mechanism for miRNA has not been elucidated in detail. This study was designed to focus on exploring its protective role in DN and whether miR-423-5p-upstream stimulating factor 2 (USF2) axis was involved in its protective mechanism. The in vivo model of rat was induced by streptozotocin (STZ) and the in vitro model of renal tubular epithelial cell (RTEC) was induced by high glucose (HG). Our in vivo study revealed that APG had different protective effects on inflammation, renal fibrosis and epithelial mesenchymal transition (EMT) in DN rats, which is mainly reflected in that the inflammatory factors (IL-6, IFN-γ, TNF-α) were obviously down-regulated, the renal fibrosis markers (IV-C, FN, Col I) were significantly inhibited, the E-cadherin (EMT factors) was significantly up-regulated, while the vimentin and α-SMA (EMT factors) were significantly down-regulated, and the renal function indexes (serum Cr, BUN) were significantly improved. In terms of mechanism, the protective effect of APG was related to the regulation of the expression of miR-423-5p-USF2 axis, and there was a targeted relationship between miR-423-5p and USF2. Down-regulating miR-423-5p or up-regulating USF2 could significantly aggravate the disease progression of in vitro model and eliminate DN resistance under APG intervention. The above results revealed that the protective role of APG on DN was mediated by miR-423-5p-USF2 axis.
Keywords: Diabetic nephropathy, apigenin, miR-423-5p, USF2, inflammation/renal fibrosis/epithelial mesenchymal transition
Introduction
DN is the most serious complication of diabetes mellitus (DM), and it is also the main factor leading to end-staged renal disease [1,2]. Statistics show that approximately 40% of patients with chronic kidney disease develop into end-staged renal disease unfortunately every year because of DN [3]. It is known that the pathological mechanism of DN is complex, which involves inflammation, renal fibrosis, EMT and other processes. Inflammation is the key factor for the progression of DM complications, accompanied by excessive secretion of various inflammatory factors [4-6]. Renal fibrosis is mainly manifested as tubular atrophy, which is the final process of DN. The EMT of renal tubules is a specific event in the process of fibrosis, which has important protective effect against kidney injury and potential apoptosis events [7]. In addition, almost all renal cells (including RTEC) respond to varying degrees under the stimulation of hyperglycemia, and RTEC is highly responsive to renal fibrosis, EMT and other pathological processes [7,8]. Therefore, RTEC is the research mediator of molecular mechanism of DN in vitro model. Apigenin (APG) is a kind of plant-derived flavonoid, which has many functions such as anti-inflammation, anti-fibrosis, anti-apoptosis and anti-DM. Moreover, it has a certain protective effect on DN-related kidney injury [9,10]. However, there are still few studies on the molecular mechanism of miRNA and its related effects and mechanisms on inflammation, renal fibrosis and EMT. This study was mainly designed to explore the influence of APG on the above pathological processes in DN and the molecular mechanism of miRNA, aiming to provide new directions and clues for the treatment of DN.
It should be added that more and more studies have revealed that miRNA is involved in the pathogenesis and protection mechanism of DN. It is an RNA molecule composed of 18-24 nucleotides, which plays a regulatory role in the physiological and pathological changes of the body at the post-transcriptional level [11]. In many reports, we have found that miRNA-mRNA axis mediates the protection mechanism against DN. For example, miR-378-TRAF5 axis is involved in the protective process of astragaloside on podocyte apoptosis of DN rat [12], and miR-497-ROCK axis mediates the therapeutic effect of melatonin on DN endothelial-interstitial glomerular endothelial cells [13]. In this study, it was found that miR-423-5p-USF2 axis might play a vital role in the anti-DN effect of APG, and both of them had potential conservative binding sites through biological analysis. miR-423-5p has been reported to have a certain therapeutic effect on podocyte injury induced by HG, which is mainly reflected in that up-regulation of its expression can inhibit oxidative stress, apoptosis and inflammatory reaction by targeting nicotinamide adenine dinucleotide phosphate oxidase 4 [14]. Upstream stimulating factor 2 (USF2) is a crucial regulator of the pathological process of DN, and its downstream target is identified as renin gene. Its transgenic mice will have typical pathological progression characteristics of DN, i.e., up-regulation of renin in kidney, increase of urinary albumin excretion and extracellular matrix deposition in glomerulus, etc. [15].
We have suspected that APG may play an anti-DN role by regulating miR-423-5p-USF2 axis, which is hereby verified.
Materials and methods
DN rat model
This animal test has been ratified by the Animal Care and Use Committee of our hospital. We purchased 30 male Wistar rats (Viton Lihua Experimental Animal Technology Co., Ltd., Beijing, China), weighing 400-450 g and aged 18 weeks. They were raised in the environment of 12 h-12 h light and dark cycle. The temperature was controlled at 24°C and the relative humidity was (60±5)%, and the rats were given water and food. The in-vivo model of rat (DN group, n=10) was induced by streptozotocin (STZ) (Kairuiji Biotechnology Co., Ltd., Beijing, China, 82342) [16], and the rats were intraperitoneally injected with STZ (50 mg/kg) dissolved in citric acid buffer (40 mg/mL) (Zeye Biotechnology Co., Ltd., Shanghai, China, ZY-N191070). Rats injected with citric acid buffer alone were selected as controls (NC group, n=10). In DN+APG group, rats were first induced by STZ and then given APG (20 mg/kg) (Kairuiji Biotechnology Co., Ltd., Beijing, China, 10806) [10] orally, once a day. DM rats were treated with insulin (4 U) (Hengdu Biotechnology Co., Ltd., Shanghai, China, I9278) every day, and the blood sugar level was maintained at 300-400 mg/dl to prevent ketoacidosis. After APG treatment for 7 days, the rats were anesthetized with pentobarbital sodium (60 mg/kg) (New Asiatic Pharmaceutical Co., Ltd., Shanghai, China, H31021725), and their heart blood was drawn and centrifuged at 1500×g and 4°C for 10 min to obtain serum for biochemical study. After that, the rats were killed by cutting their necks, and their kidneys were collected for follow-up study.
Enzyme linked immunosorbent assay (ELISA)
ELISA kits (Lvyuan Bode Biotechnology Co., Ltd., Beijing, China; rat IL-6, rat IFN-γ, rat TNF-α) were used to detect interleukin-6 (IL-6), interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) in serum of DN rats. The operation was conducted in strict accordance with the operation specifications of kit.
Western blot analysis
The protein was extracted from kidney tissue by radioimmune precipitation test (RIPA) lysis buffer (Beinuo Biotechnology Co., Ltd., Shanghai, China, Amresco N653-100ML), and BCA kit (Kairuiji Biotechnology Co., Ltd., Beijing, China, 120982) was used for quantitative detection. At first, the samples (30 μg) were separated through 10% SDS-PAGE (Lianshuobao Biotechnology Co., Ltd., Shanghai, China, N/A-271), and then transferred to polyvinylidene fluoride membrane (Kairuiji Biotechnology Co., Ltd., Beijing, China, ISEQ00011). Then, it was sealed for 1 h in membrane blocking solution (Kairuiji Biotechnology Co., LTD., Beijing, China, 121422) at ambient temperature, and then cultivated with primary antibody at 4°C overnight. Finally, the samples were incubated with secondary antibody at 37°C for 45 min. In this study, the primary antibodies included type IV collagen (IV-C), fibronectin (FN), collagen I (Col I), E-cadherin, Vimentin, α smooth muscle actin (α-SMA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (as control). The secondary antibody was horserador-peroxidase (HRP)-labeled goat anti-rabbit. Finally, the chemiluminescence substrate kit (Yaoyun Biotechnology Co., Ltd., Shanghai, China, 1B1581-KIT-100ML) was used for visualization and quantitative analysis.
Immunohistochemistry
The kidney tissue was fixed with 4% neutral paraformaldehyde (Caiyou Industrial Co., Ltd., Shanghai, China, YS-7615), embedded in paraffin, made into 3 μm slices, washed with phosphate buffered saline (PBS) (Ziqi Biotechnology Co., Ltd., Shanghai, China, 00887), and incubated with 0.01% Triton (Baiaolaibo Technology Co., Ltd., Beijing, China, GL1461-JNL) for 8 min. After that, they were contacted with 3% hydrogen dioxide solution for 10 min, and then the antigen was repaired with citrate buffer solution, and then sealed with 10% goat serum (Kairuiji Biotechnology Co., Ltd., Beijing, China, 40348). Then, it was incubated with IV-C, FN, Col I, E-cadherin, vimentin and α-SMA diluted with 1:100. The sections were washed with PBS, and then cultivated with HRP-labeled goat anti-rabbit IgG (Baiaolaibo Technology Co., Ltd., Beijing, China, WE0394-OWS) for 30 min, washed and stained with diaminobenzidine for 5 min. The optical microscope was used to observe them and obtain images.
Evaluation of renal function
In this study, the renal function indexes were mainly serum creatinine (Cr) and urea nitrogen (BUN), which were tested by automatic biochemical analyzer (Beden Medical Co., Ltd., Nanjing, China, V503629).
Assessment of renal pathological changes
After the renal tissue was made into thin sections, the hematoxylin and eosin (HE), periodic acid-schiff (PAS) staining and Masson staining were performed. Image-Pro Plus quantitative software was used to calculate the ratio of glomerular mesangial matrix area to total glomerular area (M/G) in PAS stained sections.
TUNEL
The cells of kidney tissue were stained by apoptosis in situ detection kit (Kaiji Biotechnology Co., Ltd., Nanjing, China, KGA7072). The paraffin-embedded, dewaxed and washed tissues were treated with proteinase K and incubated with TUNEL reaction mixture at 37°C for 1 h. The Leica TCS-SP laser scanning confocal microscope was used for observation and the number of TUNEL positive cells was recorded.
Purchase and transfection of RTEC and construction of DN in vitro model
RTEC (NRK-52E) (Zishi Biotechnology Co., Ltd., Shanghai, China, os100011) was purchased and cultured in DMEM (Lvyuan Bode Biotechnology Co., Ltd., Beijing, China, PM150220B) containing 10% FBS, which was grown at 37°C, 95% air and 5% CO2. RTEC was transfected with Lipo 2000 reagent (Lianshuobao Biotechnology Co., Ltd., Shanghai, China, 11668-019). The transfection agents included miR-423-5p inhibitor, control (inhibitor-NC), USF2 over-expression vector (USF2) and corresponding empty vector (pcDNA). The operation was carried out in strict accordance with the instructions. After transfection, the in-vitro model of RTEC was induced with high glucose (30 mM) (Huzhen Industrial Co., Ltd., Shanghai, China, HZ1334) [17], while rats in the NC group were intervened by normal glucose (5 mM). After 48 hours, the cells were collected to detect the transfection efficiency.
RT-PCR
In this research, the expressions of miR-423-5p, USF2 and IL-6, IFN-γ and TNF-α in renal tissue and RTEC of DN rats were measured. Firstly, the total RNA was separated by TRIzol reagent (Biolab Technology Co., Ltd., Beijing, China, QN2070-ZOG). Then, the complementary DNA was synthesized by reverse transcription system (Promega Biotechnology Co., Ltd., Beijing, China, A3500). The expression levels were tested with SYBR Premix Ex Taq II (Zhijie Fangyuan Technology Co., LTD., Beijing, China, RR820A), and GAPDH and U6 were used as internal references.
Cell migration assay
The migration level of RTEC was detected by transwell chamber. First, the cells were inoculated into the upper chamber. The RPMI1640 (500 mL) (containing 10% FBS) (Ziqi Biotechnology Co., Ltd., Shanghai, China, SH30203.05) was added to the lower chamber. After incubation for 48 hours, the RTEC in the lower chamber was immobilized with 4% paraformaldehyde for 15 minutes and dyed with 0.1% crystal violet (Mairuibo Biotechnology Co., Ltd., Beijing, China, M DZ0055) for 20 minutes. The microscope was used to observe and count.
Apoptosis assay
The ANNEXINV-FITC/PI apoptosis detection kit (Fusheng Industrial Co., Ltd., Shanghai, China, FS-79505) was used for detection. Firstly, the digested and washed RTEC were prepared into the suspension (1*106 cells/mL), and then 2 μL of AnnexinV-FITC and 5 μL of PI were added in turn, and incubated for 5 min at room temperature in the dark. After that, the apoptosis level was measured by flow cytometry (Bidake Biotechnology Co., Ltd., Changzhou, China, 1026) within 1 hour.
Detection of double luciferase gene
Wild-type and mutant fragments (USF2-WT and USF2-Mut) containing predicted binding locus of miR-423-5p were inserted into pGL3 plasmid. Then, the recombinant vector and miR-423-5p or miR-NC were co-transfected into RTEC by Lipo 2000. The luciferase activity was analyzed by double luciferase reporter gene assay system (Baiao Innovation Technology Co., Ltd., Beijing, China, E2940).
Statistical analysis
All experiments were reduplicated for three times. The data were expressed as mean number ± standard difference. The statistical significance was analyzed by student’s t-test or one-way anova. Graphpad Prism 7.0 was used to analyzed data and draw pictures. P<0.05 was considered to be statistically significant.
Results
APG could protect renal function in DN rats
Serum Cr and BUN are well-known indexes of renal function, and their abnormal up-regulation is related to abnormal renal function. We investigated the effect of APG on renal function in rats. The results revealed that the above indexes in DN group were obviously higher than those in NC group, while the above indexes were significantly reduced under the influence of APG. This indicated that APG could improve abnormal renal function by down-regulating serum Cr and BUN in DN rats (Figure 1).
Figure 1.
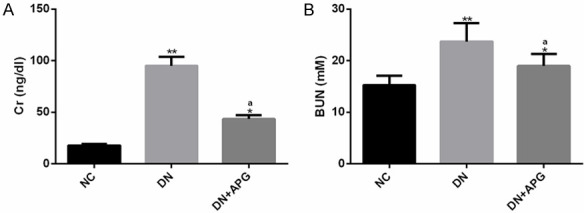
Effect of APG on renal function indexes of DN rats. It was found that (A) serum Cr and (B) BUN in DN rats were significantly higher than those in NC group, and the abnormality of these two indexes was significantly improved under the influence of APG. Note: Compared with NC group, ** means P<0.01, * means P<0.05; a means that compared with DN group, P<0.05.
APG could inhibit the pathologic changes of kidney in DN rats
We also performed HE, PAS, Masson staining analysis and TUNEL analysis on kidney of DN rats, and observed the pathological changes of kidney in each group. The results showed that compared with NC group, the rats in DN group indicated significant glomerular hypertrophy, glomerular mesangial matrix expansion and partial renal tubular atrophy under HE and PAS staining, and this pathological change was significantly improved under the influence of APG. In addition, the results of PAS staining also showed that the M/G in DN group was obviously higher than that in NC group, but this ratio was significantly reduced under the intervention of APG. Under Masson staining, the rats in DN group also showed obvious collagen fiber deposition in glomeruli and renal tubules, but this change was significantly reduced under the action of APG. TUNEL results showed that the proportion of apoptotic positive cells in DN group was obviously higher than that in NC group, and the high apoptosis level was significantly reduced under the intervention of APG. All the results suggested that APG had different degrees and aspects of kidney protection for DN rats (Figure 2).
Figure 2.
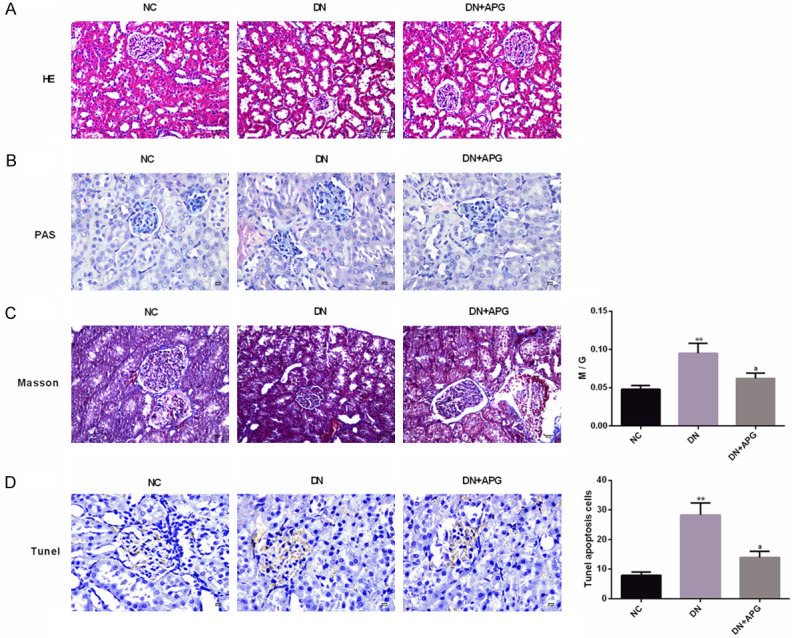
Effect of APG on pathological changes of kidney in DN rats. According to the results of (A) HE staining (scale bar: 20 μm) and (B) PAS staining (scale bar: 20 μm), we found that APG could significantly improve glomerular hypertrophy, glomerular mesangial matrix expansion and partial renal tubular atrophy in DN rats. According to (C) Masson staining (scale bar: 15 μm) results, we found that APG could significantly improve collagen fiber deposition and M/G in glomeruli and renal tubules of DN rats. According to (D) TUNEL staining (scale bar: 20 μm) results, we found that the apoptosis level of DN rats decreased significantly under the influence of APG. Note: Compared with NC group, ** means P<0.01, * means P<0.05; a means that compared with DN group, P<0.05.
APG exerted anti-inflammatory effect in DN rats
The inflammatory cytokines played a crucial role in the microenvironment of DN lesions, among which IL-6 was related to the risk of DN, IFN-γ was associated with the delay of immune response in DN, and TNF-α was bound up with the damage of glomerular permeability barrier [18-20]. We investigated the effect of APG on inflammatory reaction of DN rats. The results revealed that the concentrations of IL-6, IFN-γ and TNF-α were significantly increased in serum of DN rats compared with NC group, while the concentrations of these inflammatory indicators were significantly decreased under the intervention of APG. The above results suggested that APG could reduce the inflammatory injury of DN by inhibiting the levels of inflammatory indexes such as IL-6, IFN-γ and TNF-α (Figure 3).
Figure 3.
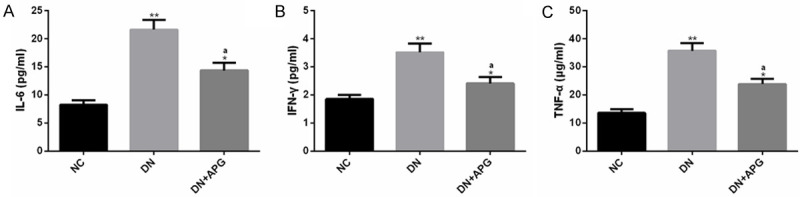
Effect of APG on inflammatory indexes in DN rats. The serum inflammatory indexes (A) IL-6, (B) IFN-γ and (C) TNF-α in DN rats were significantly higher than those in NC group, but these indexes were significantly decreased under APG intervention. Note: Compared with NC group, ** means P<0.01, * means P<0.05; a means that compared with DN group, P<0.05.
APG exerted anti-renal fibrosis effect in DN rats
The elevation of IV-C, FN and Col I levels is a marker for the deterioration of of DN fibrosis, and all of them are biomarkers of fibrosis [21,22]. We investigated the influence of APG on renal fibrosis indexes in DN rats. The protein level of renal fibrosis indexes in DN group was obviously higher than that in NC group, but the protein level of the above indexes was significantly decreased under the influence of APG. In immunohistochemistry, we also observed that the levels of IV-C, FN and Col I in DN rats were significantly increased compared with those in the NC group, and the above disorders were alleviated under the intervention of APG. This indicated that APG was helpful to resist renal fibrosis in DN (Figure 4).
Figure 4.
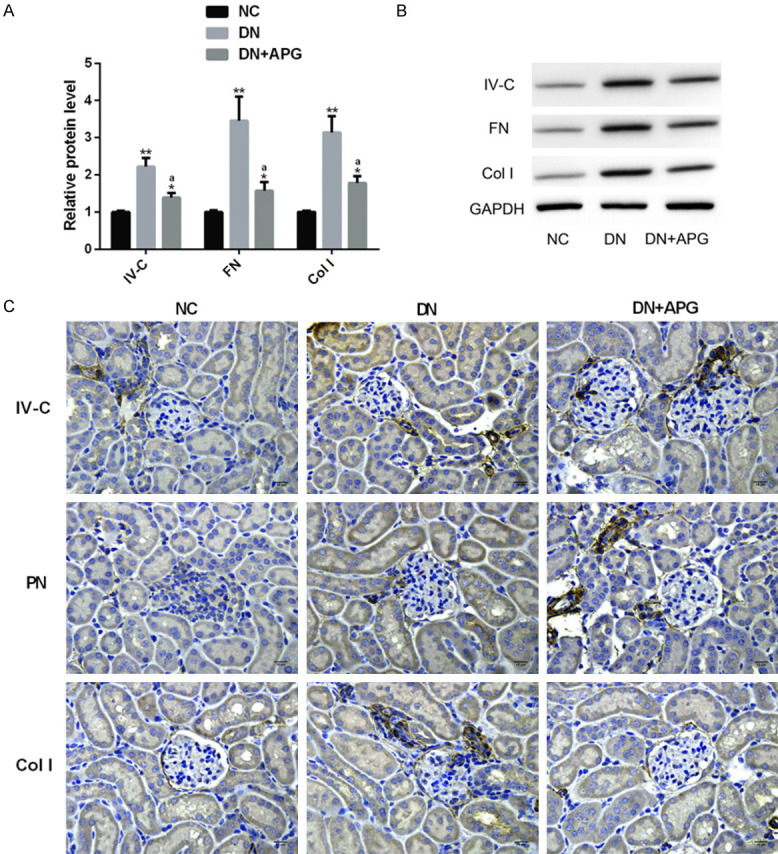
Effect of APG on renal fibrosis indexes in DN rats. The (A) IV-C, FN and Col I of rats in DN group were significantly higher than those in NC group, but the protein levels of these indexes decreased significantly under APG, which were shown in (B) protein map and (C) immunohistochemistry (×400) (scale bar: 15 μm). Note: Compared with NC group, ** means P<0.01, * means P<0.05; a means that compared with DN group, P<0.05.
APG exerted anti-EMT effect in DN rats
It is known that EMT is one of the potential pathogenic mechanisms of DN, and this pathological process will lead to the development and progression of renal fibrosis, thus aggravating the course of DN [23]. Among them, E-cadherin is an epithelial marker, while vimentin and α-SMA are mesenchymal markers. The typical feature of EMT is that the level of epithelial marker protein is significantly down-regulated, while the level of mesenchymal marker protein is significantly up-regulated [24]. We investigated the effect of APG on EMT process in DN rats. The results showed that compared with NC group, E-cadherin in DN group was significantly decreased, while vimentin and α-SMA were significantly up-regulated, and the changes were significantly improved under the influence of APG. This indicated that APG had a reversal effect on EMT process, which was mainly reflected in its change in the expression level of EMT markers (Figure 5).
Figure 5.
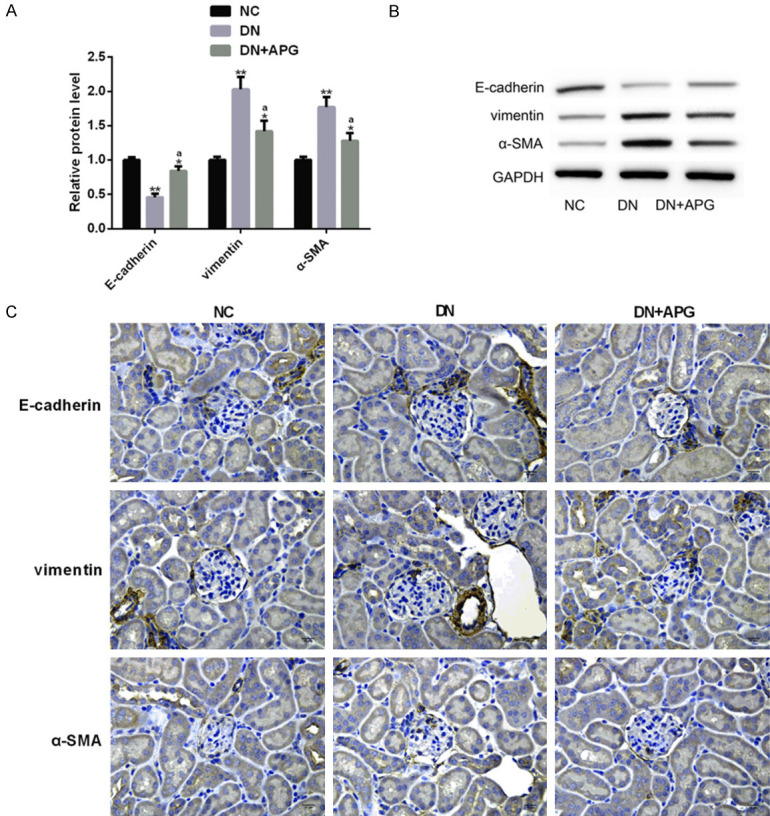
Effect of APG on EMT markers in DN rats. (A) The (A) E-cadherin of rats in DN group was significantly lower than that in DN group, while vimentin and α-SMA were significantly higher. After APG intervention, the protein level changes of these indexes were significantly improved, which were shown in (B) protein map and (C) immunohistochemistry (×400) (scale bar: 15 μm). Note: Compared with NC group, ** means P<0.01, * means P<0.05; a means that compared with DN group, P<0.05.
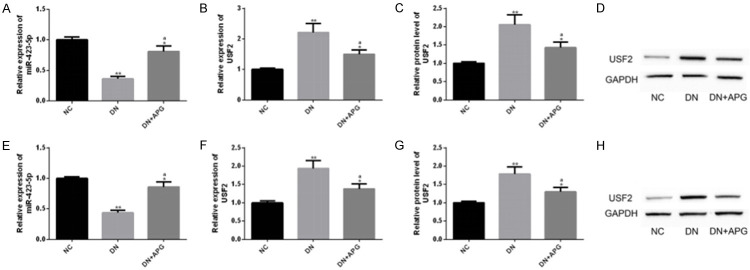
APG could adjust miR-423-5p-USF2 axis
For the purpose of understanding the underlying mechanism of APG’s anti-DN effect more deeply, we analyzed its relationship with miR-423-5p-USF2 axis. Analysis showed that DN rats had abnormally down-regulated miR-423-5p and abnormally up-regulated USF2 (transcription and protein level), and this abnormal imbalance was significantly alleviated under the interference of APG. This phenomenon was similar in the in vitro model of RTEC induced by HG. Therefore, we further explored the underlying mechanism through the in vitro model. The above results suggested that the protective effect of APG on DN might be related to miR-423-5p-USF2 axis (Figure 6).
Figure 6.
Regulatory effect of APG on miR-423-5p-USF2 axis. We found that APG could significantly adjust abnormally low levels of (A, E) miR-423-5p and abnormally high levels of (B, C, F, G) USF2 backwards in the in-vitro and in-vivo model of DN, which were shown in the (D, H) protein map of USF2.
There was a targeting relationship between miR-423-5p and USF2
According to biological analysis, miR-423-5p had a potential conservative binding site with USF2. The double luciferase report revealed that miR-423-5p only declined the level of USF2-Wt, but had no significant effect on USF2-Mut. RT-PCR and western blot analysis showed that transfection of miR-423-5p could significantly decline the expression of USF2 and its protein level. The above results indicated that there was a targeting relationship between them (Figure 7).
Figure 7.
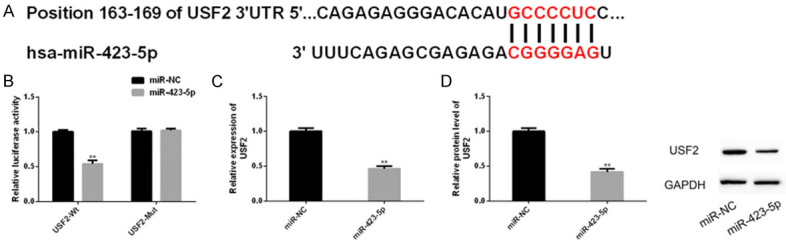
Relationship between miR-423-5p and USF2. The online prediction site of miRNA target genes showed (A) the binding site of miR-423-5P and USF2, and (B) the double luciferase report showed that miR-423-5p only knocked down USF2-Wt (rather than USF2-Mut). (C, D) RT-PCR and western blot analysis also showed that transfection of miR-423-5p could reduce the expression and protein level of USF2.
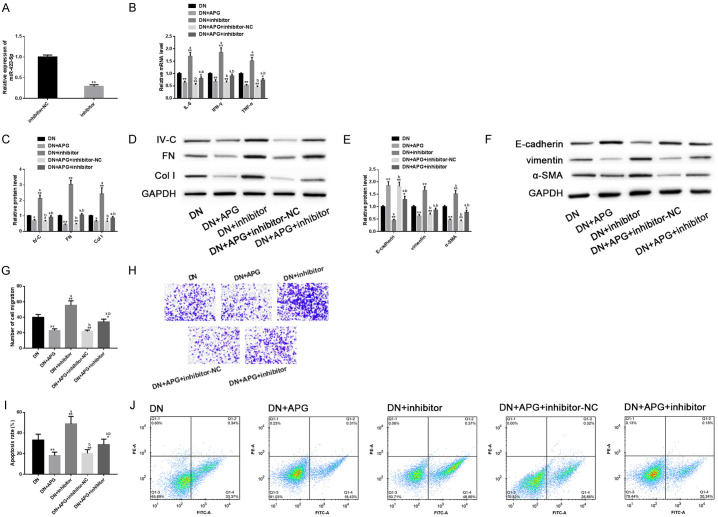
Down-regulation of miR-423-5p could aggravate the progression of DN in vitro model and eliminate the protective effect of APG in vitro
For the purpose of exploring the role of miR-423-5p-USF2 axis in APG’s anti-DN protection mechanism more deeply, we successively analyzed the effect of miR-423-5p and USF2 in DN and their influence on the protection of APG. First, the miR-423-5P was down-regulated by transfecting miR-423-5p inhibitors. Then, we found that down-regulating miR-423-5p could aggravate inflammation, fibrosis, EMT and other processes of DN model in vitro, improve its migration level and induce its apoptosis. In addition, down-regulating miR-423-5p could also significantly eliminate the improvement of APG on DN in-vitro model in these aspects. This revealed that down-regulation of miR-423-5p not only aggravated the disease progression of DN model in vitro, but also significantly interfered with the protective effect of APG in vitro (Figure 8).
Figure 8.
Effect of down-regulating miR-423-5p on DN model in vitro and protective effect of APG in vitro. (A) miR-423-5p was down-regulated by inhibitor transfection. Down-regulation of miR-423-5p had different degrees of negative effects on (B) inflammation, (C, D) fibrosis, (E, F) EMT, (G, H) cell migration (scale bar: 20 μm) and (I, J) cell apoptosis of DN model in vitro, and it could also offset the improvement of APG in the above aspects. Note: Compared with inhibitor-NC/DN, ** means P<0.01, * means P<0.05; a represents that compared with DN+APG, P<0.05; b represents that compared with DN+inhibitor, P<0.05.
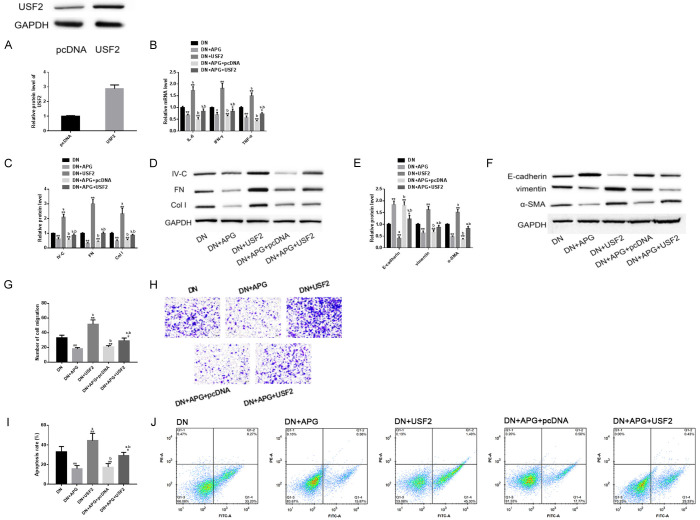
Up-regulation of USF2 could aggravate the progression of DN model in vitro and eliminate the protective effect of APG in vitro
Similarly, USF2 was over-expressed by transfection. We found that up-regulation of USF2 and down-regulation of miR-423-5p had similar performances, that is, they had negative effects on inflammation, fibrosis, EMT process, cell migration and apoptosis of DN, which made the pathological changes of DN in-vitro model more serious than before. On the other hand, this effect also threatened the in-vitro protective effect of APG, and almost eliminated the in-vitro protective effect of APG in the above aspects. The above results suggested that up-regulating USF2 could also aggravate DN and reduce the anti-DN performance of APG (Figure 9).
Figure 9.
Effect of upregulation of USF2 on DN model in vitro and protective effect of APG in vitro. (A) USF2 was up-regulated by transfection. Up-regulation of USF2 had different degrees of negative effects on (B) inflammation, (C, D) fibrosis, (E, F) EMT, (G, H) cell migration (scale bar: 20 μm) and (I, J) apoptosis of DN in-vitro model, and it could also reduce the protective effects of APG in these aspects. Note: Compared with pcDNA/DN, ** means P<0.01, * means P<0.05; a represents that compared with DN+APG, P<0.05; b represents that compared with DN+USF2, P<0.05.
Discussion
DN is the most common factor of DM’s morbidity and death, as well as the main risk factor of cardiovascular disease, which has a great negative impact on human health and social and economic burden [25,26]. APG is also one of the components of Ginkgo biloba extract, which has a positive effect on the in-vitro biotransformation of intestinal bacterial matrix in DN rats [27]. In this study, an in vivo and in vitro study of DN was carried out around APG, and three conclusions were mainly drawn. First, APG had anti-inflammatory, anti-fibrosis and anti-EMT effects, and it could ameliorate the pathologic changes of kidney in DN rats. Secondly and thirdly, down-regulating miR-423-5p or up-regulating USF2 could aggravate the pathological process of DN model in vitro, and it could reduce the in-vitro protective effect of APG. These last two points are also the novelty of the results of this study. These conclusions revealed the key effects and potential mechanisms of APG and miR-423-5p-USF2 axis in DN process, which might provide new clues for the treatment of DN.
Our in-vivo study first revealed that APG had different protective effects on inflammation, renal fibrosis and EMT in DN rats. APG is known to have similar functions in other diseases. For example, the studies by Dang [28] have reported that it can suppress the inflammatory cascade reaction by inhibiting the activation of NF-κB, and play an anti-inflammatory and anti-apoptosis role in testicular cells. Jiao et al. [29] have revealed that it can improve the epidural fibrosis of fibroblasts by regulating Wnt3a/β-catenin signaling pathway. In addition, Qin et al. [30] have revealed that it can resist EMT process and metastasis of cancer cells by inhibiting NF-κB-Snai pathway in liver cancer. miR-423-5p has been revealed to promote disease progression by activating NF-κB in lupus nephritis [31]. In the molecular mechanism based on myocardial cells in cardiovascular diseases, depletion of miR-423-5p can stimulate activation of Wnt3a/β-catenin pathway by targeting Myb-related protein B (MYBL2), thereby improving myocardial apoptosis and mitochondrial dysfunction [32]. In addition, miR-423-5p has also been proved to mediate the regulation of EMT process [33]. The above studies indicate that APG is closely related to miR-423-5p, and the former may affect the expression of miR-423-5p through one or more of the above molecular pathways.
The above studies have also revealed the positive performance and therapeutic effect of APG in diseases from different angles. In addition, there are many studies on APG in DN or DM. Previous studies have shown that APG is also anti-DM, which can play a therapeutic role by relieving STZ-related oxidative damage of islet β cells. APG can also alleviate vascular dysfunction of type 2 DM rats by affecting glucose and lipid metabolism [9,34]. Sun et al. [10] have reported that APG can inhibit oxidative stress, fibrosis and other pathological changes in DN rats by regulating the signal transduction of MAPK-NF-κB-TNF-α and TGF-β1-MAPK-Fn. Zhang [35] and others have pointed out that it can also play the protective role of DN in vitro, mainly by regulating the activation of NF-E2-related factor 2 (Nrf2) pathway to protect the RTEC injury induced by HG. Our in-vitro study also revealed that APG could play an anti-DN role in inflammation, fibrosis, EMT and other aspects.
Through further experiments, we explored the underlying molecular mechanism of APG’s protective effect in DN. We found that the protective effect of APG was related to the expression regulation of the miR-423-5p-USF2 axis, which was mainly manifested as that APG could improve abnormally down-regulated miR-423-5p and abnormally up-regulated USF2 in DN in-vitro model. It has been reported that miR-423-5p is significantly declined in kidney tissues of patients with DN, which is similar to the results of our research. In addition, up-regulating miR-423-5p can improve cell damage induced by HG by improving podocyte vitality and inhibiting apoptosis and inflammatory state, and this protective effect is related to its targeted inhibition of Nox4 and suppression of ROS activity [14]. Our research also showed that miR-423-5p had a target-controlled relationship with USF2, and the expression or protein level of USF2 was negatively regulated by miR-423-5p. It is known that miR-423-5p is also abnormally down-regulated in ovarian cancer, growth hormone cell adenoma and other diseases, and the restoration of miR-423-5p expression has shown positive effects to varying degrees [36,37]. However, USF2 has different pathogenic mechanisms in DN or DM, such as being significantly up-regulated under the stimulation of HG, and this abnormality may be triggered by the activation of angiotensin II-AT1-dependent cAMP response element binding protein in RTEC, thus promoting the renal fibrosis process of DM [38]. For another example, its transcription and protein level are significantly higher in mesangial cells of DN, and this change may be related to the stimulation of glycated albumin and the dependent reverse effect of its promoter on NF-κB [39]. All the above evidences have revealed that USF2 is highly expressed in DM or DN and has certain pathogenicity, which is similar to the results of our research. On the other hand, we also revealed that down-regulation of miR-423-5p or up-regulation of USF2 could significantly aggravate the disease progression of in-vitro model and eliminate DN resistance under the intervention of APG, both of which were reflected in five aspects, namely inflammation, fibrosis, EMT, cell migration and apoptosis. Therefore, miR-423-5p-USF2 axis not only mediated the pathological process of DN, but also had a certain regulatory capacity for the DN treatment of APG.
The novelty of this study lies in proving that the anti-inflammatory, anti-renal fibrosis and anti-EMT mechanism of APG in DN is related to the regulation of it APG the miR-423-5p-USF2 axis. Our research has revealed the protective effect of APG in DN and it has shown that its protective mechanism are related to miR-423-5p-USF2 axis. However, there are still some shortcomings that can be improved in our research. First of all, we can increase the exploration of the upstream target factors of miR-423-5p, which is of great significance for further expanding the potential regulation mechanism of APG. Secondly, we can explore whether the molecular network regulated by APG is accompanied by the regulation of a certain pathway, which is of great value for further improving the molecular mechanism. We will carry out supplementary research based on the above points in the future, hoping to contribute to the new molecular therapy strategy of DN.
In a word, we have proposed for the first time that APG alleviates inflammation, renal fibrosis and EMT in DN by regulating miR-423-5p-USF2 axis, which may be a new opportunity for the treatment of DN.
Disclosure of conflict of interest
None.
References
- 1.Donate-Correa J, Luis-Rodriguez D, Martin-Nunez E, Tagua VG, Hernandez-Carballo C, Ferri C, Rodriguez-Rodriguez AE, Mora-Fernandez C, Navarro-Gonzalez JF. Inflammatory targets in diabetic nephropathy. J Clin Med. 2020;9:458. doi: 10.3390/jcm9020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhbari M, Khalili M, Shahrabi-Farahani M, Biglari A, Bandarian F. Expression level of circulating cell free miR-155 gene in serum of patients with diabetic nephropathy. Clin Lab. 2019;65 doi: 10.7754/Clin.Lab.2019.190209. [DOI] [PubMed] [Google Scholar]
- 3.Yuan CM, Nee R, Ceckowski KA, Knight KR, Abbott KC. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clin Kidney J. 2017;10:257–262. doi: 10.1093/ckj/sfw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang YY, Tan RZ, Zhang XQ, Yu Y, Yu C. Calycosin ameliorates diabetes-induced renal inflammation via the NF-kappaB pathway in vitro and in vivo. Med Sci Monit. 2019;25:1671–1678. doi: 10.12659/MSM.915242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamed R, Jayakumar C, Chen F, Fulton D, Stepp D, Gansevoort RT, Ramesh G. Low-dose IL-17 therapy prevents and reverses diabetic nephropathy, metabolic syndrome, and associated organ fibrosis. J Am Soc Nephrol. 2016;27:745–765. doi: 10.1681/ASN.2014111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao J, Wang W, Wang F, Guo C. LncRNA-NR_033515 promotes proliferation, fibrogenesis and epithelial-to-mesenchymal transition by targeting miR-743b-5p in diabetic nephropathy. Biomed Pharmacother. 2018;106:543–552. doi: 10.1016/j.biopha.2018.06.104. [DOI] [PubMed] [Google Scholar]
- 7.Yang G, Zhao Z, Zhang X, Wu A, Huang Y, Miao Y, Yang M. Effect of berberine on the renal tubular epithelial-to-mesenchymal transition by inhibition of the Notch/snail pathway in diabetic nephropathy model KKAy mice. Drug Des Devel Ther. 2017;11:1065–1079. doi: 10.2147/DDDT.S124971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He M, Wang J, Yin Z, Zhao Y, Hou H, Fan J, Li H, Wen Z, Tang J, Wang Y, Wang DW, Chen C. MiR-320a induces diabetic nephropathy via inhibiting MafB. Aging (Albany NY) 2019;11:3055–3079. doi: 10.18632/aging.101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang N, Yi WJ, Tan L, Zhang JH, Xu J, Chen Y, Qin M, Yu S, Guan J, Zhang R. Apigenin attenuates streptozotocin-induced pancreatic beta cell damage by its protective effects on cellular antioxidant defense. In Vitro Cell Dev Biol Anim. 2017;53:554–563. doi: 10.1007/s11626-017-0135-4. [DOI] [PubMed] [Google Scholar]
- 10.Malik S, Suchal K, Khan SI, Bhatia J, Kishore K, Dinda AK, Arya DS. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-kappaB-TNF-alpha and TGF-beta1-MAPK-fibronectin pathways. Am J Physiol Renal Physiol. 2017;313:F414–F422. doi: 10.1152/ajprenal.00393.2016. [DOI] [PubMed] [Google Scholar]
- 11.Sun J, Zhao F, Zhang W, Lv J, Lv J, Yin A. BMSCs and miR-124a ameliorated diabetic nephropathy via inhibiting notch signalling pathway. J Cell Mol Med. 2018;22:4840–4855. doi: 10.1111/jcmm.13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei X, Zhang BD, Ren JG, Luo FL. Astragaloside suppresses apoptosis of the podocytes in rats with diabetic nephropathy via miR-378/TRAF5 signaling pathway. Life Sci. 2018;206:77–83. doi: 10.1016/j.lfs.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Zhang S, Xu R, Gao S, Yin J. Melatonin attenuates endothelial-to-mesenchymal transition of glomerular endothelial cells via regulating miR-497/ROCK in diabetic nephropathy. Kidney Blood Press Res. 2018;43:1425–1436. doi: 10.1159/000493380. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Zhang J, Fan L, He X. miR-423-5p suppresses high-glucose-induced podocyte injury by targeting Nox4. Biochem Biophys Res Commun. 2018;505:339–345. doi: 10.1016/j.bbrc.2018.09.067. [DOI] [PubMed] [Google Scholar]
- 15.Wang S. Role of upstream stimulatory factor 2 in diabetic nephropathy. Front Biol (Beijing) 2015;10:221–229. doi: 10.1007/s11515-015-1359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiao CC, Huang WH, Cheng KH, Lee CT. Low-energy extracorporeal shock wave therapy ameliorates kidney function in diabetic nephropathy. Oxid Med Cell Longev. 2019;2019:8259645. doi: 10.1155/2019/8259645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Yin Z, Li H, Fan J, Yang S, Chen C, Wang DW. MiR-30c protects diabetic nephropathy by suppressing epithelial-to-mesenchymal transition in db/db mice. Aging Cell. 2017;16:387–400. doi: 10.1111/acel.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhamodharan U, Viswanathan V, Krishnamoorthy E, Rajaram R, Aravindhan V. Genetic association of IL-6, TNF-alpha and SDF-1 polymorphisms with serum cytokine levels in diabetic foot ulcer. Gene. 2015;565:62–67. doi: 10.1016/j.gene.2015.03.063. [DOI] [PubMed] [Google Scholar]
- 19.Guo H, Pan C, Chang B, Wu X, Guo J, Zhou Y, Liu H, Zhu Z, Chang B, Chen L. Triptolide improves diabetic nephropathy by regulating Th cell balance and macrophage infiltration in rat models of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2016;124:389–398. doi: 10.1055/s-0042-106083. [DOI] [PubMed] [Google Scholar]
- 20.Navarro JF, Mora-Fernandez C. The role of TNF-alpha in diabetic nephropathy: pathogenic and therapeutic implications. Cytokine Growth Factor Rev. 2006;17:441–450. doi: 10.1016/j.cytogfr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Hou X, Tian J, Geng J, Li X, Tang X, Zhang J, Bai X. MicroRNA-27a promotes renal tubulointerstitial fibrosis via suppressing PPARgamma pathway in diabetic nephropathy. Oncotarget. 2016;7:47760–47776. doi: 10.18632/oncotarget.10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang ZH, Tang YZ, Song HN, Yang M, Li B, Ni CL. miRNA342 suppresses renal interstitial fibrosis in diabetic nephropathy by targeting SOX6. Int J Mol Med. 2020;45:45–52. doi: 10.3892/ijmm.2019.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Q, Chen YB, Yang H, Wang WW, Li CC, Wang L, Wang J, Du L, Yin XX. Inactivation of TSC1 promotes epithelial-mesenchymal transition of renal tubular epithelial cells in mouse diabetic nephropathy. Acta Pharmacol Sin. 2019;40:1555–1567. doi: 10.1038/s41401-019-0244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng Q, Zhai X, Yuan Y, Ji Q, Zhang P. lncRNA ZEB1-AS1 inhibits high glucose-induced EMT and fibrogenesis by regulating the miR-216a-5p/BMP7 axis in diabetic nephropathy. Braz J Med Biol Res. 2020;53:e9288. doi: 10.1590/1414-431X20209288. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Guo C, Ding G, Huang W, Wang Z, Meng Z, Xiao W. Total saponin of Dioscoreae hypoglaucae rhizoma ameliorates streptozotocin-induced diabetic nephropathy. Drug Des Devel Ther. 2016;10:799–810. doi: 10.2147/DDDT.S99670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kretschmar C, Oyarzun C, Villablanca C, Jaramillo C, Alarcon S, Perez G, Diaz-Encarnacion MM, Pastor-Anglada M, Garrido W, Quezada C, San Martin R. Reduced adenosine uptake and its contribution to signaling that mediates profibrotic activation in renal tubular epithelial cells: implication in diabetic nephropathy. PLoS One. 2016;11:e0147430. doi: 10.1371/journal.pone.0147430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang D, Yu Y, Zheng X, Wu J, Li Y, Wu X, Du Q, Yin X. Comparative investigation of in vitro biotransformation of 14 components in Ginkgo biloba extract in normal, diabetes and diabetic nephropathy rat intestinal bacteria matrix. J Pharm Biomed Anal. 2014;100:1–10. doi: 10.1016/j.jpba.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Dang Y, Li Z, Wei Q, Zhang R, Xue H, Zhang Y. Protective effect of apigenin on acrylonitrile-induced inflammation and apoptosis in testicular cells via the NF-kappaB pathway in rats. Inflammation. 2018;41:1448–1459. doi: 10.1007/s10753-018-0791-x. [DOI] [PubMed] [Google Scholar]
- 29.Jiao R, Chen H, Wan Q, Zhang X, Dai J, Li X, Yan L, Sun Y. Apigenin inhibits fibroblast proliferation and reduces epidural fibrosis by regulating Wnt3a/beta-catenin signaling pathway. J Orthop Surg Res. 2019;14:258. doi: 10.1186/s13018-019-1305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin Y, Zhao D, Zhou HG, Wang XH, Zhong WL, Chen S, Gu WG, Wang W, Zhang CH, Liu YR, Liu HJ, Zhang Q, Guo YQ, Sun T, Yang C. Apigenin inhibits NF-kappaB and snail signaling, EMT and metastasis in human hepatocellular carcinoma. Oncotarget. 2016;7:41421–41431. doi: 10.18632/oncotarget.9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Gao J, Wang F. MiR-663a/MiR-423-5p are involved in the pathogenesis of lupus nephritis via modulating the activation of NF-kappaB by targeting TNIP2. Am J Transl Res. 2017;9:3796–3803. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu X, Lu X. MiR-423-5p inhibition alleviates cardiomyocyte apoptosis and mitochondrial dysfunction caused by hypoxia/reoxygenation through activation of the wnt/beta-catenin signaling pathway via targeting MYBL2. J Cell Physiol. 2019;234:22034–22043. doi: 10.1002/jcp.28766. [DOI] [PubMed] [Google Scholar]
- 33.Oak SR, Murray L, Herath A, Sleeman M, Anderson I, Joshi AD, Coelho AL, Flaherty KR, Toews GB, Knight D, Martinez FJ, Hogaboam CM. A micro RNA processing defect in rapidly progressing idiopathic pulmonary fibrosis. PLoS One. 2011;6:e21253. doi: 10.1371/journal.pone.0021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren B, Qin W, Wu F, Wang S, Pan C, Wang L, Zeng B, Ma S, Liang J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur J Pharmacol. 2016;773:13–23. doi: 10.1016/j.ejphar.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Zhao X, Zhu H, Wang J, Ma J, Gu M. Apigenin protects against renal tubular epithelial cell injury and oxidative stress by high glucose via regulation of NF-E2-related factor 2 (Nrf2) pathway. Med Sci Monit. 2019;25:5280–5288. doi: 10.12659/MSM.915038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang X, Zeng X, Huang Y, Chen S, Lin F, Yang G, Yang N. miR-423-5p serves as a diagnostic indicator and inhibits the proliferation and invasion of ovarian cancer. Exp Ther Med. 2018;15:4723–4730. doi: 10.3892/etm.2018.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao S, Li J, Feng J, Li Z, Liu Q, Lv P, Wang F, Gao H, Zhang Y. Identification of serum miRNA-423-5p expression signature in somatotroph adenomas. Int J Endocrinol. 2019;2019:8516858. doi: 10.1155/2019/8516858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visavadiya NP, Li Y, Wang S. High glucose upregulates upstream stimulatory factor 2 in human renal proximal tubular cells through angiotensin II-dependent activation of CREB. Nephron Exp Nephrol. 2011;117:e62–70. doi: 10.1159/000320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Wang S. Glycated albumin upregulates upstream stimulatory factor 2 gene transcription in mesangial cells. Am J Physiol Renal Physiol. 2010;299:F121–127. doi: 10.1152/ajprenal.00074.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]