Abstract
Objective: To study swimming exercise’s effect on caspase-3 expression in chondrocytes in osteoarthritis (OA). Methods: 36 SD rats were randomly separated into normal group (n = 12), OA model group (n = 12) and swimming exercise group (n = 12). After modeling, rats received no intervention in model group and swimming exercise once a day (15 min/time) for 4 consecutive weeks in swimming exercise group. After intervention for 4 weeks, specimens were taken to analyze tissue morphology by H&E staining, caspase-3 expression by Western blot and qPCR, chondrocytes apoptosis by TUNEL assay. Results: HE staining revealed abnormal bone tissue morphology in model group and swimming exercise group with improved morphology after swimming exercise. Model group and swimming exercise group all showed significantly higher Caspase-3 protein level than normal group with lower level after swimming exercise (P < 0.05). Consistently, qPCR showed similar expression profile of caspase-3 mRNA level to the protein level. Conclusion: Swimming exercise can inhibit caspase-3 level and chondrocytes apoptosis in OA, thus improving the joint morphology.
Keywords: Swimming exercise, osteoarthritis, chondrocyte, caspase-3, apoptosis
Introduction
Osteoarthritis (OA) is a kind of common arthritic disease in clinic, which frequently occurs in the finger joint, shoulder joint, knee joint, etc. [1]. The major pathological changes of OA are cartilage destruction and osteoproliferation, and arthralgia and activity limitation are the most common clinical manifestations of patients, seriously affecting the quality of life of patients [2,3]. Currently, the exact cause and pathogenesis of OA remain unclear, but age, genetic factors and mechanical force are considered as major risk factors for OA [4]. Studies have demonstrated that [5,6] apoptosis of articular chondrocytes is observed in OA, and there is a close correlation between them. Caspase-3 is an important participant and executor of the apoptotic process [7]. Moreover, studies have revealed that [8] caspase-3 is highly expressed in cartilages in OA, and is positively correlated with the severity of OA. Previous study indicated that age, but not short-term intensive swimming, affects chondrocyte turnover in zebrafish vertebral cartilage [9]. The role of swimming exercise in chondrocyte remains to be further determined. Swimming exercise is a commonly-used rehabilitation method, and its advantages include the higher accuracy of biological regulation in active exercise and rehabilitation via underwater exercise, which is often clinically used in the rehabilitation therapy of OA with an excellent curative effect. This study aims to assess whether swimming exercise affects caspase-3 level in chondrocytes in OA, so as to clarify the mechanism of swimming exercise.
Materials and methods
Laboratory animals and grouping
A total of 36 adult SD rats purchased from Shanghai SLAC Laboratory Animal Co., Ltd. were equally and randomly separated into normal group, model group and swimming exercise group using a random number table. All operations and protocols on animals were approved by the Laboratory Animal Ethics Committee of the First Hospital of Jilin University.
Experimental reagents and instruments
Primary antibodies: anti-caspase-3 antibody (Abcam, USA) and anti-β-actin antibody, immunohistochemistry kit (Maxim, Fuzhou), hematoxylin-eosin (HE) staining kit (Solarbio, Beijing), and fluorescence qPCR instrument (ABI 7500, USA).
Construction of rat model of OA
After anesthesia with 7% chloral hydrate (5 mL/kg), rats were fixed on the operating table under a supine position, the knee joint was exposed, the hair was shaved off and the joint was disinfected. Then 0.1 mL 2% papain and 0.05 mL 0.05 mmol/L L-cysteine were injected once into the knee joint, and they were injected again after 4 days to construct the rat model of OA.
Treatment in each group
Rats in normal group were fed normally without any treatment. The OA model was prepared using the above method in model group without any intervention. The OA model was also prepared using the above method in swimming exercise group, and rats were treated with swimming exercise at 3 days after modeling, and the specific method is as follows: the rats were placed in a 36 cm-deep pool at 32-36°C, and swam at a speed of 3 cm/s once a day (15 min/time) for 4 consecutive weeks.
Sampling
After anesthesia, cartilage tissues were collected from 6 rats in each group, fixed in 4% paraformaldehyde at 4°C, and decalcified with ethylene diaminetetraacetic acid (EDTA) decalcifying solution. The decalcifying solution was replaced once every 3 d till the complete decalcification of tissues. Then tissues were prepared into paraffin sections used for immunohistochemical detection. The knee cartilage tissues were isolated directly from the remaining 6 rats for measuring caspase-3 level.
Detection method
HE staining
The paraffin sections (5 μm-thick) were routinely dewaxed and soaked in water, followed by HE staining using the HE staining kit according to the instructions to observe the morphology of knee joint of rats.
TUNEL assay
The paraffin sections were routinely dewaxed and soaked in water, followed by analysis of chondrocytes apoptosis by TUNEL kit.
Immunohistochemistry
The paraffin sections were routinely dewaxed and soaked in water, and the citric acid buffer was added and heated for antigen retrieval followed by endogenous peroxidase blockage and addition of anti-caspase-3 antibody (1:200) for overnight incubation at 4°C and then secondary antibody for 10 min. After incubation with streptavidin-peroxidase solution and DAB addition for development, the sections were counterstained with hematoxylin followed by observation under a microscope.
Western blot
The tissue protein was isolated using lysis solution and quantified by BCA assay followed by separation on SDS-PAGE for western blot using anti-caspase-3 antibody (1:2000 dilution). The membrane was developed after addition of chemiluminescence reagent.
qPCR
RNA was extracted for cDNA synthesis followed by qPCR with conditions: 96°C 10 min, 40 cycles of 96°C 10 s, 60°C 30 s. Gene expression was analyzed using GAPDH as a reference. The primer sequences were shown in Table 1.
Table 1.
Primer sequences
| Name | Primer sequence |
|---|---|
| Caspase-3 | Forward: 5’-TATTCCACAGCACCTGGTTA-3’ |
| Reverse: 5’-CAATACATGGAATCTGTTTCTT-3’ | |
| GADPH | Forward: 5’-ACGGCAAGTTCAACGGCACAG-3’ |
| Reverse: 5’-GAAGACGCCAGTAGACTCCACGAC-3’ |
Statistical methods
Data in this study were presented as mean ± standard deviation; Statistical Product and Service Solutions (SPSS) 19.0 software (SPSS Inc., Chicago, IL, USA) was used for data processing. Continuous data from multiple groups were analyzed by using one-way ANOVA, with the Tukey’s post hoc test. P-values < 0.05 were considered statistically significant.
Results
Observation of tissue morphology via HE staining
In normal group, the knee joint had normal morphology, complete structure and normal chondrocytes arranged closely and in order, and there were complete tidal lines a little far away from the joint surface. In model group, the knee cartilages were damaged with abnormal morphology, incomplete structure and partial loss, the number of chondrocytes was reduced and disorganized, and tidal lines were incomplete and near to the joint surface. In swimming exercise group, the knee cartilages were partially damaged, but the morphology and structure were improved, the number of chondrocytes was larger and they were arranged in well order, and there were complete tidal lines which were a little far away from the joint surface (Figure 1).
Figure 1.

Observation of tissue morphology via HE staining (scale bar 20 μm).
Immunohistochemical staining
Caspase-3 positive expression showed dark brown color and was lower in normal group and higher in model group and swimming exercise group (Figure 2) with a significantly reduced level after swimming exercise than model group (P < 0.05) (Figure 3).
Figure 2.
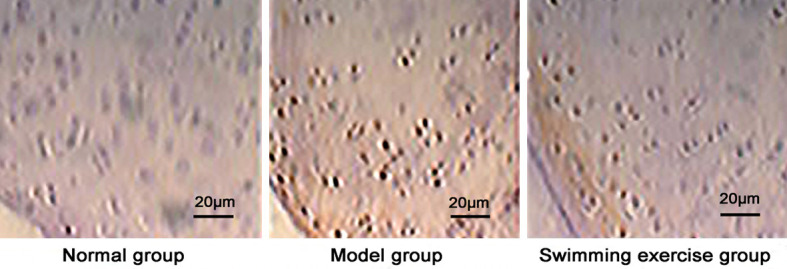
Immunohistochemical detection of caspase-3 expression (scale bar 20 μm).
Figure 3.
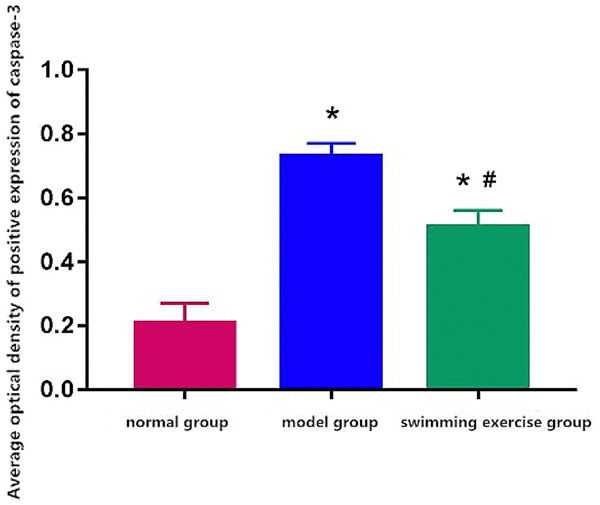
Average optical density of positive expression of caspase-3. Note: *P < 0.05 vs. normal group, #P < 0.05 vs. model group.
Caspase-3 protein expression
Caspase-3 protein expression was significantly higher in model group and swimming exercise group than normal group (Figures 4, 5) and it was significantly decreased after swimming exercise in comparison to the model group (P < 0.05) (Figure 5).
Figure 4.

Detection of caspase-3 protein expression via Western blotting. A. normal group. B. model group. C. swimming exercise group.
Figure 5.
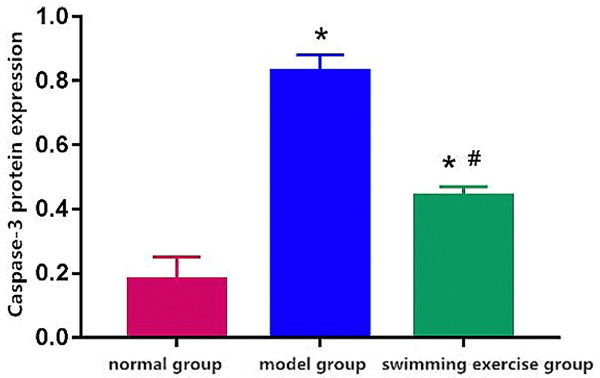
Caspase-3 protein expression. Note: *P < 0.05 vs. normal group, #P < 0.05 vs. model group.
Caspase-3 mRNA expression
Caspase-3 mRNA level was lower in normal group and higher in model group and swimming exercise group with a declined level after swimming exercise in comparison to model group (P < 0.05) (Figure 6).
Figure 6.

Caspase-3 mRNA expression. Note: *P < 0.05 vs. normal group, #P < 0.05 vs. model group.
TUNEL apoptosis detection
The apoptotic rate was lower in normal group and higher in model group and swimming exercise group with a decreased level in swimming exercise group in comparison to model group (P < 0.05) (Figure 7).
Figure 7.
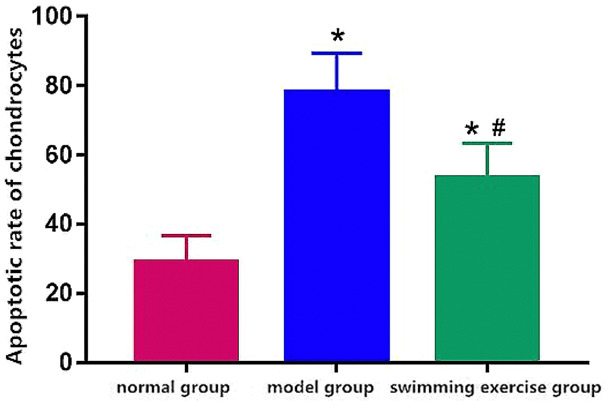
Apoptotic rate of chondrocytes. Note: *P < 0.05 vs. normal group, #P < 0.05 vs. model group.
Discussion
The major pathological reactions of OA, a common degenerative joint disease in clinic, are degradation of articular cartilage and chondrocyte apoptosis [10]. OA is thought to be related to a variety of factors, including chondrocyte apoptosis, genetic factors and immune response [11,12]. Studies have demonstrated that [13,14] the apoptosis degree of chondrocytes in OA patients is significantly increased compared with normal chondrocytes, indicating a close correlation between chondrocyte apoptosis and OA. At the same time, some studies have further confirmed [15,16] that the results of in-situ hybridization are consistent between experimental rabbit model of OA and human OA, and TUNEL assay has shown that the degree of chondrocyte apoptosis in OA model was significantly higher than that of normal chondrocytes, further suggesting that chondrocyte apoptosis is one of the important pathological reactions of OA. Moreover, studies have confirmed [17,18] that the progression of OA is accelerated by chondrocyte apoptosis after OA occurs, so chondrocyte apoptosis is involved in the process of OA progression. Capase-3-induced apoptosis, one of the known important components of death receptor signal transduction, is known to have a close correlation with OA [19,20]. Caspase-3 inhibitors can effectively reduce the expression of caspase-3 in chondrocytes in OA and inhibit the chondrocyte apoptosis, indicating caspase-3’s role in OA and chondrocyte apoptosis [21,22]. Currently, it has been confirmed that the high expression of caspase-3 and chondrocyte apoptosis can be found in OA patients and rodent OA caused by modeling [23,24]. Therefore, caspase-3 can serve as one of the important targets for inhibiting the chondrocyte apoptosis in OA. It has been indicated that reduced Rspo-2 levels in OA osteoblasts are responsible, at least in part, for their reduced Wnt/beta-catenin signaling and abnormal mineralization [25]. Spermidine activates RIP1 deubiquitination to inhibit TNF-alpha-induced NF-kappaB/p65 signaling pathway in osteoarthritis [26]. The limitation of the study therefore exists that the mechanisms of swimming exercise need further investigation besides caspase-3, which may lead to potential new avenues of combined treatment of OA.
The advantages of swimming exercise are as follows: (1) the buoyancy of water reduces the weight load on the joint during exercise, (2) the resistance of water against joint movement enhances the exercise, and (3) the appropriate water temperature can alleviate the muscle spasm, ease the pain and improve the blood circulation in the joint. At the same time, studies have revealed that [27,28] the effects of exercise on the body’s metabolism and structural morphology are highly specific, and only the exercise load of the specific type, intensity, frequency and duration can exert a positive effect on maintaining the normal function of articular cartilage. Swimming exercise is a typical cyclic motion of limbs, which has an excellent rehabilitation effect in clinic. During swimming exercise, the complex biological resistance and discomfort in active motion of joint in OA can be perceived by the fine proprioceptive sensation, thus adjusting the body motion in time, so that the body function and pathological changes can be compatible with the swimming status [29,30].
Conclusion
Results of this study indicate that swimming exercise can significantly inhibit the expression of caspase-3, an apoptotic gene in OA, thus inhibiting chondrocyte apoptosis in OA, which may be one of the reasons for the good rehabilitation effect of swimming exercise on OA.
Disclosure of conflict of interest
None.
References
- 1.Berenbaum F, Walker C. Osteoarthritis and inflammation: a serious disease with overlapping phenotypic patterns. Postgrad Med. 2020;132:377–384. doi: 10.1080/00325481.2020.1730669. [DOI] [PubMed] [Google Scholar]
- 2.Bullock GS, Collins GS, Peirce N, Arden NK, Filbay SR. Playing sport injured is associated with osteoarthritis, joint pain and worse health-related quality of life: a cross-sectional study. BMC Musculoskelet Disord. 2020;21:111. doi: 10.1186/s12891-020-3136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takada S, Nakamura E, Sabanai K, Tsukamoto M, Otomo H, Kanoh S, Murai T, Fukuda H, Okada Y, Uchida S, Sakai A. Attenuation of post-traumatic osteoarthritis after anterior cruciate ligament injury via inhibition of hedgehog signaling. J Orthop Res. 2020;38:609–619. doi: 10.1002/jor.24494. [DOI] [PubMed] [Google Scholar]
- 4.Jimenez G, Cobo-Molinos J, Antich C, Lopez-Ruiz E. Osteoarthritis: trauma vs. disease. Adv Exp Med Biol. 2018;1059:63–83. doi: 10.1007/978-3-319-76735-2_3. [DOI] [PubMed] [Google Scholar]
- 5.He Z, Li H, Han X, Zhou F, Du J, Yang Y, Xu Q, Zhang S, Zhang S, Zhao N, Yan M, Yu Z. Irisin inhibits osteocyte apoptosis by activating the Erk signaling pathway in vitro and attenuates ALCT-induced osteoarthritis in mice. Bone. 2020;141:115573. doi: 10.1016/j.bone.2020.115573. [DOI] [PubMed] [Google Scholar]
- 6.Tarquini C, Pucci S, Scioli MG, Doldo E, Agostinelli S, D’Amico F, Bielli A, Ferlosio A, Caredda E, Tarantino U, Orlandi A. Clusterin exerts a cytoprotective and antioxidant effect in human osteoarthritic cartilage. Aging (Albany NY) 2020;12:10129–10146. doi: 10.18632/aging.103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida FDJF, Carvalho CAD, Nina VJDS, Mochel EG, Almeida FDJF, Carvalho CAD. Application of kinesiotherapy and electrothermotherapy in the treatment of elderly with knee osteoarthrosis: a comparative study. Fisioter Mov. 2016;29:325–334. [Google Scholar]
- 8.Baharuddin MY, Ooi CA, Cao Y. Mathematical modeling of caspase activated DNAase (CAD) regulation towards apoptosis. IFMBE Proc. 2008;21:52–55. [Google Scholar]
- 9.Jian QL, HuangFu WC, Lee YH, Liu IH. Age, but not short-term intensive swimming, affects chondrocyte turnover in zebrafish vertebral cartilage. PeerJ. 2018;6:e5739. doi: 10.7717/peerj.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsson H, Manolescu I, Stefansson SE, Ingvarsson T, Jonsson HH, Manolescu A, Gulcher J, Stefansson K. The inheritance of hand osteoarthritis in Iceland. Arthritis Rheum. 2003;48:391–395. doi: 10.1002/art.10785. [DOI] [PubMed] [Google Scholar]
- 11.Lynch TS, O’Connor M, Minkara AA, Westermann RW, Rosneck JT. Biomarkers for femoroacetabular impingement and hip osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2019;47:2242–2250. doi: 10.1177/0363546518803360. [DOI] [PubMed] [Google Scholar]
- 12.Ingvarsson T, Stefánsson SE, Hallgrímsdóttir IB, Frigge ML, Jónsson H Jr, Gulcher J, Jónsson H, Ragnarsson JI, Lohmander LS, Stefánsson K. The inheritance of hip osteoarthritis in iceland. Arthritis Rheum. 2000;43:2785–2792. doi: 10.1002/1529-0131(200012)43:12<2785::AID-ANR19>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16:26035–26054. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang S, Lv ZT, Zhang JM, Wang YT, Dong YH, Wang ZG, Chen K, Cheng P, Yang Q, Guo FJ, Lu WW, Zhu WT, Chen AM. Necrostatin-1 attenuates trauma-induced mouse osteoarthritis and IL-1beta induced apoptosis via HMGB1/TLR4/SDF-1 in primary mouse chondrocytes. Front Pharmacol. 2018;9:1378. doi: 10.3389/fphar.2018.01378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takayama K, Kawakami Y, Lee S, Greco N, Lavasani M, Mifune Y, Cummins JH, Yurube T, Kuroda R, Kurosaka M, Fu FH, Huard J. Involvement of ERCC1 in the pathogenesis of osteoarthritis through the modulation of apoptosis and cellular senescence. J Orthop Res. 2014;32:1326–1332. doi: 10.1002/jor.22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song B, Song H, Wang W, Wang H, Peng H, Cui J, Wang R, Huang H, Wang W, Wang L. Beclin 1 overexpression inhibits chondrocyte apoptosis and downregulates extracellular matrix metabolism in osteoarthritis. Mol Med Rep. 2017;16:3958–3964. doi: 10.3892/mmr.2017.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Lima DD, Hashimoto S, Chen PC, Colwell CW Jr, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9:712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 18.Aigner T, Kim HA. Apoptosis and cellular vitality: issues in osteoarthritic cartilage degeneration. Arthritis Rheum. 2002;46:1986–1996. doi: 10.1002/art.10554. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda N, Takano Y, Kageyama S, Hatakeyama N, Shakunaga K, Kitajima I, Yamazaki M, Hattori Y. Silencing of caspase-8 and caspase-3 by RNA interference prevents vascular endothelial cell injury in mice with endotoxic shock. Cardiovasc Res. 2007;76:132–140. doi: 10.1016/j.cardiores.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Zou Y, Liu Q, Guo P, Huang Y, Ye Z, Hu J. Antichondrocyte apoptosis effect of genistein in treating inflammationinduced osteoarthritis. Mol Med Rep. 2020;22:2032–2042. doi: 10.3892/mmr.2020.11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abed E, Chan TF, Delalandre A, Martel-Pelletier J, Pelletier JP, Lajeunesse D. R-spondins are newly recognized players in osteoarthritis that regulate Wnt signaling in osteoblasts. Arthritis Rheum. 2011;63:3865–3875. doi: 10.1002/art.30625. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Lin CX, Song B, Li CC, Qiu JX, Li SX, Lin SP, Luo WQ, Fu Y, Fang GB, Wei-Ping L, Saw PE, Ding Y. Spermidine activates RIP1 deubiquitination to inhibit TNF-alpha-induced NF-kappaB/p65 signaling pathway in osteoarthritis. Cell Death Dis. 2020;11:503. doi: 10.1038/s41419-020-2710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelletier JP, Jovanovic DV, Lascau-Coman V, Fernandes JC, Manning PT, Connor JR, Currie MG, Martel-Pelletier J. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum. 2010;43:1290–1299. doi: 10.1002/1529-0131(200006)43:6<1290::AID-ANR11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Xu C, Jiang T, Ni S, Chen C, Li C, Zhuang C, Zhao G, Jiang S, Wang L, Zhu R, van Wijnen AJ, Wang Y. FSTL1 promotes nitric oxide-induced chondrocyte apoptosis via activating the SAPK/JNK/caspase3 signaling pathway. Gene. 2020;732:144339. doi: 10.1016/j.gene.2020.144339. [DOI] [PubMed] [Google Scholar]
- 25.Matsuo M, Nishida K, Yoshida A, Murakami T, Inoue H. Expression of caspase-3 and -9 relevant to cartilage destruction and chondrocyte apoptosis in human osteoarthritic cartilage. Acta Med Okayama. 2001;55:333–340. doi: 10.18926/AMO/32000. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Li M, Ma X, Zhou SL, Ren ZW, Qiu YS. GADD45beta-I attenuates oxidative stress and apoptosis via Sirt3-mediated inhibition of ER stress in osteoarthritis chondrocytes. Chem Biol Interact. 2018;296:76–82. doi: 10.1016/j.cbi.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Xu Z, Buckley MJ, Evans CH, Agarwal S. Cyclic tensile strain acts as an antagonist of IL-1 beta actions in chondrocytes. J Immunol. 2000;165:453–460. doi: 10.4049/jimmunol.165.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gosset M, Berenbaum F, Levy A, Pigenet A, Thirion S, Cavadias S, Jacques C. Mechanical stress and prostaglandin E2 synthesis in cartilage. Biorheology. 2008;45:301–20. [PubMed] [Google Scholar]
- 29.Torres-Hernández BA, Colón LR, Rosa-Falero C, Torrado A, Miscalichi N, Ortíz JG, González-Sepúlveda L, Pérez-Ríos N, Suárez-Pérez E, Bradsher JN, Behra M. Reversal of pentylenetetrazole-altered swimming and neural activity-regulated gene expression in zebrafish larvae by valproic acid and valerian extract. Psychopharmacology (Berl) 2016;233:2533–2547. doi: 10.1007/s00213-016-4304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolarevic J, Aas-Hansen Ø, Espmark Å, Baeverfjord G, Terjesen BF, Damsgård B. The use of acoustic acceleration transmitter tags for monitoring of Atlantic salmon swimming activity in recirculating aquaculture systems (RAS) Aquacult Eng. 2016;72-73:30–39. [Google Scholar]


