Abstract
Objective: To investigate the effect of dexmedetomidine (DMED) on acute kidney injury in children undergoing congenital heart surgery (CHS) with cardiopulmonary bypass (CPB). Methods: The children undergoing CHS with CPB were randomized to the control and the DMED groups. The children in the DMED group were injected with DMED (1 µg/kg) followed by DMED infusion (0.5 µg/kg/h) until 12 h after operation; the controls received normal saline. Markers were detected before operation (T0), 30 min after anesthesia induction (T1), and at 24 h, 48 h, and 72 h after operation (T2, T3, T4). Results: The heart rate and mean arterial pressure in the DMED group decreased at T1 and differed from controls at T1-T3 (all P<0.05). No intergroup differences were observed in the central venous pressure and caspase-3 level (all P>0.05). The DMED group had higher central venous pressure at T3 than at T0 (P<0.05). At T2-T4, the DMED group had lower percentages of TLR3+ cells than the controls (all P<0.05). In the DMED group, the percentagesof TLR3+ cells decreased with time; whereas in the control group, the percentage increased with time (all P<0.05). Compared with the controls, the DMED group had lower levels of NF-κB and TLR3 at T2-T4, lower levels of sCr, IL-1β, and TNF-α at T3-T4, and lower incidence of AKI at T3 (all P≤0.01). Conclusion: DMED can reduce the risk of AKI in children undergoing CHS with CPB, which may be because DMED can inhibit TLR3/NF-κB signaling and its downstream inflammatory mediators.
Keywords: Dexmedetomidine, cardiopulmonary bypass, acute kidney injury, NF-kappa B
Introduction
The incidence rate of acute kidney injury (AKI) in children undergoing surgical correction of congenital heart disease (CHD) with cardiopulmonary bypass (CPB) is 10%-45% [1]. The occurrence of AKI in this operation is mainly related to systemic inflammatory response and renal tubular cell apoptosis induced by CPB [2-4]. It has been reported that dexmedetomidine (DMED), as an α2-adrenergic receptor agonist, can markedly reduce the risk of AKI after CPB, but its detailed mechanism remains unclear [5-7]. The inflammatory response can be mediated by some members of the toll-like receptor (TLR) family through various channels. Luo et al. reported that DMED can reduce the inflammatory response following CPB by inhibiting the expression levels of TLR2 and TLR4 in the peripheral blood mononuclear cells (PBMCs) of patients [8]. Other studies also documented that DMED can reduce the expressions of TLR2 and TLR4 in PBMCs in patients [9,10]. In recent years, researchers have found that TLR3/nuclear factor kappa-B (NF-κB) signaling pathway in PBMCs and its downstream inflammatory mediators such as TNF-α and IL-1β participate in the pathogenesis of AKI [11]. Since there is a lack of reports on the correlation between DMED and TLR3, we aimed to investigate whether the renal protective effect of DMED on children receiving congenital heart surgery with CPB is associated with the inhibition of TLR3 expression.
Materials and methods
Participants
The sample size was calculated based on the fact that the incidence of AKI in children undergoing congenital heart surgery with CPB is about 30%, and we assumed that the pre-infusion with DMED reduces the incidence of AKI in children after congenital heart surgery with CPB by 20%. Based on α=0.05, β=0.10, we estimated that 82 patients need to be enrolled in each group. Therefore, a total of 170 children who received surgical correction of CHD with CPB in The Second Affiliated Hospital of Soochow University between June 2017 and June 2019 were selected for this study. Of them, 42 patients had congenital atrial septal defect (ASD) and 128 patients had congenital ventricular septal defect. The age of the children ranged from 1 to 6 years (4.3±0.6 years). Inclusion criteria were 1) Children who would undergo elective surgery for ASD or ventricular septal defect repair; 2) children with American Society of Anesthesiologists (ASA) class I-II; 3) the heart surgery would be performed under CPB; 4) children who had a complete clinical data record. Exclusion criteria were: 1) Children whose serum creatinine (sCr) level was over 7 mg/L before surgery; 2) children with cyanotic heart disease; 3) children who had arrhythmia; 4) children with patent ductus arteriosus; 5) children with coarctation of the aorta; 6) children with severe left ventricular dysfunction (left ventricular ejection fraction (LVEF) <50%); 7) children with right-sided heart failure; 8) children who took cardiotonic drugs or vasoactive drugs before surgery; 9) children who had respiratory diseases; 10) children who had contraindications for DMED; 11) newborns less than 12 months old. The patients were divided into the control group and the DMED group of 85 cases each according to a random number table. Age, gender, percentage of ASD in the control group were not different from those in the DMED group (male 40, female 45 vs. male 43, female 42; 4.5±0.3 years vs. 4.1±0.5 years; 20 cases of ASD vs. 22 cases of ASD, all P>0.05). All the parents or the legal guardians of the children signed the informed consent. This study was approved by the Ethics Committee of The Second Affiliated Hospital of Soochow University and complied with the Helsinki Declaration. All participants were registered at Chinese Clinical Trial Registry before enrollment to the study.
Methods
The pulse oxygen saturation, heart rate, and blood pressure were measured in all children. According to the literature, the recommended dose of DMED for renal protection is 0.2-0.7 µg/kg/h, thus we chose a dose of 0.5 µg/kg/h DMED for this study [12]. At 15 min before routine anesthesia induction, the patients in the DMED group were given intravenous injection of DMED (Yangtze River Pharmaceutical, China) at a dose of 1 µg/kg. Fifteen minutes later, the patients were given a continuous infusion of DMED at a dose of 0.5 µg/kg/h until 12 h after operation; meanwhile, the patients in the control group received intravenous infusion of normal saline (Otsuka Pharmaceutical, China) until 12 h after operation. During anesthesia induction, the patients were injected intravenously with 0.1-0.2 mg/kg midazolam (Jiangsu Nhwa Pharmaceutical, China), 0.6 mg/kg rocuronium (Sino Biopharma, China), 1-2 µg/kg sufentanil (Yichang Humanwell Pharmaceutical, China), and 2 mg/kg propofol (Fresenius-kabi, Germany). After anesthesia induction, the patients underwent tracheal intubation for mechanical ventilation under the laryngoscope. The tidal volume was maintained at 8-10 mL/kg, the frequency was 20-30 times/min, and the postapneic end-tidal carbon dioxide pressure (PETCO2) was maintained at 30-35 mmHg. The patients were catheterized through radial artery and right internal jugular vein guided under ultrasound. Compound electrolytes (2-4 mL/kg/h) were continuously pumped to the patients. Sufentanil (1-2 µg/kg/h) and rocuronium (1 mg/kg/h) were used for anesthesia maintenance. The patients inhaled the mixture of sevoflurane (1.5%-3%, 1 L/min, Shanghai Hengrui Pharmaceutical, China), air (1 L/min), and oxygen (1 L/min) before starting CPB. During the operation, the bispectral index was maintained between 40 and 60. The whole procedure was performed by the same group of thoracic surgeons. If necessary, diuretics were used to correct urinary flow velocity. When CPB was stopped, the patients were given milrinone, dopamine, and epinephrine according to their conditions. Patients were dropped from the study if their heart failed to beat automatically after opening of the ascending aorta, they had severe arrhythmia (including atrioventricular block) during operation, or diuretics were used. The patients who dropped out were not included in the statistical analyses.
Outcome measures
Main outcome measures: the peripheral venous blood (3 mL) was collected into anticoagulation tubes before operation (T0), 30 min after anesthesia induction (T1), 24 h after operation (T2), 48 h after operation (T3), and 72 h after operation (T4), respectively. The blood samples were centrifuged (8,000 rpm, 5 min) at room temperature, and the serum was collected from the upper layer and stored at -80°C. The sCr level was detected with an automated biochemical analyzer (Beckman Coulter, USA) in the clinical laboratory of The Second Affiliated Hospital of Soochow University. ELISA was employed to detect interleukin (IL)-1β and tumor necrosis factor (TNF)-α. Flow cytometry was performed to detect the percentage of TLR3+ cell, and western blot was performed to detect the expression levels of NF-κB (p65), TLR3, and caspase-3 in PBMCs. The intraoperative fluid volume and urine output at different time points were also measured. According to the 2012 standard by the Kidney Disease: Improving Global Outcomes, the diagnostic criteria of AKI were as follows: the renal function decreased rapidly within 48 hours, the sCr level increased by ≥26.5 µmol/L or ≥1.5 times from baseline, or urine output <0.5 mL/kg/h for more than 6 hours [13]. Secondary outcome measures: the general information of all the patients during the perioperative period were recorded, including the time from anesthesia induction to skin incision, operation duration, CPB duration, aorta blocking period, anesthesia duration, and values of hemodynamic markers such as heart rate, mean arterial pressure, and central venous pressure. The use of epinephrine, dopamine, and milrinone during the surgery was also recorded.
Percentage of TLR3+ cells by flow cytometry
The peripheral venous blood at the same time point from each patient (2 mL) was collected into the anticoagulant tubes (Beijing Solarbio, China) containing heparin sodium (Jiangsu Wanbang Biopharmaceutical, China). The PBMCs were separated using Ficoll lymphocyte separation solution (Meijing Biotechnology, China). The obtained PBMCs were washed in phosphate buffer (HyClone, USA), and the cell concentration was adjusted to 2*106 cells/mL. The cell suspension (100 µL) was added with 20 µL of CD14-PC5 (Miltenyi Biotec, Germany) to label PBMCs followed by incubation with 10 µL of TLR3 antibody (Abcam, UK) at 4°C overnight. After centrifugation, the samples were incubated with 30 µL of NF-κB antibody (Abcam, UK) at 4°C overnight, and the sample volume was fixed for testing. Subsequently, flow cytometry (BD FACSCanto, BD Biosciences, USA) was performed to determine the percentage of TLR3+ cells in the samples.
Detection of levels of IL-1β and TNF-α in serum by ELISA
Serum inflammatory factors IL-1β and TNF-α in children were detected using ELISA according to the manufacturer’s instructions of the test kits (Shanghai Beyotime Biotechnology, China).
Detection of protein expression levels of NF-κB (p65), TLR3, and Caspase-3 in PBMCs by western blot
The total protein in PBMCs was extracted and placed in 1.5 mL Eppendorf tubes. The samples were treated with 500 µL of radio immunoprecipitation assay lysis buffer (Shanghai Beyotime Biotechnology, China) containing phenylmethylsulfonyl fluoride and protease inhibitor on ice for 30 min. Next, the samples were centrifuged at 12,000 rpm (centrifugal radius: 4 cm) for 20 min at 4°C. The supernatant was collected by micropipette (Eppendorf, Germany) into a new EP tube and stored at -80°C. After gel preparation, 5 µL of the protein samples and the marker (Shanghai EpiZyme, China) were loaded unto the gel for electrophoresis. The separated proteins were then transferred from the gel unto a polyvinylidene fluoride membrane (Roche Diagnostics, USA) in western transfer buffer. The membrane was blocked in milk (Bright Food, China) followed by incubation with rabbit anti-human antibody (1:1,000, Cell Signaling Technology, USA) at 4°C overnight. The membrane was washed and incubated with sheep anti-rabbit secondary antibody (1:1,000, Shanghai Beyotime Biotechnology, China) for 1 h. The grayscale value of the protein band was detected using chemiluminescence. The relative protein expression level was calculated as the ratio of the grayscale of the target protein to the grayscale of β-actin. All experiments were repeated three times.
Statistical analysis
SPSS 22.0 software was used for data analysis. Measured data are presented as mean ± standard deviation (x̅ ± sd). Comparison between the groups was conducted by independent samples t-test. Comparison within a group at different time points was conducted by repeated-measures analysis of variance. If there was a difference, Bonferroni test was performed for pairwise comparison. Count data are presented as number or percentage and were examined by Chi-square test or Fisher’s exact test. P<0.05 was considered a significant difference.
Results
Baseline data in the two groups
One patient in the DMED group and one in the control group were dropped from the study as they were treated with diuretics. There were no significant differences in the baseline data between the two groups as displayed in Table 1 (all P>0.05).
Table 1.
Baseline data
| Baseline data | DMED group (n=84) | Control (n=84) | t/χ2 | P |
|---|---|---|---|---|
| Gender (n, %) | 0.095 | 0.758 | ||
| Male | 42 (50.00) | 40 (47.62) | ||
| Female | 42 (50.00) | 44 (52.38) | ||
| Age (month) | 33.2±18.4 | 32.3±15.3 | 0.391 | 0.696 |
| BMI (kg/m2) | 11.6±3.1 | 11.9±2.9 | 0.648 | 0.518 |
| Height (cm) | 86.28±15.34 | 89.11±18.04 | 1.173 | 0.242 |
| BSA (m2) | 0.60±0.15 | 0.57±0.17 | 1.213 | 0.227 |
| ASD (number, %) | 22 (26.19) | 20 (23.81) | 0.525 | 0.469 |
| VSD (number, %) | 62 (73.81) | 64 (76.19) | ||
| Anesthesia duration (min) | 237.89±51.64 | 248.55±56.23 | 1.331 | 0.185 |
| Operation duration (min) | 186.65±45.21 | 192.62±46.44 | 0.855 | 0.394 |
| CPB duration (min) | 105.83±35.65 | 108.21±38.36 | 0.532 | 0.595 |
| Aorta blocking period (min) | 68.38±28.95 | 66.32±35.72 | 0.409 | 0.683 |
| Cooling threshold (°C) | 32.61±1.28 | 32.35±1.47 | 1.491 | 0.138 |
| Vasoactive drugs used in the surgery (n, %) | ||||
| Epinephrine | 1 (1.19) | 2 (2.38) | 0.339 | 0.560 |
| Dopamine | 2 (2.38) | 4 (4.76) | 0.173 | 0.678 |
| Milrinone | 6 (7.14) | 5 (5.95) | 0.097 | 0.755 |
| Intraoperative fluid volume (mL) | 172.54±52.57 | 169.61±49.85 | 0.367 | 0.714 |
| Urine output | ||||
| During CPB (mL/kg.h) | 2.23±1.18 | 2.41±1.19 | 1.289 | 0.199 |
| After CPB (mL/kg.h) | 2.56±1.92 | 2.24±2.13 | 0.971 | 0.333 |
| T2 (mL/kg.h) | 2.94±1.04 | 2.56±2.32 | 0.365 | 0.715 |
| T3 (mL/kg.h) | 3.72±1.36 | 3.35±1.47 | 1.440 | 0.152 |
| T4 (mL/kg.h) | 3.85±1.27 | 3.63±1.56 | 0.365 | 0.615 |
Notes: T2: 24 h after operation; T3: 48 h after operation; T4: 72 h after operation; BSA: body surface area; CPB: cardiopulmonary bypass; ASD: atrial septal defect; VSD: ventricular septal defect; DMED: dexmedetomidine; BMI body mass index.
Perioperative hemodynamics in the two groups
There was no intergroup difference in the heart rate and mean arterial pressure at T0, but there were intergroup differences in these markers at T1-T3 (all P<0.05). In the DMED group, the heart rate and mean arterial pressure decreased at T1 compared with T0 (both P<0.05). No intergroup differences were observed in central venous pressure at different time points (all P>0.05). In the DMED group, the central venous pressure increased at T3 compared with T0 (P<0.05). See Table 2.
Table 2.
HR, MAP, and CVP in the perioperative period
| Control (n=84) | DMED group (n=84) | t | P | |
|---|---|---|---|---|
| HR (beat/min) | ||||
| T0 | 121.26±25.13 | 123.05±20.51 | 0.506 | 0.614 |
| T1 | 118.31±18.52 | 106.90±13.57a | 4.554 | 0.000 |
| T2 | 125.30±20.13 | 110.42±12.44 | 5.763 | 0.000 |
| T3 | 130.46±17.28 | 118.59±13.17 | 6.272 | 0.000 |
| T4 | 123.28±14.53 | 120.49±11.66 | 1.815 | 0.071 |
| F | 4.637 | 16.880 | ||
| P | 0.001 | 0.000 | ||
| MAP (mmHg) | ||||
| T0 | 77.16±9.56 | 78.53±8.47 | 1.170 | 0.244 |
| T1 | 76.35±10.02 | 70.26±11.38a | 3.687 | 0.000 |
| T2 | 82.43±8.39 | 75.44±10.32 | 4.817 | 0.000 |
| T3 | 80.49±8.45 | 76.87±9.99 | 2.536 | 0.012 |
| T4 | 76.48±6.36 | 77.69±7.02 | 1.171 | 0.243 |
| F | 7.572 | 8.918 | ||
| P | 0.000 | 0.000 | ||
| CVP (cmH2O) | ||||
| T0 | 6.53±2.11 | 6.42±1.15 | 0.420 | 0.675 |
| T1 | 6.98±1.18 | 7.25±1.37 | 1.369 | 0.173 |
| T2 | 7.02±1.43 | 7.19±1.18 | 0.791 | 0.430 |
| T3 | 7.21±1.46 | 7.54±1.30b | 1.547 | 0.124 |
| T4 | 6.89±2.52 | 7.03±2.85 | 0.337 | 0.736 |
| F | 1.619 | 5.034 | ||
| P | 0.168 | 0.000 |
Note: 1 mmHg = 0.133 KPa; 1 cmH2O = 0.098 KPa. T0: before operation; T1: 30 min after anesthesia induction; T2: 24 h after operation; T3: 48 h after operation; T4: 72 h after operation.
In the comparison of T1 and T0;
P<0.05.
In the comparison of T3 and T0;
P<0.05.
HR: heart rate; MAP: mean arterial pressure; CVP: central venous pressure; DMED: dexmedetomidine.
Percentage of TLR3+ cells in PBMCs in the two groups at different time points
At T0 and T1, there were no intergroup differences in the percentage of TLR3+ cells in PBMCs (both P>0.05). At T2, T3 and T4, the percentages of TLR3+ cells in PBMCs in the DMED group were lower than those in the control group (all P<0.05). In the DMED group, the percentage of TLR3+ cells decreased with time, and the percentages of TLR3+ cells in PBMCs at T2, T3, and T4 were lower than that at T0 (all P<0.05). In the control group, the percentage of TLR3+ cells increased with time, and the percentages of TLR3+ cells in the PBMCs at T2, T3, and T4 were higher than that at T0 (all P<0.05). See Table 3; Figures 1 and 2.
Table 3.
Percentage of TLR3+ cells in PBMCs
| TLR3+ cells (%) | Control (n=84) | DMED group (n=84) | t | P |
|---|---|---|---|---|
| T0 | 3.45±0.32 | 3.52±0.25 | 1.580 | 0.116 |
| T1 | 3.51±0.18 | 3.43±0.41 | 1.637 | 0.103 |
| T2 | 4.23±0.24 | 3.06±0.20 | 40.617 | 0.000 |
| T3 | 4.31±0.15 | 2.98±0.42 | 27.332 | 0.000 |
| T4 | 4.62±0.14 | 2.85±0.37 | 41.007 | 0.000 |
| F | 480.50 | 64.34 | ||
| P | 0.000 | 0.000 |
Notes: T0: before operation; T1: 30 min after anesthesia induction; T2: 24 h after operation; T3: 48 h after operation; T4: 72 h after operation; DMED: dexmedetomidine; TLR: toll-like receptor; PBMC: peripheral blood mononuclear cells.
Figure 1.
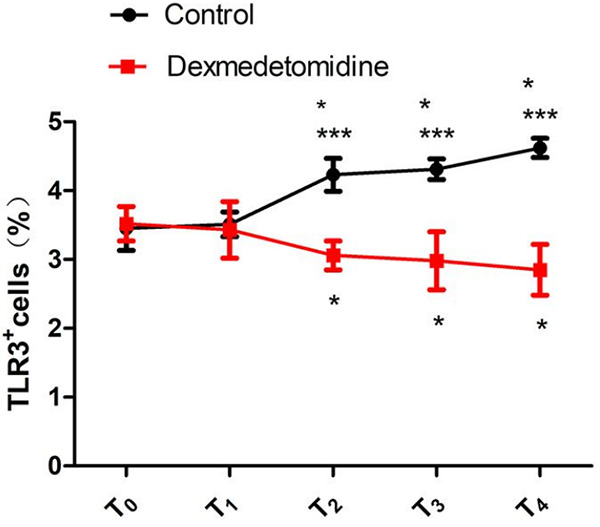
Percentage of TLR3+ cells in PBMCs. Compared with the control group, ***P<0.001; compared with T0 within the same group, *P<0.05. T0: before operation; T1: 30 min after anesthesia induction; T2: 24 h after operation; T3: 48 h after operation; T4: 72 h after operation; TLR: toll-like receptor; PBMC: peripheral blood mononuclear cells.
Figure 2.
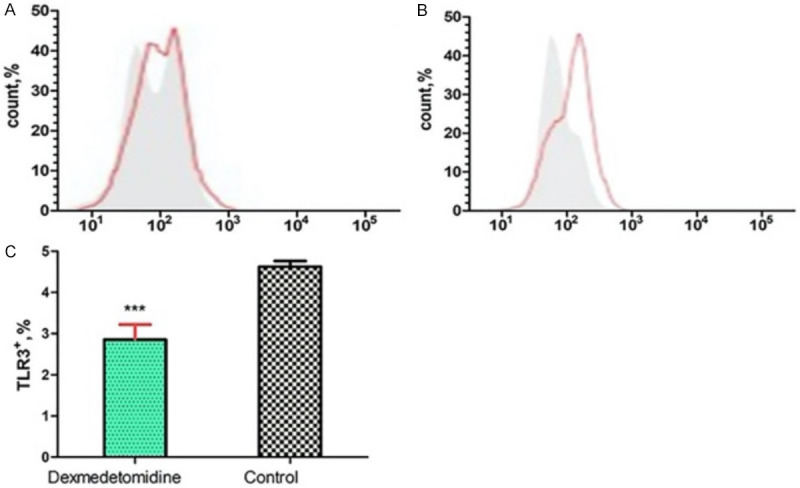
Flow cytometry results of the percentage of TLR3+ cells. A: Flow cytometry results of the percentage of TLR3+ cells at T4 in the DMED group; B: Flow cytometry results of the percentage of TLR3+ cells at T4 in the control group; C: Comparison of the percentage of TLR3+ cells between the two groups at T4. Compared with the control group, ***P<0.001. TLR: toll-like receptor; DMED: dexmedetomidine; T4: 72h after operation.
Expression levels of sCr, NF-κB, caspase-3, TLR3, IL-1β, and TNF-α in the two groups at different time points
At T3 and T4, the sCr levels in the control group was much higher than those in the DMED group (both P<0.01). The expression levels of NF-κB and TLR3 in the PBMCs in the DMED group were lower than those in the control group at T2, T3, and T4, and the serum levels of IL-1β and TNF-α in the DMED group were lower than those in the control group at T3 and T4 (all P<0.001). There were no intergroup differences in the protein expression levels of caspase-3 in PBMCs at all time points (all P>0.05). See Table 4; Figures 3 and 4.
Table 4.
Expression levels of sCr, NF-κB, caspase-3, TLR3, IL-1β, and TNF-α
| Control (n=84) | DMED group (n=84) | t | P | |
|---|---|---|---|---|
| sCr (mol/L) | ||||
| T0 | 79.12±9.75 | 78.18±8.84 | 0.655 | 0.514 |
| T1 | 77.15±10.02 | 79.28±11.46 | 1.282 | 0.202 |
| T2 | 84.26±15.54 | 83.48±17.26 | 0.308 | 0.759 |
| T3 | 93.46±17.28 | 85.43±16.24 | 3.103 | 0.002 |
| T4 | 106.28±14.53 | 87.49±14.61 | 4.799 | 0.000 |
| NF-κB | ||||
| T0 | 9.68±1.12 | 9.25±2.36 | 1.509 | 0.133 |
| T1 | 14.25±2.86 | 13.69±3.23 | 1.189 | 0.235 |
| T2 | 20.18±3.83 | 16.47±6.54 | 4.486 | 0.000 |
| T3 | 34.28±4.54 | 20.67±5.44 | 17.604 | 0.000 |
| T4 | 54.19±5.75 | 24.08±7.72 | 28.668 | 0.000 |
| Caspase-3 | ||||
| T0 | 2.35±0.56 | 2.42±0.76 | 0.679 | 0.498 |
| T1 | 2.41±0.12 | 2.39±0.23 | 0.707 | 0.481 |
| T2 | 2.92±0.25 | 2.87±0.35 | 1.065 | 0.288 |
| T3 | 3.02±0.81 | 2.98±0.63 | 0.357 | 0.721 |
| T4 | 3.11±0.43 | 2.97±0.85 | 1.347 | 0.179 |
| IL-1β (ng/mL) | ||||
| T0 | 62.49±10.03 | 59.63±12.85 | 1.608 | 0.109 |
| T1 | 70.15±12.54 | 72.59±13.69 | 1.204 | 0.230 |
| T2 | 82.55±14.49 | 83.49±13.82 | 0.430 | 0.667 |
| T3 | 100.06±14.48 | 86.49±11.33 | 6.764 | 0.000 |
| T4 | 123.59±16.26 | 90.48±16.69 | 13.023 | 0.000 |
| TNF-α (ng/mL) | ||||
| T0 | 87.54±12.26 | 90.36±17.82 | 1.195 | 0.233 |
| T1 | 103.25±16.66 | 108.53±24.49 | 1.634 | 0.104 |
| T2 | 145.62±20.15 | 151.87±29.54 | 1.602 | 0.111 |
| T3 | 263.49±26.58 | 195.49±20.15 | 18.685 | 0.000 |
| T4 | 350.48±27.69 | 261.45±24.44 | 22.093 | 0.000 |
| TLR3 | ||||
| T0 | 8.56±1.21 | 8.34±1.43 | 1.076 | 0.283 |
| T1 | 13.18±2.35 | 12.96±2.26 | 0.618 | 0.537 |
| T2 | 19.32±4.51 | 11.46±5.68 | 9.933 | 0.000 |
| T3 | 22.37±4.57 | 10.67±5.57 | 14.883 | 0.000 |
| T4 | 28.38±6.52 | 9.09±6.76 | 18.824 | 0.000 |
Notes: T0: before operation; T1: 30 min after anesthesia induction; T2: 24 h after operation; T3: 48 h after operation; T4: 72 h after operation; sCr: serum creatinine; IL: interleukin; TLR: toll-like receptor; TNF: tumor necrosis factor; DMED: dexmedetomidine; NF-κB: nuclear factor kappa B.
Figure 3.
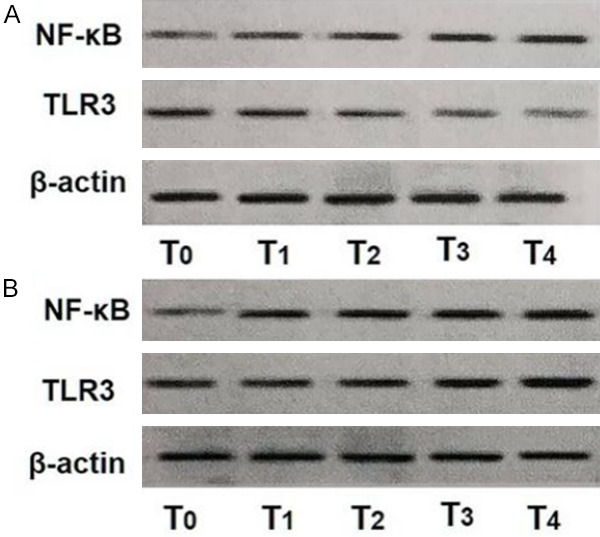
Expression levels of NF-κB (p65) and TLR3 detected by western blot. A: DMED group; B: Control group. T0: before operation; T1: 30 min after anesthesia induction; T2: 24 h after operation; T3: 48 h after operation; T4: 72 h after operation; TLR: toll-like receptor; DMED: dexmedetomidine; NF-κB: nuclear factor kappa-B.
Figure 4.
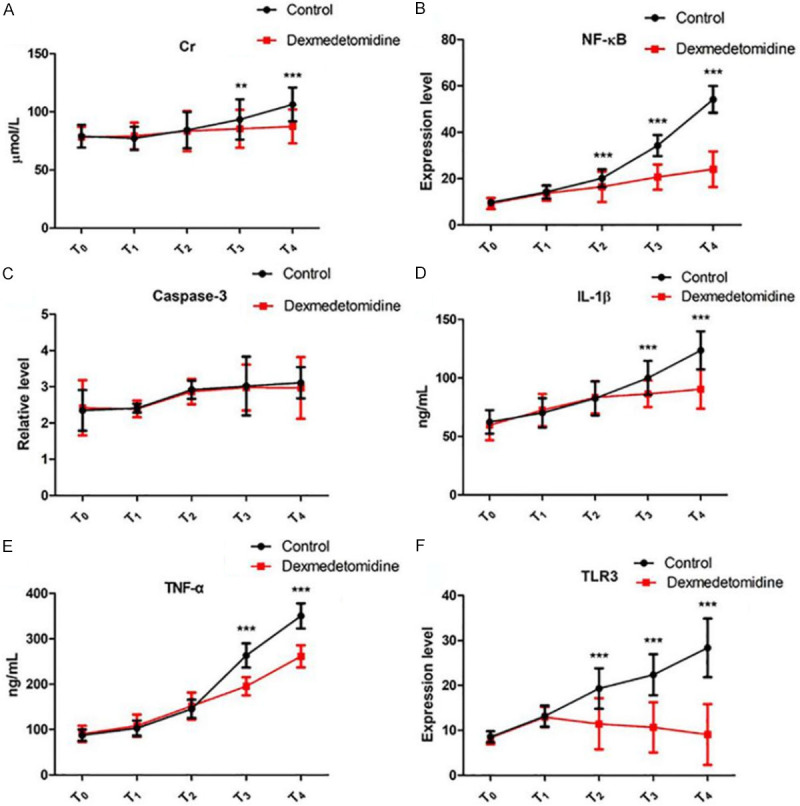
Expression levels of sCr, NF-κB, Caspase-3, IL-1β, TNF-α, and TLR3. A: sCr levels at different time points in the two groups; B: NF-κB levels at different time points in the two groups; C: Caspase-3 levels at different time points in the two groups; D: IL-1β levels at different time points in the two groups; E: TNF-α levels at different time points in the two groups; F: TLR3 levels at different time points in the two groups. Compared with the control group, **P<0.01; compared with the control group, ***P<0.001. T0: before operation; T1: 30 min after anesthesia induction; T2: 24 h after operation; T3: 48 h after operation; T4: 72 h after operation; sCr: serum creatinine; IL: interleukin; TLR: toll-like receptor; TNF: tumor necrosis factor; NF-κB: nuclear factor kappa-B.
Incidence rate of AKI between the two groups after operation
At 48 h after operation, the incidence rate of AKI in the control group was higher than that in the DMED group (19.05%, 16/84 vs. 5.95%, 5/84, χ2=6.585, P=0.010).
Discussion
Studies have demonstrated that the incidence rate of acute kidney injury (AKI) in children undergoing congenital heart surgery with CPB is about 34%, and the mortality of these AKI patients is up to 15.25% [14]. Since the incidence of AKI following congenital heart surgery can severely affect the clinical prognosis of children, it is essential to clarify the pathogenesis of AKI. Many studies have reported that the occurrence AKI after CPB in children may be related to factors such as blood glucose level, age, CPB duration, and aortic blocking period, but it is believed that postoperative systemic inflammatory reaction plays an even more important role in the pathogenesis of AKI [2,3,15]. Kwon et al. found that IL-18 is a key predictor of postoperative AKI and the level of IL-18 is associated with patients’ mortality rate [16]. Jacob et al. reported that the anti-inflammatory pretreatment with high-dose of DMED can significantly reduce the risk of AKI in heart surgery [17]. Moreover, the use of a leukocyte filter in heart surgery can alleviate the deterioration of patients’ renal function [18]. These findings suggest that inflammatory response may play an essential role in the pathogenesis of AKI. At present, most of the studies only discussed the effect of DMED on the expression levels of TLR2 and TLR4 [8-10]. The reports on the correlation between DMED and TLR3 are few. On the other hand, TLR3/NF-κB signaling pathway serves a key role in the pathogenesis of AKI, and DEMD has renal protective effect [11]. Therefore, we systematically analyzed the effects of DMED on the expression levels of TLR3 and NF-κB in PBMCs and its downstream signals TNF-α, IL-1β, and caspase-3 in children undergoing CPB.
The results in this study showed that DMED could significantly reduce the percentage of TLR3+ cells in PBMCs of children after CPB. The binding of TLR3 to the TIR domain-containing adaptor inducing interferon β can induce the activation of NF-κB through the N-terminal binding domain of tumor necrosis factor receptor-associated factor 6 [19]. NF-κB can mediate the inflammatory response through various ways, especially through regulating the expression of its downstream inflammatory factors TNF-α and IL-1β [20]. Li. et al. found that the protein expression levels of NF-κB and TNF-α can increase markedly in the renal tissues of rats with paraquat-induced AKI [21]. Ding et al. also documented there are high expression levels of IL-1β and TNF-α in the serum and renal tissues of rats with lipopolysaccharide-induced AKI [22]. In the present study, we found that the DMED group had a much lower level of NF-κB at 24 h after operation and much lower levels of TNF-α and IL-1β at 48 h and 72 h after operation than the control group. Some studies revealed that continuous infusion of 0.6 µg/kg/h DMED in the operation can markedly reduce the TNF-α and IL-1β concentration in children after CPB, indicating DMED can inhibit the inflammatory reaction of infants after CPB [23]. NF-κB can further activate caspase signal transduction pathway through Fas-associated death domain, which can eventually lead to cellular apoptosis, and caspase-3 is the main protein in the cascade reaction of caspase [24]. It has been confirmed that the protein and mRNA expression levels of caspase-3 are high in the renal tissues of an AKI animal model, and there is a correlation between the level of caspase-3 in PMBCs and the level of caspase-3 in the renal tissues [22,25]. In this study, DMED had no significant effect on the protein expression level of caspase-3 in PBMC in children. Due to ethical issues, we were unable to detect the protein expression level of caspase-3 in the renal tissue of children. However, Li et al. reported that DMED can greatly reduce the protein expression level of caspase-3 in the lung tissues in a dose-dependent manner [26]. Therefore, the effect of DMED pretreatment on the protein expression level of caspase-3 in the renal tissue of children after CPB needs to be further investigated.
At 48 h after operation, the incidence rate of AKI in the DMED group was much lower than that of the control group (5.95% vs. 19.05%), indicating that DMED has a protective effect on the renal function of children undergoing CPB. A study by Kwiatkowski et al. revealed that the incidence rate of AKI after treatment with DMED was lower than those in the control group (14% vs. 26%) in children undergoing congenital heart surgery with CPB [12]. However, the incidence rates of AKI reported in the study by Kwiatkowski et al. were higher than ours, which may be due to the fact that the children enrolled in that study had complex congenital heart disease, whereas our study only investigated children with simple congenital heart disease. At present, the level of sCr is often used as the diagnostic criteria of AKI. sCr is a common marker of renal function, but it is not sensitive as the increase of sCr level can only be detected at least 48 h after renal injury. In this study, no difference was observed in the sCr level between the two groups 24 h after operation, while the sCr in the DMED group was much lower than that in the control group 48 h after operation. This result aligned with the previous studies [27].
DMED at a dose of 0.5 µg/kg/h can achieve marked protective effect on the renal function of children undergoing congenital heart surgery with CPB, which may be related to the inhibition of the TLR3/NF-κB signaling pathway and its downstream inflammatory factors. However, we did not detect the related proteins in the renal tissues of children and did not set up different doses of DMED for comparison. Moreover, other possible mechanisms by which DMED protects the kidney in children undergoing CPB need to be investigated.
Disclosure of conflict of interest
None.
References
- 1.Kumar TK, Allen Ccp J, Spentzas Md T, Berrios Ccp L, Shah Md S, Joshi Md VM, Ballweg Md JA, Knott-Craig Md CJ. Acute kidney injury following cardiac surgery in neonates and young infants: experience of a single center using novel perioperative strategies. World J Pediatr Congenit Heart Surg. 2016;7:460–466. doi: 10.1177/2150135116648305. [DOI] [PubMed] [Google Scholar]
- 2.Sawhney S, Mitchell M, Marks A, Fluck N, Black C. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open. 2015;5:e006497. doi: 10.1136/bmjopen-2014-006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ling GX, Luo C, Li YG, Zheng BS. Research progress of acute kidney injury after cardiac surgery. Med Recapitulate. 2018;6:1103–1108. [Google Scholar]
- 4.Gao D, Jing S, Zhang Q, Wu G. Pterostilbene protects against acute renal ischemia reperfusion injury and inhibits oxidative stress, inducible nitric oxide synthase expression and inflammation in rats via the Toll-like receptor 4/nuclear factor-κB signaling pathway. Exp Ther Med. 2018;15:1029–1035. doi: 10.3892/etm.2017.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinese society of cardiothoracic anesthesia. Expert consensus on application of dexmedetomidine in cardiovascular anesthesia and perioperative period (2018) J Clin Anesthesiol. 2018:914–917. [Google Scholar]
- 6.Shi QX, Han DN, Jia M, Hou XT. Risk factors of acute kidney injury after cardiopulmonary bypass. Chin J Evi-based Cardiovasc Med. 2017;9:452–455. [Google Scholar]
- 7.Zhai M, Kang F, Han M, Huang X, Li J. The effect of dexmedetomidine on renal function in patients undergoing cardiac valve replacement under cardiopulmonary bypass: a double-blind randomized controlled trial. J Clin Anesth. 2017;40:33–38. doi: 10.1016/j.jclinane.2017.03.053. [DOI] [PubMed] [Google Scholar]
- 8.Luo L, Wei X, Li MX. Effect of dexmedetomidine on peripheral blood monocyte signaling pathway in patients undergoing open heart surgery under cardiopulmonary bypass. Chin J Extracorpor Circ. 2018;1:33–37. [Google Scholar]
- 9.Liu YF, Cong L, Shi F, Wang B. Effect of dexmedetomidine on the expression of TLR2 and TLR4 in peripheral blood mononuclear cells of patients undergoing pulmonary lobectomy. Chin J Anesthesiol. 2015;35:1044–1046. [Google Scholar]
- 10.Chen XF, Hu JT, Zhang C, Pan YP, Tian DS, Kuang FF, Tang ZH. Pulmonary protective effect of dexmedetomidine sedation on sepsis complicated with ARDS. Chin Crit Care Med. 2018;30:151–155. [Google Scholar]
- 11.Li SS, Luo DQ. Effect of dexmedetomidine on acute kidney injury after operation of congenital heart disease in infants. Chin J Cardiovasc Res. 2018;16:840–844. [Google Scholar]
- 12.Kwiatkowski DM, Axelrod DM, Sutherland SM, Tesoro TM, Krawczeski CD. Dexmedetomidine is associated with lower incidence of acute kidney injury after congenital heart surgery. Pediatr Crit Care Med. 2016;17:128–134. doi: 10.1097/PCC.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 13.Vives M, Hernandez A, Parramon F, Estanyol N, Pardina B, Muñoz A, Alvarez P, Hernandez C. Acute kidney injury after cardiac surgery: prevalence, impact and management challenges. Int J Nephrol Renovasc Dis. 2019;12:153–166. doi: 10.2147/IJNRD.S167477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan L, Hu GH, Jiang M, Zhang CL. Clinical characteristics and prognosis of children with acute kidney injury after cardiopulmonary bypass for congenital heart disease. Chin J Contemp Pediatr. 2017;19:1196–1201. doi: 10.7499/j.issn.1008-8830.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Yi LW, Yang GX, Chen RW, Huang P. Risk factors of acute kidney injury after cardiopulmonary bypass in children with noncyanotic congenital heart disease. Chin Pediatr Integr Tradit Western Med. 2017;9:155–158. [Google Scholar]
- 16.Kwon JT, Jung TE, Lee DH. Predictive risk factors of acute kidney injury after on-pump coronary artery bypass grafting. Ann Transl Med. 2019;7:44. doi: 10.21037/atm.2018.12.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob KA, Leaf DE, Dieleman JM, van Dijk D, Nierich AP, Rosseel PM, van der Maaten JM, Hofland J, Diephuis JC, de Lange F, Boer C, Kluin J, Waikar SS. Intraoperative high-dose dexamethasone and severe AKI after cardiac surgery. J Am Soc Nephrol. 2015;26:2947–2951. doi: 10.1681/ASN.2014080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scrascia G, Guida P, Rotunno C, de Luca Tupputi Schinosa L, Paparella D. Anti-inflammatory strategies to reduce acute kidney injury in cardiac surgery patients: a meta-analysis of randomized controlled trials. Artif Organs. 2014;38:101–112. doi: 10.1111/aor.12127. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Zhou N, Wu XN, Cao HJ, Sun YJ, Zhang TZ, Chen KY, Yu DM. Role of the Toll-like receptor 3 signaling pathway in the neuroprotective effect of sevoflurane pre-conditioning during cardiopulmonary bypass in rats. Mol Med Rep. 2015;12:7859–7868. doi: 10.3892/mmr.2015.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong HR, Lei C, Xiong LZ. Establishment of risk prediction model for acute kidney injury after cardiac surgery: literature analysis. Chin J Anesthesiol. 2020;40:18–26. [Google Scholar]
- 21.Li J, Zhang JF, Lu JY, Ye Z, Zhou ZB, Luo YF, Li S. Changes of renal histopathology, expression of TNF-α and NF-κB and serum IL-6 level in rats with acute kidney injury induced by paraquat poisoning after intragastric administration of captopril. Shandong Med J. 2018;47:51–55. [Google Scholar]
- 22.Ding RY, Zhao DM, Hu ZW, Wang L, Li X, Sun YN, Zhang ZD, Ma XC. Rho kinase inhibitor attenuates lipopolysaccharide induced renal injury by inhibiting toll like receptor 4 and nuclear factor kappa B signaling pathway. J Chin Med Univ. 2018;47:1–5. [Google Scholar]
- 23.Gao Y, Shi L, Wang JX, Zhao HT, Gao JG. Effects of different doses of dexmedetomidine on inflammatory factors in infants after cardiopulmonary bypass. Hebei Med J. 2016;38:177–180. [Google Scholar]
- 24.Rathnasamy G, Sivakumar V, Rangarajan P, Foulds WS, Ling EA, Kaur C. NF-κB-mediated nitric oxide production and activation of caspase-3 cause retinal ganglion cell death in the hypoxic neonatal retina. Invest Ophthalmol Vis Sci. 2014;55:5878–5889. doi: 10.1167/iovs.13-13718. [DOI] [PubMed] [Google Scholar]
- 25.Adil M, Kandhare AD, Visnagri A, Bodhankar SL. Naringin ameliorates sodium arsenite-induced renal and hepatic toxicity in rats: decisive role of KIM-1, Caspase-3, TGF-β, and TNF-α. Ren Fail. 2015;37:1396–1407. doi: 10.3109/0886022X.2015.1074462. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Chen Q, He X, Alam A, Ning J, Yi B, Lu K, Gu J. Dexmedetomidine attenuates lung apoptosis induced by renal ischemia-reperfusion injury through α(2)AR/PI3K/Akt pathway. J Transl Med. 2018;16:78. doi: 10.1186/s12967-018-1455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YH, Fang F. Acute kidney injury and biomarkers for early diagnosis. Chin J Pract Pediatri. 2018;33:109–113. [Google Scholar]


