Abstract
Objective: To explore the intervention effect of exercise rehabilitation therapy on patients with type 2 diabetic osteoporosis. Methods: From August 2017 to November 2019, 117 patients with type 2 diabetic osteoporosis who received treatment in Nanhua Hospital affiliated to Nanhua University were selected. Among them, 54 cases were given routine treatment in the control group (CG), and 63 cases were given exercise rehabilitation therapy on the basis of routine treatment in the study group (SG). The blood glucose level, bone mineral density, quality of life, VAS score, therapeutic effect and adverse reactions were compared between the two groups. Results: After treatment, the blood glucose level and VAS score in the SG were obviously lower than those in the CG, while the bone mineral density, quality of life and clinical total effective rate were obviously higher than those in the CG, and the adverse reactions were lower than those in the CG. Conclusion: Exercise rehabilitation therapy can significantly improve the symptoms and the quality of life of patients with type 2 diabetic osteoporosis.
Keywords: Exercise rehabilitation therapy, type 2 diabetic osteoporosis, bone mineral density, VAS
Introduction
Around the world, approximately 1 in every 11 adults has diabetes, which is considered as the ninth primary cause of death [1]. Type 2 diabetes accounts for more than 90% of patients with diabetes mellitus (T2D) and results in microvascular and macro-vascular complications, which brings profound psychological and physiological troubles to patients and nursing staff and a heavy burden to the medical system [2]. Postmenopausal Osteoporosis (PMO) is an increasingly serious metabolic bone disease in the world, which generally develops in middle-aged and elderly women and postmenopausal women, and it is characterized by low bone mass and microstructure degradation of bone tissues [3]. Studies have shown that there are complex pathophysiological effects between them: T2D directly affects bone metabolism and strength. Some antidiabetic drugs affect bone metabolism, and diabetes complications are related to the risk of falls and subsequent fractures [4]. Patients with T2D have higher trabecular density and lower cortical bone density, which leads to lower bone intensity [5].
Diabetes is not only related to mortality, but also to the decrease of patients’ activities. Patients with type 2 diabetes have an increased risk of muscular atrophy, especially senile patients. Therefore, elderly patients with T2D have a higher risk of fractures, which is directly related to the reduction of activities of daily living [6]. Studies have revealed that physical exercise or proper exercise can prevent the development of osteoporosis, and the exercise has been used as the prevention and treatment of non-drug osteoporosis by WHO [7]. The exercise can successfully maintain or increase bone mineral density (BMD). In addition, it can enhance muscle strength around bones, thus improving posture stability and muscle strength and reducing the incidence of harmful falls [8]. At the same time, the increase of exercise can improve cardiopulmonary function, muscular strength, insulin resistance, glucose and lipids metabolism, and reduce body weight and fat, blood pressure and inflammation cytokines [9]. Exercise is also a crucial factor to prevent muscular atrophy in patients with T2D. Appropriate training has been proved to be effective in improving gait speed, balance, muscular strength and joint flexibility in diabetic patients [10]. Studies have revealed that an aerobic endurance comprehensive exercise for 12 weeks is very effective for patients with T2DM in improving their muscle strength and fatigue, blood sugar control and health-related quality of life (HRQoL) [11]. These data suggest better clinical care in T2DM patients with aerobic exercise and resistance exercise.
This study was designed to intervene the patients with type 2 diabetic osteoporosis through moderate intensity aerobic exercise to understand the influence of aerobic exercise, so as to provide more clinical data for the diagnosis and treatment of type 2 diabetic osteoporosis.
Materials and methods
Research objects
From August 2017 to November 2019, 117 patients with type 2 diabetic osteoporosis who received treatment in Nanhua Hospital affiliated to Nanhua University were selected and randomly divided into study (SG) and control (CG) group. Among them, 54 cases were treated with conventional therapy in CG, including 23 male subjects and 31 female subjects, with a mean age of (62.04±5.33) years old. In addition, 63 patients in SG were treated with exercise rehabilitation therapy on the basis of routine treatment, including 29 male patients and 34 female patients, with an average age of (63.73±5.64) years old. Inclusion criteria: The patient was diagnosed as type 2 diabetes in accordance with the world Health Organization (WHO) criteria for diagnosis and classification of diabetes: symptoms+random blood sugar ≥11.1 mmol /L, fasting blood sugar (FPG) ≥7.0 mmol/L or plasma glucose ≥11. 1mmol/L in OGTT for 2 hours. The patient was diagnosed as T2DM. The patients had no long-term bedridden history and did not take glucocorticoid, vitamin D, calcium and other drugs that affected bone metabolism. The patients had no limb dysfunction and were able to complete the exercise training in this experiment. The osteoporosis criteria were based on the T score of the BMD as assessed by dual-energy X-ray absorptiometry (DXA) at the femoral neck or spine. The T-score (BMD of hip and spine) was divided into: A T score of -1.0 or above was normal; A T score between -1.0 and -2.5 was considered low bone mass (osteopenia); A T score of -2.5 or less was considered osteoporosis. Exclusion criteria were as below: patients with other types of diabetes; patients with secondary osteoporosis; patients who took drugs that affected bone metabolism; patients with severe cardio-cerebrovascular disease, liver, kidney and psychosis within 3 months; patients with diabetes ketoacidosis and other acute metabolic disorders and complicated by infections, pregnancy and lactation in recent 1 month; patients with severe hypertension; patients with hyperuricemia. In this study, the family members have signed informed consent, and this experiment has been approved by the hospital ethics committee.
Outcome measures
In the two groups, the changes of blood glucose, glycosylated hemoglobin, bone mineral density, serum bone metabolism indexes (tartrate-resistant acid phosphatase-5b (TRACP-5b), bone-specific alkaline phosphatase (BALP) level and osteocalcin (OC) level) and pain score were observed and recorded before and after exercise therapy, and the changes of quality of life and adverse drug reactions were observed.
Routine detection
(1) Routine experimental tests were as follows: blood and urine routine; liver and kidney function; fasting blood glucose (FPG); blood glucose (2hPG) after meal for 2 h.
(2) Detection of glycosylated hemoglobin (HbA1c): The level of glycosylated hemoglobin was measured by D-10 automatic glycated hemoglobin analyzer produced by American Bio-Rad Company and related supporting reagents.
(3) Evaluation of bone metabolism index: The levels of blood calcium, phosphorus, BALP and OC were measured by electrochemiluminescence automatic immune analyzer. The enzyme-linked immunosorbent assay was applied to measure the level of tartrate-resistant acid phosphatase 5b (TRACP-5b).
(4) Evaluation of bone mineral density: The GE LunarProdigy AdvancePA+300164 dual-energy X-ray absorptiometry (DEXA) (USA) was applied. The subjects were measured in supine position, and the positions were in the 2nd to 4th lumbar vertebrae of the anterior position (L2~4), the left femoral neck (Neck) and the triangle of the Wards. The differences between groups were compared.
Treatment methods
In both groups, patients received conventional medication, routine nursing and health education. They were given routine life intervention (adjusting diet structure and arranging meals reasonably). Patients were orally taken hypoglycemic drugs (acarbose tablets 50 mg once a day) or subcutaneously injection with insulin at 10:00 PM (insulin glargine 8~30 U·d-1). At the same time, patients were orally taken calcium carbonate D3 tablets (Wyeth, USA) once a day, two tablets each time (each tablet contains 600 mg calcium and D125IU vitamin D) and alendronate sodium (Foshanmei, Hangzhou Merck Pharmaceutical Co., Ltd.) (SFDA Approval No. J20130085) 70 mg/week. The alendronate sodium should be taken with a full glass of white water before breakfast, and should be avoided to stay in bed and eat for at least 30 minutes after taking the medicine. The exercise therapy was not required in CG.
In SG, patients exercised on the basis of the original treatment plan. First, family members or caregivers were trained as assistants to help patients complete their training. The exercise program was developed according to the specific conditions of the patient. The amount of daily exercise was determined on the basis of a fixed diet, which was determined by three factors: intensity, time and frequency of exercise. The intensity of exercise was measured by self-measured pulse, and the suitable pulse rate of exercise was 170 minus age (years old). Considering the older intervention subjects, the actual exercise pulse rate was adjusted to 85% of the appropriate pulse rate in this study.
Exercise mode: Patients arranged exercise modes according to their own habits and characteristics, including running, gymnastics, dance, mountain climbing, swimming, playing ball, shadow boxing, qigong, dancing, climbing stairs and other sports.
Exercise time: It was usually arranged in 60-90 minutes after meal, 2-3 times a day, about 1-1.5 hours of exercise per day, mainly aerobic exercise.
Exercise intensity: It could stimulate muscles to a certain extent and patients needed to have a sense of movement. Patients needed to maintain a moderate intensity of exercise, and the simple method was applied: the pulse rate during exercise = (170-age) × 85%. The exercise time span was 6 months, and the exercise therapy was supervised by both doctors and patients to complete and keep its regularity and persistence.
Criteria of pain score
The Visual analogue scale (VAS) was used. A score of 0-10 meant different levels of pain: A score of 0 meant no pain; A score of 1-3 meant mild and tolerable pain; A score of 4-6 meant that the patient pained, the sleep were affected and the patient could tolerate it. A score of 7 to 10 indicated that the patient had intense pain, which was unbearable, and affected appetite and sleep.
Efficacy evaluation
Evaluation criteria of clinical efficacy
Markedly effective: The symptoms disappeared, the bone mineral density was increased by > 2% by DXA, and biochemical indexes of bone metabolism improved obviously; FPG and 2hPG decreased to normal range, or FPG or 2hPG decreased more than 40% before treatment; HbA1c was less than or equal to 6.2%, or decreased by more than 30% before treatment.
Effective: The symptoms were obviously alleviated, the bone mineral density was increased by 1%~2% by DXA, and the biochemical indexes of bone metabolism were improved to some extent; FPG and 2hPG decreased by more than 20% before treatment, but did not reach the standard of markedly effective; HbA1c decreased by more than 10% before treatment, but did not reach the standard of markedly effective.
Ineffective: After treatment, the symptoms did not change significantly, the bone mineral density was increased by < 1% by DXA, and the biochemical indexes of bone metabolism did not change. The FPG, 2hPG and HbA1c did not decrease, or the decrease did not reach the effective standard.
Quality of life survey
The sF-36 health survey questionnaire was used for investigation. It was composed of 8 dimensions and 36 items in total, including social function (SF), emotional function (RE), physiological function (PF), physiological role (RP), general health (GH), physical pain (BP), vitality (VT) and mental health (MH). When scoring, each item was processed accordingly and scored according to each item. Then, the score was converted to a standard score of 0-100 according to the standard points conversion formula. The higher the score, the better the quality of life is. In both groups, patients were followed up before the study and at 6 months after intervention for SF-36 health Survey scale scores.
Adverse reactions
In the two groups, the adverse drug reactions during treatment and the adverse reactions during exercise were observed and recorded.
Statistical methods
SPSS 19.0 (Asia Analytics Formerly SPSS China) was applied to complete the relevant data analysis. The counting data were recorded and represented by percentage and tested via χ2 test. The measurement data were evaluate and represented as mean ± sd and tested via t test. P < 0.05 was statistically significant.
Results
Baseline data
Before treatment, there was no statistical difference in gender, age, body mass index (BMI), cholesterol and triglyceride between the two groups (P > 0.05), which was comparable (Table 1).
Table 1.
Baseline data
| Factors | SG (n=63) | CG (n=54) | χ2/t | P |
|---|---|---|---|---|
| Gender | 0.139 | 0.709 | ||
| Male | 29 (46.03) | 23 (42.59) | ||
| Female | 34 (53.97) | 31 (57.41) | ||
| Age/years old | 63.73±5.64 | 62.04±5.33 | 1.657 | 0.100 |
| BMI (kg/m2) | 24.64±3.81 | 24.73±3.74 | 0.128 | 0.898 |
| Cholesterol (mmol/L) | 5.55±0.91 | 5.45±0.69 | 0.661 | 0.510 |
| Triglyceride (mmol/L) | 1.83±0.87 | 1.75±0.78 | 0.520 | 0.604 |
| High density lipoprotein (mmol/L) | 0.99±0.21 | 0.97±0.19 | 0.536 | 0.593 |
| Urinary albumin (mmol/L) | 21.41±1.21 | 21.02±1.42 | 1.604 | 0.111 |
Changes of diabetes control indexes before and after treatment in both groups
After treatment for 6 months, all the control indexes of diabetes were obviously improved, and FPG, 2hPG and HbA1c were obviously decreased, with statistical significance (P < 0.05). The improvement of patients in the SG was obviously better than that of patients in the CG (P < 0.05) (Figure 1).
Figure 1.
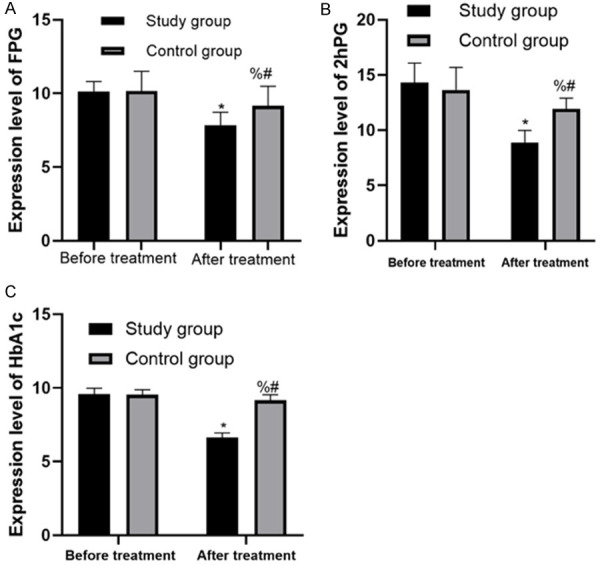
Changes of diabetes control indexes before and after treatment in the two groups. A. Changes of FPG in the two groups before and after treatment; B. Changes of 2hPG in the two groups before and after treatment; C. Changes of HbA1c in the two groups before and after treatment. * represents the comparison with the SG before treatment, P < 0.05; % represents the comparison with the SG after treatment, P < 0.05; # represents the comparison with the CG before treatment, P < 0.05.
Changes of biochemical indexes related to bone mineral density and bone metabolism before and after treatment
There was no statistic difference in serum TRACP-5, BALP, blood calcium, blood phosphorus and OC between the two groups before intervention (P > 0.05). After intervention for 6 months, the levels of serum TRACP-5 and blood calcium were significantly decreased, while the levels of serum BALP, blood phosphorus and OC were significantly increased compared with those before intervention (P < 0.05). The improvement of patients in the SG was obviously better than that of patients in the CG (P < 0.05). We found that the bone density of L2-4, femoral neck and Wards triangle of patients in the two groups increased to varying degrees after treatment for 6 months, and the increase in bone density was more significant in the SG, and the change rate was statistically different from that in the CG (P < 0.05) (Table 2).
Table 2.
Comparison of biochemical indexes related to BMD and bone metabolism before and after treatment
| Factors | SG (n=63) | t | P | CG (n=54) | t | P | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Before treatment | After treatment | Before treatment | After treatment | |||||
| Serum phosphorus (mmol/L) | 1.03±0.12 | 1. 46±0.22 | 13.619 | < 0.001 | 1. 06±0.15 | 1.22 ±0.21a | 4.556 | < 0.001 |
| Blood calcium (mmol/L) | 2.61±0.48 | 2. 23±0.15 | 5.998 | < 0.001 | 2.59±0.58 | 2.38±0.29a | 2.380 | 0.019 |
| BALP (mmol/L) | 12.95±2.43 | 19.12±3.20 | 12.188 | < 0.001 | 13.13±2.21 | 16.42±2.63a | 7.037 | < 0.001 |
| TRACP-5b (U·L-1) | 9.04±2.19 | 4.84±1.06 | 13.702 | < 0.001 | 8.96±1.95 | 6.12±1.26a | 8.989 | < 0.001 |
| Osteocalcin (ng·ml-1) | 6.55±1.41 | 9.43±2.85 | 7.189 | < 0.001 | 6.67±1.39 | 8.20±2.05a | 4.539 | < 0.001 |
| Femoral neck BMD | 0.858±0.130 | 0.967±0.145 | 4.442 | < 0.001 | 0.858±0.121 | 0.859±0.135a | 0.041 | 0.968 |
| W triangle bone mineral density | 0.751±0.159 | 0.875±0.153 | 4.460 | < 0.001 | 0.755±0.122 | 0.765±0.132a | 0.409 | 0.683 |
| L2-4 bone mineral density | 0.868±0.118 | 1.022±0.137 | 6.760 | < 0.001 | 0.862±0.139 | 0.866±0.118a | 0.161 | 0.872 |
means the comparison with the SG after treatment, P < 0.05.
Comparison of pain scores between the two groups before and after treatment
Before therapy, there was no statistic difference in VAS pain scores in both groups (P > 0.05). After therapy for 6 months, the VAS pain scores were improved in both groups compared with those before treatment, and the improvement of patients in the SG was significantly better than that of patients in the CG, and the difference was statistically significant (P < 0.01) (Figure 2).
Figure 2.
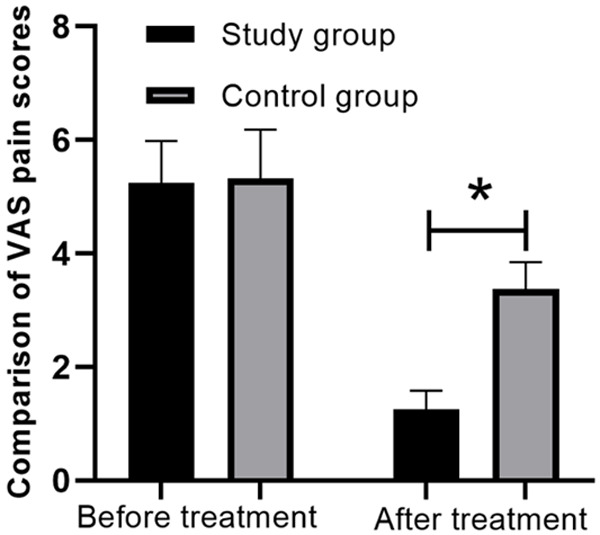
Comparison of bone pain scores between the two groups before and after treatment. *P < 0.05.
Quality of life scores in both groups before and after treatment
There was no statistical difference in the scores of quality of life in both groups before treatment (P > 0.05). After treatment for 6 months, there was no statistical difference in the scores before treatment in CG (P > 0.05). The scores of general health, vitality, social function, emotional function and mental health increased in SG, and there were statistical differences between the SG and the CG (P < 0.05) (Table 3).
Table 3.
Quality of life scores in both groups before and after treatment
| Groups | Physiological function (PF) | Physiological role (RP) | Physical pain (BP) | General health (GH) | Vitality (VT) | Social function (SF) | Emotional function (RE) | Mental health (MH) | |
|---|---|---|---|---|---|---|---|---|---|
| CG (n=54) | Before treatment | 68.64±16.55 | 39.33±20.71 | 52.27±15.17 | 46.75±10.85 | 48.38±19.51 | 64.39±18.98 | 54.10±25.53 | 57.68±16.17 |
| After treatment | 71.24±14.19 | 41.19±19.87 | 50.54±10.70 | 44.25±11.06 | 47.42±18.93 | 63.57±15.98 | 49.04±19.36 | 55.15±19.74 | |
| t | 0.876 | 0.476 | 0.689 | 1.186 | 0.260 | 0.243 | 1.161 | 0.729 | |
| P | 0.383 | 0.635 | 0.495 | 0.238 | 0.796 | 0.809 | 0.248 | 0.468 | |
| SG (n=63) | Before treatment | 70.24±13.98 | 39.21±19.78 | 54.35±14.68 | 46.92±12.15 | 47.30±20.30 | 62.81±20.77 | 52.58±28.20 | 54.08±18.22 |
| After treatment | 71.35±13.54 | 42.36±15.37 | 53.49±17.09 | 75.29±14.95a | 68.64±17.41a | 75.77±18.50a | 71.22±17.93a | 73.69±14.40a | |
| t | 0.453 | 0.998 | 0.303 | 11.689 | 6.334 | 3.698 | 4.427 | 6.702 | |
| P | 0.652 | 0.320 | 0.762 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
means the comparison with the CG after treatment, P < 0.05.
Comparison of clinical efficacy between the two groups after treatment
In CG, there were 4 cases with markedly effective, 35 cases with effective and 15 cases with ineffective after conventional treatment. After exercise therapy intervention for 6 months, there were 13 cases with markedly effective, 46 cases with effective and 4 cases with ineffective in SG. The total clinical effective rate of patients in the SG (93.65%) was significantly higher than that of patients in the CG (72.22%), and the difference was statistically significant (P < 0.01) (Table 4).
Table 4.
Efficacy in the two groups after treatment (n, %)
| Groups | Markedly effective | Effective | Ineffective | Total effective rate |
|---|---|---|---|---|
| SG (n=63) | 13 (20.63%) | 46 (73.02%) | 4 (6.35%) | 93.65% |
| CG (n=54) | 4 (7.41%) | 35 (64.81%) | 15 (27.78%) | 72.22% |
Comparison of adverse effects between the two groups
There were 6 cases in SG and 7 cases in CG who had adverse reactions during drug treatment in the two groups, all of which were nausea and upper abdominal discomfort, with mild symptoms and no interruption of treatment. There was no statistically obvious difference in both groups (P > 0.05). During exercise, 7 patients had mild hypoglycemia reactions such as palpitation and hunger, and their symptoms improved after taking sugar water orally, without serious adverse consequences (Table 5).
Table 5.
Comparison of adverse reactions between the two groups (n, %)
| Adverse reactions | SG | CG | P |
|---|---|---|---|
| Nausea | 3 (4.76) | 4 (7.41) | 0.702 |
| Upper abdominal discomfort | 3 (4.76) | 3 (5.56) | > 0.999 |
| Palpitation | 4 (6.35) | 0 | 0.123 |
| Hypoglycemic reaction | 3 (4.76) | 0 | 0.248 |
Discussion
The incidence of chronic illnesses such as diabetes is increasing year by year [12,13]. China is the country with the largest population of diabetes in the world, and the age-standardized incidence rates of male and female diabetic patients are 9.6 and 9.2 per 1,000 people per year, respectively [14]. T2DM is also a metabolic disease characterized by chronic hyperglycemia and various complications, such as cardio-vascular disease, obesity, microangiopathy and renal failure [15]. At the same time, the damage of glucose metabolism has many adverse effects on bone remodeling, such as reducing bone mass and increasing fracture risk [16]. The development of microvascular and macrovascular diseases in diabetes may increase the fracture risk of osteoporosis patients [17].
At present, exercise therapy has been reported as an effective method to ameliorate blood sugar control and quality of life [18]. Diet and exercise therapy constitute the basis of the treatment of type 2 diabetes. However, dietary adjustment alone cannot reduce fat and improve insulin resistance, while exercise therapy lasting at least 6 months is helpful to improve HbA1c of patients with T2D [19]. Therefore, this paper was designed to intervene in type 2 diabetic osteoporosis in combination with exercise therapy on the basis of conventional treatment and explore whether exercise therapy can improve the symptoms of type 2 diabetic osteoporosis. Previous studies have shown that aerobic exercise and melatonin can improve type 2 diabetic osteoporosis. By increasing the exercise time of rats with type 2 diabetes, glucose metabolism can be regulated, thus effectively reducing calcium, improving bone density and relieving osteoporosis [20]. In addition, Rehling T et al. have reported that exercise training may not only have a positive impact on the pain of musculoskeletal system, but also have a positive impact on blood sugar control for patients with type 2 diabetes and osteoporosis, osteoarthritis or rheumatoid arthritis. Therefore, exercise training may reduce the diabetic complications and mortality of patients with type 2 diabetes [21]. In our research, through the intervention of exercise therapy, we found that the diabetes indexes (blood sugar and glycosylated hemoglobin) in the SG were significantly lower than those in the CG, while BMD was significantly higher, and the improvement range of effective biochemical indexes related to bone metabolism was significantly better than that in the CG. These results suggested that the symptoms of type 2 diabetic osteoporosis could be improved by exercise rehabilitation therapy, which was consistent with the conclusions of the above scholars. We also analyzed VAS score and quality of life score, and found that the improvement range in the SG was obviously better than that in the CG, and the total clinical effective rate in the SG was higher than that in the CG. Therefore, the application of routine treatment and proper exercise training in the treatment of type 2 diabetic osteoporosis was conducive to improving BMD level, lowering blood sugar and improving the quality of life of patients.
To sum up, the exercise rehabilitation therapy can effectively improve the quality of life in patients with type 2 diabetic osteoporosis and improve the symptoms of type 2 diabetic osteoporosis, so as to prevent the fractures. This method may become an effective means for early non-surgical treatment of type 2 diabetic osteoporosis. However, there are some limitations in this paper. For example, the sample is relatively insufficient, so more patients will be enrolled in the future to analyze the predictive factors of exercise effect.
Disclosure of conflict of interest
None.
References
- 1.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 3.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, Hope S, Kanis JA, McCloskey EV, Poole KES, Reid DM, Selby P, Thompson F, Thurston A, Vine N National Osteoporosis Guideline Group (NOGG) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12:43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dede AD, Tournis S, Dontas I, Trovas G. Type 2 diabetes mellitus and fracture risk. Metabolism. 2014;63:1480–1490. doi: 10.1016/j.metabol.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Ho-Pham L, Chau P, Do A, Nguyen H, Nguyen T. Type 2 diabetes is associated with higher trabecular bone density but lower cortical bone density: the Vietnam Osteoporosis Study. Osteoporosis International. 2018;29:2059–2067. doi: 10.1007/s00198-018-4579-5. [DOI] [PubMed] [Google Scholar]
- 6.Akagawa M, Miyakoshi N, Kasukawa Y, Ono Y, Yuasa Y, Nagahata I, Sato C, Tsuchie H, Nagasawa H, Hongo M, Shimada Y. Effects of activated vitamin D, alfacalcidol, and low-intensity aerobic exercise on osteopenia and muscle atrophy in type 2 diabetes mellitus model rats. PLoS One. 2018;13:e0204857. doi: 10.1371/journal.pone.0204857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong X, Chen X, Zhang S, Huang M, Shen X, Xu J, Zou J. The effect of exercise on the prevention of osteoporosis and bone angiogenesis. BioMed Res Int. 2019;2019:8171897. doi: 10.1155/2019/8171897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues IB, Armstrong JJ, Adachi JD, MacDermid JC. Facilitators and barriers to exercise adherence in patients with osteopenia and osteoporosis: a systematic review. Osteoporos Int. 2017;28:735–745. doi: 10.1007/s00198-016-3793-2. [DOI] [PubMed] [Google Scholar]
- 9.Yanai H, Adachi H, Masui Y, Katsuyama H, Kawaguchi A, Hakoshima M, Waragai Y, Harigae T, Hamasaki H, Sako A. Exercise therapy for patients with type 2 diabetes: a narrative review. J Clin Med Res. 2018;10:365–369. doi: 10.14740/jocmr3382w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mavros Y, Kay S, Anderberg KA, Baker MK, Wang Y, Zhao R, Meiklejohn J, Climstein M, O’Sullivan A, de Vos N, Baune BT, Blair SN, Simar D, Rooney K, Singh N, Fiatarone Singh MA. Changes in insulin resistance and HbA1c are related to exercise-mediated changes in body composition in older adults with type 2 diabetes: interim outcomes from the GREAT2DO trial. Diabetes Care. 2013;36:2372–2379. doi: 10.2337/dc12-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomas-Carus P, Ortega-Alonso A, Pietilainen KH, Santos V, Goncalves H, Ramos J, Raimundo A. A randomized controlled trial on the effects of combined aerobic-resistance exercise on muscle strength and fatigue, glycemic control and health-related quality of life of type 2 diabetes patients. J Sports Med Phys Fitness. 2016;56:572–578. [PubMed] [Google Scholar]
- 12.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 13.Harding JL, Andes LJ, Gregg EW, Cheng YJ, Weir HK, Bullard KM, Burrows NR, Imperatore G. Trends in cancer mortality among people with vs without diabetes in the USA, 1988-2015. Diabetologia. 2020;63:75–84. doi: 10.1007/s00125-019-04991-x. [DOI] [PubMed] [Google Scholar]
- 14.Gotham K, Unruh K, Lord C. Depression and its measurement in verbal adolescents and adults with autism spectrum disorder. Autism. 2015;19:491–504. doi: 10.1177/1362361314536625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14:1483–1496. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdulameer SA, Sahib MN, Sulaiman SAS. The prevalence of osteopenia and osteoporosis among malaysian type 2 diabetic patients using quantitative ultrasound densitometer. Open Rheumatol J. 2018;12:50–64. doi: 10.2174/1874312901812010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldshtein I, Nguyen AM, dePapp AE, Ish-Shalom S, Chandler JM, Chodick G, Shalev V. Epidemiology and correlates of osteoporotic fractures among type 2 diabetic patients. Archives of Osteoporosis. 2018;13:15. doi: 10.1007/s11657-018-0432-x. [DOI] [PubMed] [Google Scholar]
- 18.Nicolucci A, Balducci S, Cardelli P, Cavallo S, Fallucca S, Bazuro A, Simonelli P, Iacobini C, Zanuso S, Pugliese G Italian Diabetes Exercise Study Investigators. Relationship of exercise volume to improvements of quality of life with supervised exercise training in patients with type 2 diabetes in a randomised controlled trial: the Italian Diabetes and Exercise Study (IDES) Diabetologia. 2012;55:579–588. doi: 10.1007/s00125-011-2425-9. [DOI] [PubMed] [Google Scholar]
- 19.Miyauchi M, Toyoda M, Kaneyama N, Miyatake H, Tanaka E, Kimura M, Umezono T, Fukagawa M. Exercise therapy for management of type 2 diabetes mellitus: superior efficacy of activity monitors over pedometers. J Diabetes Res. 2016;2016:5043964. doi: 10.1155/2016/5043964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jing HF, Wang XM. Effects of aerobic exercise combined with melatonin on osteoporosis of type II diabetic rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2017;33:252–256. doi: 10.12047/j.cjap.5395.2017.062. [DOI] [PubMed] [Google Scholar]
- 21.Rehling T, Bjørkman AD, Andersen MB, Ekholm O, Molsted S. Diabetes is associated with musculoskeletal pain, osteoarthritis, osteoporosis, and rheumatoid arthritis. J Diabetes Res. 2019;2019:6324348. doi: 10.1155/2019/6324348. [DOI] [PMC free article] [PubMed] [Google Scholar]


