Abstract
Background: To investigate the effect of bevacizumab combined with chemotherapy on the metastasis response rate, survival time of patients with metastatic colorectal cancer (mCRC), the incidence of complications, and the efficacy and safety of bevacizumab for mCRC were recorded. Methods: Of 87 patients with mCRC, 42 were treated without bevacizumab (control group, CG) and 45 were treated with bevacizumab (observation group, OG). Baseline characteristics, resectability of metastases, quality of life (QOL), and short- and long-term curative effect were compared to evaluate the safety of the treatment plan in the two groups. Results: After 6 months of treatment, the overall response rate (ORR) and disease control rate (DCR) of the CG were 28.57% and 59.52%, respectively, whereas the ORR and DCR of the OG were notably higher at 48.89% and 86.67%, respectively (P < 0.05). The resectability rate of metastases in the OG increased from 8.89% pretreatment to 40.00% posttreatment, whereas that of metastases in the CG increased from 11.90% pretreatment to 23.81% posttreatment. In the OG, the median survival time was 23.0 (range, 19.7-26.3) months, and the median progression-free survival (PFS) was 11.0 (range, 9.4-12.6) months. These results were all superior to those of the CG, which were 14.0 (range, 12.6-15.4) months and 6.0 (range, 4.9-7.2) months, respectively. Conclusion: Bevacizumab combined with first-line chemotherapy can significantly prolong survival and PFS, improve QOL, increase the resectability rate of metastases, and improve survival outcomes of patients with mCRC.
Keywords: Bevacizumab, chemotherapy, colorectal cancer, tumor metastasis, efficacy
Introduction
The incidence of colorectal cancer (CRC) is ranked the third with respect to the incidence of malignant tumors worldwide, and it ranks the fifth with respect to cancer-related deaths in the Chinese population. The incidence rate has increased significantly in recent years. Approximately 50% of patients have metastasized CRC, and they have lost the opportunity to undergo surgical treatment at the time of diagnosis [1]. The 5-year survival rate of CRC patients can reach 90% in the early stage of surgical treatment, but that of patients with metastatic CRC (mCRC) is only 10% [2]. Therefore, clinical research for identifying methods to improve the mCRC survival rate is ongoing [3].
With recent developments in molecular biology, targeted therapy has gradually gained attention in treating malignant tumors. Numerous investigations have shown that targeted therapy can remarkably extend overall survival (OS) and progression-free survival (PFS) for patients. The current targeted drugs for CRC are bevacizumab and cetuximab. Cetuximab is a monoclonal antibody against epidermal growth factor receptor (EGFR) [4]. However, following the results of the OPUS and CRYSTAL trials in 2013, the effective use of cetuximab was limited to patients with KRAS (Kirsten rat sarcoma virus oncogene) wild-type CRC [5]. Patients with KRAS mutations will not benefit from cetuximab, and the efficacy of cetuximab may even be decreased [6]. As such, the clinical application of cetuximab in CRC treatment is limited. Bevacizumab is a humanized monoclonal antibody of vascular endothelial growth factor (VEGF), which exerts an anti-tumor effect by blocking the generation of tumor blood vessels and regulating the immune function of patients [7]. In 2004, the FDA approved the use of bevacizumab in combination with chemotherapeutic drugs as the first-line treatment of mCRC. Numerous surveys have shown that bevacizumab can remarkably prolong the overall response rate (ORR), PFS, and OS in patients with advanced CRC, and it has positive effects in the maintenance treatment of CRC after progression [8]. However, a previous study on bevacizumab mostly focused on the survival rate and less on its efficacy in treating mCRC metastases.
In the present study, we retrospectively investigated the effect of bevacizumab combined with chemotherapy on the metastasis response rate, survival time of patients with mCRC, and incidence of complications, and aiming to assess the efficacy and safety of bevacizumab in treating mCRC.
Material and methods
General information
The clinical data of 87 patients with mCRC who were admitted to First People’s Hospital of Fuyang Hangzhou from March 2012 to September 2015 were retrospectively analyzed. The inclusion criteria: patients who were diagnosed with mCRC through clinical, laboratory, and imaging examinations, followed by pathological confirmation; patients with mCRC who had an unresectable metastasis; and patients with mCRC who did not receive any molecular-targeted drug therapy. The exclusion criteria: patients with other primary cancers; with serious infection; with severe gastrointestinal bleeding that may be life-threatening; with mental illness, disturbance of consciousness, and/or who were unable to complete follow-up; patients with incomplete clinical data. Among 87 patients, 46 were male patients and 41 were female patients, and they were aged between 45 and 78 years. The average age was 63.56±13.7 years. Forty-two patients were confirmed with adenocarcinoma and 5 were confirmed with undifferentiated carcinoma. With regard to tumor staging, there were 15 stage III patients and 32 stage IV patients. The patients were grouped into two groups according to the treatment plan as follows: 42 patients underwent treatment without bevacizumab (control group, CG) and 45 patients were treated with bevacizumab (observation group, OG). This study has obtained the approval of the Ethics Committee of the First People’s Hospital of Fuyang Hangzhou. All patients provided written informed consent prior to participating in the study.
Treatment method
The CG received a combination chemotherapy regimen: Oxaliplatin [Sanofi (Hangzhou) Pharmaceutical Co., Ltd., Item No.: 17G03] 85 mg/m2, intravenous drop infusion, d1; Irinotecan (Jiangsu Hengrui Medicine Co., Ltd., Item No.: 160912) 180 mg/m2, intravenous drop infusion, d1; 5-FU (Shanghai Xudong Haipu Pharmaceutical Co., Ltd., Art. No.: 170130) 600 mg/m2, intravenous injection, continuous pumping for 22 h, d1, d2; Calcium folinate (H. Faulding & Co., Ltd. Trading as David Bull Lab., Item No.: 171210) 200 mg/m2, 2 weeks as a course of treatment. In the OG, bevacizumab (Shanghai TheraMabs Bio-technology Co., Ltd., Item No.: TM-BAVA-00002-1) was added at the same time as the combination chemotherapy, and it was administered 2 weeks after chemotherapy by means of a 15 mg/kg intravenous drip, once every 2 weeks, and 4 weeks for 1 cycle. CT or MRI examinations were performed every 2 cycles to assess efficacy.
Patient follow-up occurred via a specialist outpatient review or through telephone follow-up inquiries. The follow-up period was from the date of treatment to the last follow-up day or disease progression, and all patients were followed up for 6-39 months, with median follow-up period of 22 months.
Observation indicators
(1) Baseline characteristics: age, sex, duration of disease, location of primary tumor, pathological type, TNM grade, number of metastatic foci, location of metastatic foci, body mass index [BMI = body weight (kg)/height (m2)], chemotherapy programs, and other relevant information were compared in both groups.
(2) Quality of life (QOL): the QOL of patients pre- and post-6 cycles of treatment was evaluated using the World Health Organization Quality of Life Summary. The higher the field score, the better the QOL.
(3) Resectability of metastases: the resectability of metastases was compared in both groups pre- and post-treatment.
(4) Evaluation of curative effect: after 6 months of treatment, curative effect was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) efficacy test, including complete remission (CR), partial remission (PR), disease stabilization (SD), and progression (PD). The patient’s ORR and disease control rate (DCR) were counted. ORR = (CR+PR)/total number of patients, and DCR = (CR+PR+SD)/total number of patients. If the disease progressed or new lesions occurred, the drug was discontinued. If patients could not tolerate adverse reactions, the drug was discontinued.
(5) Safety evaluation: changes in blood routine, liver and kidney function, and tumor markers after each cycle were recorded. The occurrence of adverse events during the treatment was observed and recorded, including the incidence of adverse reactions and the extent of drug-related adverse reactions. Almost all patients (98.2%) had at least one adverse event (any grade) during the first-line treatment. There was at least a 3/4 event in more than half (55.8%) of the patients. The most common grade 3/4 events were hematologic adverse reactions (23.9%) including neutropenia in 18.5% of patients, digestive system adverse reactions in 15.7% of patients, and skin reactions in 15.2% of patients, as well as general conditions/fatigue. Adverse events were evaluated according to CTCAE version 3.0 evaluation criteria and were divided into grades 1 and 2 (light-to-moderate), grades 3 and 4 (severely threatening a patient’s life), and grade 5 (death).
(6) Follow-up: according to the efficacy evaluation criteria, the patient’s PFS was recorded. PFS refers to the time from the beginning of treatment to disease progression or to the last follow-up time. OS refers to the time from the beginning of treatment to death or the time of the last follow-up. The follow-up time referred to the time from the beginning of treatment to the end of follow-up or death.
Statistical analysis
An SPSS 13.0 statistical software package was applied for data analysis. Measured data were expressed as mean ± standard deviation (x̅ ± sd). Two independent sample t-tests were used for the comparison of the mean of the two groups, and paired t-tests were used to compare the mean of the two groups pre- and post-intervention. The count data was represented as “n”, and the rate comparison was performed using the χ2 test. Rank data are compared using rank sum tests. The skewed distribution is represented with a median and a 95% confidence interval. The Kaplan-Meier method was adopted for survival analysis. P < 0.05 was considered statistically significant.
Results
Patient baseline characteristics
No significant difference was observed in baseline data such as mean age, sex, ECOG score, primary tumor site, pathological type, TNM stage, metastatic sites, number of metastatic foci, chemotherapy schedule, and chemotherapy cycle between the two groups (P > 0.05) (Table 1).
Table 1.
Baseline characteristics of the two groups of patients
| Index | Control group n=42 | Observation group n=45 | Statistic value | P |
|---|---|---|---|---|
| Age | 63.45±11.52 | 63.67±12.17 | 0.086 | 0.931 |
| Gender: Male Female | 24/18 | 22/23 | ||
| BMI | 19.32±2.71 | 19.18±3.26 | 0.217 | 0.829 |
| ECOG score | ||||
| 0~1 | 33 (78.57) | 37 (82.22) | ||
| 2~4 | 9 (21.43) | 8 (17.78) | 0.427 | 0.670 |
| Primary tumor site | ||||
| colon | 29 (69.05) | 30 (66.67) | ||
| rectum | 13 (30.95) | 15 (33.33) | 0.056 | 0.812 |
| Pathological type | ||||
| Adenocarcinoma | 38 (90.48) | 40 (88.89) | ||
| Mucinous adenocarcinoma | 3 (7.14) | 3 (6.67) | ||
| Villous adenoma canceration | 1 (2.38) | 2 (4.44) | 0.282 | 0.869 |
| TNM stage | ||||
| III | 11 (26.19) | 10 (22.22) | ||
| IV | 31 (73.81) | 35 (77.78) | 0.430 | 0.667 |
| Number of metastases | ||||
| 1 | 22 (52.38) | 21 (46.67) | ||
| 2 | 14 (33.33) | 16 (35.56) | ||
| 3 | 5 (11.90) | 6 (13.38) | ||
| 4 | 1 (2.38) | 2 (4.44) | 0.594 | 0.552 |
| Metastasis site | ||||
| Localized liver metastasis | 17 (40.48) | 15 (33.33) | ||
| Non-restricted liver metastasis | 15 (35.71) | 19 (42.22) | ||
| Non-liver metastasis | 10 (23.81) | 11 (24.44) | 0.540 | 0.763 |
| Chemotherapy | ||||
| Irinotecan Based | 23 (54.76) | 25 (55.56) | ||
| Oxaliplatin-based | 14 (33.33) | 17 (37.78) | ||
| Irinotecan + oxaliplatin based | 3 (7.14) | 2 (4.44) | ||
| fluoropyrimidine alone | 2 (4.76) | 1 (2.22) | 0.804 | 0.848 |
| Chemotherapy cycle | 5.26±2.75 | 5.71±2.93 | 0.737 | 0.463 |
Quality of life (QOL)
The two groups showed no significant difference in the QOL pre-treatment (P > 0.05). The improvement in QOL in the OG post-treatment was remarkably better than that of the CG, with a significant difference (P < 0.05) (Table 2).
Table 2.
Comparison of the quality of life between the two groups before and after treatment (x̅ ± sd)
| Group | Life quality | Health status | Physiological field | Psychological field | Social relations field | Environmental field | |
|---|---|---|---|---|---|---|---|
| Control group n=42 | Before treatment | 11.13±3.16 | 10.15±3.29 | 10.46±2.71 | 10.06±2.53 | 12.61±2.76 | 12.59±1.93 |
| After treatment | 12.65±3.04* | 11.97±2.11* | 12.15±2.39* | 11.73±2.32* | 13.25±2.12 | 13.16±1.74 | |
| Difference | 1.52±2.13 | 1.82±1.76 | 1.69±2.15 | 1.67±1.93 | 0.64±1.12 | 0.57±0.36 | |
| Observation group n=45 | Before treatment | 11.16±3.21 | 10.13±3.35 | 10.45±2.31 | 10.05±2.41 | 12.58±2.53 | 12.48±1.85 |
| After treatment | 14.67±3.19* | 13.39±3.28* | 14.26±2.17* | 13.93±2.75* | 15.37±2.61* | 14.57±1.76* | |
| Difference | 3.51±2.08# | 3.26±2.03# | 3.81±2.11# | 3.88±1.76# | 2.79±1.38# | 2.09±1.13# |
Note: Compared with before treatment;
P < 0.05.
Compared with the control group;
P < 0.05.
Metastasis resectability
The interval between the start of treatment and the first surgical intervention was 5.6 (range, 3.6-7.2) months in the CG and 5.1 (range, 3.4-7.8) months in the OG. In the CG and the OG, 12 and 15 patients, respectively, were treated for primary tumor and metastasis. Resection of metastases includes either surgery with/without radiofrequency ablation or radiofrequency ablation alone. Portal vein embolization was performed in 5 patients with liver metastases, and 3 patients underwent a 2-stage hepatectomy. Post-treatment, the resectability rate of metastasis in the CG was higher than the pre-treatment rate, without statistically significant difference (P > 0.05) (Table 3).
Table 3.
Comparison of resectability rates of metastases in the two groups (%)
| Groups | n | Resectability before treatment | Resection rate after treatment | χ2 | P | ||
|---|---|---|---|---|---|---|---|
| Control group | 42 | Localized liver metastasis | 17 | 3 (17.65) | 5 (29.41) | 0.654 | 0.419 |
| Non-restricted liver metastasis | 15 | 1 (6.67) | 3 (20.00) | 1.154 | 0.283 | ||
| Non-liver metastasis | 10 | 1 (10.00) | 2 (20.00) | 0.392 | 0.531 | ||
| Total | 5 (11.90) | 10 (23.81) | 2.029 | 0.154 | |||
| Observation group | 45 | Localized liver metastasis | 17 | 2 (11.76) | 10 (58.82) | 8.242 | 0.004 |
| Non-restricted liver metastasis | 15 | 1 (6.67) | 5 (33.33) | 3.333 | 0.068 | ||
| Non-liver metastasis | 10 | 1 (10.00) | 3 (30.00) | 1.250 | 0.264 | ||
| total | 4 (8.89) | 18 (40.00) | 11.791 | 0.001 |
Short-term efficacy evaluation
The average chemotherapy cycle in the CG and OG was 5.26±2.75 and 5.71±2.93, respectively. After 6 months of treatment, the ORR and DCR of the CG were 28.57% and 59.52%, respectively. The ORR and DCR of the OG were 48.89% and 86.67%, respectively. The ORR and DCR of the OG were notably higher than those of the CG, showing statistical significance (P < 0.05) (Table 4).
Table 4.
Comparison of the curative effect between the two groups [n (%)]
| Groups | n | CR | PR | SD | PD | ORR | DCR |
|---|---|---|---|---|---|---|---|
| Control group | 42 | 2 (4.76) | 9 (21.43) | 14 (33.33) | 17 (40.48) | 12 (28.57) | 25 (59.52) |
| Observation group | 45 | 5 (11.11) | 17 (37.78) | 19 (42.22) | 6 (13.33) | 22 (48.89) | 39 (86.67) |
| χ2 | 4.754 | 8.230 | |||||
| P | 0.029 | 0.004 |
Follow-up
The CG was followed up for 8-27 months. The median survival time was 14.0 (ranged 12.6-15.4) months, and the median PFS was 6.0 (ranged 4.9-7.2) months. The OG was followed up for 11-35 months. The median survival time was 23.0 (ranged 19.7-26.3) months, and the median PFS was 11.0 (ranged 9.4-12.6) months. The median survival time and the median PFS in the OG were significantly longer than those in the CG and the difference was statistically significant (Log-rank method, χ2=35.645, P=0.000, χ2=18.437, P=0.000) (Figure 1).
Figure 1.
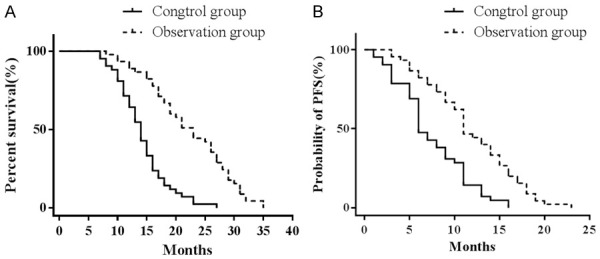
A. Overall survival curves of the two groups. B. Progression-free survival curves of the two groups.
Safety evaluation
A total of 32 adverse reactions occurred in the CG with an adverse reaction rate of 76.19%. There were 39 adverse reactions in the OG with an adverse reaction rate of 86.67%. The two groups showed no significant difference in the incidence of adverse reactions (χ2=1.589, P=0.208). Adverse reactions mainly included myelosuppression, gastrointestinal reactions, hemorrhage, liver damage, and hypertension. No serious adverse reactions such as gastrointestinal perforation occurred (Table 5).
Table 5.
Comparison of the incidence of adverse reactions in the two groups [n (%)]
| Adverse reactions | Control group (n=42) | Observation group (n=45) | χ2 | P | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 1+2 level | 3+4 level | total | 1+2 level | 3+4 level | total | |||
| Hematological toxicity | ||||||||
| Hematological toxicity | 13 (30.95) | 3 (7.14) | 16 (38.09) | 19 (42.22) | 2 (4.44) | 21 (46.67) | 0.653 | 0.419 |
| Thrombocytopenia | 3 (7.14) | 1 (23.8) | 4 (9.52) | 4 (8.89) | 0 | 4 (8.89) | 0.010 | 0.918 |
| anemia | 10 (23.81) | 5 (11.90) | 15 (35.71) | 13 (28.89) | 4 (8.89) | 17 (37.78) | 0.040 | 0.842 |
| Non-hematological toxicity | ||||||||
| Fatigue | 7 (16.67) | 0 | 7 (16.67) | 6 (13.33) | 0 | 6 (13.33) | 0.190 | 0.663 |
| Proteinuria | 6 (14.29) | 1 (2.38) | 7 (16.67) | 5 (11.11) | 0 | 5 (11.11) | 0.564 | 0.453 |
| hematuria | 5 (11.90) | 1 (2.38) | 6 (14.29) | 8 (17.78) | 1 (2.22) | 9 (20.00) | 0.497 | 0.481 |
| Nosebleed | 2 (4.76) | 1 (2.38) | 3 (7.14) | 3 (6.67) | 0 | 3 (6.67) | 0.008 | 0.930 |
| Hematemesis | 1 (2.38) | 0 | 1 (2.38) | 0 | 2 (4.44) | 2 (4.44) | 0.278 | 1.000 |
| hypertension | 2 (4.76) | 0 | 2 (4.76) | 6 (13.33) | 3 (6.67) | 9 (20.00) | 0.497 | 0.481 |
| Nausea and vomiting | 23 (54.76) | 3 (7.14) | 26 (61.90) | 21 (46.67) | 4 (8.89) | 25 (55.56) | 0.361 | 0.548 |
| Transaminase elevation | 12 (28.57) | 1 (2.38) | 13 (30.95) | 10 (22.22) | 0 | 10 (22.22) | 0.851 | 0.356 |
| Venous thrombosis | 0 | 0 | 0 | 0 | 1 (2.22) | 1 (2.22) | 0.001 | 1.000 |
Discussion
Approximately 50% of patients have metastasized CRC at the time of diagnosis and have lost their chance to undergo surgery. Therefore, the main treatment of mCRC is still based on chemotherapy. By the late 1990s, owing to the emergence of irinotecan and oxaliplatin, the effective treatment rate for mCRC increased by approximately 20% [9,10]. In recent years, with developments in molecular biology, targeted therapies have played an important role in the treatment of malignant tumors. Current targeted drugs for CRC include angiogenic factor inhibitors and EGFR inhibitors, such as bevacizumab [11], cetuximab [12], and panitumumab [12]. Numerous studies have demonstrated that cetuximab plus chemotherapy can significantly improve the clinical efficacy of mCRC and prolong PFS and OS. However, only patients with wild-type KRAS tumors have demonstrated clinical benefits [5], whereas patients with KRAS mutations have not, and among them the efficacy may decrease [13]. As such, the application of rituximab in CRC is limited. Panitumumab and cetuximab are both EGFR inhibitors. Some studies have reported that panitumumab combined with first-line chemotherapy for KRAS-mutant patients is less effective than chemotherapy alone [14].
In the process of tumor metastasis, the formation of new blood vessels is a crucial mechanism [15], and the purpose of tumor reduction can be achieved by inhibiting the formation of tumor neovascularization. VEGF plays an important part in angiogenesis, and blocking VEGF is a key anti-tumor angiogenesis target therapy [16]. Bevacizumab is the first human-based monoclonal antibody acting on VEGF, which acts on VEGF-A to block the signal transduction of VEGF, thereby inhibiting the growth and metastasis of tumor cells [17]. The FDA approved bevacizumab for first-line treatment of mCRC in 2004. It has been reported that bevacizumab combined with oxaliplatin or irinotecan-based chemotherapy can improve the efficacy of treatment in advanced CRC and prolong survival [18], and its efficacy is not affected by the KRAS genotype [19]. In the KRAS wild-type patient population, the median survival and PFS with bevacizumab as the first-line treatment were significantly better than those with bevacizumab as the second-line treatment [20]. Therefore, patients who do not have the KRAS gene mutation or KRAS gene mutation can undergo bevacizumab as a first-treatment option [21].
The results of this study revealed that the ORR and DCR were 48.89% and 86.67% in the OG post-treatment with bevacizumab and chemotherapy, which was significantly higher than pretreatment ORR and DCR of 28.57% and 59.52%, respectively, indicating that combination of chemotherapy and bevacizumab can significantly improve the efficacy of mCRC. Liu et al. [22] pointed out that the ORR of bevacizumab group was 29.4%, the DCR was 64.7%, and the effective rate of first-line treatment was higher than second-line treatment, which were similar to the results of this study. This may be related to the synergistic effect of bevacizumab and chemotherapy drugs. After treatment, the improvement scores in various areas of QOL in the OG were significantly better than those in the CG (P < 0.05). During follow-up, the median survival time of the OG was 23.0 (range, 19.7-26.3) months, and the median PFS was 11.0 (ranged 9.4-12.6) months, which were significantly better than the 14.0 (ranged 12.6-15.4) months and 6.0 (ranged 4.9-7.2) months in the CG. It appears that bevacizumab combined with chemotherapy in the first-line treatment of mCRC can significantly improve the QOL of patients and prolong survival time.
The EREBUS cohort study indicated that a combination of cetuximab plus chemotherapy for the treatment of KRAS wild-type mCRC can significantly increase the resection rate of unresectable metastases, and the resection rate is related to the complete remission rate of patients, especially for patients with localized hepatic metastases. Therefore, observing the resectability of metastases can reflect the survival outcome to some extent [23,24]. In this study, the resectability of the two mCRC groups pre- and post-treatment was observed. The results suggested that the resectability rate of metastases in the OG increased from 8.89% pre-treatment to 40.00% post-treatment, while the resectability rate of metastasis in the CG increased from 11.90% pre-treatment to 23.81% post-treatment. This shows that bevacizumab combined with chemotherapy can significantly improve the resection of metastatic lesions, thereby further improving the survival outcome of patients, and its mechanism may be related to bevacizumab inhibiting the formation of tumor blood vessels, thereby achieving tumor reduction. In the metastatic sites, the resectability rate of localized liver metastases had improved markedly. Localized liver metastases are associated with higher PFS.
In the safety evaluation, the incidence of adverse reactions of the OG was 86.67%, which was not significant different from that of the CG (76.19%, χ2=1.589, P=0.208). The main adverse reactions were myelosuppression, digestive tract reaction, hemorrhage, liver damage, and hypertension. No serious adverse reactions occurred including gastrointestinal perforation. This suggested that bevacizumab was safe, and its combination with chemotherapy did not increase the adverse reactions of patients.
However, there are still some shortcomings in this study. First, the small number of cases may lead to certain bias in the data. In the next study, the number of cases will be expanded to further confirm the accuracy of the results. In addition, there were two types of adenocarcinoma and undifferentiated carcinoma in the selected cases, and the influence of two different pathological types on the efficacy and drug resistance of bevacizumab has not been thoroughly discussed. In the next step, the influence of pathological types on drug efficacy will be further explored.
In summary, bevacizumab combined with chemotherapy can significantly prolong the survival and PFS of patients with mCRC, and improve QOL, resectability rate of metastatic lesions, and survival outcomes.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 3.Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, Saba NF, Weiss J, Wirth L, Sukari A. Pembrolizumab for platinum-and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J. Clin. Oncol. 2017;35:1542–1549. doi: 10.1200/JCO.2016.70.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houts AC, Ogale S, Sommer N, Satram-Hoang S, Walker MS. Treatment patterns and outcomes in patients with KRAS wild-type metastatic colorectal cancer treated in first line with bevacizumab- or cetuximab-containing regimens. J Gastrointest Cancer. 2017;50:69–77. doi: 10.1007/s12029-017-0027-6. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 6.Manzoni M, Rovati B, Ronzoni M, Loupakis F, Mariucci S, Ricci V, Gattoni E, Salvatore L, Tinelli C, Villa E, Danova M. Immunological effects of bevacizumab-based treatment in metastatic colorectal cancer. Oncology. 2010;79:187–196. doi: 10.1159/000320609. [DOI] [PubMed] [Google Scholar]
- 7.Goey KKH, Elias SG, van Tinteren H, Laclé MM, Willems SM, Offerhaus GJA, de Leng WWJ, Strengman E, Ten Tije AJ, Creemers GM, van der Velden A, de Jongh FE, Erdkamp FLG, Tanis BC, Punt CJA, Koopman M. Maintenance treatment with capecitabine and bevacizumab versus observation in metastatic colorectal cancer: updated results and molecular subgroup analyses of the phase 3 CAIRO3 study. Ann Oncol. 2017;28:2128–2134. doi: 10.1093/annonc/mdx322. [DOI] [PubMed] [Google Scholar]
- 8.Jeng LB, Kumar Velmurugan B, Chen MC, Hsu HH, Ho TJ, Day CH, Lin YM, Padma VV, Tu CC, Huang CY. Fisetin mediated apoptotic cell death in parental and oxaliplatin/irinotecan resistant colorectal cancer cells in vitro and in vivo. J Cell Physiol. 2018;233:7134–7142. doi: 10.1002/jcp.26532. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, Wang T, Yang S, Xiao Y, Song Y, Zhang N, Garg S. Development and evaluation of oxaliplatin and irinotecan co-loaded liposomes for enhanced colorectal cancer therapy. J Control Release. 2016;238:10–21. doi: 10.1016/j.jconrel.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Passardi A, Nanni O, Tassinari D, Turci D, Cavanna L, Fontana A, Ruscelli S, Mucciarini C, Lorusso V, Ragazzini A, Frassineti GL, Amadori D. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: final results for first-line treatment from the ITACa randomized clinical trial. Ann Oncol. 2015;26:1201–1207. doi: 10.1093/annonc/mdv130. [DOI] [PubMed] [Google Scholar]
- 11.Hong DS, Morris VK, El Osta B, Sorokin AV, Janku F, Fu S, Overman MJ, Piha-Paul S, Subbiah V, Kee B, Tsimberidou AM, Fogelman D, Bellido J, Shureiqi I, Huang H, Atkins J, Tarcic G, Sommer N, Lanman R, Meric-Bernstam F, Kopetz S. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov. 2016;6:1352–1365. doi: 10.1158/2159-8290.CD-16-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baba Y, Tamura T, Satoh Y, Gotou M, Sawada H, Ebara S, Shibuya K, Soeda J, Nakamura K. Panitumumab interaction with TAS-102 leads to combinational anticancer effects via blocking of EGFR-mediated tumor response to trifluridine. Mol Oncol. 2017;11:1065–1077. doi: 10.1002/1878-0261.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, Blasinska-Morawiec M, Smakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J. Clin. Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Q, Zhu Y, Wei X, Zhou J, Chang L, Sui H, Han Y, Piao D, Sha R, Bai Y. MiR-590-5p inhibits colorectal cancer angiogenesis and metastasis by regulating nuclear factor 90/vascular endothelial growth factor A axis. Cell Death Dis. 2016;7:e2413. doi: 10.1038/cddis.2016.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akagi K, Ikeda Y, Miyazaki M, Abe T, Kinoshita J, Maehara Y, Sugimachi K. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer. 2000;83:887–891. doi: 10.1054/bjoc.2000.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendell JC, Bekaii-Saab TS, Cohn AL, Hurwitz HI, Kozloff M, Tezcan H, Roach N, Mun Y, Fish S, Flick ED, Dalal D, Grothey A. Treatment patterns and clinical outcomes in patients with metastatic colorectal cancer initially treated with FOLFOX-bevacizumab or FOLFIRI-bevacizumab: results from ARIES, a bevacizumab observational cohort study. Oncologist. 2012;17:1486–1495. doi: 10.1634/theoncologist.2012-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinemann V, Hoff PM. Bevacizumab plus irinotecan-based regimens in the treatment of metastatic colorectal cancer. Oncology. 2010;79:118–128. doi: 10.1159/000314993. [DOI] [PubMed] [Google Scholar]
- 20.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmuller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Muller S, Link H, Niederle N, Rost A, Hoffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 21.Loupakis F, Cremolini C, Salvatore L, Masi G, Sensi E, Schirripa M, Michelucci A, Pfanner E, Brunetti I, Lupi C, Antoniotti C, Bergamo F, Lonardi S, Zagonel V, Simi P, Fontanini G, Falcone A. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur J Cancer. 2014;50:57–63. doi: 10.1016/j.ejca.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Liu XW, Cheng LW, Chai SM, Li Z. Comparison of clinical efficacy of cetuximab and bevacizumab in the treatment of patients with KRAS wild-type metastatic colorectal cancer. Chin J Gerontol. 2014:6003–6005. [Google Scholar]
- 23.Petrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Barni S. Cetuximab and panitumumab in KRAS wild-type colorectal cancer: a meta-analysis. Int J Colorectal Dis. 2011;26:823–833. doi: 10.1007/s00384-011-1149-0. [DOI] [PubMed] [Google Scholar]
- 24.Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet JB, Lecomte T, Rougier P, Lievre A, Landi B, Boige V, Ducreux M, Ychou M, Bibeau F, Bouche O, Reid J, Stone S, Penault-Llorca F. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J. Clin. Oncol. 2009;27:5924–5930. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]


